Introduction
Colorectal cancer (CRC) is the third most common
malignancy worldwide, accounting for ~10% of all cancer cases and
CRC is one of the most common causes of death related to
gastrointestinal cancers (1–3).
Although the incidence rates of colon cancer have declined
somewhat, current therapies are associated with serious
side-effects, high cost and recurrence rates exceeding 50%,
primarily due to the development of acquired chemoresistance to
conventional chemotherapeutics (4,5).
Emerging data suggest that malignant tumors contain
a small distinct population of cancer stem cells (CSCs), which are
responsible for tumor initiation and propagation (6). Stem cell research and the cancer stem
cell (CSC) hypothesis have shown that colonic stem cells or CSCs
are involved in tissue regeneration and colonic carcinogenesis
(7–9). Drug-resistant CSCs are thought to be
one of the key causes of CRC treatment failure, and it is
hypothesized that these cells are ultimately the likely cause of
metastasis and tumor recurrence (10–12).
Most modern treatments are ineffective against solid tumors and
this may be the result of the increased resistance of CSCs
(13). Therefore, it is vital to
find novel therapeutic methods to eradicate CSCs and enable the
development of more effective treatment protocols (14).
Salinomycin is a 751-Da monocarboxylic polyether
antibiotic, which was initially used to eliminate bacteria, fungi
and parasites and is fed to ruminants to improve nutrient
absorption and feeding efficiency (15,16).
This compound is now considered an important anticancer drug
candidate (17,18). It has recently been reported that
salinomycin can selectively kill human breast cancer stem cells,
and is 100-fold more effective at reducing the proportion of CSCs
than paclitaxel, albeit by an unknown mechanism (19). Salinomycin was also found to be a
selective inhibitor of human lung, gastric, osteosarcoma, squamous
cell carcinoma, prostate and pancreatic CSCs (20–25).
However, since the mechanism involved in the salinomycin anti-CSC
activity is poorly understood, it is necessary to conduct more
in-depth research into the activity of salinomycin in different
types of human CSCs. In the present study, we found that
salinomycin selectively induced apoptosis in human colorectal
cancer stem cells (CRSCs) by activating a distinct apoptotic
pathway. The apoptosis was accompanied by caspase activation,
increased DNA damage, loss of membrane potential and regulation of
the Bcl-2/Bax ratio. Our results indicate that salinomycin may be a
novel therapy for CRC.
Materials and methods
Cell lines and culture
HCT-116 human CRC cells were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China), and
maintained in McCoy's 5A (Sigma-Aldrich, St. Louis, MO, USA). Media
contained 100 IU penicillin, 100 µg/ml streptomycin
(Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum (FBS;
Gibco-BRL, Grand Island, NY, USA). The cells were incubated at 37°C
in 5% CO2. CD44+EpCAM+ HCT-116
cells (CRSCs) were maintained in Dulbecco's modified eagle's medium
(DMEM)/F12 (Sigma-Aldrich) containing 2% B27 (Invitrogen), 20 ng/ml
epidermal growth factor, and 20 ng/ml basic fibroblast growth
factor (both from PeproTech, Rocky Hill, NJ, USA) in an incubator
at 37°C in 5% CO2.
Drugs and antibodies
Salinomycin (Sigma-Aldrich) was stored as a 500 mM
dimethylsulfoxide (DMSO; Gibco, CA, USA) solution in the dark at
−20°C. For experiments, salinomycin solutions were prepared by
diluting the stock solution with DMEM/F12. The antibodies used for
western blotting were as follows: rabbit anti-caspase-3 and
anti-caspase-6, mouse anti-Bcl-2, anti-Bax, anti-caspase-8
anti-caspase-9 and anti-β-actin. All antibodies were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). CD44 and epithelial
cell adhesion molecule (EpCAM) antibodies (eBioscience, San Diego,
CA, USA) were used for magnetic-activated cell sorting (MACS).
MACS
MACS was performed using a CELLection™ Biotin Binder
kit according to the manufacturer's instructions (Invitrogen). In
brief, HCT-116 cells were collected and incubated with the CD44
antibody for 10 min. Dynabeads (750 µl) were added to the
cells, which were then incubated for 20 min and separated using a
magnet. Subsequently, releasing buffer (DNase I; 120 µl) was
added, and the cells were incubated for 15 min at room temperature
with gentle tilting and rotation to collect target cells
(CD44+ cells). CD44+ cells were incubated
with the EpCAM antibody for 10 min, and Dynabeads (25 µl)
were added to the cells. The cells were incubated for 20 min and
then separated using a magnet. Subsequently, releasing buffer
(DNase I; 4 µl) was added and the cells were incubated for
15 min at room temperature with gentle tilting and rotation to
collect target cells (EPCAM+CD44+ cells,
CRSCs).
Serum-induced differentiation
To induce differentiation, CRSCs were collected and
maintained in DMEM/F12 medium supplemented with 10% FBS and
incubated for 3 days. Results were analyzed using an inverted
microscope.
Soft agar colony formation
One hundred living cells mixed with 0.3% agar liquor
were immediately plated onto a 0.5% solidified agar-based 6-well
plate. Cells were incubated for 3 weeks on soft agar culture
medium. The resulting colonies were photographed with an optical
microscope.
Cell viability
HCT-116 cells and CRSCs were plated at a density of
5,000 cells/well in flat-bottom 96-well plates (100 µl
medium/well). After 24 h, the cells were treated with salinomycin
at various concentrations (1, 10 and 25 µM), 100 µM
cisplatin (DDP) as a positive parallel control or 0.1% DMSO as a
solvent control. After 48 h, the Cell Counting Kit-8 (CCK-8;
Dojindo, Tokyo, Japan) was used according to the manufacturer's
instructions, optical density was measured using a microplate
reader at 450 nm, and the cell viability was calculated. All
experimental concentrations were assessed in triplicate. Inhibition
ratio was calculated using the formula: Inhibition ratio (%) = 1 −
ODtreatment group/ODsolvent control ×
100.
Invasion assay
An invasion assay was performed using 6.5-mm
Transwell® plates with sterile 8.0-µm pore
polycarbonate membrane inserts (Corning, Steuben County, NY, USA).
In brief, 5,000 CRSCs in DMEM/F12 medium were seeded in the insert
and treated with salinomycin (0–25 µM). The lower chamber
was filled with DMEM/F12 medium supplemented with 10% FBS as a
chemotactic factor, and the plates were subsequently incubated at
37°C in 5% CO2 for 48 h. The insert chamber contained an
8-µm pore polycarbonate membrane covered with a thin layer
of BD Matrigel™ (BD, San Diego, CA, USA). The Matrigel layer blocks
pores in the membrane, which prevents the migration of non-invasive
cells; however, invading cells can migrate through the Matrigel
layer and eventually attach themselves to the bottom of the
polycarbonate layer. After 48 h, the number of cells in the lower
chamber was quantified using the CCK-8 assay as described
above.
DNA ladder assay
Apoptotic response was evaluated by detecting DNA
fragmentation using an apoptosis DNA ladder detection kit (Keygen,
Nanjing, China). CRSCs were treated with salinomycin (1, 10 or 25
µM), 100 µM DDP as a positive parallel control, or
0.1% DMSO as solvent control. After 48 h, CRSCs were collected and
washed with PBS, resuspended in 20 µl lysis buffer, and
lysed for 10 min on ice. Subsequently, enzyme A (10 µl) was
added and incubated at 37°C for 1 h. Then samples were incubated
with enzyme B (10 µl) at 50°C for 90 min. After 2% agarose
gel electrophoresis, DNA was stained with ethidium bromide (EB) and
photographed.
Acridine orange (AO) and EB assay
AO is used to stain normal cells and EB indicates
apoptotic cells. In brief, CRSCs were plated into 6-well plates and
treated with salinomycin (1, 10 and 25 µM), 100 µM
DDP as a positive parallel control and 0.1% DMSO as a solvent
control. After 48 h, each well was treated with AO (5 µl)
and EB (5 µl), and subsequently incubated for 5 min at room
temperature. The stained cells were analyzed using a fluorescence
microscope (Olympus, Tokyo, Japan). The experiments were repeated 3
times.
Annexin V analysis
Annexin V analysis was performed using an Annexin
V-fluorescein isothiocyanate (FITC) kit (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer's instructions.
Briefly, after CRSCs were incubated with 1, 10 or 25 µM
salinomycin, 100 µM DDP as a positive parallel control or
0.1% DMSO as a solvent control, they were harvested by quick
trypsinization to minimize potentially high Annexin V background
levels in adherent cells. Cells were then washed twice with cold
phosphate-buffered saline (PBS) and re-suspended in binding buffer
at a concentration of 1×106 cells/ml. Cells (100
µl) were stained with Annexin V-FITC (5 µl) and
propidium iodide (PI; 5 µl) and incubated in the dark at
room temperature for 15 min. Then, binding buffer (400 µl)
was added, and the cells were analyzed using a flow cytometer
(Beckman Coulter, Salt Lake, UT, USA) and a fluorescence microscope
(Olympus). Cells negative for both Annexin V and PI were viable,
Annexin V+/PI− cells were in early apoptosis
and Annexin V+/PI+ cells were necrotic or in
late apoptosis. The experiments were repeated 3 times.
JC-1 assay
JC-1 and FITC staining were carried out using a
commercial mitochondrial membrane potential detection (JC-1) kit
(BD Biosciences). Briefly, CRSCs were incubated with salinomycin
(1, 10 or 25 µM), 100 µM DDP as a positive parallel
control or 0.1% DMSO as a solvent control for 48 h. CRSCs were
harvested, then washed twice with cold PBS and re-suspended in
binding buffer at a concentration of 1×106 cells/ml.
Cells (100 µl) were stained with JC-1 (5 µl) and pI
(5 µl) and incubated in the dark at room temperature for 15
min. Then, binding buffer (400 µl) was added and cells were
analyzed using a flow cytometer (Beckman Coulter). Cells negative
for both JC-1 and PI were viable, JC-1+/PI+
cells were in early apoptosis and JC-1+/PI−
cells were necrotic or in late apoptosis. The experiments were
repeated 3 times.
Quantitative real-time reverse
transcriptase-polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen). First-strand cDNA was reverse transcribed using
PrimeScript RT kit (Takara, Otsu, Shiga, Japan) according to the
manufacturer's protocol. Relative mRNA levels were quantitatively
determined using a real-time PCR system. The primer sequences used
for quantitative real-time PCR are shown in Table I. glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as the endogenous reference. cDNA
was subjected to PCR for 40 cycles of 94°C for 30 sec, 60°C for 30
sec and 72°C for 45 sec. qPCR was performed using a
Thermo® PikoReal 96 system (Thermo, Waltham, MA, USA).
Real-time RT-qPCR was performed using FastStart universal
SYBR-green Master (ROX) (Roche, Basle, Switzerland) and analyzed
using PikoReal software 2.1 (Thermo). Experiments were performed in
triplicate and the average CT values of target genes were
normalized to control as ΔCT. Changes in expression levels are
shown either as a fold increase or as a ratio (target gene/control
gene).
 | Table IReal-time PCR primer sequences. |
Table I
Real-time PCR primer sequences.
| Gene | Primer sequence
(5′→3′) |
|---|
| Bcl-2 | F
CATGTGTGTGGAGAGCGTCAA |
| R
GCCGGTTCAGGTACTCAGTCA |
| Bax | F
GATCCAGGATCGAGCAGA |
| R
AAGTAGAAGAGGGCAACCAC |
| Caspase-3 | F
CAGAACTGGACTGTGGCATTGAG |
| R
GGATGAACCAGGAGCCATCCT |
| Caspase-6 | F
AGAAAGATAGCAGCAGTGCCTCA |
| R
ATTGCCAGTAGAAGTCTTCATGGTT |
| Caspase-8 | F
CAAGTTCCTGAGCCTGGACTACATT |
| R
GACAGATTGCTTTCCTCCAACATT |
| Caspase-9 | F
GCGAACTAACAGGCAAGCAGC |
| R
CGACATCACCAAATCCTCCAGAAC |
| GAPDH | F
CATCAGCAATGCCTCCTGCAC |
| R
TGAGTCCTTCCACGATACCAAAGTT |
Western blotting
Western blotting was carried out to test for
caspase-3, -6, -8 and -9, Bcl-2 and Bax. To collect whole protein,
cells were lysed with RIPA buffer containing protease inhibitor
cocktail (Roche), and protein concentrations were determined using
a BCA assay kit (Beyotime, Nanjing, Jiangsu, China). Protein bands
were separated by 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and were then transferred to membranes
(Millipore, Bedford, MA, USA) at 100 V for 45 min at room
temperature. After blocking in 4% non-fat dry milk in tris-buffered
saline (TBS), the membranes were incubated with primary antibodies
at a 1:1,000 dilution in TBS overnight at 4°C, washed 3 times with
TBS containing 0.5% Tween-20, and then incubated with secondary
antibodies conjugated with horseradish peroxidase (HRP) at a
1:5,000 dilution in TBS for 1 h at room temperature. Membranes were
washed again in TBS containing 0.5% Tween-20 for 3 times at room
temperature. Protein bands were visualized on X-ray film using
enhanced chemiluminescence (ECL; GE Healthcare, Bethesda, MD,
USA).
CRSC xenograft studies
Severe combined immunodeficiency (SCID) mice
(CB17/Icr-Prkdcscid/IcrlcoCrlVr) were bred in
specific-pathogen-free microisolator cages that were purchased from
the Animal Institute of the Chinese Academy of Medical Science. All
experiments were performed according to the regulations of the
Animal Care Committee of Jilin University. To generate CRSC
xenografts, 1×105 CRSCs were resuspended in PBS (100
µl) and injected subcutaneously into the right flank of the
mice. The weight and size of the tumors were measured every other
day. When tumors reached a volume of 40–60 mm3, the mice
were randomized into therapy and control groups. After that, the
mice were treated with vehicle (DMSO), 5-fluorouracil (5-Fu),
salinomycin or a combination of salinomycin and 5-Fu. 5-Fu was
administered once a week (100 mg/kg) 3 times, while salinomycin was
injected every other day (4 mg/kg) 5 times. Both agents were
injected intraperitoneally. The animal weight and tumor volumes
were monitored every other day. Using Vernier calipers, tumor
volume was calculated according to the formula A × B × B/2, where A
is the length of the tumor and B is the width.
Statistical analysis
All analyses were performed using Origin8 (Origin8
Technologies Ltd., London, UK) and data are presented as means ±
standard deviation (SD). Values of p<0.05 were considered to be
statistically significant and were evaluated using the Student's
t-test.
Results
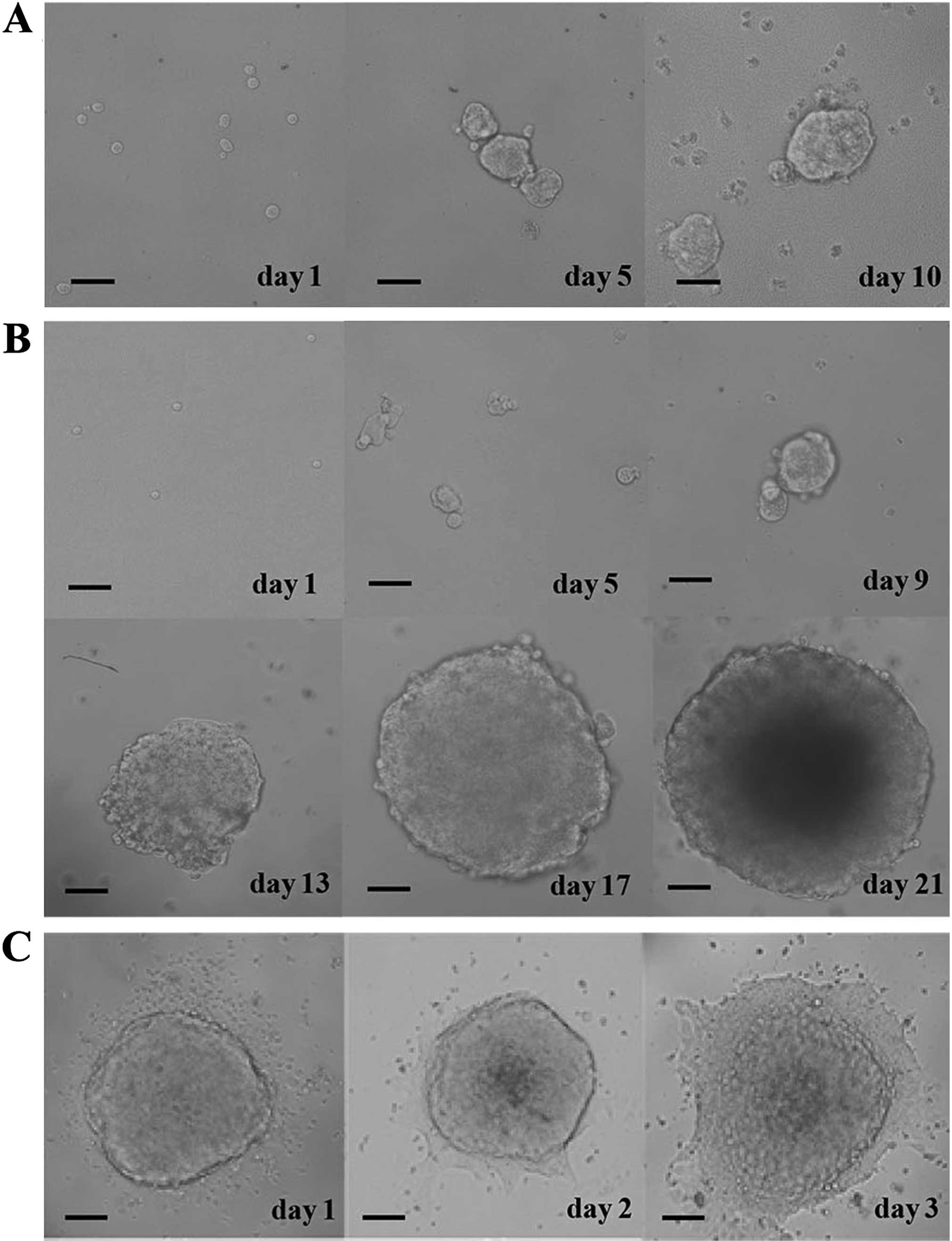
Biological characterization of CRSCs
The proportion of CRSCs in HCT-116 cells was found
to be 3%, and the CRSCs formed spheres after culturing for 10 days
in CRSC medium (Fig. 1A). The
generation of spheres was observed over the next 3 weeks in soft
agar using an inverted microscope (Fig.
1B). CRSC spheroid cells became adherent cells when serum was
added to the culture medium (Fig.
1C).
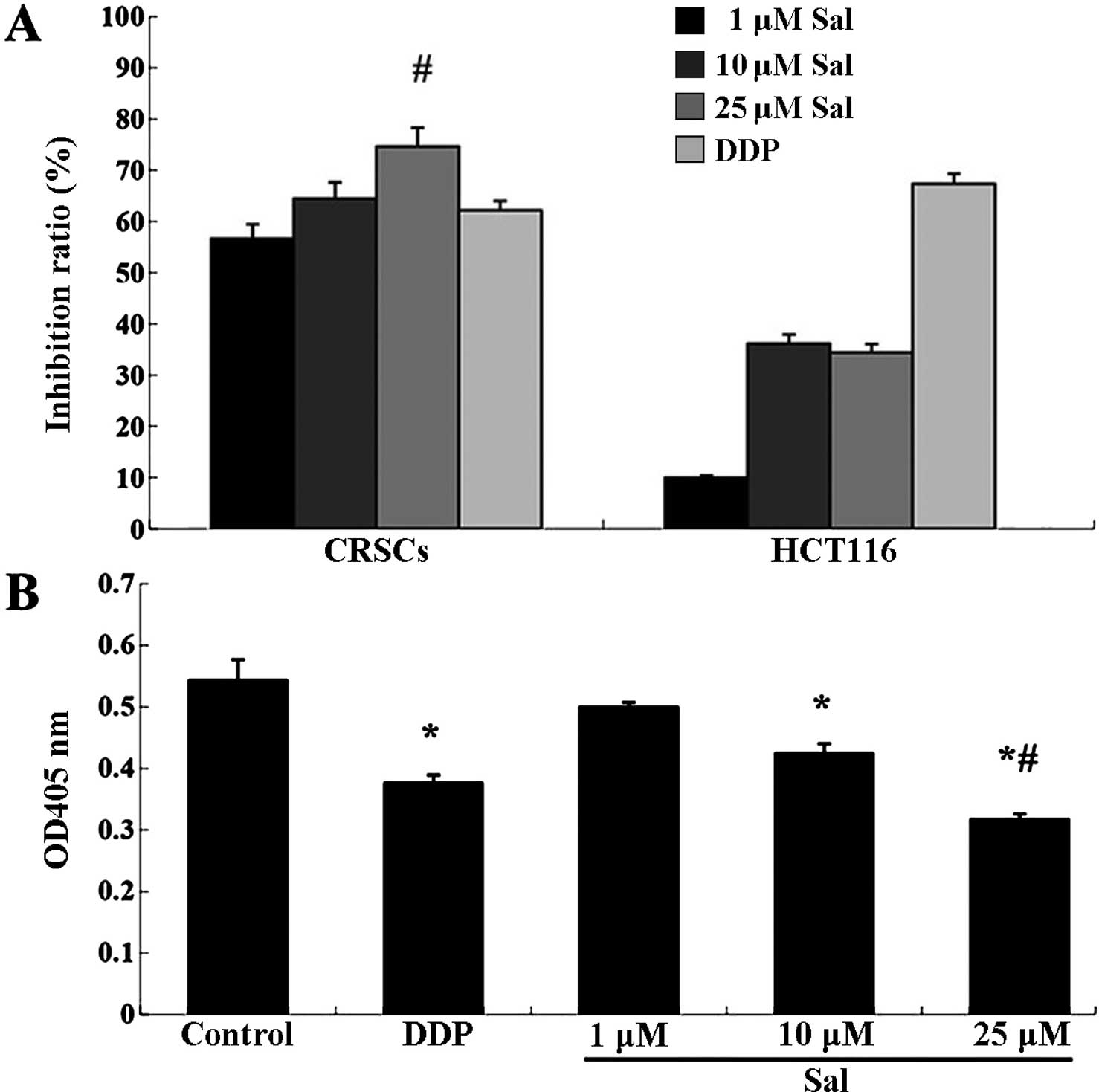
Salinomycin inhibits the viability and
invasion of CRSCs
To initially assess the anticancer effect of
salinomycin on CRSCs, a CCK-8 assay was performed on the treated
cells. Salinomycin reduced the cell viability of HCT-116 cells and
CRSCs in a concentration-dependent manner (Fig. 2A). Additionally, the Transwell
migration assay indicated that treatment with 25 µM
salinomycin significantly reduced the number of invasive cells
(Fig. 2B).
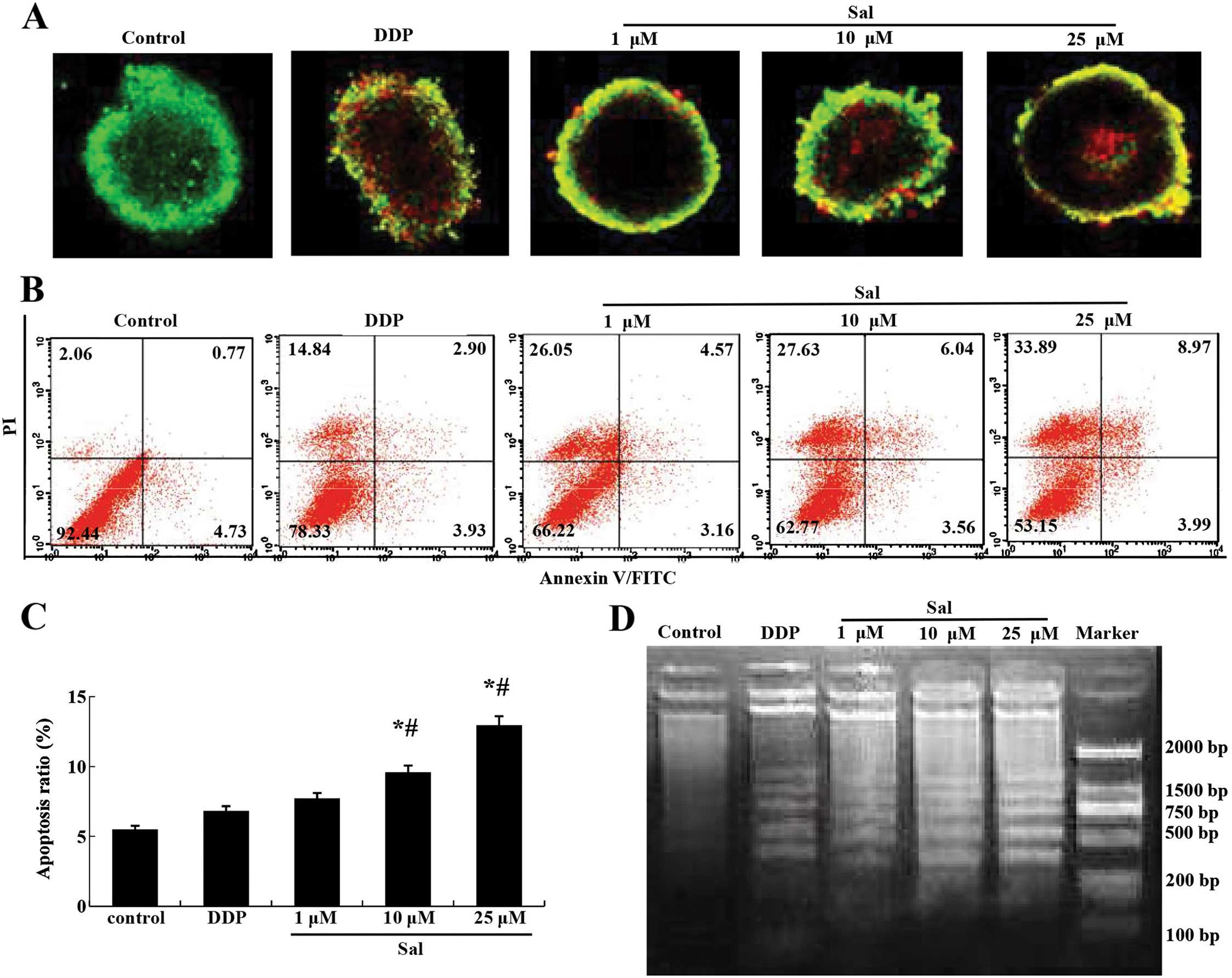
Salinomycin induces the apoptosis of
CRSCs
AO/EB staining indicated that the number of
apoptotic CRSCs increased in response to salinomycin treatment in a
concentration-dependent manner (Fig.
3A). The Annexin V/PI double staining assay revealed that
salinomycin treatment increased the percentage of Annexin
V-positive cells in the CRSCs (Fig. 3B
and C). A DNA ladder assay indicated that severe nuclear
fragmentation took place in the CRSCs following treatment with 10
or 25 µM salinomycin, but not in the cells treated with DMSO
or 1 µM salinomycin (Fig.
3D).
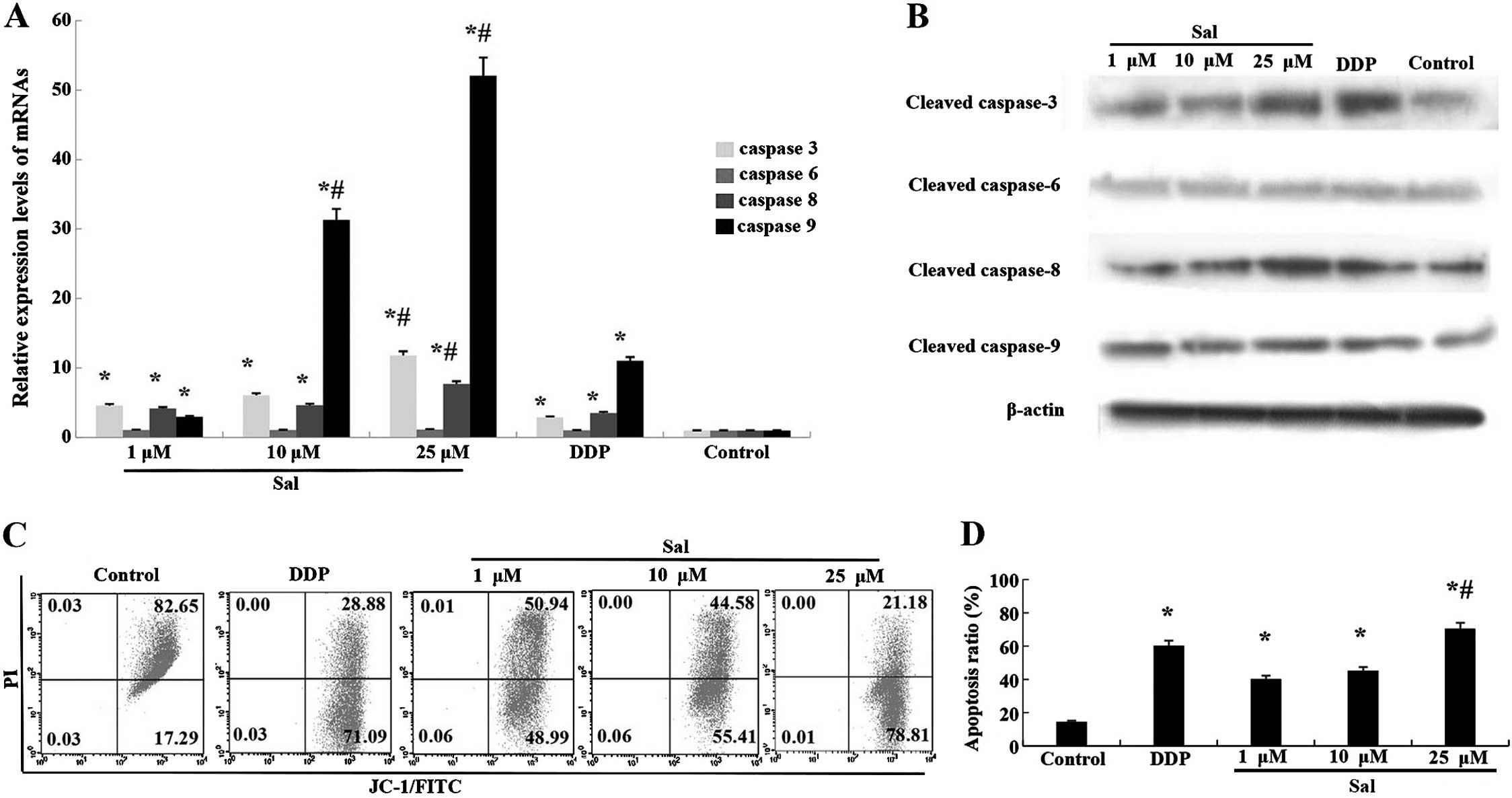
Salinomycin-induced apoptosis of CRSCs is
caspase-dependent
To further clarify the mechanism of
salinomycin-induced apoptosis, the expression of a number of
apoptosis-related proteins and their mRnAs were analyzed.
Upregulation of caspase-3, -8 and -9 mRNA was observed in CRSCs
treated with salinomycin for 48 h (Fig.
4A). Additionally, the protein expression of cleaved caspase-3,
-8 and -9 was enhanced following treatment with salinomycin for 48
h (Fig. 4B).
Salinomycin induces cell apoptosis via
the mitochondrial pathway
An increased level of cleaved caspase-9 implied the
breakdown of mitochondria in the salinomycin-induced apoptosis. The
JC-1 staining assay showed that the percentage of cells
experiencing a loss of mitochondrial membrane potential increased
from 17.29 to 78.81% in the CRSCs following treatment with 25
µM salinomycin. The loss of mitochondrial membrane
potential, along with increased cleaved caspase-9 (Fig. 4C and D), suggests that
salinomycin-induced CRSCs death via the mitochondrial apoptosis
pathway.
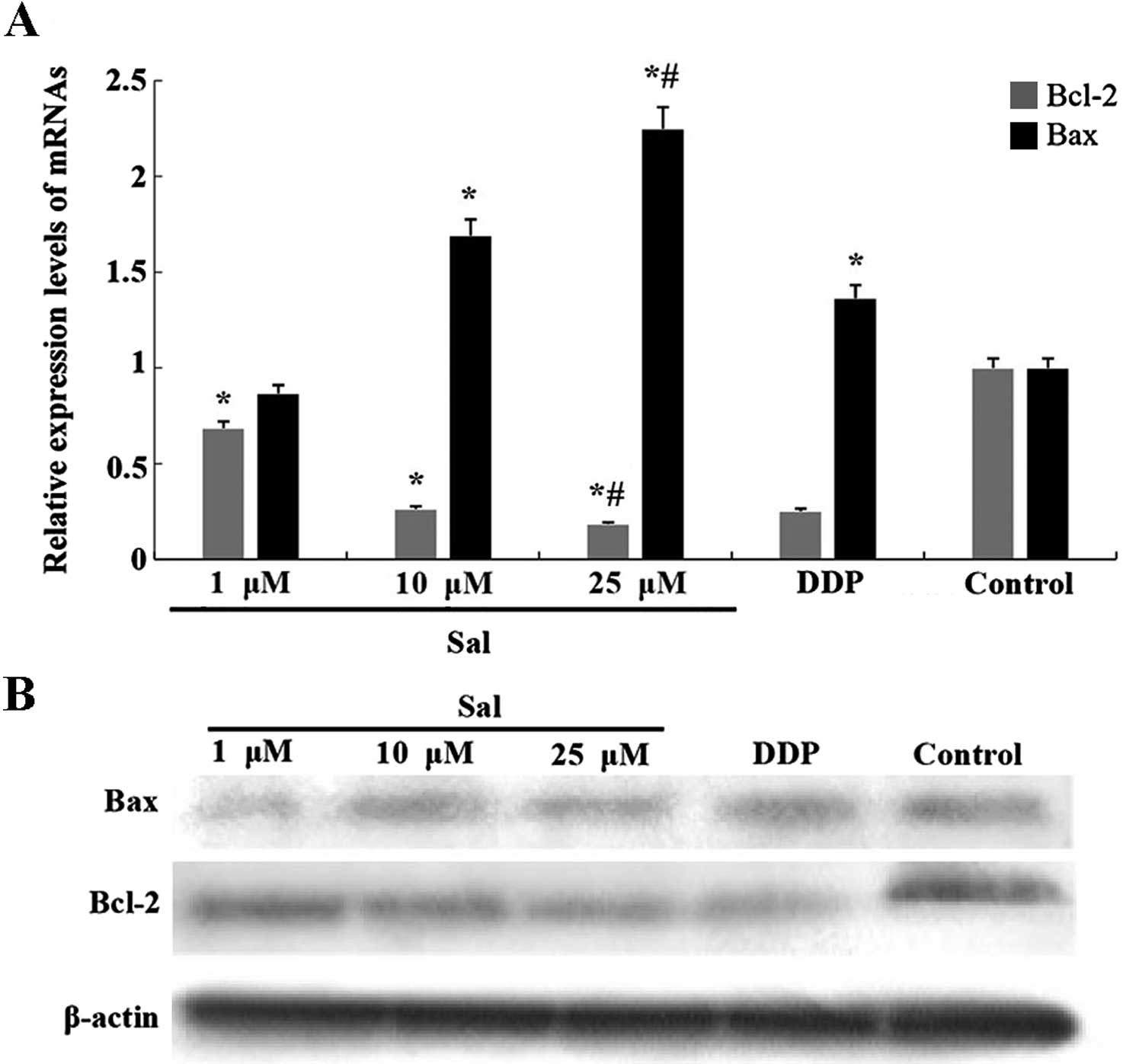
Salinomycin-induced apoptosis of CRSCs is
dependent on the Bcl-2 family
We measured the expression of Bcl-2 and Bax using
western blotting and real-time RT-qPCR. Salinomycin treatment for
48 h significantly suppressed the expression of Bcl-2 and
upregulated the expression of Bax in the CRSCs (Fig. 5A and B).
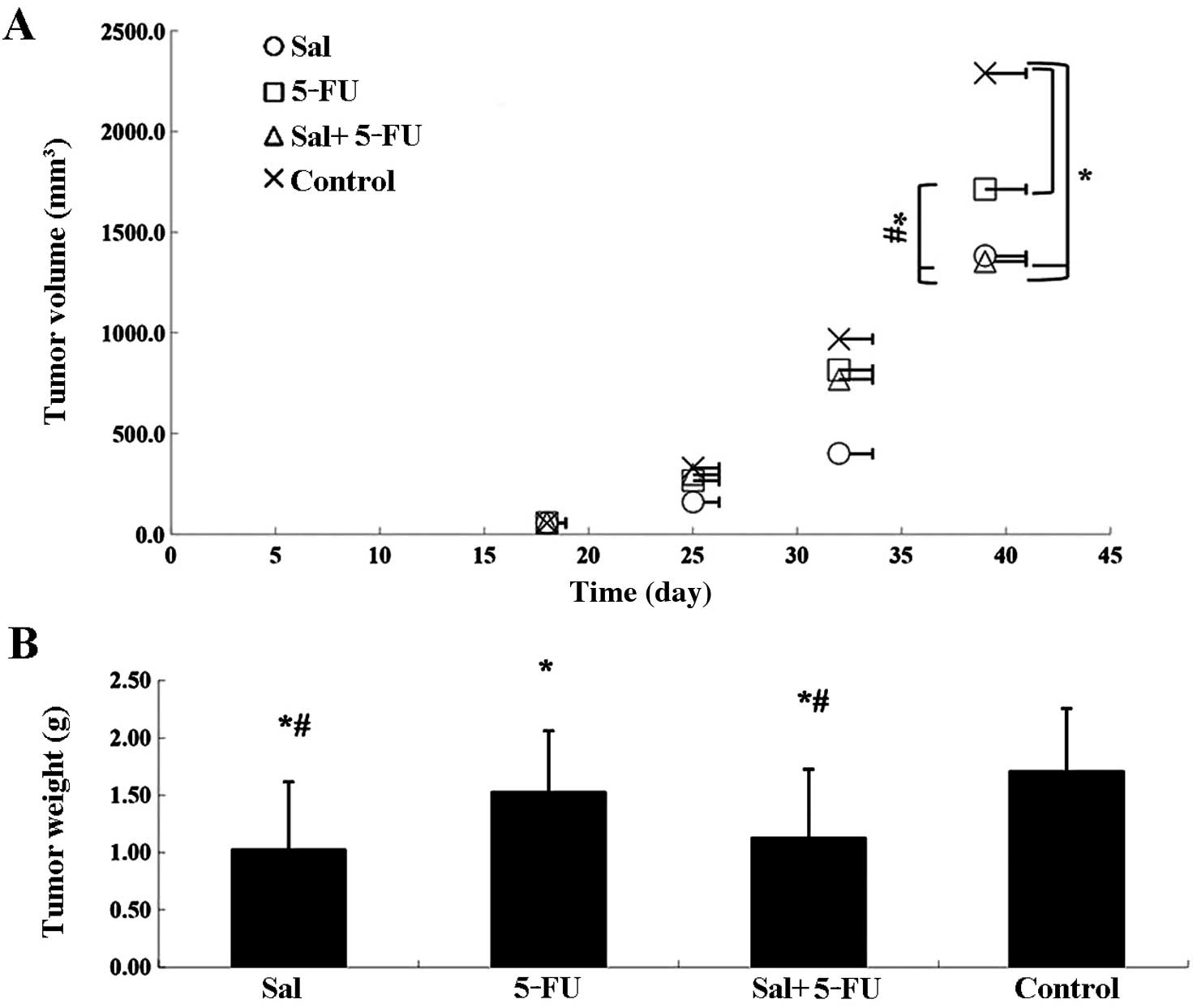
Salinomycin inhibits tumor growth in
vivo
To evaluate the in vivo anticancer activity
of salinomycin, CRSCs were subcutaneously injected into SCID mice
in the right flank. Delayed tumor growth was observed in the
salinomycin-treated group, the 5-Fu-treated group and the combined
treatment group as compared to the control group. Tumor volumes in
the salinomycin-treated group and the combined treatment group
decreased significantly compared to the 5-Fu-treated group
(Fig. 6A). After 3 weeks of
treatment, the animals were sacrificed and CRSC xenografts were
dissected and weighed (Fig. 6B).
The weight of tumors in the salinomycin-treated group and the
combined treatment group were both significantly smaller than those
in the 5-Fu-treated and control groups (P<0.05).
Discussion
Recently salinomycin has been shown to induce
apoptosis in several types of malignant cancer cells (26,27).
In the present study, we found that salinomycin inhibited the
proliferation of CRSCs in vitro and reduced tumor growth
in vivo. The pharmacologic action of salinomycin has
attracted increased attention in recent years in view of its
potential as a new cancer chemotherapeutic based on its activity as
a selective inhibitor of breast cancer stem cells. Salinomycin
treatment was also found to reduce the formation of metastatic
nodules by CSCs (19,28). Compared with drugs that kill general
cancer cells, such as paclitaxel (3) and oxaliplatin (29), salinomycin selectively kills cancer
stem cells, providing a new strategy for cancer therapy.
Our results demonstrated that salinomycin decreased
the viability and proliferation of CRSCs in a time- and
dose-dependent manner. The results also indicated that the
anti-CRSC properties of salinomycin are a result of apoptosis
initiation. The results of the Annexin V-FITC and JC-1 staining
assays provided evidence for early and late apoptosis, and necrosis
in CRSCs treated with different concentrations of salinomycin.
Research has shown that resistance to apoptosis is one of the main
causes of tumorigenesis and tumor drug resistance (30), and the caspase and the Bcl-2
families play a significant role in the regulation of apoptosis.
Caspase-3 is a downstream molecule that is activated by upstream
molecules such as caspase-8 or -9, leading to apoptosis.
pro-apoptotic Bax boosts essential apoptosis by forming oligomers
in the mitochondrial outer membrane and promoting the release of
apoptogenic molecules, while anti-apoptotic Bcl-2 blocks
mitochondrial apoptosis by blocking the release and oligomerization
of Bax (31). In the present study,
molecular biology assays indicated that salinomycin
dose-dependently activated cleaved caspase-3, -8 and -9 at both the
mRNA and protein levels; salinomycin treatment also decreased the
expression of the apoptotic protein Bcl-2 and increased expression
of the proapoptotic protein Bax. It was therefore demonstrated that
salinomycin remedies the apoptosis resistance of CRSCs in
vitro and in vivo, which may make it an effective
chemotherapeutic agent for treating CRC.
In conclusion, we demonstrated that salinomycin
suppressed the proliferation of CRSCs, vital for tumor development.
In the present study, we demonstrated that salinomycin inhibited
proliferation, induced apoptosis by increasing the activity of the
caspase family (caspase-3, -8 and -9) and Bax, and by
downregulating the activity of Bcl-2. Although the exact mechanism
of the antitumor activity of salinomycin remains unclear, this
research represents an important first step in the development of
salinomycin-related colon cancer therapy.
Acknowledgments
The present study was supported by the Jilin
province Science Foundation (20120960).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, Ward E and Thun MJ:
Cancer occurrence. Methods Mol Biol. 471:3–29. 2009. View Article : Google Scholar
|
|
3
|
Nautiyal J, Kanwar SS, Yu Y and Majumdar
AP: Combination of dasatinib and curcumin eliminates
chemo-resistant colon cancer cells. J Mol Signal. 6:72011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: Mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang WQ, Fu FF, Li YX, Wang WB, Wang HH,
Jiang HP and Teng LS: Molecular biomarkers of colorectal cancer:
Prognostic and predictive tools for clinical practice. J Zhejiang
Univ Sci B. 13:663–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simeone DM: pancreatic cancer stem cells:
Implications for the treatment of pancreatic cancer. Clin Cancer
Res. 14:5646–5648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boman BM and Huang E: Human colon cancer
stem cells: A new paradigm in gastrointestinal oncology. J Clin
Oncol. 26:2828–2838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vermeulen L, Sprick MR, Kemper K, Stassi G
and Medema JP: Cancer stem cells - old concepts, new insights. Cell
Death Differ. 15:947–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shipitsin M and Polyak K: The cancer stem
cell hypothesis: In search of definitions, markers, and relevance.
Lab Invest. 88:459–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fabrizi E, di Martino S, Pelacchi F and
Ricci-Vitiani L: Therapeutic implications of colon cancer stem
cells. World J Gastroenterol. 16:3871–3877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ong CW, Kim LG, Kong HH, Low LY, Iacopetta
B, Soong R and Salto-Tellez M: CD133 expression predicts for
non-response to chemotherapy in colorectal cancer. Mod Pathol.
23:450–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saigusa S, Tanaka K, Toiyama Y, Yokoe T,
Okugawa Y, Kawamoto A, Yasuda H, Morimoto Y, Fujikawa H, Inoue Y,
et al: Immunohistochemical features of CD133 expression:
Association with resistance to chemoradiotherapy in rectal cancer.
Oncol Rep. 24:345–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarkar B, Dosch J and Simeone DM: Cancer
stem cells: A new theory regarding a timeless disease. Chem Rev.
109:3200–3208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mueller MT, Hermann PC, Witthauer J,
Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M,
Bartenstein P, D'Haese JG, et al: Combined targeted treatment to
eliminate tumorigenic cancer stem cells in human pancreatic cancer.
Gastroenterology. 137:1102–1113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahmoudi N, de Julián-Ortiz JV, Ciceron L,
Gálvez J, Mazier D, Danis M, Derouin F and García-Domenech R:
Identification of new antimalarial drugs by linear discriminant
analysis and topological virtual screening. J Antimicrob Chemother.
57:489–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitani M, Yamanishi T, Miyazaki Y and
Otake N: Salinomycin effects on mitochondrial ion translocation and
respiration. Antimicrob Agents Chemother. 9:655–660. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naujokat C, Fuchs D and Opelz G:
Salinomycin in cancer: A new mission for an old agent. Mol Med Rep.
3:555–559. 2010. View Article : Google Scholar
|
|
18
|
Huczynski A: Salinomycin: A new cancer
drug candidate. Chem Biol Drug Des. 79:235–238. 2012. View Article : Google Scholar
|
|
19
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y: effects of salinomycin on cancer
stem cell in human lung adenocarcinoma A549 cells. Med Chem.
7:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
|
|
22
|
Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ,
Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, et al: Salinomycin
inhibits osteosarcoma by targeting its tumor stem cells. Cancer
Lett. 311:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basu D, Montone KT, Wang LP, Gimotty PA,
Hammond R, Diehl JA, Rustgi AK, Lee JT, Rasanen K, Weinstein GS, et
al: Detecting and targeting mesenchymal-like subpopulations within
squamous cell carcinomas. Cell Cycle. 10:2008–2016. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ketola K, Hilvo M, Hyötyläinen T, Vuoristo
A, Ruskeepää AL, Orešič M, Kallioniemi O and Iljin K: Salinomycin
inhibits prostate cancer growth and migration via induction of
oxidative stress. Br J Cancer. 106:99–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang GN, Liang Y, Zhou LJ, Chen SP, Chen
G, Zhang TP, Kang T and Zhao YP: Combination of salinomycin and
gemcitabine eliminates pancreatic cancer cells. Cancer Lett.
313:137–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim KY, Yu SN, Lee SY, Chun SS, Choi YL,
Park YM, Song CS, Chatterjee B and Ahn SC: Salinomycin-induced
apoptosis of human prostate cancer cells due to accumulated
reactive oxygen species and mitochondrial membrane depolarization.
Biochem Biophys Res Commun. 413:80–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Salinomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maitland NJ and Collins AT: prostate
cancer stem cells: A new target for therapy. J Clin Oncol.
26:2862–2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong TT, Zhou HM, Wang LL, Feng B, Lv B
and Zheng MH: Salinomycin selectively targets 'CD133+'
cell subpopulations and decreases malignant traits in colorectal
cancer lines. Ann Surg Oncol. 18:1797–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee S and Schmitt CA: Chemotherapy
response and resistance. Curr Opin Genet Dev. 13:90–96. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leibowitz B and Yu J: Mitochondrial
signaling in cell death via the Bcl-2 family. Cancer Biol Ther.
9:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|