Introduction
Osteosarcoma comprises tumor osteoblasts and
bone-like tissue, with malignant tumors originating for
osteogenesis organization. Osteosarcoma is a primary malignant bone
tumor (1). The disease is
characterized by a high degree of tumor malignancy and poor
prognosis, while the 3- to 5-year survival rate after amputation is
only 5–20% (1). Advances in
treatment such as chemotherapy and surgery have improved the
patient survival rate. However, even with combinatorial treatment
comprising chemotherapy and surgery, the 5-year survival rate
remains at 55–68% (2). Metastatic
spread is the main cause for the poor prognosis of osteosarcoma.
However, transfer therapies currently available are not
satisfactory. Additionally, the side effects of systemic
chemotherapy cause damage to internal organs of the human body
(3,4). Invasion and metastasis of osteosarcoma
seriously affect the quality of life and prognosis of patients.
Gene therapy remains a hotspot in the study of osteosarcoma, while
the specific mechanism involved remains to be determined. Gene
therapy continues to be considered an effective form of treatment
for osteosarcoma (5).
Ginkgo biloba is the only species in the Ginkgo
genus of the family of ginkgoaceae, and comprises the components
ginkgolides and bilobalide, which are present in the leaf and
velamen of the plant (6). Ginkgetin
is a biflavone isolated from the ginkgo biloba leaves (7) and its effects on cyclooxygenase and
anti-inflammatory activity in the body have been previously
investigated (8). Ginkgetin exerts
anti-inflammatory, antioxidant, as well as anticancer effects
(6,8,9). Thus,
in the present study, the possible inhibitory and apoptotic effects
of ginkgetin in osteosarcoma cells, as well as the underlying
mechanisms involved were investigated.
Materials and methods
Chemical reagents
Dulbecco's modified Eagle's medium/Ham's F-12 medium
(DMEM/F-12) and fetal bovine serum (FBS) were obtained from
Invitrogen (Grand Island, NY, USA). Trypsin,
3.3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) and lactate dehydrogenase (LDH) were obtained from the
Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). The
Annexin V-FITC/propidium iodide (PI) kit was obtained from BD
Biosciences (San Jose, CA, USA).
Cell culture and cell proliferation
assay
The present study was approved by the regional
Ethics Committee of the Affiliated Dongfeng Hospital, Hubei
University of Medicine (Hubei, China). Written informed consent was
obtained from all the patients. Giant cell tumor samples were
collected from patients at the Affiliated Dongfeng Hospital, Hubei
University of Medicine. The samples were separated and sectioned in
medium containing DMEM/F-12, supplemented with 10% FBS and 100 U/ml
penicillin and 100 mg/ml streptomycin. The giant cell tumor samples
were digested with 0.01% trypsin (Beyotime Institute of
Biotechnology) at room temperature for 10–30 min. The samples were
transferred into 25-cm2 flasks, and the cell samples
were incubated at 37°C in a water-saturated atmosphere of 95% air
and 5% CO2. The culture medium was replaced half with
fresh complete medium every 2 days. Primary cultures were
subcultured and stored in liquid nitrogen at 37°C in a
water-saturated atmosphere of 95% air and 5% CO2 until
cell confluence was reached.
MTT assay
To determine cell growth, osteosarcoma cells were
seeded in a 96-well plate (1×103 cells/well) and
cultured with ginkgetin (0, 5, 10, 20, 30, 40, 50 and 60 µM)
for 24 h at room temperature of 37°C in a humidified atmosphere of
5% CO2. MTT solution (20 µl) was added to each
well and the samples were incubated for 4 h at a temperature of
37°C in a humidified atmosphere of 5% CO2.
Dimethylsulfoxide (DMSO) solution) 150 µl) was added to each
well followed by gentle agitation for 20 min. The cell growth of
each well was measured at λ=570 nm using a multiscanner (XL-818;
Bio-Tek, Winooski, VT, USA).
LDH assay
To determine cytotoxicity, osteosarcoma cells were
seeded in a 96-well plate (1×103 cells/well) and
cultured with ginkgetin (0, 5, 10, 20, 30, 40, 50 and 60 µM)
for 24 h at a room temperature of 37°C in a humidified atmosphere
of 5% CO2. LDH solution (100 µl) was added into
each well and incubated for 30 min at a temperature of 37°C in a
humidified atmosphere of 5% CO2. The cell growth of each
well was measured at λ=490 nm using a multiscanner (XL-818).
Flow cytometry
To determine cell apoptosis, osteosarcoma cells were
seeded in a 6-well plate (1.5×106 cells/well) and
cultured with ginkgetin (0, 20, 30 and 40 µM) for 24 h at a
room temperature of 37°C in a humidified atmosphere of 5%
CO2. Annexin-V/FITC (10 µl) and 10 µl of
PI were added into each well and incubated for 30 min in the dark.
Cell apoptosis was detected using flow cytometry (EPICS®
Altra™, Brea, CA, USA).
Western blot analysis
Osteosarcoma cells were seeded in a 6-well plate
(1.5×106 cells/well) and cultured with ginkgetin (0, 20,
30 and 40 µM) for 24 h at a room temperature of 37°C in a
humidified atmosphere of 5% CO2. The osteo sarcoma cells
were resuspended using lysis buffer containing RIPA lysis buffer
(Beyotime Institute of Biotechnology) for 30 min on ice. The
protein concentration was determined with Coomassie blue staining.
Protein sample (20 µg) was used to load the sodium dodecyl
sulfate (SDS)-polyacrylamide gel and the samples were transferred
onto a cellulose nitrate film (Hybond™-C; Amersham Biosciences,
Piscataway, NJ, USA). The cellulose nitrate film was blocked with
tris-buffered saline (TBS) containing 5% non-fat milk to block
non-specific binding sites. The cellulose nitrate film was
incubated with anti-p-STAT3 (phosphorylation-STAT3, 1:1,000),
anti-Bcl-2 (1:2,000), anti-Bcl-xL (1:1,000), anti-cyclin D1
(1:1,000), anti-survivin (1:1,500), anti-total PARP (1:2,000) (all
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
anti-β-actin (1:500; Sangon Biotech, Shanghai, China) overnight at
4°C. The cellulose nitrate film was washed three times with 0.1%
(v/v) Tween-20 in tris-buffered saline (TTBS) and incubated with
the secondary antibody (1:5,000; Santa Cruz Biotechnology, Inc.).
The film was subsequently washed using an ECL Advanced Western Blot
Detection kit (Beyotime Biotech, Nanjing, China). The resultant
bands were detected using the gel imaging system (GDS8000; Ultra
Violet Products, Upland, CA, USA).
Caspase-3/9 activation
Osteosarcoma cells were seeded in a 6-well plate
(1.5×106 cells/well) and cultured with ginkgetin (0, 20,
30 and 40 µM) for 24 h at a room temperature of 37°C in a
humidified atmosphere of 5% CO2. Osteosarcoma cells were
resuspended using lysis buffer containing RIPA lysis buffer for 30
min on ice. The protein concentration was determined with Coomassie
blue staining. The caspase-3/9 activation was visualized using
fluorescence and was detected at the wavelength of 405 nm with the
caspase-3 and -9 colorimetric assay kits (Beyotime Institute of
Biotechnology).
Statistical analysis
Data were presented as means ± SD and the degree of
significance was analyzed by the Student's t-test. Differences were
considered to indicate a statistically significant result at
P=0.05.
Results
Inhibitory growth effect of ginkgetin in
osteosarcoma cells
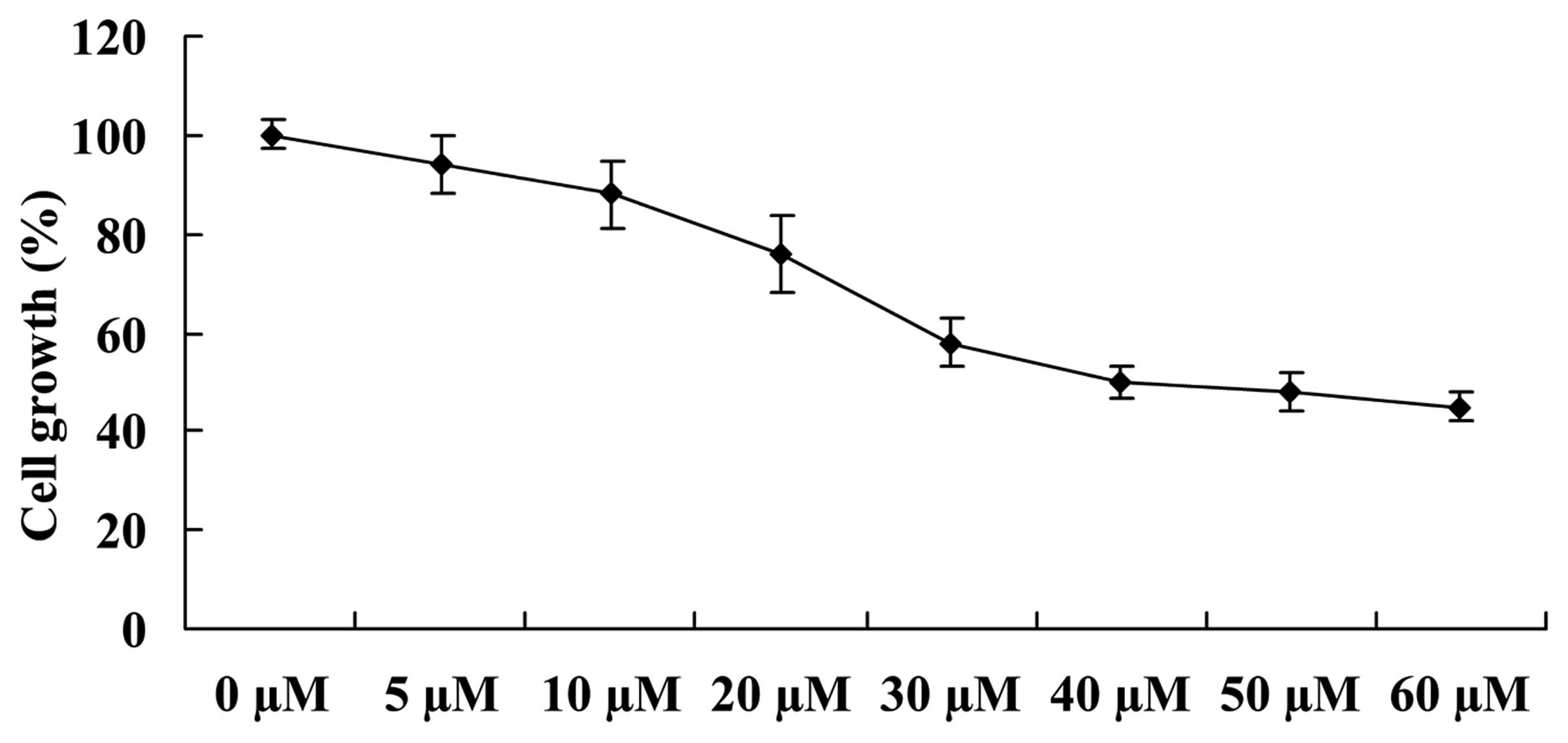
To identify the growth inhibitory effect of
ginkgetin in osteosarcoma cells, the cell growth was measured using
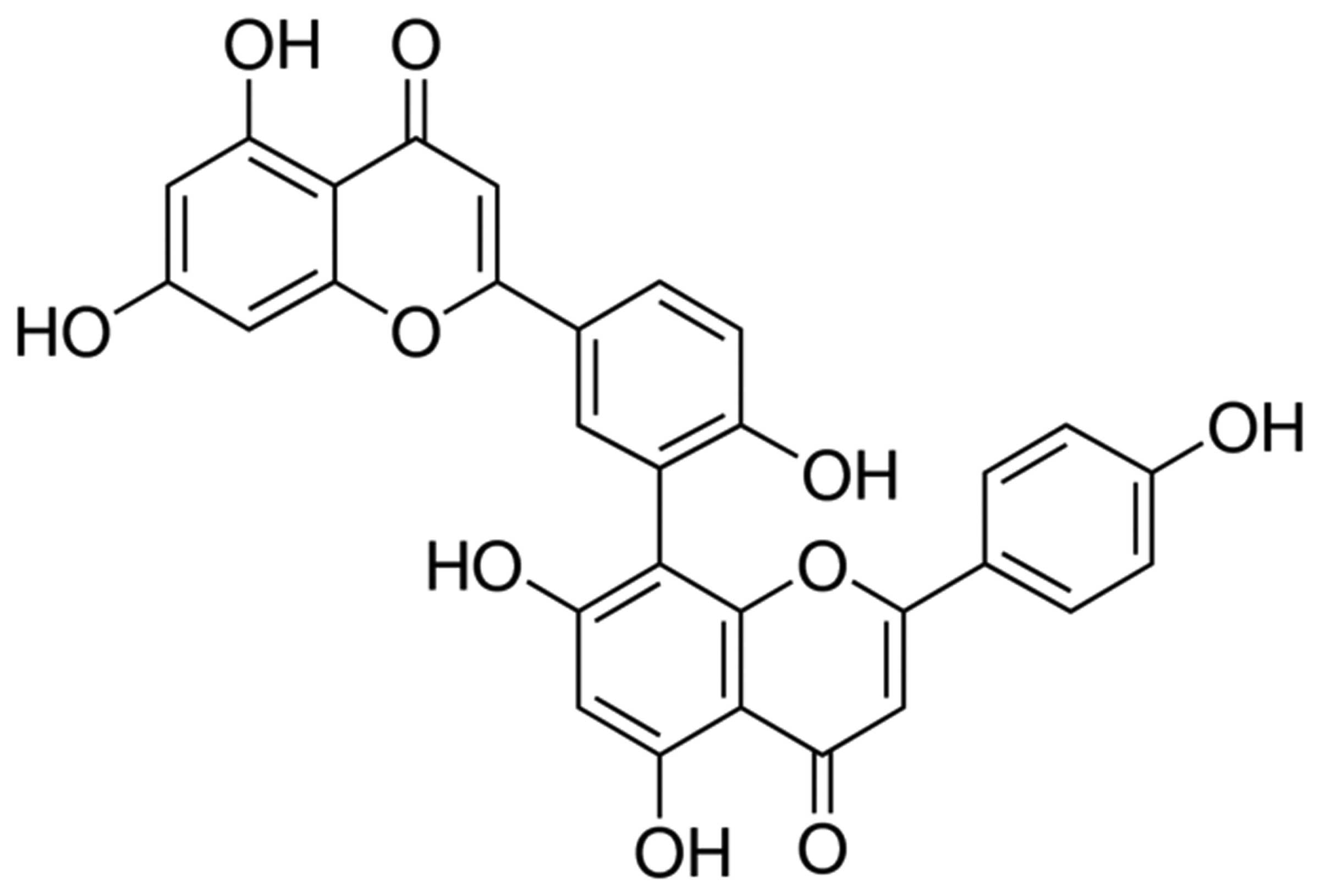
an MTT assay. The structure of ginkgetin is shown in Fig. 1. Ginkgetin was identified as
exerting a potential anticancer effect on osteosarcoma cells by
inhibiting the growth of osteosarcoma cells in a dose-dependent
manner. Thus, 35.5 µM of ginkgetin exerted a 50% inhibitory
cell growth effect on osteosarcoma cells (Fig. 2).
Growth inhibitory effect of ginkgetin
results in an increase in the cytotoxicity of osteosarcoma
cells
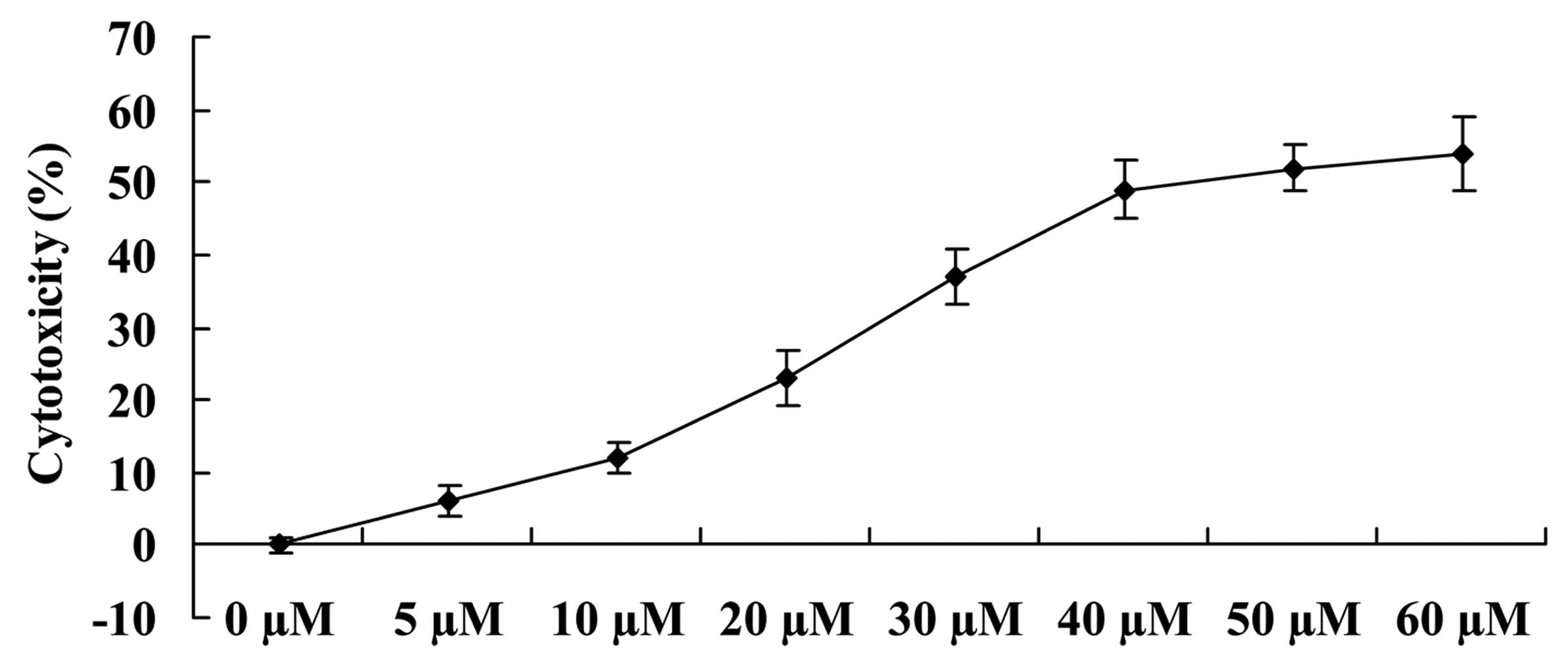
We examined whether the growth inhibitory effects of
ginkgetin increased the cytotoxicity of osteosarcoma cells. As
shown in Fig. 3, ginkgetin
effectively induced the cytotoxicity of osteosarcoma cells in a
dose-dependent manner. Thus, 41.2 µM of ginkgetin resulted
in a 50% increase of cytotoxicity of osteosarcoma cells, compared
to the 0 µM ginkgetin-treated group.
Apoptotic effect of ginkgetin on
osteosarcoma cells
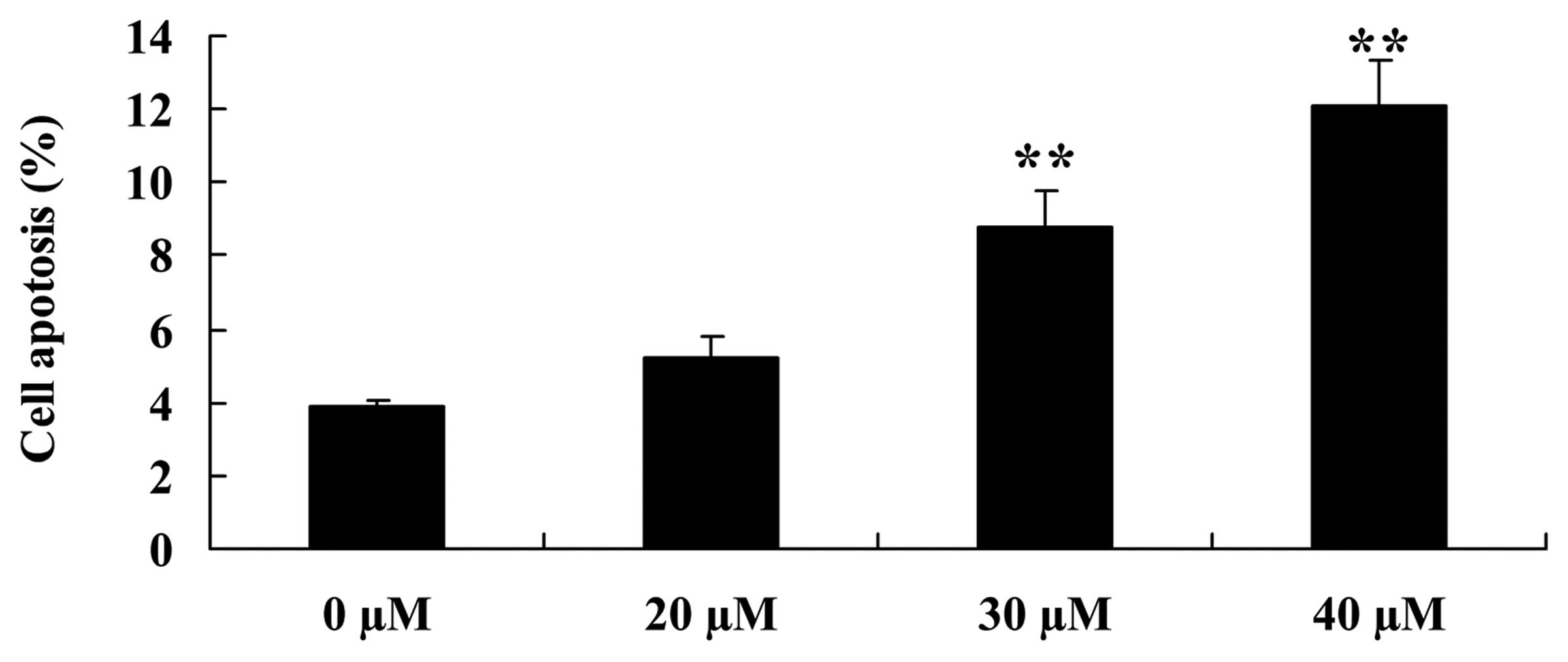
To examine the apoptotic effect of ginkgetin on
osteosarcoma cells, flow cytometry was used to analyze the
apoptosis of osteosarcoma cells. Osteosarcoma cells were treated
with ginkgetin at 0, 20, 30 and 40 µM. The results showed
that ginkgetin markedly induced the apoptosis of osteosarcoma cells
in a concentration-dependent manner, suggesting 30 or 40 µM
of ginkgetin induced apoptosis of osteosarcoma cells, and the
result was statistically significant (Fig. 4).
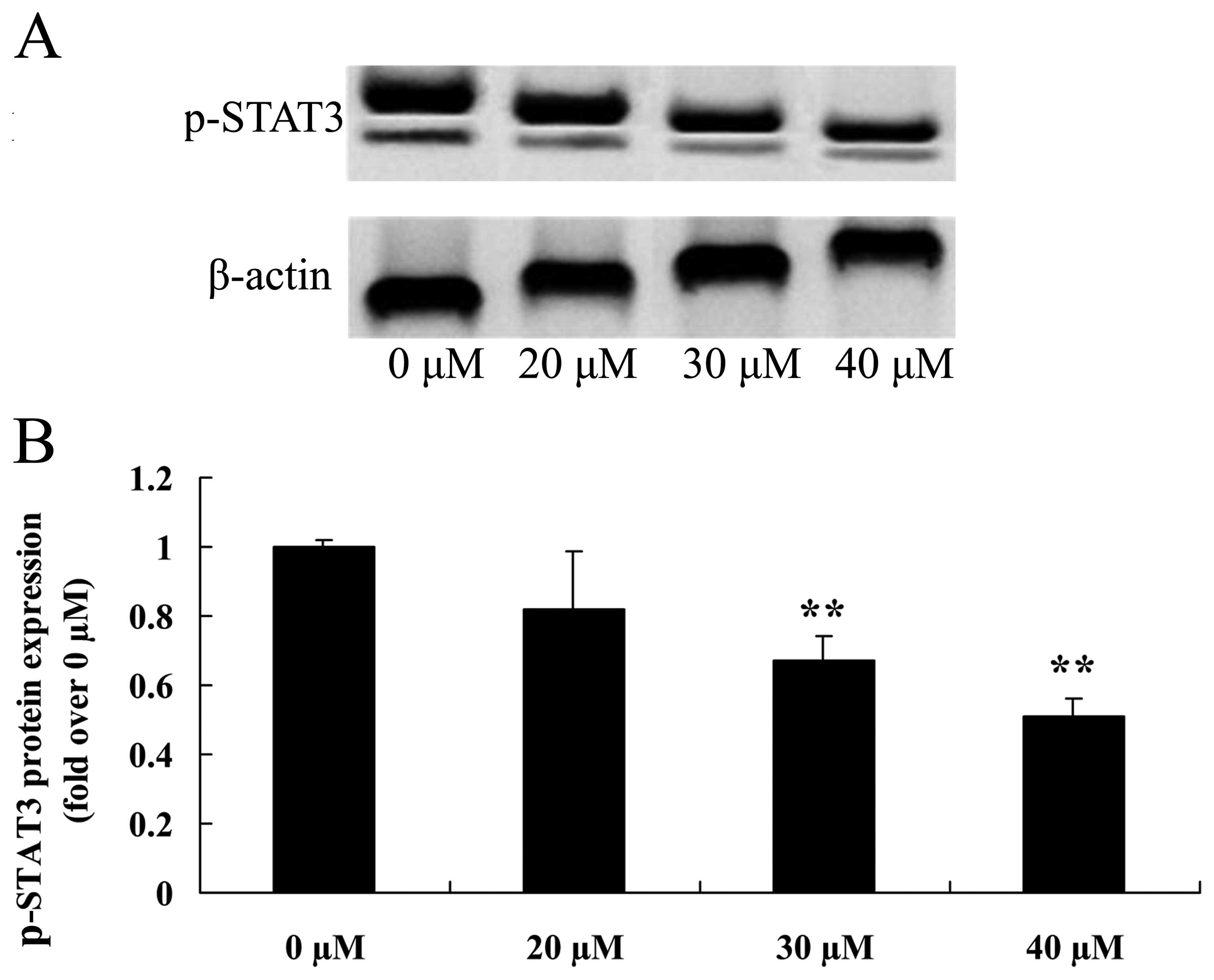
Inhibitory growth effect suppresses STAT3
of osteosarcoma cells
As shown in Fig. 5A and
B, pretreatment with ginkgetin markedly suppressed the p-STAT3
protein expression of osteosarcoma cells in a dose-dependent
manner. Thus, 30 or 40 µM of ginkgetin suppressed the
p-STAT3 protein expression in osteosarcoma cells, and the result
was statistically significant.
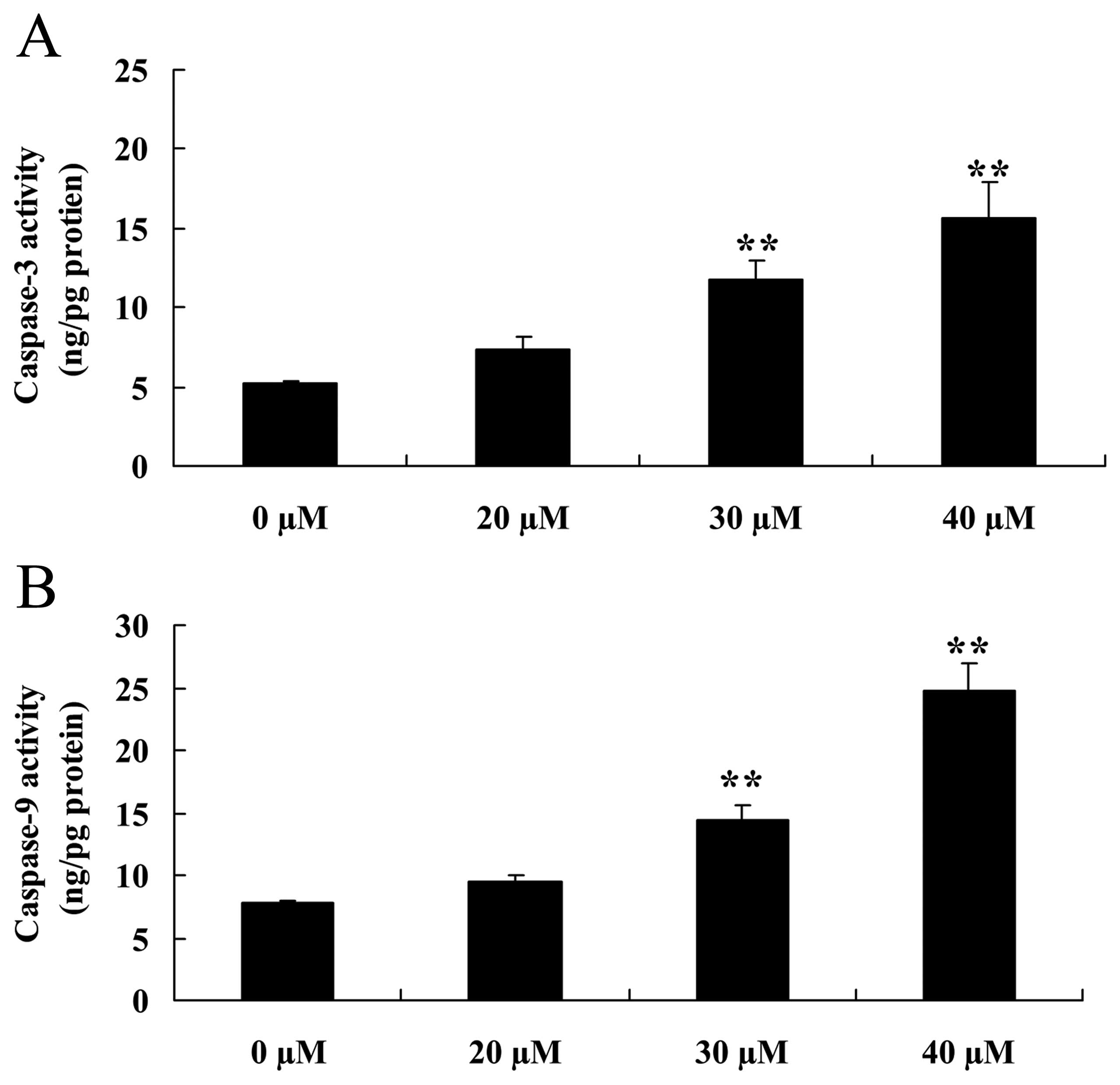
Inhibitory effect of ginkgetin induces
caspase-3/9 activation of osteosarcoma cells
To confirm the effect of the caspase-3/9 pathway on
the inhibition of ginkgetin on osteosarcoma cells, osteosarcoma
cells were treated with ginkgetin and the activation of caspase-3/9
was measured. The results showed a marked increase in the
activation of caspase-3 and -9 of osteosarcoma cells treated with
ginkgetin (30 or 40 µM, Fig.
6).
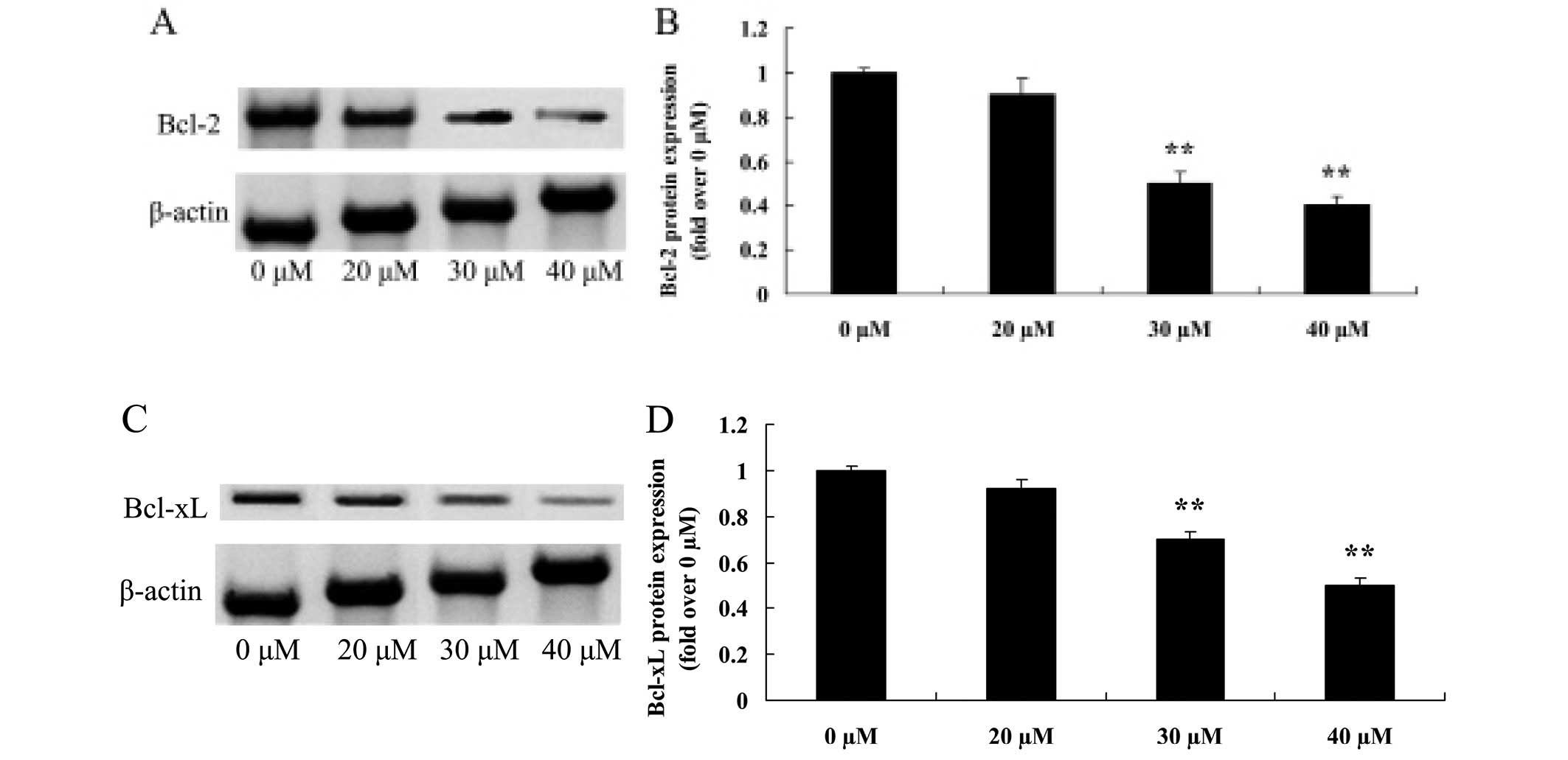
Growth inhibitory effect of ginkgetin
suppresses Bcl-2 and Bcl-xL in osteosarcoma cells
Regulatory proteins such as Bcl-2 and Bcl-xL, are
involved in the apoptotic signaling pathway (10). Fig. 7A
and C shows the suppression of the two proteins following
treatment with ginkgetin. Fig. 7B and
D shows that 30 or 40 µM of ginkgetin markedly reduced
the protein expression of Bcl-2 and Bcl-xL in osteosarcoma cells in
a dose-dependent manner.
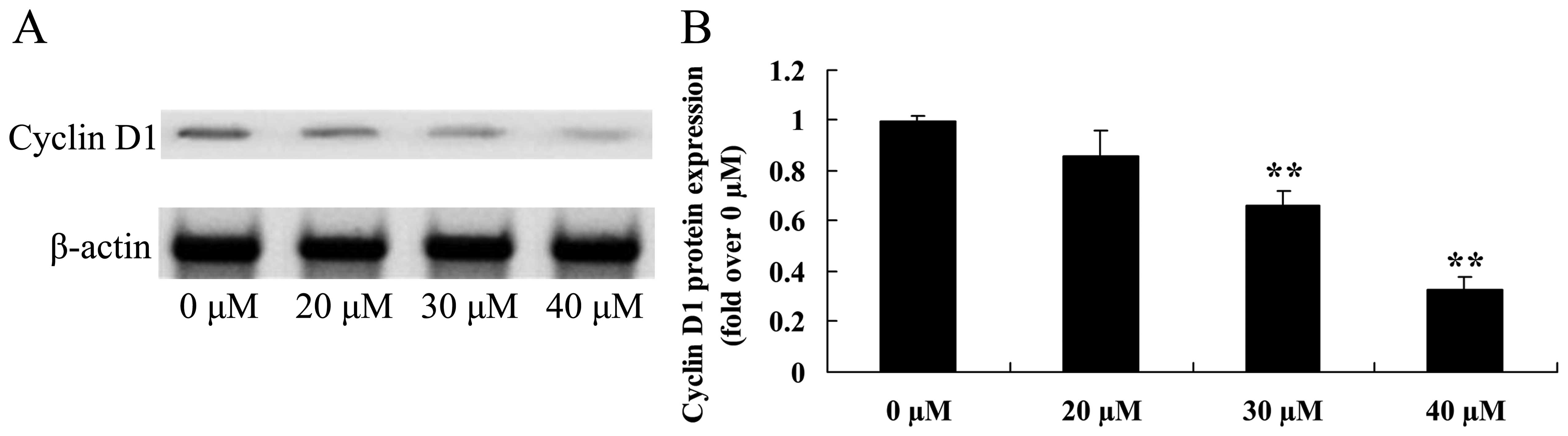
Growth inhibitory effect of ginkgetin
suppresses cyclin D1 in osteosarcoma cells
Induced apoptotic regulatory protein cyclin D1 was
assessed using western blot analysis (Fig. 8A). Treatment with ginkgetin (30 or
40 µM) markedly reduced the protein expression of cyclin D1
of osteosarcoma cells (Fig.
8B).
Growth inhibitory effect of ginkgetin
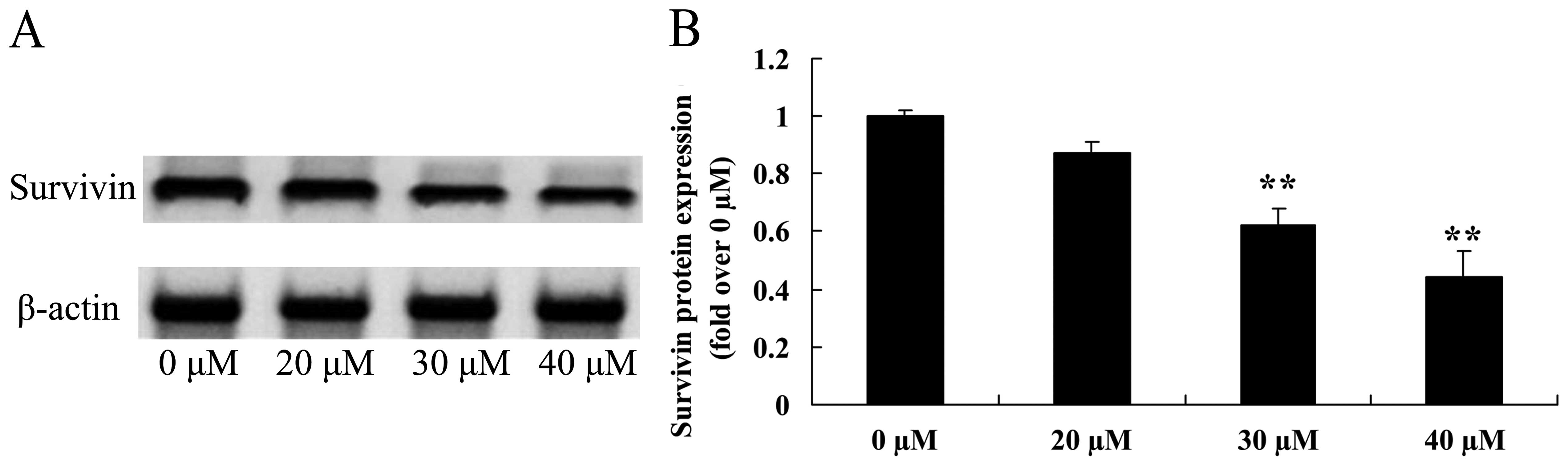
suppresses survivin in osteosarcoma cells
We also measured the induced apoptotic regulatory
protein survivin, using western blot analysis. The expression of
survivin protein was suppressed by treatment with 30 or 40
µM of ginkgetin in osteosarcoma cells (Fig. 9).
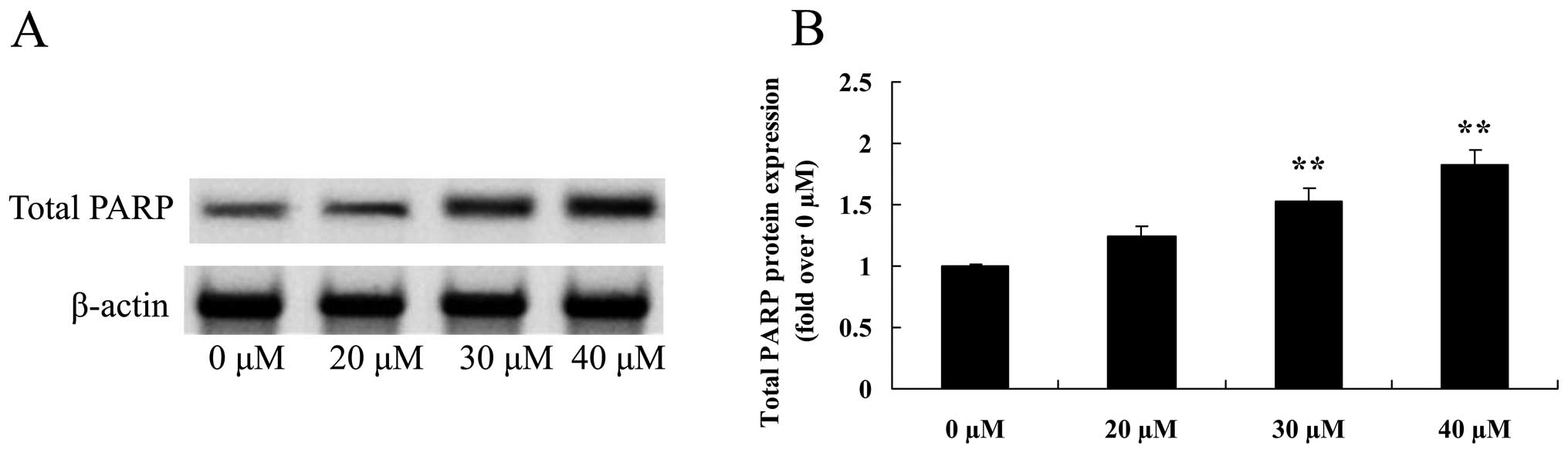
Growth inhibitory effect of ginkgetin
suppresses total PARP of osteosarcoma cells
To assess whether the growth inhibitory effect of
ginkgetin suppressed total PARP in osteosarcoma cells, total PARP
protein expression was measured using western blot analysis. The
results revealed that the total PARP protein expression was
significantly suppressed following the treament of ginkgetin at 30
or 40 µM in osteosarcoma cells (Fig. 10).
Discussion
Osteogenesis is one of the most common malignant
osseous tumors. Osteogenesis is characterized by tumor cells that
produce osteoid matrix, which is produced directly by the sarcoma
of osteoblasts, bone tissue and new bone, and is simultaneously
combined with osteogenesis and bone destruction in different
proportions, resulting in osteogenesis and osteolysis types often
occurring in lung metastasis (1).
Osteosarcoma is highly malignant and has a poor prognosis, and even
with surgical treatment combined with chemotherapy, the 5-year
survival rate is only 55–68% (11).
The main reason for the poor prognosis is early metastatic spread
of osteosarcoma. However, current anti-metastatic therapy remains
unsatisfactory, with side-effects of systemic chemotherapy causing
damage to internal organs of the human body. Additionally, surgical
treatment is usually considered unacceptable by patients due to
potential loss of limb (12).
Therefore, identification of treatment for osteosarcoma is
imperative. You et al have identified that ginkgetin induces
the apoptosis of PC-3 prostate cancer cells through the activation
of caspase (10). In the present
study, 30 or 40 µM of ginkgetin inhibited cell growth,
increased cytotoxicity and induced the cell apoptosis of
osteosarcoma cells. Thus, a new potential anticancer effect of
ginkgetin has been identified involving the inhibition of cell
growth and induction of apoptosis of osteosarcoma cells. However,
the mechanisms underlying its anticancer activities remain to be
elucidated.
SATA3 is a key signal transduction and
transcriptional activation protein found in the body. The
STAT3 coding gene in human is located in chromosome 12
(13). The STAT3 signaling pathway
is a signal transduction system that is transduced from the
membrane to the nucleus, through the activation of the receptor
tyrosine kinase STAT3 target genes (14). STAT3 is mainly stimulated through
tyrosine phosphorylation, triggering the activation of STAT3
receptor, thereby forming the cytoplasm of homologous or
heterologous dimers (14). The
STAT3 dimer enters the nucleus, and combines to the specific gene
promoter, thereby inducing gene expression (15). In normal tissue cells, the
expression of STAT3 is rapid and short, while in a variety of
malignant tumors, such as liver cancer, gastric cancer, lung
cancer, head and neck squamous cell carcinoma, breast and prostate
cancer, STAT3 expression is characterized by persistent activation
and a high expression. Additionally, its expression level is
closely associated with the degree of malignancy and tumor
prognosis (16). Osteosarcoma
organization is highly expressed in STAT3, while the expression of
STAT3 is associated with tumor staging as well as tumor presence of
soft tissue infiltration (17).
STAT3 signal plays an important role in the occurrence and
development of osteosarcoma (17).
In the present study, we elucidated the anticancer mechanism of
ginkgetin against osteosarcoma cells through the suppression of
p-STAT3 expression. Additionally, Jeon et al reported that
ginkgetin inhibits the cell growth by suppressing STAT3 protein
expression in human prostate DU-145 cancer cells (18).
Our mechanistic study indicates that ginkgetin
reduced the activation of caspase-3 and -9 in osteosarcoma cells.
Caspase-3/9 is a cysteine protease that is distributed in tissues
and cell lines of bone and cartilage. It is involved in the
apoptosis mechanism due to its structure domain having a FADD-like
death effect, proceeding into the death domain and FADD effect
structure. Caspase-3/9 is involved in apoptosis primarily through
the death-induced signal complex. The caspase protease family is
situated in a central position in the cell apoptotic process, and
is directly involved in early apoptosis, signal transmission and
late apoptosis, including caspase-3/9, which is identified in the
top of the cascade reaction. The expression of caspase-3/9 reflects
the level of the cell apoptotic reaction as well as the existence
of initiation of apoptotic factors. Su et al have identified
the ginkgetin-induced apoptosis of human ovarian adenocarcinoma
cells via the activation of caspase-3 (6). Thus, a possible anticancer effect of
apoptosis occurring following activation of caspase-3/9 in
osteosarcoma cells has been identified.
Mitochondrial cell apoptotic pathways are regulated
by Bcl-2 family proteins (19,20).
The Bcl-2 family is a polygenic family comprising at least 25
family members in mammals. It includes subfamilies such as the
Bcl-2 subfamily, whose members Bcl-2, Bcl-xl, Mcl-1, A1 and Bcl-w
inhibit cell apoptosis; the Bax subfamily, whose members Bax and
Bak promote cell apoptosis; and the BH3-only protein families,
which promote cell apoptosis. Bcl-2 subfamily members including
Bcl-xl, Mcl-1 and A1, can be used as targets for gene therapy for
tumors of the digestive system (20,21).
Our results revealed that ginkgetin has antitumor activity against
the reduction of the protein expression of Bcl-2 and Bcl-xL in
osteosarcoma cells in a dose-dependent manner. Jeon et al
also reported that ginkgetin inhibits cell growth through
anti-apoptotic proteins (Bcl-2 and Bcl-xL) in prostate DU-145
cancer cells (18). This result
shows that Bcl-2 and Bcl-xL are biomarkers involved in the
anticancer effect of ginkgetin on osteosarcoma.
Cyclin D1 is considered an oncogene whose encoded
protein accelerates the regulation of cell proliferation. However,
Overexpression of this oncogene as well as loss of control leads to
an abnormal cell cycle, thus causing cancer (22). The expression of survivin protein in
the human body and its biological effects are not expressed in
healthy adult tissue except for the outer thymus, placenta,
CD34+ stem and epithelial cells on the basal portion of
the colon (23). However, survivin
has been shown to be expressed in lung, breast and colon cancer,
and soft tissue sarcoma and malignant cells in the blood. In
addition, retrieval of the wide gene expression profile confirmed
that compared with normal tissue, there were statistically
significant differences in survivin expression (19). We observed that 30 or 40 µM
of ginkgetin treatment markedly reduced the protein expression of
cyclin D1 and survivin of osteosarcoma cells. Jeon et al
also reported that ginkgetin inhibits cell growth through cell
survival-related genes (cyclin D1 and survivin) in prostate DU-145
cancer cells (18). Therefore, the
results provide new insight into the inhibition of cell
survival-related genes (cyclin D1 and survivin) in the process of
ginkgetin on osteosarcoma cells.
There are 18 subtypes in the PARP family, with
PARP-1 having the largest proportion of the family and over 90%
functions. These functions include mediated DNA repair and cell
energy consumption pool, causing cellular dysfunction and death,
and promoting inflammation gene transcription (24). Griffin et al (25) first reported that the cytotoxic
activity of sulfuric acid dimethyl ester can be enhanced in the
process of DNA excision repair by attenuating the activity of PARP,
which shows that it can be used as a sensitizer in cancer therapy
together with cytotoxic drugs (25,26).
Administration of ginkgetin at 30 or 40 µM
significantly suppressed the total PARP protein expression in the
osteosarcoma cells. You et al reported that ginkgetin
induces apoptosis through cleavages of PARP in PC-3 prostate cancer
cells (10). Our findings suggest
that ginkgetin induces apoptosis through suppression of PARP in
osteosarcoma cells.
To the best of our knowledge, this is the first
study to identify that ginkgetin is highly effective in
osteosarcoma cells, inhibits cell growth, increases cytotoxicity
and induces the cell apoptosis of osteosarcoma cells. These results
provide new insight into the action of ginkgetin, which potently
inhibits the STAT3, caspase, cyclin D1, survivin and PARP signaling
pathway. Therefore, our findings indicate that ginkgetin has a
potential role in the treatment of osteosarcoma cells.
References
|
1
|
Bacci G, Gherlinzoni F, Picci P, et al:
Adriamycin-methotrexate high dose versus adriamycin-methotrexate
moderate dose as adjuvant chemotherapy for osteosarcoma of the
extremities: a randomized study. Eur J Cancer Clin Oncol.
22:1337–1345. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burdach S, van Kaick B, Laws HJ, Ahrens S,
Haase R, Körholz D, Pape H, Dunst J, Kahn T, Willers R, et al:
Allogeneic and autologous stem-cell transplantation in advanced
Ewing tumors. An update after long-term follow-up from two centers
of the European Intergroup study EICESS Stem-Cell Transplant
Programs at Düsseldorf University Medical Center, Germany and St
Anna Kinderspital, Vienna, Austria. Ann Oncol. 11:1451–1462. 2000.
View Article : Google Scholar
|
|
3
|
Kong CB, Kim MS, Lee SY, Cho WH, Song WS,
Lee JA, Yoo JY, Chung SH and Jeon DG: Prognostic effect of
diaphyseal location in osteosarcoma: A cohort case-control study at
a single institute. Ann Surg Oncol. 16:3094–3100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al Italian and Scandinavian Sarcoma Groups: Neoadjuvant
chemotherapy with high-dose ifosfamide, high-dose methotrexate,
cisplatin, and doxorubicin for patients with localize1d
osteosarcoma of the extremity: A joint study by the Italian and
Scandinavian Sarcoma Groups. J Clin Oncol. 23:8845–8852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JA, Choi SY, Kang HJ, Lee JW, Kim H,
Kim JH, Sung KW, Shin HY, Ahn HS and Park KD: Treatment outcome of
osteosarcoma after bilateral retinoblastoma: A retrospective study
of eight cases. Br J Ophthalmol. 98:1355–1359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su Y, Sun CM, Chuang HH and Chang PT:
Studies on the cytotoxic mechanisms of ginkgetin in a human ovarian
adenocarcinoma cell line. Naunyn Schmiedebergs Arch Pharmacol.
362:82–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YQ, Wang MY, Fu XR, Peng-Yu, Gao GF,
Fan YM, Duan XL, Zhao BL, Chang YZ and Shi ZH: Neuroprotective
effects of ginkgetin against neuro-injury in Parkinson's disease
model induced by MPTP via chelating iron. Free Radic Res. Jul
1–2015.Epub ahead of print.
|
|
8
|
Lim H, Son KH, Chang HW, Kang SS and Kim
HP: Effects of anti-inflammatory biflavonoid, ginkgetin, on chronic
skin inflammation. Biol Pharm Bull. 29:1046–1049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Shi S, Wang Y and Huang K:
Target-guided isolation and purification of antioxidants from
Selaginella sinensis by offline coupling of DPPH-HPLC and HSCCC
experiments. J Chromatogr B Analyt Technol Biomed Life Sci.
879:191–196. 2011. View Article : Google Scholar
|
|
10
|
You OH and Kim SH, Kim B, Sohn EJ, Lee HJ,
Shim BS, Yun M, Kwon BM and Kim SH: Ginkgetin induces apoptosis via
activation of caspase and inhibition of survival genes in PC-3
prostate cancer cells. Bioorg Med Chem Lett. 23:2692–2695. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwata S, Ishii T, Kawai A, Hiruma T,
Yonemoto T, Kamoda H, Asano N and Takeyama M: Prognostic factors in
elderly osteosarcoma patients: A multi-institutional retrospective
study of 86 cases. Ann Surg Oncol. 21:263–268. 2014. View Article : Google Scholar
|
|
12
|
Boye K, Del Prever AB, Eriksson M, Saeter
G, Tienghi A, Lindholm P, Fagioli F, Skjeldal S, Ferrari S and Hall
KS: High-dose chemotherapy with stem cell rescue in the primary
treatment of metastatic and pelvic osteosarcoma: Final results of
the ISG/SSG II study. Pediatr Blood Cancer. 61:840–845. 2014.
View Article : Google Scholar
|
|
13
|
Wang X, Goldstein D, Crowe PJ and Yang JL:
Impact of STAT3 inhibition on survival of osteosarcoma cell lines.
Anticancer Res. 34:6537–6545. 2014.PubMed/NCBI
|
|
14
|
Salas S, Jiguet-Jiglaire C, Campion L,
Bartoli C, Frassineti F, Deville JL, Maues De Paula A, Forest F,
Jézéquel P, Gentet JC, et al: Correlation between ERK1 and STAT3
expression and chemoresistance in patients with conventional
osteosarcoma. BMC Cancer. 14:6062014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Aksoy I, Gonnot F, Osteil P, Aubry
M, Hamela C, Rognard C, Hochard A, Voisin S, Fontaine E, et al:
Reinforcement of STAT3 activity reprogrammes human embryonic stem
cells to naive-like pluripotency. Nat Commun. 6:70952015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Wei W, Guo CA, Han N, Pan JF, Fei T
and Yan ZQ: Stat3 upregulates leucine-rich repeat-containing g
protein-coupled receptor 4 expression in osteosarcoma cells. Biomed
Res Int. 2013:3106912013.
|
|
18
|
Jeon YJ, Jung SN, Yun J, Lee CW, Choi J,
Lee YJ, Han DC and Kwon BM: Ginkgetin inhibits the growth of DU-145
prostate cancer cells through inhibition of signal transducer and
activator of transcription 3 activity. Cancer Sci. 106:413–420.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Lyu H, Wang J and Liu B: MicroRNA
regulation and therapeutic targeting of survivin in cancer. Am J
Cancer Res. 5:20–31. 2015.PubMed/NCBI
|
|
20
|
Nemec KN and Khaled AR: Therapeutic
modulation of apoptosis: Targeting the BCL-2 family at the
interface of the mitochondrial membrane. Yonsei Med J. 49:689–697.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rautureau GJ, Day CL and Hinds MG:
Intrinsically disordered proteins in bcl-2 regulated apoptosis. Int
J Mol Sci. 11:1808–1824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang L, Wang P, Chen L and Chen H:
Down-regulation of FoxM1 by thiostrepton or small interfering RNA
inhibits proliferation, transformation ability and angiogenesis,
and induces apoptosis of nasopharyngeal carcinoma cells. Int J Clin
Exp Pathol. 7:5450–5460. 2014.PubMed/NCBI
|
|
23
|
Ma YP, Zou P, Xiao J and Huang SA:
Expression of survivin in cord blood CD34+
stem/progenitor cells and its significance. Zhonghua Xue Ye Xue Za
Zhi. 24:238–240. 2003.In Chinese. PubMed/NCBI
|
|
24
|
Langelier MF and Pascal JM: PARP-1
mechanism for coupling DNA damage detection to poly(ADP-ribose)
synthesis. Curr Opin Struct Biol. 23:134–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Griffin RJ, Pemberton LC, Rhodes D,
Bleasdale C, Bowman K, Calvert AH, Curtin NJ, Durkacz BW, Newell
DR, Porteous JK, et al: Novel potent inhibitors of the DNA repair
enzyme poly(ADP-ribose)polymerase (PARP). Anticancer Drug Des.
10:507–514. 1995.PubMed/NCBI
|
|
26
|
Yang F, Nam S, Zhao R, Tian Y, Liu L,
Horne DA and Jove R: A novel synthetic derivative of the natural
product berbamine inhibits cell viability and induces apoptosis of
human osteosarcoma cells, associated with activation of JNK/AP-1
signaling. Cancer Biol Ther. 14:1024–1031. 2013. View Article : Google Scholar : PubMed/NCBI
|