Introduction
Cancer stem cells (CSCs) have been found in various
tumors including breast, gastric and colon, as well as lung cancer
(1). They are the driving force of
tumor progression, recurrence and drug resistance (2,3). CSCs
and non-CSCs are two distinctive populations with different
properties (4,5). CSCs constitute only a small fraction
of tumor cells, however, establishing a method to effectively and
economically enrich CSCs may be useful to gain a better
understanding of CSCs.
There is controversy over the markers of lung CSCs.
Eramo et al reported that the tumorigenic cells in human
lung cancer were a rare undifferentiated cell population expressing
CD133 (6). Meng et al
reported that both CD133+ and CD133−
subpopulations of A549 and H446 cells contained CSCs (7). Cui et al found that CD133 may
be used as a marker for CSCs in H446 cells but not in A549 cells
(8). Additionally, based on a
higher expression of ATP-binding cassette (ABC) transporters and
higher activity of aldehyde dehydrogenase (ALDH) in CSCs, side
population (SP) and ALDHhigh population are also
enriched with lung CSCs (9).
However, none of the methods mentioned above is used exclusively to
isolate lung CSCs, emphasizing the need to define more specific
markers (10).
CSCs can be enriched in the spheres formed after
culturing in the serum-free medium supplemented with mitogens such
as epidermal growth factor (EGF) and basic fibroblast growth factor
(bFGF) (11). However, this process
is usually time-consuming and inefficient. Recently, a new method
known as 'The Modified Non-Adhesive Culture System', which improved
these drawbacks, was used to isolate CSCs from the human oral
squamous cell carcinoma cell lines (SAS and OECM-1) and human
cervical carcinoma cell line (HeLa) (12,13).
However, whether this isolation method can be applied to lung
cancer remains to be determined. In the present study, the
nonadhesive culture system was used to generate tumor spheres from
the A549 cell line. The CSCs characteristics of isolated sphere
cells were verified in vitro and in vivo. A reliable
model of enriching CSCs from A549 in vitro was established
that may be used in the selection of new drugs targeting lung
CSCs.
Materials and methods
Reagents
The A549 human non-small cell lung cancer (NSCLC)
cell line was obtained from the Shanghai Cell Biology Institute of
the Chinese Academy of Sciences (Shanghai, China). The antibodies
against CD31 (sc-1506), Oct4 (sc-5279) and Sox2 (sc-17320) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The FITC-labeled rabbit anti-mouse IgG antibody (ab97045) and the
Cy5-labeled donkey anti-goat IgG antibody (ab6566) were purchased
from Abcam (Cambridge, MA, USA).
Cell culture
A549 cells were maintained in RPMI-1640 medium
(Gibco-Life Technologies, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin
(100 µg/ml) in a humidified atmosphere of 5% CO2
at 37°C.
Tumor sphere formation and culture
Tumor spheres were formed in the modified
non-adhesive culture system reported previously by Chen et
al (13). Briefly, parental
A549 adherent cells were dissociated with trypsin into single-cell
suspension and seeded in a 10-cm cell culture dish coated with a
thin film of 1.2% agarose (dissolved in deionized water) at the
density of 8×104 cells/dish. Until tumor spheres were
formed, the culture medium was changed every other day during the
incubation period.
Limiting dilution analysis (LDA) and MTT
assay
The 96-well cell culture plates were made
non-adhesive for cells by coating with agarose. Parental adherent
cells and tumor spheres were trypsinized and seeded at serial
concentrations. Cultures were maintained for a week and scored for
wells that had no spheres. The results were processed using the
web-based extreme LDA (ELDA) software (http://bioinf.wehi.edu.au/software/elda/). The
experiments were repeated three times independently. MTT assay was
performed as reported in a previous study (12).
RT-PCR
Total RNA (1 µg) was used as a template for
the synthesis of cDNA using a RevertAid First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Beijing, China) following the
manufacturer's instructions. From the 5′ to the 3′ end, the
sequence of primers used in the reactions was as follows: Oct4
forward, GAAAGCGAACCAGTATCGAGAAC and reverse, CCCCTGAGAAAGGAGACCCA;
Sox2 forward, GGTTACCTCTTCCTCCCACTCC and reverse,
CCCTCCCATTTCCCTCGTTT; CD133 forward, GCATTGGCATCTTCTATGGTT and
reverse, CGCCTTGTCCTTGGTAGTGT; and GAPDH forward,
CGGATTTGGTCGTATTGGG and reverse, CTGGAAGATGGTGATGGGATT.
Cell invasion assay
The cell invasion assay was performed using 24
Transwell chambers (Corning Inc., Corning, NY, USA) as previously
described (14). The single cells
were suspended in serum-free RPMI-1640 medium at the concentration
of 3×105 cells/ml. Cell suspension (150 µl) was
loaded into the upper chamber insert coated with Matrigel while the
lower chamber was loaded with 500 µl RPMI-1640 with 10% FBS.
After being cultured in the incubator for 48 h, the cells in the
upper chamber were removed with a cotton swab. The lower chamber
filter was fixed with 4% paraformaldehyde and stained with crystal
violet. The cells that migrated to the lower side of the membrane
were viewed under an inverted microscope.
Immunohistochemistry (IHC) and
immunofluorescence staining (IFS)
Tumors or spheres were fixed in 4% formalin and
embedded in paraffin. IHC was carried out as previously described
(13). For the quantification of
microvessel density (MVD), any endothelial cell cluster
immunoreactive for CD31, clearly separated from adjacent tumor
cells, was considered a countable microvessel. Microvessels were
quantified in three random fields (magnification, ×200) within the
areas of high-density staining. For the IFS staining of spheres,
after blocking in PBS containing 5% bovine serum albumin (BSA),
slides were incubated with primary antibodies overnight at 4°C.
After being washed with PBS, the slides were incubated with the
secondary antibody for 30 min at 37°C. DAPI was then added for
nuclear staining. An Axio Vert A1 inverted fluorescence microscope
was used to capture the images.
Oil red O staining
Fresh frozen tumor tissues were cut (5–10 µm)
and mounted on slides. The slides were air-dried and maintained for
60 min at room temperature and fixed in cold formalin for 15 min.
After being air-dried for another 60 min, the slides were placed in
absolute propylene glycol for 5 min and then stained in 0.5% Oil
red O solution for 10 min at 60°C. The slides were placed in 85%
propylene glycol solution for 2 min and rinsed with distilled
water. The slides were subsequently stained in hematoxylin and
mounted.
In vivo tumorigenicity study
Experiments were performed as per the Guide for the
Care and Use of Laboratory Animals. Mice were kept at 25°C, 50%
humidity, in an air-conditioned environment under a 12-h dark/light
cycle. The cell suspension (100 µl) was injected
subcutaneously into the right flank of 6-week-old BALB/c nude mice
at the dose of 1×103, 1×104 and
1×105 cells. At 8 weeks after inoculation, the mice were
euthanized under anesthesia and the tumors removed and
measured.
Statistical analysis
Statistical results were analyzed using SPSS 13.0
statistical software. The significance of difference between two
samples of the same group were analyzed using the t-test. The
result was considered statistically significant when P<0.05.
P<0.01 was considered to indicate a stati stically highly
significant result.
Results
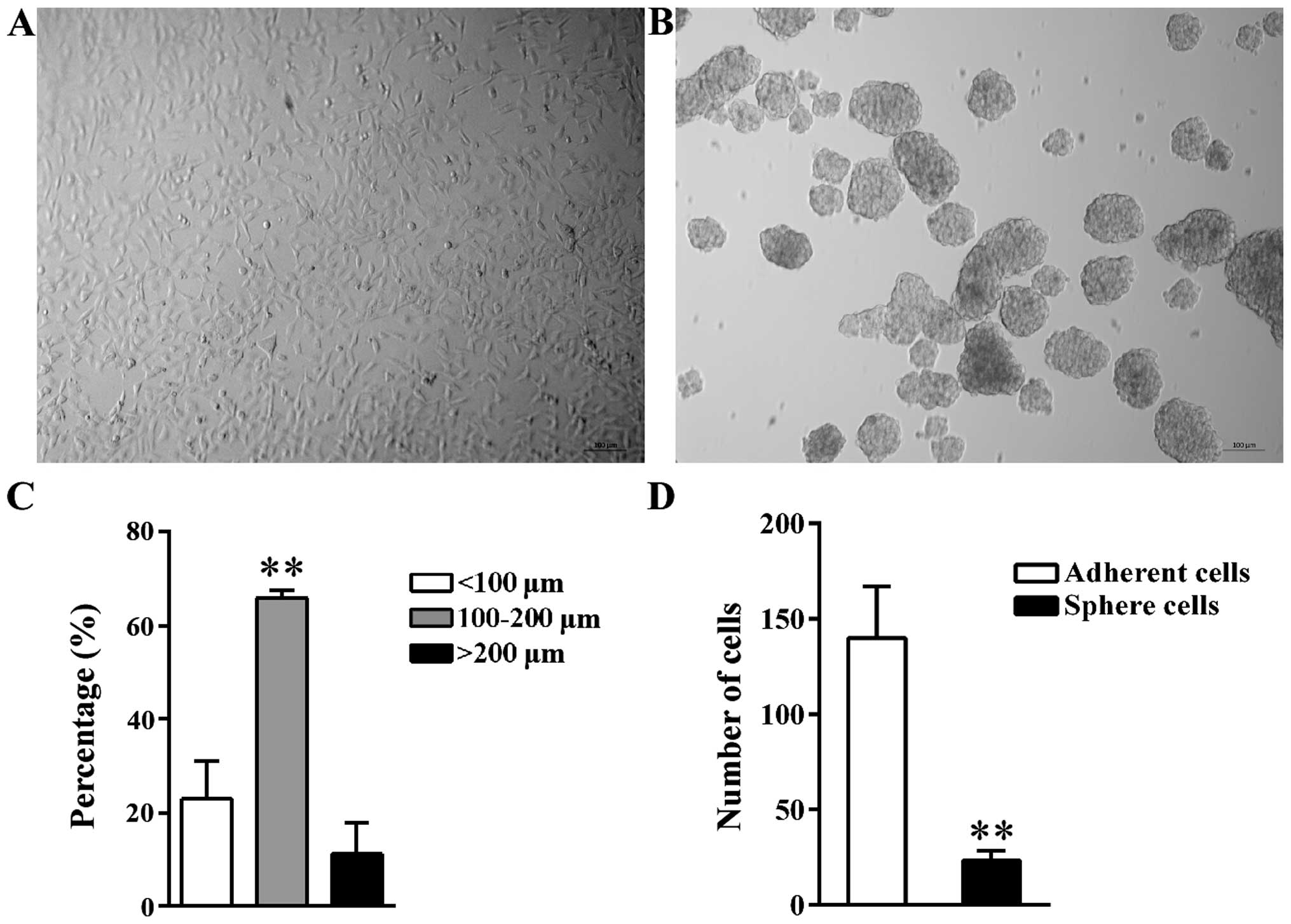
Morphology of tumor spheres
A549 tumor spheres were formed after incubation
under the non-adhesive culture condition. Compared to parental A549
adherent cells (Fig. 1A), the
suspended spheres were cluster of cells that had an oval or round
shape (Fig. 1B). The diameter of
spheres ranged from 30 to 250 µm; the diameter of 65.93%
spheres was between 100 and 200 µm; 22.95% spheres were
<100 µm, while the remaining 11.12% spheres were >200
µm (Fig. 1C). Spheres with a
diameter >200 µm may result from the fusion between two
small individual spheres based on observation.
The self-renewal potential of sphere
cells is higher than that in adherent cells
LDA was used to compare the sphere-forming ability
of parental adherent cells and tumor sphere cells cultured under
the non-adhesive condition. Data analysis with ELDA software showed
that, on average, the frequency of sphere-initiating cells was 1 in
24 for tumor spheres but 1 in 140 for adherent cells (Fig. 1D). In contrast to adherent cells,
the spheres contained a 5.83-fold higher frequency of
sphere-initiating cells contributing to its higher self-renewal
potential.
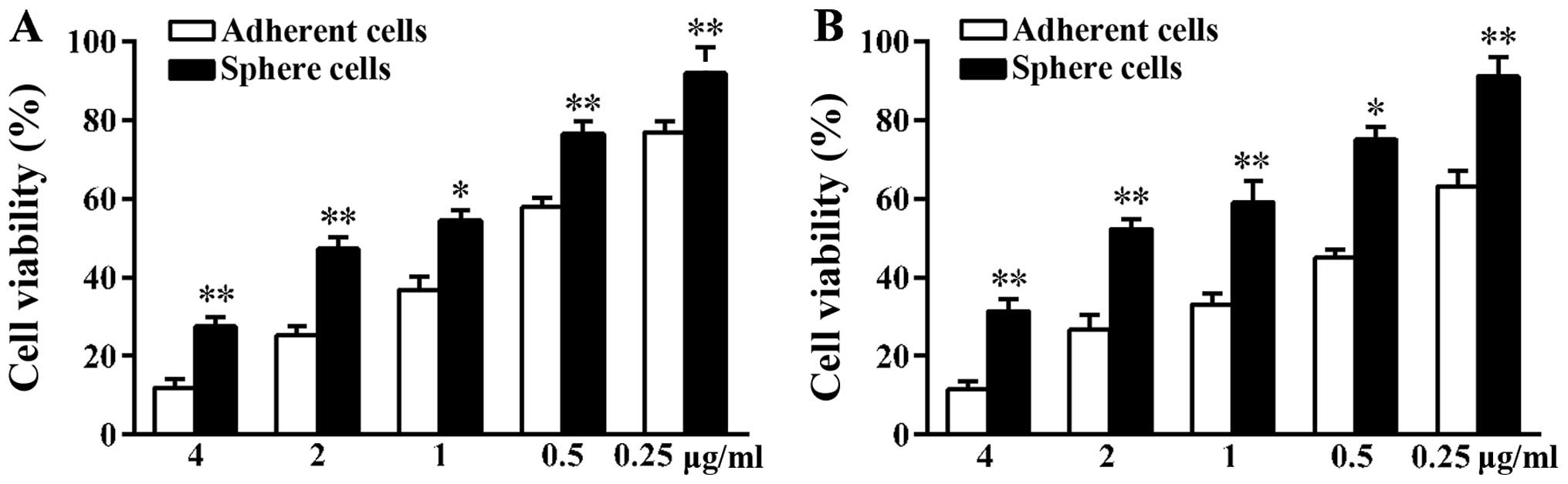
Sphere cells are less sensitive to
chemotherapy drugs than adherent A549 cells
Many tumors develop drug resistance during
treatment. The reason is that CSCs can survive even in the presence
of cytotoxic drugs (15). Since the
activities of ABC transporters on the surface of CSCs are higher
than that of non-CSCs, CSCs can discharge drugs leading to tumor
regenesis (16). The A549 adherent
and sphere cells were treated with the same concentration of
docetaxel and cisplatin for 48 h. At the end of treatment, the MTT
assay was used to validate cell viability. As shown in the results,
in contrast to adherent cells, more sphere cells survived under
high concentration of drugs (Fig.
2). The number of sphere cells that survived was 2.33-fold that
of adherent cells when treated with 4 µg/ml docetaxel. When
treated with 4 µg/ml cisplatin, the number of sphere cells
that survived was 2.73-fold that of adherent cells. Tumor sphere
cells showed higher chemoresistance ability compared to adherent
cells suggesting more CSCs were in the sphere cells.
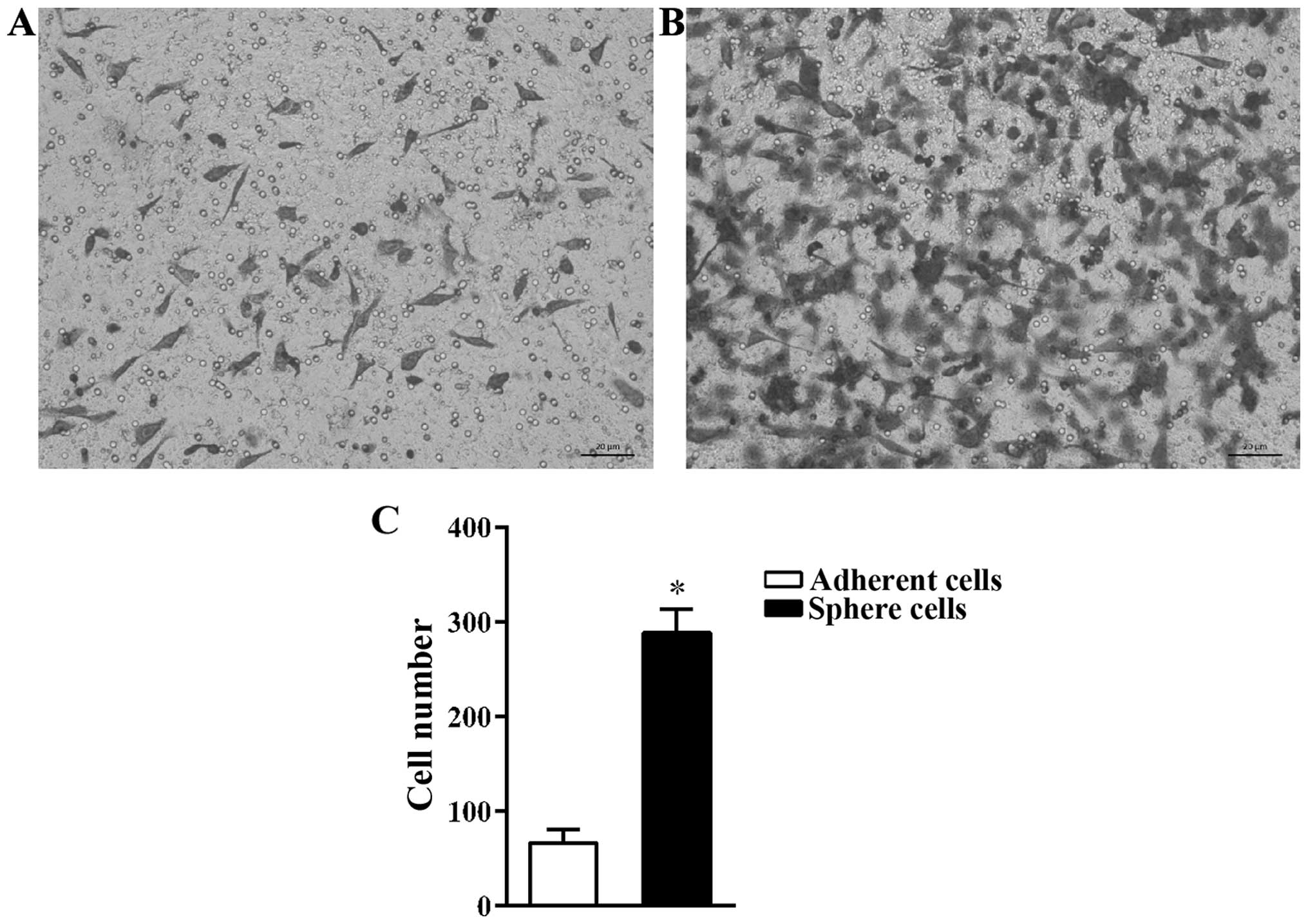
Migration ability of sphere cells is
higher than that of adherent cells
CSCs are the main cause of tumor metastasis
(3,17). The cell invasion assay was used to
compare the migration capacity of A549 adherent and sphere cells.
It was found that, more sphere cells migrated through the Transwell
membrane than adherent cells (Fig. 3A
and B). The number of adherent cells that migrated was
66.67±14.57/field while the number of sphere cells that migrated
was 288.67±25.11/field (Fig.
3C).
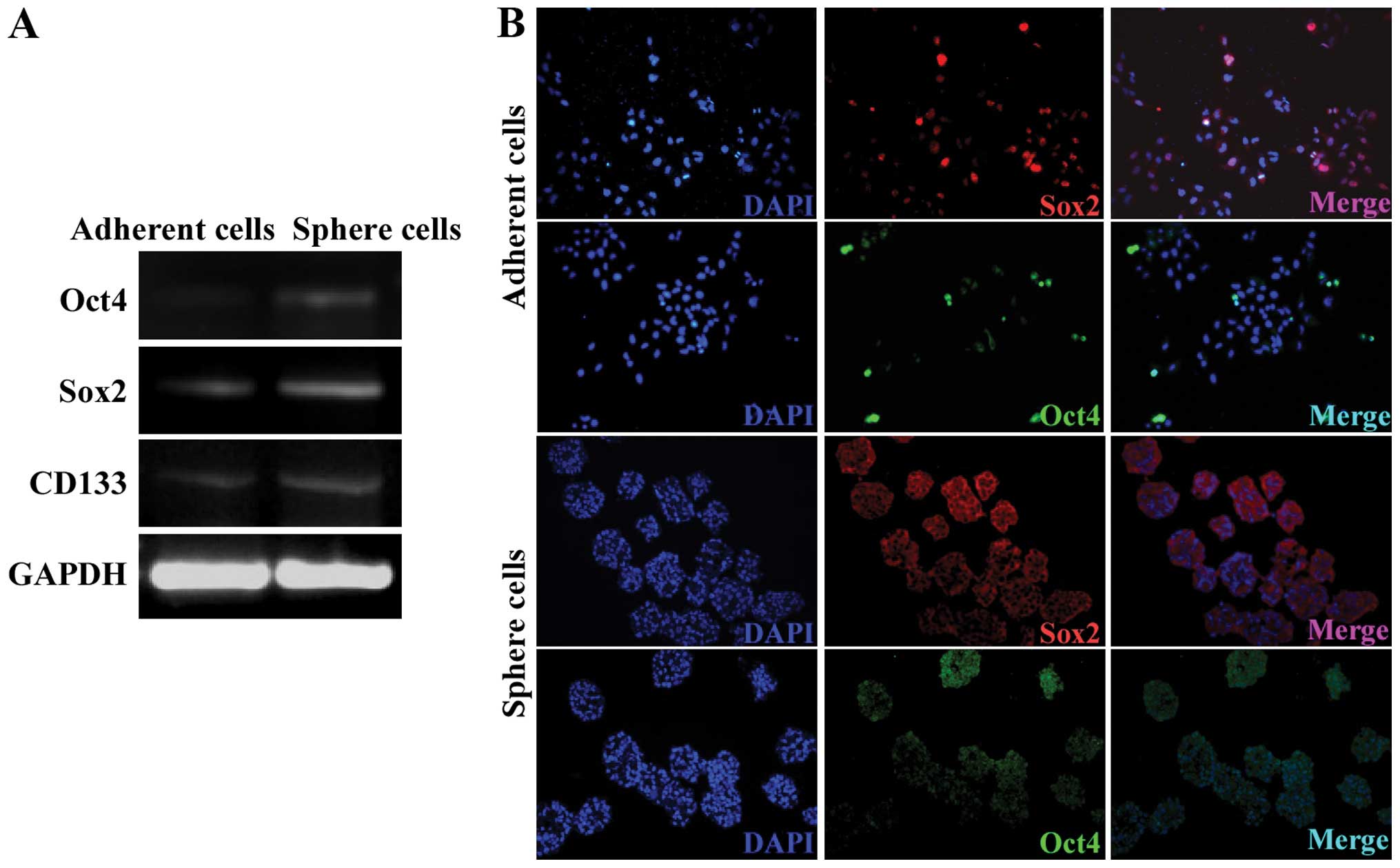
Sphere cells express high levels of Oct4,
Sox2 and CD133
Oct4 and Sox2 are two important transcription
factors that are essential to embryonic stem cells (18,19).
Previous findings have identified that Oct4 and Sox2 are important
in the regene ration of many CSCs (20–22).
RT-PCR and IFS assays were used to validate the expression of Oct4
and Sox2 in the A549 spheres. The mRNA expression of Oct4 and Sox2
was higher in the A549 sphere cells compared to the adherent cells
(Fig. 4A). In spite of the
controversial studies over whether CD133 could be used as a marker
for lung CSCs, the expression of CD133 was upregulated in the A549
sphere cells (Fig. 4A). Consistent
with the RT-PCR assay result, Oct4 and Sox2 protein was also
detected in the sphere cells colocalized with the nuclei (Fig. 4B). The expression level of Oct4 and
Sox2 was not uniform among the sphere cells suggesting that the
sphere cells were at different stages. Sphere cells with a low
expression of Oct4 and Sox2 may result from the differentiation of
CSCs to non-CSCs.
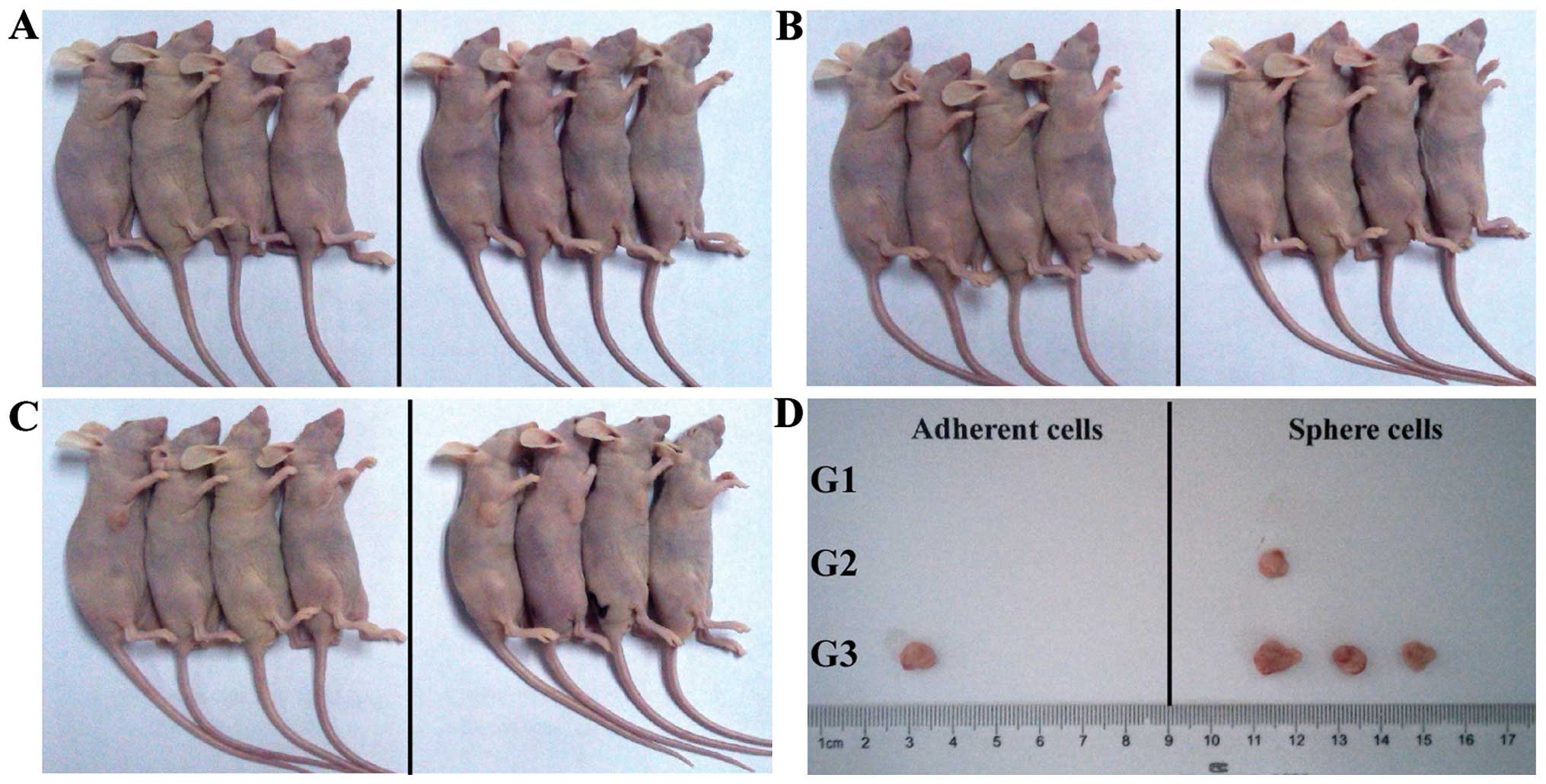
Sphere cells are more tumorigenic in
vivo
The A549 adherent and sphere cells were
subcutaneously injected into nude mice. The A549 sphere cells
induced tumor when only 1×104 cells were injected into
mice (one out of four mice, Fig. 5A and
B). By contrast, 1×105 parental A549 adherent cells
were needed to generate tumor (one out of four mice, Fig. 5C). When 1×105 cells were
injected, the sphere cells had a higher tumor formation rate (three
out of four mice) than the adherent cells (one out of four mice)
suggesting that A549 sphere cells were enriched with
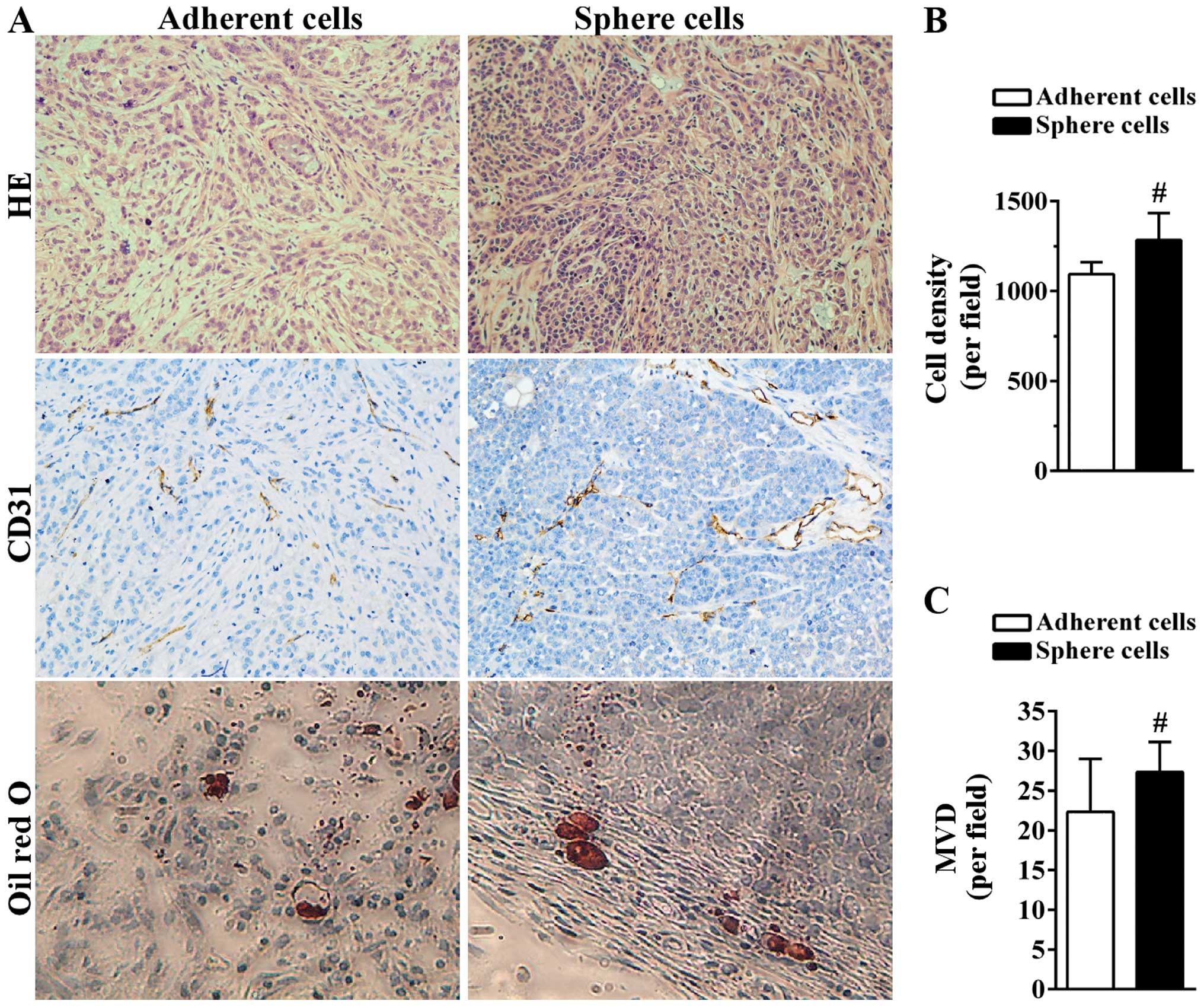
tumor-initiating cells. The histological results showed that
compared to tumors derived from adherent A549 cells, tumor tissue
derived from sphere cells was more compact with less space between
tumor cells (Fig. 6A and B).
Angiogenesis plays a critical role in the growth of tumor by
supplying tumor cells with necessary oxygen and nutrients. Blood
vessels were formed in the two tumor tissues as indicated by the
CD31-positive area (Fig. 6A). The
MVD of tumors derived from adherent cells was 22.33±6.66/field
while the MVD of tumors generated from sphere cells was
27.33±3.79/field (Fig. 6C). The
presence of more CSCs in sphere cells may lead to the formation of
more blood vessels. Certain CSCs still maintain the ability to
differentiate into multiple types of cells (23). Intracellular lipid vesicles were
detected on the edge of the two tumor tissues as shown in the Oil
red O staining, suggesting the in vivo differentiation
ability of A549 sphere cells into adipocytes (Fig. 6A).
Discussion
The CSCs theory sheds light on the origin of tumor,
tumor development, metastasis, relapse and drug resistance.
Therefore, the establishment of a reliable and efficient model of
enriching CSCs is necessary for basic and clinical research.
Compared to other methods, the non-adhesive culture system holds
great advantage as it does not require the specific surface markers
or chemical drugs (24). In this
study, human NSCLC stem-like cells were enriched by the A549 cell
line using the non-adhesive culture system. To the best of our
knowledge, this was the first study involving the enrichment of
A549 CSCs using this method.
The transcription profiles of some high-grade tumors
and stem cells (SCs) are similar (25). The expression of transcription
factors Oct4 and Sox2, which were essential to maintain the
pluripotency of stem cells was upregulated in the sphere cells. The
similarity between SCs and CSCs suggested that important signaling
pathways may be shared. Our results also show that CD133 expression
was upregulated in sphere cells suggesting that CD133+
cells were enriched in A549 sphere cells. There has been
controversy regarding whether CD133 can be used as the lung CSCs
marker, which may result from the interchange between CSCs and
differentiated tumor cells as suggested by the stochastic model
(26). In addition, it has been
found that sorted CD133+ and CD133− cells are
capable of regenerating CD133+ and CD133−
cells (7). Considering the
heterogeneity of lung cancer, CD133 alone may not be sufficient for
the identification of lung CSCs.
The tumorigenic ability of sphere cells was
confirmed in vivo. In contrast to A549 adherent cells, fewer
sphere cells were needed to generate tumor in mice. More blood
vessels were detected in the tumors derived from sphere cells. It
is a complex and bidirectional interaction between CSCs and the
blood vessels around them. In a previous study it was found that
brain tumor CSCs can promote blood vessel formation by secreting
VEGF (27). Since CSCs depend on
the blood vessel to supply oxygen and nutrients, angiogenesis
inhibitors such as bevacizumab have been used in combination with
chemotherapeutic drugs in the treatment of lung cancer (28). The intracellular lipid vesicles
detected in the tumor tissue suggested the differentiation
potential of sphere cells in vivo. The induction of lung
CSCs to adipogenic cells in vitro has been reported
(29). Induction of the
differentiation of CSCs to non-tumorigenic cells that are
vulnerable to chemotherapeutic drugs is a new option in the
treatment of lung cancer (30).
In conclusion, the human NSCLC stem-like cells were
successfully enriched by the A549 cell line using the non-adhesive
culture system. Sphere cells exhibited the characteristics of CSCs
in vitro and in vivo and may be used as a model for
screening substances targeting CSCs for the preclinical research of
human NSCLC. Combined therapy targeting different signaling
pathways of CSCs may achieve a better clinical outcome considering
the characteristics of CSCs.
Acknowledgments
We would like to thank Professor Jialiang Hu for the
valuable suggestions during the writing of the manuscript. This
study was supported by the 863 High-Technology Development Planning
(no. SQ2011SF11B02030), the Project Program of the State Key
Laboratory of Natural Medicines (no. SKLNMBZ201403) and the
National Science and Technology Major Projects of New Drugs (nos.
2012ZX09103301-004 and 2014ZX09508007) in China.
References
|
1
|
Medema JP: Cancer stem cells: The
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li S and Li Q: Cancer stem cells and tumor
metastasis (Review). Int J Oncol. 44:1806–1812. 2014.PubMed/NCBI
|
|
4
|
Tang DG: Understanding cancer stem cell
heterogeneity and plasticity. Cell Res. 22:457–472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larzabal L, El-Nikhely N, Redrado M,
Seeger W, Savai R and Calvo A: Differential effects of drugs
targeting cancer stem cell (CSC) and non-CSC populations on lung
primary tumors and metastasis. PLoS One. 8:e797982013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
7
|
Meng X, Li M, Wang X, Wang Y and Ma D:
Both CD133+ and CD133− subpopulations of A549
and H446 cells contain cancer-initiating cells. Cancer Sci.
100:1040–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui F, Wang J, Chen D and Chen YJ: CD133
is a temporary marker of cancer stem cells in small cell lung
cancer, but not in non-small cell lung cancer. Oncol Rep.
25:701–708. 2011.
|
|
9
|
Akunuru S, James Zhai Q and Zheng Y:
Non-small cell lung cancer stem/progenitor cells are enriched in
multiple distinct phenotypic subpopulations and exhibit plasticity.
Cell Death Dis. 3:e3522012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tirino V, Desiderio V, Paino F, De Rosa A,
Papaccio F, La Noce M, Laino L, De Francesco F and Papaccio G:
Cancer stem cells in solid tumors: An overview and new approaches
for their isolation and characterization. FASEB J. 27:13–24. 2013.
View Article : Google Scholar
|
|
11
|
Roudi R, Madjd Z, Ebrahimi M, Samani FS
and Samadikuchaksaraei A: CD44 and CD24 cannot act as cancer stem
cell markers in human lung adenocarcinoma cell line A549. Cell Mol
Biol Lett. 19:23–36. 2014. View Article : Google Scholar
|
|
12
|
Wang L, Guo H, Lin C, Yang L and Wang X:
Enrichment and characterization of cancer stem like cells from a
cervical cancer cell line. Mol Med Rep. 9:2117–2123.
2014.PubMed/NCBI
|
|
13
|
Chen SF, Chang YC, Nieh S, Liu CL, Yang CY
and Lin YS: Nonadhesive culture system as a model of rapid sphere
formation with cancer stem cell properties. PLoS One. 7:e318642012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng HH, Kuo CC, Yan JL, Chen HL, Lin WC,
Wang KH, Tsai KK, Guvén H, Flaberg E, Szekely L, et al: Control of
cyclooxygenase-2 expression and tumorigenesis by endogenous
5-methoxytryptophan. Proc Natl Acad Sci USA. 109:13231–13236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niero EL, Rocha-Sales B, Lauand C, Cortez
BA, de Souza MM, Rezende-Teixeira P, Urabayashi MS, Martens AA,
Neves JH and Machado-Santelli GM: The multiple facets of drug
resistance: One history, different approaches. J Exp Clin Cancer
Res. 33:372014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di C and Zhao Y: Multiple drug resistance
due to resistance to stem cells and stem cell treatment progress in
cancer (Review). Exp Ther Med. 9:289–293. 2015.PubMed/NCBI
|
|
17
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tay Y, Zhang J, Thomson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, Oct4 and Sox2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakatsugawa M, Takahashi A, Hirohashi Y,
Torigoe T, Inoda S, Murase M, Asanuma H, Tamura Y, Morita R,
Michifuri Y, et al: SOX2 is overexpressed in stem-like cells of
human lung adenocarcinoma and augments the tumorigenicity. Lab
Invest. 91:1796–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beltran AS, Rivenbark AG, Richardson BT,
Yuan X, Quian H, Hunt JP, Zimmerman E, Graves LM and Blancafort P:
Generation of tumor-initiating cells by exogenous delivery of OCT4
transcription factor. Breast Cancer Res. 13:R942011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling GQ, Chen DB, Wang BQ and Zhang LS:
Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in
human breast cancer cell lines. Oncol Lett. 4:1264–1268.
2012.PubMed/NCBI
|
|
23
|
Liu H, Zhang W, Jia Y, Yu Q, Grau GE, Peng
L, Ran Y, Yang Z, Deng H and Lou J: Single-cell clones of liver
cancer stem cells have the potential of differentiating into
different types of tumor cells. Cell Death Dis. 4:e8572013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tirino V, Desiderio V, Paino F, Papaccio G
and De Rosa M: Methods for cancer stem cell detection and
isolation. Methods Mol Biol. 879:513–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y and Laterra J: Cancer stem cells:
Distinct entities or dynamically regulated phenotypes? Cancer Res.
72:576–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borovski T, De Sousa E Melo F, Vermeulen L
and Medema JP: Cancer stem cell niche: The place to be. Cancer Res.
71:634–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Twelves C, Chmielowska E, Havel L, Popat
S, Swieboda-Sadlej A, Sawrycki P, Bycott P, Ingrosso A, Kim S,
Williams JA, et al: Randomised phase II study of axitinib or
bevacizumab combined with paclitaxel/carboplatin as first-line
therapy for patients with advanced non-small-cell lung cancer. Ann
Oncol. 25:132–138. 2014. View Article : Google Scholar
|
|
29
|
Zakaria N, Yusoff NM, Zakaria Z, Lim MN,
Baharuddin PJ, Fakiruddin KS and Yahaya B: Human non-small cell
lung cancer expresses putative cancer stem cell markers and
exhibits the transcriptomic profile of multipotent cells. BMC
Cancer. 15:842015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu X, Chen H and Wang X: Can lung cancer
stem cells be targeted for therapies? Cancer Treat Rev. 38:580–588.
2012. View Article : Google Scholar : PubMed/NCBI
|