Introduction
According to National Vital Statistics System
(2013), cancer is the second major cause of death in US. Despite
improvements in diagnostic techniques, clinical intervention and
increased public concern, the prevalence of cancer in developed
countries continues to rise (1).
The standard medical treatment for cancer includes surgery of the
cancerous tumor followed by radiotherapy and/or chemotherapy to
kill the remaining cancerous cells. Most therapeutic strategies
focus on overcoming two key hallmarks of cancer: i) excessive
proliferation and ii) apoptosis resistance (2). However, a more certain cure is
induction of apoptosis rather than inhibition of proliferation
because the former is able to eliminate cancer cells while later
may only limit tumor outgrowth. For this reason, the development of
apoptotic agents has become of great interest in cancer
research.
The tumor necrosis factor (TNF)-related apoptosis
inducing ligand (TRAIL) was first identified based on its sequence
homology to TNF and CD95L (3,4), and
was found to induce apoptosis in a wide range of human cancer cell
lines (3–6) while leaving normal tissues intact
(7–9). Although five receptors have been
identified as TRAIL recognition partner, only 2 receptors, DR4
(TRAIL-R1) and DR5 (TRAIL-R2), have cytoplasmic death domains that
participate in apoptotic event upon TRAIL binding (10). Based on this knowledge, a fully
human monoclonal agonist antibody against human DR5 has been
developed as an anticancer therapy (11). However, cancer cells frequently
acquire TRAIL resistance during the progression of the tumor
(12). TRAIL resistance mechanisms
have not yet been clearly elucidated but numerous mechanisms to
manage TRAIL-induced apoptosis have been reported. Dysfunction of
DR4 and DR5, and defects of essential component for assembly of the
death-inducing signaling complex such as Fas-associated death
domain (FADD) and caspase-8, can lead to TRAIL resistance (13–15).
Overexpression of cellular FADD-like interleukin-1β-converting
enzyme inhibitory protein (cFLIP) (16,17),
X-linked inhibitor of apoptosis (XIAP) (18), Mcl-1, nuclear factor (NF)-κB
(19,20), Bcl-2 and Bcl-xL (21), and loss of Bax and Bak function also
contribute to TRAIL resistance in cancerous cell (22). Thus, agents capable of evading
resistance mechanisms to TRAIL-induced apoptosis are receiving
significant attention from cancer therapy community.
Hispolon, a phenol compound isolated from
Phellinus linteus (PL) and used as traditional medicinal
mushroom in Asia, possesses various functions including
anti-inflammatory, anti-proliferative and antioxidant effects
(23–25). Hispolon also has an antitumor effect
inhibiting tumor cell growth or metastasis in various tumor types
(23,26–30).
For example, hispolon showed anti-proliferative effects in breast
and bladder cancer cells (26),
proapoptotic effect on human epidermoid KB cell and suppression of
human hepatoma cell metastasis (27). Studies on its antitumor mechanism
show downregulation of MDM2 via activated extracellular
signal-regulated kinase 1/2 (ERK1/2) can induce the death of KB
cells through a mitochondria-mediated apoptotic pathway and reduce
expression of matrix metalloproteinase-2 (MMP-2), (MMP-9) and
urokinase-type plasminogen activator (uPA).
In the present study, the ability of hispolon to
modulate TRAIL-induced apoptosis in human colon cancer cells was
investigated, as well as the subsequent mechanism of action.
Hispolon was found to enhance TRAIL-induced apoptosis through the
upregulation of pro-apoptotic proteins, downregulation of cell
survival proteins and upregulation of death receptors.
Materials and methods
Materials
Hispolon was kindly provied by Dr B.B. Aggarwal, MD
Anderson Cancer Center. Soluble recombinant human TRAIL was
purchased from PeproTech (Rocky Hill, NJ, USA). Penicillin,
streptomycin, Dulbecco's modified Eagle's medium, RPMI-1640 and
fetal bovine serum were obtained from Invitrogen (Carlsbad, CA,
USA). Soluble antibodies against Bcl-2, c-FLIP, Bcl-xL, DR4, Bid,
Bax, CAAT enhancer binding protein homologous protein (CHOP), p53,
procaspase-3 and procaspase-8 were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The DR5 antibody was purchased
from ProSci, Inc. (Poway, CA, USA), anti XIAP antibody was from BD
Biosciences. Antibodies against caspase-9 and cleaved caspase-8
were purchased from Cell Signaling Technology Inc. (Danvers, MA,
USA). Mouse monoclonal anti-β-actin antibody was purchased from
Sigma (St. Louis, MO, USA).
Cell lines
Human colon adenocarcinoma HCT-116, embryonic kidney
carcinoma A293, multiple myeloma U-266 cells were obtained from
American Type Culture Collection (Manassas, VA, USA). Human myeloid
leukemia KBM-5 cells were kindly supplied by Dr Nicholas Donato
(University of Michigan Comprehensive Cancer Center). Human colon
cancer cell line HCT-116 was cultured in McCoy's 5A medium
supplemented with 10% fetal calf serum and penicillin/streptomycin
(Invitrogen). KBM-5 cells were cultured in Iscove's modified
Dulbecco's medium with 15% fetal bovine serum. U-266 cells were
cultured in RPMI-1640 with 10% fetal bovine serum, and A293 cells
were cultured in Dulbecco's modified Eagle's medium, 100 U/ml
penicillin and 100 mg/ml streptomycin.
Live/dead assay
To measure apoptosis of cells, we used the live/dead
assay (Invitrogen), which assesses intracellular esterase activity
and plasma membrane integrity. It is a two color fluorescence assay
that simultaneously examines live and dead cells. The details of
this assay were described before (31).
Cytotoxicity assay
The effects of hispolon on the cytotoxic potential
of TRAIL were detected by measuring mitochondrial dehydrogenase
activity using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) as the substrate. This assay is based on the
conversion of soluble MTT to purple colored insoluble formazan by
mitochondrial dehydrogenases of viable cells. To examine the
synergy between hispolon and TRAIL, cells were treated with
hispolon alone (25 µM) and TRAIL alone (10, 25 and 50 ng/ml)
and the combination at fixed ratio. Cell viability was examined by
MTT assay.
Western blot analysis
The effect of hispolon on the level of protein
expression was studied by western blot analysis. The prepared whole
cell extract was separated by SDS-PAGE. The separated proteins on
the acrylamide gel were electro-transferred onto nitrocellulose
membrane, stained with specific antibodies, and detected by an ECL
regent (GE Healthcare, Pittsburgh, PA, USA).
Assay for cell surface expression of DR4
and DR5
HCT116 cells were treated with hispolon and washed
with 1X PBS supplemented with 0.5% bovine serum albumin (BSA) after
detachment with EDTA. Then cells were stained with phycoerythrin
(PE)-conjugated mouse monoclonal anti-human DR4 and DR5 (clone
69036 and 71908, respectively) (R&D Systems, Minneapolis, MN,
USA) for 45 min at 4°C according to the manufacturer's instructions
before washing and resuspension in a fluorescence-activated cell
sorting buffer (1X PBS + 0.5% BSA). The cells were analyzed by flow
cytometry using an excitation wavelength of 488 nm.
Results
Hispolon upregulates TRAIL-induced
apoptosis
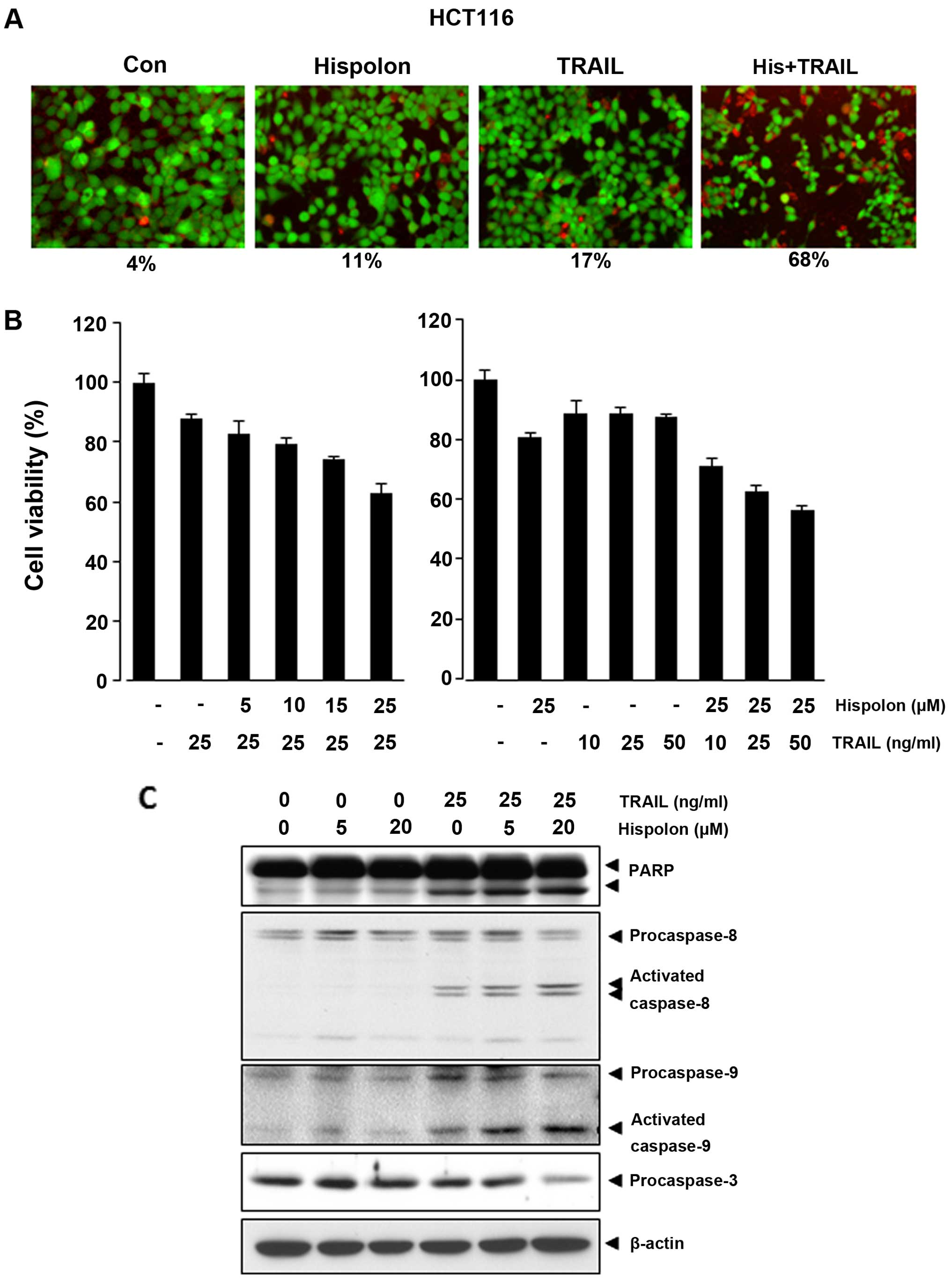
Hispolon enhancement of TRAIL-induced apoptosis was
examined and detected by live/dead assay in human colon cancer
cells. As shown in Fig. 1A, when
hispolon was added to HCT-116 cells already treated with TRAIL, a
number of apoptotic cells were increased. The population of
apoptotic cells, however, was not significantly influenced by
adding either TRAIL or hispolon alone. TRAIL-induced apoptosis was
upregulated by adding hispolon from 17 to 68% in HCT-116 cells.
According to MTT assay, we found that hispolon reduced the
viability of HCT-116 cells synergistically after pretreated with
TRAIL (Fig. 1B, right panel).
TRAIL is known to mediate apoptosis through the
activation of caspase-8, caspase-9 and caspase-3, so the effect of
hispolon on activation of these caspases and poly(ADP-ribose)
polymerase (PARP) cleavage enhanced by TRAIL in HCT-116 cells was
investigated. Although neither TRAIL nor hispolon had significant
effect on the activation of these caspases or on cleavage of PARP,
treatment of cells with combination TRAIL and hispolon enhanced
activation of all caspases and ensuing PARP cleavage (Fig. 1C).
Hispolon downregulates the expression of
anti-apoptotic proteins
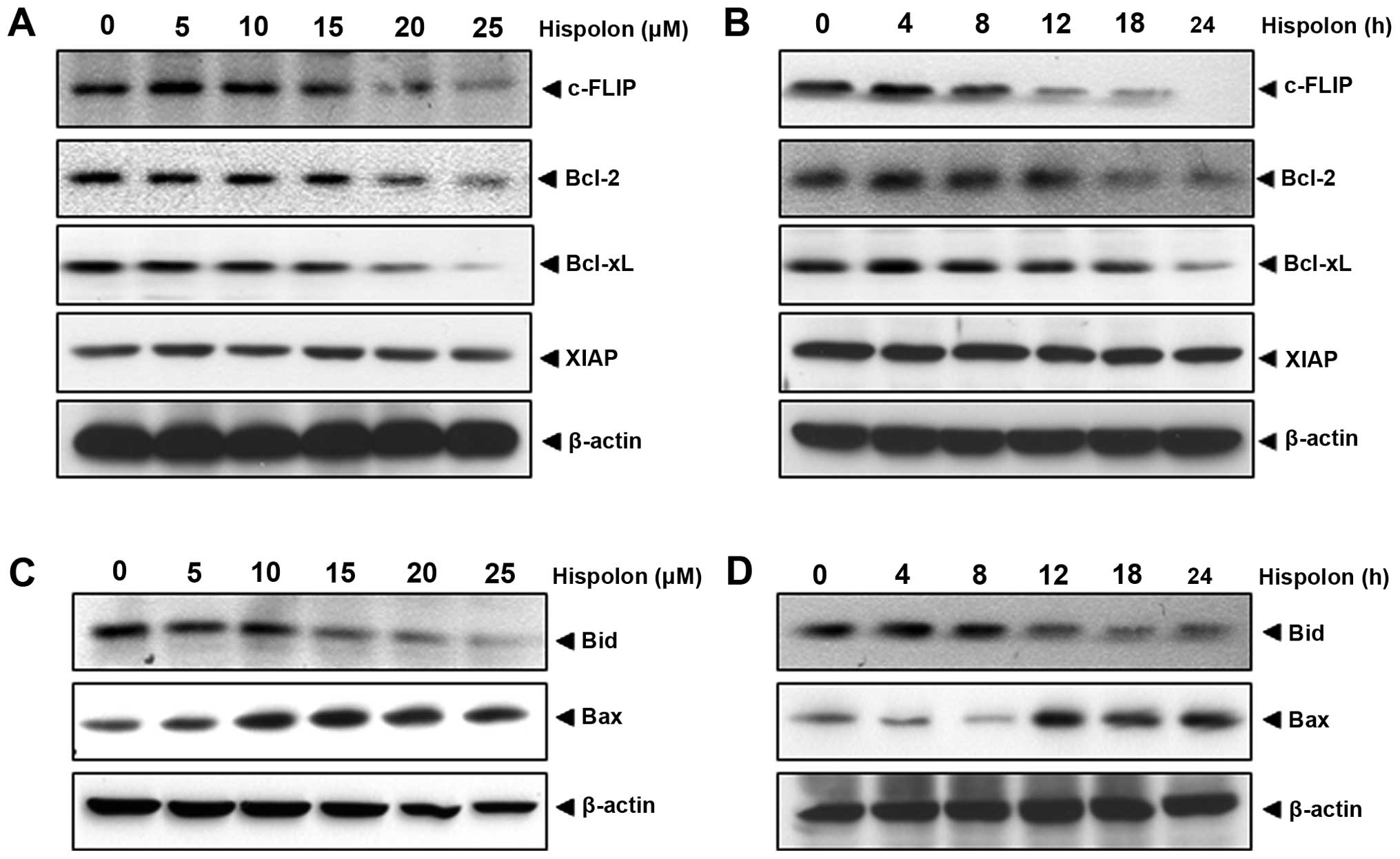
Hispolon modulation of anti-apoptotic proteins
linked to TRAIL resistance in human colon cancer cells was
examined. Results indicate the expression of c-FLIP, Bcl-2 and
Bcl-xL was strongly downregulated in a concentration-dependent
manner, while the downregulation of XIAP was not visible (Fig. 2A). Time-dependent effect of hispolon
on the expression of these cell survival proteins was also
examined. As shown in Fig. 2B,
c-FLIP, Bcl-2 and Bcl-xL were downregulated by hispolon in a
time-dependent manner.
Hispolon upregulates the apoptotic
progression
Whether hispolon regulates the expression of
pro-apoptotic proteins, Bid and Bax, was examined. Results showed
that hispolon upregulated the expression of Bax in a concentration-
and time-dependent manner in HCT-116 cells (Fig. 2C and D). The pro-apoptotic protein
Bid, in which N-terminal helices are cleaved by caspase-8 to the
truncated active form for apoptosis was downregulated
concentration- and time-dependently. These results suggest that
hispolon can upregulate the apoptotic progression.
Hispolon upregulates expression of the
death receptor
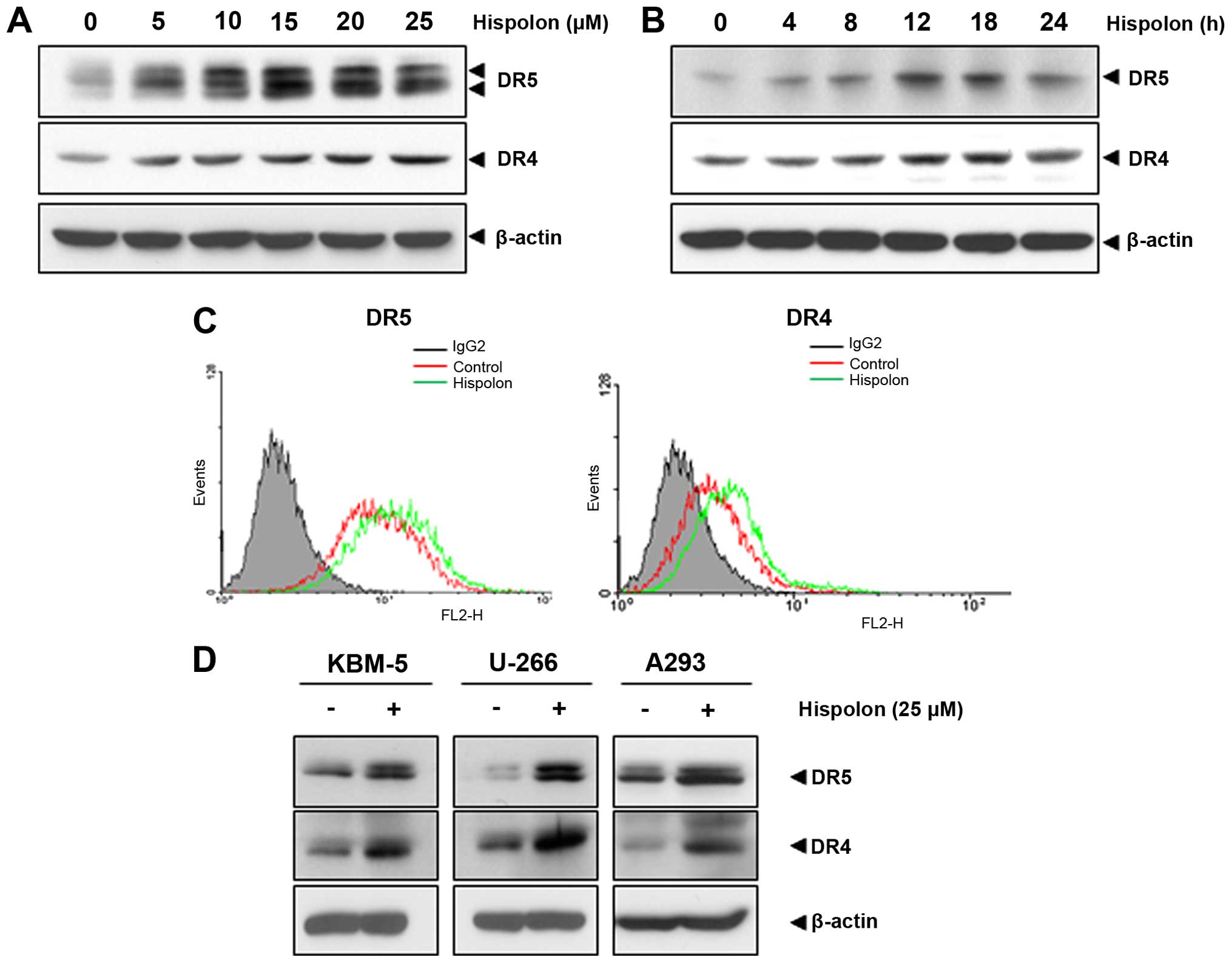
To understand further possible mechanisms of
regulation of TRAIL-induced apoptosis by hispolon, the effect of
hispolon on expression of the death receptor was investigated in
human colon cancer cells. As shown in Fig. 3A and B, hispolon upregulated DR4 and
DR5 expression in HCT116 cells concentration- and a
time-dependently, but upregulation of DR4 was less pronounced.
Result also indicated that treatment of cells with 25 µM
hispolon for 12–18 h was optimal for inducing DR4 and DR5. As shown
in Fig. 3C, hispolon also increased
expression of the death receptors on the cell surface, investigated
by flow cytometry.
Upregulation of the death receptor is not
cell type-dependent
We investigated whether upregulation of death
receptor by hispolon is specific to human colon cancer cells. As
shown in Fig. 3D, the results
indicated that hispolon upregulated both DR4 and DR5 in chronic
myeloid leukemia cell (KBM-5), multiple myeloma cell (U-266) and
embryonic kidney carcinoma (A293). These results suggest that the
upregulation of DRs by hispolon is not cell type-specific.
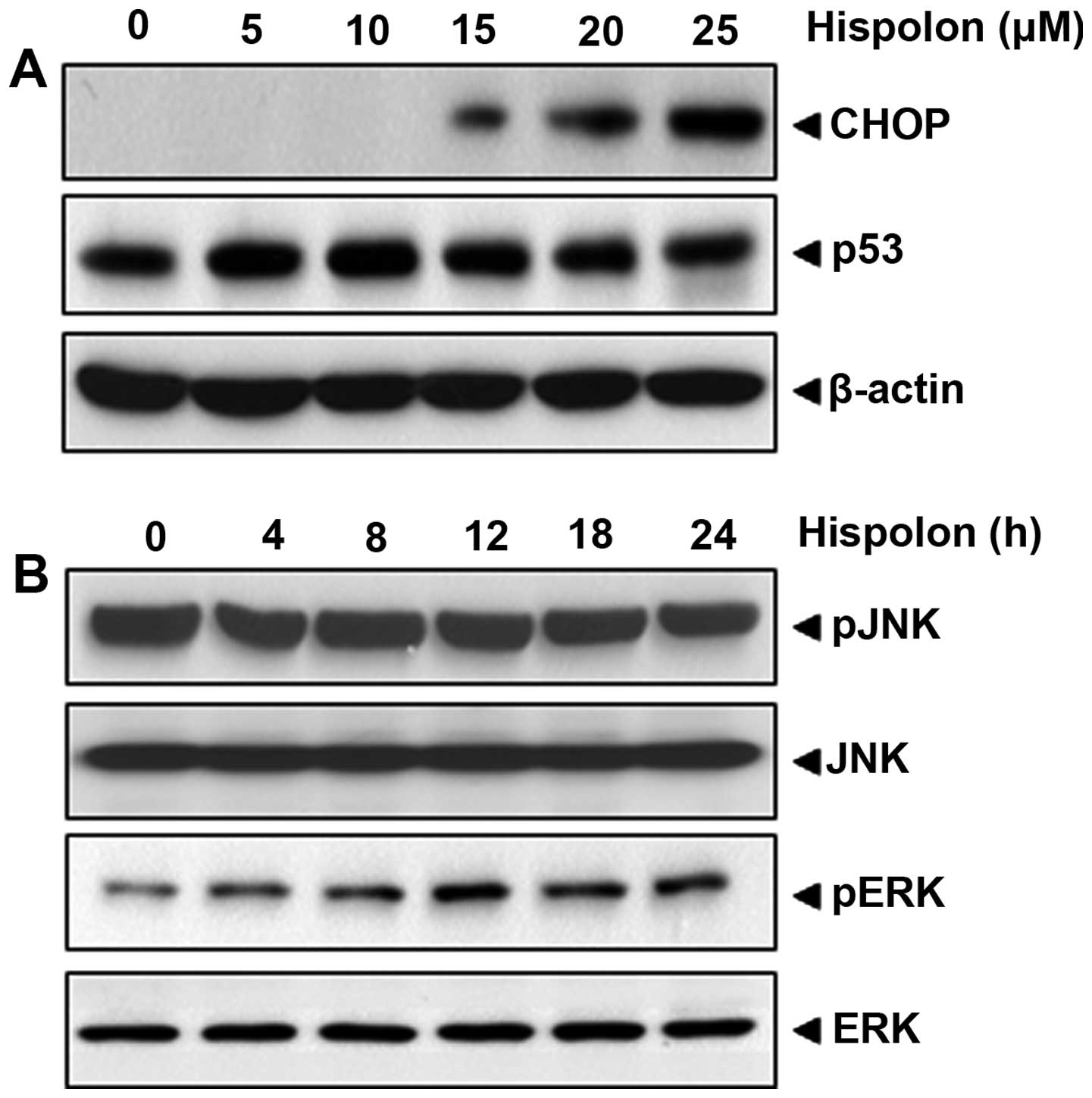
Hispolon upregulates CHOP
CHOP has been previously linked with the
upregulation of DR5 expression (32,33),
thus CHOP in relation to hispolon-induced DRs was investigated. Our
result indicated that hispolon induced the expression of CHOP, with
optimum induction occurring at over 20 µM (Fig. 4A).
Hispolon-induced upregulation of the
death receptor is p53 independent
Because p53 is known to induce the TRAIL receptor,
hispolon modulation of the expression of p53 in HCT-116 cells was
investigated. As shown in Fig. 4A,
the expression of p53 was not changed in colon cancer cells,
suggesting that p53 is unnecessary for hispolon-induced
upregulation of TRAIL receptors.
Activation of JNK by hispolon is
unnecssary but activation of ERK is linked to the role of
hispolon
Various studies reported the importance of MAPK
activation in TRAIL receptor induction (34,35).
Thus, we investigated the hispolon-induced activation of ERK and
JNK, respectively. When cells were treated with hispolon,
phosphor-ERK was increased in a time-dependent manner, whereas
hispolon could not induce JNK activation (Fig. 4B). These results suggest that
hispolon-induced apoptosis is possibly linked to the ERK pathway,
but not to the JNK pathway.
Discussion
TRAIL is a very promising cytokine molecule for
anticancer therapy, but it has a limitation caused by the
resistance developed by certain cancer types. The development of
agents that sensitize cancer cells to TRAIL is important for the
success of the promising TRAIL-based cancer therapy. In the present
study, we showed that hispolon enhances TRAIL-induced apoptosis of
human colon cancer cells and investigate the molecular mechanism of
sensitizing human cancer cells to TRAIL.
Hispolon was identified to obviously enhance the
TRAIL-induced apoptosis in HCT-116, human colon cancer cells,
through live/dead and MTT assays. HCT-116 belongs to type II cells,
classified based on how apoptosis induction is employed upon DISC
activation (36). Type II cells
have been defined to be dependent on mitochondria for the induction
of the Fas death receptor-mediated apoptosis, while type I cells
are mitochondria-independent (37).
For this reason, there is a significant possibility of a
complicated mechanism for TRAIL-induced apoptosis in HCT-116.
Considering the pivotal role of caspases in the initiation and
execution of apoptosis, caspase activation by hispolon was
examined. We found that hispolon activated caspase-8, caspase-9 and
caspase-3, enzymes known to be involved in type II TRAIL-induced
apoptosis pathway. This activation of caspase was also supported by
PARP cleavage, a known hallmark of apoptosis and caspase
activation. Downregulation of cFLIP, an inhibitor of caspase-8, was
also found and suggested that upregulation of caspase-8 activity
could be from reduced expression of cFLIP. However, XIAP, known to
be a potent inhibitor of caspase-3 and caspase-9, was not regulated
by hispolon, thus ruling out the possibility that upregulations of
caspase-3 and caspase-9 are caused by downregulation of XIAP.
The expression of Bcl-2 and Bcl-xL was also
downregulated by hispolon. These proteins have been linked to
suppression of apoptosis by TRAIL, frequently found in TRAIL
resistance in a variety of cancer cells including colon (38), breast (39) and kidney cancers (40). The reasons for downregulation of
anti-apoptotic proteins including Bcl-2 and Bcl-xL have not been
clearly elucidated. Previously, it was reported that hispolon
downregulates NF-κB (41) as most
anti-apoptotic proteins are regulated by NF-κB. Taken together,
hispolon-induced downregulation of these anti-apoptotic proteins
may also be regulated by the downregulation of NF-κB in colon
cancer cells. Our findings suggest pro-apoptosis proteins, Bax and
Bid, also contribute to TRAIL-induced apoptosis. The results that
upregulation of Bax and downregulation of Bid can be explained by
the mitochondrial-dependent apoptosis mechanism through
caspase-8-Bid-tBid-Bax pathway.
We further found that hispolon significantly induced
the expression of both the TRAIL receptors, DR4 and DR5. We also
demonstrated the upregulation of death receptors on the cell
surface by hispolon. Moreover, hispolon-induced death receptor
upregulation is not tissue-specific, which means hispolon can be
applied in treatment to various cancer patients as a combination
therapy with TRAIL. A few agents have been shown to upregulate the
death receptor in human colon cancer cells including capsazepine
(42) and azadirone (43). These results suggested that the
death receptor is essential for TRAIL-induced apoptosis.
Upregulation of the death receptor in HCT-116 by hispolon may play
an important role in TRAIL-induced apoptosis similarly to other
agents mentioned above.
The molecular mechanism of DR4 and DR5 induction in
colon cancer cells was also investigated. Numerous mechanisms have
been suggested for induction of this death receptor, including ROS
generation, p53 induction and NF-κB, DNA damage-inducible
transcript 3 (DDIT3), peroxisome proliferator-activated receptor
and MAPK activation (33,44–46).
CHOP is also known to be a regulator of the death receptor via
binding of CHOP to the death receptor promoter (32,33,47).
We found that CHOP was upregulated by hispolon in HCT-116 cells and
hispolon can induce the death receptor through CHOP mediated
mechanism. We also found that hispolon-induced DR5 was related to
ERK activation, but independent of JNK. In agreement of these
observations, a previous study reported the important role of ERK
in upregulation of death receptors by zerumbone (48). In addition, azadirone and gossypol
also demonstrated that these molecules induce death receptor
through activation of the ROS-ERK-CHOP pathway in human colon
cancer cells. ROS was not examined when we explored this mechanism,
however, ROS is known to be an up-stream regulator of JNK, p38 and
ERK. In addition, hispolon induction of apoptosis through
ROS-mediated mitochondria pathway was reported (28). Considering the information now
available, hispolon may induce TRAIL-induced apoptosis through
ROS-ERK-CHOP-mediated upregulation of the death receptor.
Another mechanism, induction of apoptosis through
p53 was also investigated because of the importance of this pathway
in the response to cell stress such as chemotherapy and
radiotherapy. In this pathway, the upregulation of p53 is essential
for an apoptosis event, however, hispolon could not induce p53 in
HCT-116 cells. In other words, hispolon-induced apoptosis is not
involved in the extrinsic apoptosis pathway, which corresponds to
the type II classification of HCT-116.
Overall, our studies provide strong evidence that
hispolon could potentiate TRAIL-induced apoptosis hypothetically
through ROS-, ERK- and CHOP-mediated upregulation of death
receptors. The enhancement of apoptosis by hispolon was also shown
to be related to the upregulation of cell survival proteins and the
upregulation of pro-apoptotic proteins. Hispolon combined with
TRAIL may be a good candidate for anticancer therapy, however,
further studies using animal models are needed to realize this
combination anticancer therapy.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(NRF-2013R1A1A1062064).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka M, Suda T, Yatomi T, Nakamura N and
Nagata S: Lethal effect of recombinant human Fas ligand in mice
pretreated with Propionibacterium acnes. J Immunol. 158:2303–2309.
1997.PubMed/NCBI
|
|
6
|
Walczak H, Degli-Esposti MA, Johnson RS,
Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA,
Smith CA, et al: TRAIL-R2: A novel apoptosis-mediating receptor for
TRAIL. EMBO J. 16:5386–5397. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
French LE and Tschopp J: The TRAIL to
selective tumor death. Nat med. 5:146–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gura T: How TRAIL kills cancer cells, but
not normal cells. Science. 277:7681997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhardwaj A and Aggarwal BB:
Receptor-mediated choreography of life and death. J Clin Immunol.
23:317–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Doi T, Murakami H, Ohtsu A, Fuse N,
Yoshino T, Yamamoto N, Boku N, Onozawa Y, Hsu CP, Gorski KS, et al:
Phase 1 study of conatumumab, a pro-apoptotic death receptor 5
agonist antibody, in Japanese patients with advanced solid tumors.
Cancer Chemother Pharmacol. 68:733–741. 2011. View Article : Google Scholar
|
|
12
|
Maksimovic-Ivanic D, Stosic-Grujicic S,
Nicoletti F and Mijatovic S: Resistance to TRAIL and how to
surmount it. Immunol Res. 52:157–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fulda S, Küfer MU, Meyer E, Van Valen F,
Dockhorn-Dworniczak B and Debatin KM: Sensitization for death
receptor- or drug-induced apoptosis by re-expression of caspase-8
through demethylation or gene transfer. Oncogene. 20:5865–5877.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eggert A, Grotzer MA, Zuzak TJ, Wiewrodt
BR, Ho R, Ikegaki N and Brodeur GM: Resistance to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
in neuroblastoma cells correlates with a loss of caspase-8
expression. Cancer Res. 61:1314–1319. 2001.PubMed/NCBI
|
|
15
|
Bodmer JL, Holler N, Reynard S,
Vinciguerra P, Schneider P, Juo P, Blenis J and Tschopp J: TRAIL
receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell
Biol. 2:241–243. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tepper CG and Seldin MF: Modulation of
caspase-8 and FLICE-inhibitory protein expression as a potential
mechanism of Epstein-Barr virus tumorigenesis in Burkitt's
lymphoma. Blood. 94:1727–1737. 1999.PubMed/NCBI
|
|
17
|
Okano H, Shiraki K, Inoue H, Kawakita T,
Yamanaka T, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Fujikawa K,
et al: Cellular FLICE/caspase-8-inhibitory protein as a principal
regulator of cell death and survival in human hepatocellular
carcinoma. Lab Invest. 83:1033–1043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schimmer AD, Welsh K, Pinilla C, Wang Z,
Krajewska M, Bonneau MJ, Pedersen IM, Kitada S, Scott FL,
Bailly-Maitre B, et al: Small-molecule antagonists of apoptosis
suppressor XIAP exhibit broad antitumor activity. Cancer Cell.
5:25–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ricci MS, Kim SH, Ogi K, Plastaras JP,
Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, et al:
Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib
sensitizes resistant human cancer cells to TRAIL-induced death.
Cancer Cell. 12:66–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ravi R and Bedi A: Requirement of BAX for
TRAIL/Apo2L-induced apoptosis of colorectal cancers: Synergism with
sulindac-mediated inhibition of Bcl-xL. Cancer Res. 62:1583–1587.
2002.PubMed/NCBI
|
|
21
|
Hinz S, Trauzold A, Boenicke L, Sandberg
C, Beckmann S, Bayer E, Walczak H, Kalthoff H and Ungefroren H:
Bcl-xL protects pancreatic adenocarcinoma cells against CD95- and
TRAIL-receptor-mediated apoptosis. Oncogene. 19:5477–5486. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kandasamy K, Srinivasula SM, Alnemri ES,
Thompson CB, Korsmeyer SJ, Bryant JL and Srivastava RK: Involvement
of proapoptotic molecules Bax and Bak in tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced
mitochondrial disruption and apoptosis: Differential regulation of
cytochrome c and Smac/DIABLO release. Cancer Res. 63:1712–1721.
2003.PubMed/NCBI
|
|
23
|
Huang GJ, Deng JS, Chiu CS, Liao JC, Hsieh
WT, Sheu MJ and Wu CH: Hispolon protects against acute liver damage
in the rat by inhibiting lipid peroxidation, proinflammatory
cytokine, and oxidative stress and downregulating the expressions
of iNOS, COX-2, and MMP-9. Evid Based Complement Alternat med.
2012:4807142012. View Article : Google Scholar
|
|
24
|
Chien YC, Huang GJ, Cheng HC, Wu CH and
Sheu MJ: Hispolon attenuates balloon-injured neointimal formation
and modulates vascular smooth muscle cell migration via AKT and ERK
phosphorylation. J Nat Prod. 75:1524–1533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ali NA, Lüdtke J, Pilgrim H and Lindequist
U: Inhibition of chemiluminescence response of human mononuclear
cells and suppression of mitogen-induced proliferation of spleen
lymphocytes of mice by hispolon and hispidin. Pharmazie.
51:667–670. 1996.PubMed/NCBI
|
|
26
|
Lu TL, Huang GJ, Lu TJ, Wu JB, Wu CH, Yang
TC, Iizuka A and Chen YF: Hispolon from Phellinus linteus has
antiproliferative effects via MDM2-recruited ERK1/2 activity in
breast and bladder cancer cells. Food Chem Toxicol. 47:2013–2021.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang GJ, Deng JS, Huang SS and Hu ML:
Hispolon induces apoptosis and cell cycle arrest of human
hepatocellular carcinoma Hep3B cells by modulating ERK
phosphorylation. J Agric Food Chem. 59:7104–7113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Zhao Z, Li L, Wu B, Chen SF, Zhou
H, Wang Y and Li YQ: Hispolon induces apoptosis in human gastric
cancer cells through a ROS-mediated mitochondrial pathway. Free
Radic Biol Med. 45:60–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang GJ, Yang CM, Chang YS, Amagaya S,
Wang HC, Hou WC, Huang SS and Hu ML: Hispolon suppresses SK-Hep1
human hepatoma cell metastasis by inhibiting matrix
metallo-proteinase-2/9 and urokinase-plasminogen activator through
the PI3K/Akt and ERK signaling pathways. J Agric Food Chem.
58:9468–9475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen W, He FY and Li YQ: The apoptosis
effect of hispolon from Phellinus linteus (Berkeley & Curtis)
Teng on human epidermoid KB cells. J Ethnopharmacol. 105:280–285.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sung B, Park B, Yadav VR and Aggarwal BB:
Celastrol, a triterpene, enhances TRAIL-induced apoptosis through
the downregulation of cell survival proteins and upregulation of
death receptors. J Biol Chem. 285:11498–11507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshida T, Shiraishi T, Nakata S, Horinaka
M, Wakada M, Mizutani Y, Miki T and Sakai T: Proteasome inhibitor
MG132 induces death receptor 5 through CCAAT/enhancer-binding
protein homologous protein. Cancer Res. 65:5662–5667. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ichijo H: From receptors to
stress-activated MAP kinases. Oncogene. 18:6087–6093. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sarker M, Ruiz-Ruiz C and López-Rivas A:
Activation of protein kinase C inhibits TRAIL-induced caspases
activation, mitochondrial events and apoptosis in a human leukemic
T cell line. Cell Death Differ. 8:172–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ozoren N, Kim K, Burns TF, Dicker DT,
Moscioni AD and El-Deiry WS: The caspase-9 inhibitor Z-LEHD-FMK
protects human liver cells while permitting death of cancer cells
exposed to tumor necrosis factor-related apoptosis-inducing ligand.
Cancer Res. 60:6259–6265. 2000.PubMed/NCBI
|
|
37
|
Krammer PH: CD95's deadly mission in the
immune system. Nature. 407:789–795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cummins JM, Kohli M, Rago C, Kinzler KW,
Vogelstein B and Bunz F: X-linked inhibitor of apoptosis protein
(XIAP) is a nonredundant modulator of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human
cancer cells. Cancer Res. 64:3006–3008. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee TJ, Lee JT, Park JW and Kwon TK:
Acquired TRAIL resistance in human breast cancer cells are caused
by the sustained cFLIP(L) and XIAP protein levels and ERK
activation. Biochem Biophys Res Commun. 351:1024–1030. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs
BS, Lindner DJ and Borden EC: Downregulation of Bcl-2, FLIP or IAPs
(XIAP and survivin) by siRNAs sensitizes resistant melanoma cells
to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 11:915–923.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ravindran J, Subbaraju GV, Ramani MV, Sung
B and Aggarwal BB: Bisdemethylcurcumin and structurally related
hispolon analogues of curcumin exhibit enhanced prooxidant,
anti-proliferative and anti-inflammatory activities in vitro.
Biochem Pharmacol. 79:1658–1666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sung YH, Park KH, Lee YB, Park HM, Shin
DJ, Park JS, Oh MS, Ma HI, Yu KH, Kang SY, et al: Midbrain atrophy
in subcortical ischemic vascular dementia. J Neurol. 256:1997–2002.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gupta SC, Francis SK, Nair MS, Mo YY and
Aggarwal BB: Azadirone, a limonoid tetranortriterpene, induces
death receptors and sensitizes human cancer cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) through a p53
protein-independent mechanism: Evidence for the role of the
ROS-ERK-CHOP-death receptor pathway. J Biol Chem. 288:32343–32356.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ravi R, Bedi GC, Engstrom LW, Zeng Q,
Mookerjee B, Gélinas C, Fuchs EJ and Bedi A: Regulation of death
receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB.
Nat Cell Biol. 3:409–416. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shenoy K, Wu Y and Pervaiz S: LY303511
enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via
hydrogen peroxide-mediated mitogen-activated protein kinase
activation and upregulation of death receptors. Cancer Res.
69:1941–1950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu GS, Burns TF, McDonald ER III, Jiang W,
Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu M, Xia L, Hua H and Jing Y:
Acetyl-keto-beta-boswellic acid induces apoptosis through a death
receptor 5-mediated pathway in prostate cancer cells. Cancer Res.
68:1180–1186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yodkeeree S, Sung B, Limtrakul P and
Aggarwal BB: Zerumbone enhances TRAIL-induced apoptosis through the
induction of death receptors in human colon cancer cells: Evidence
for an essential role of reactive oxygen species. Cancer Res.
69:6581–6589. 2009. View Article : Google Scholar : PubMed/NCBI
|