Introduction
Recently, complementary and alternative medicine
(CAM) has received great interest among cancer patients as an
alternative therapeutic method (1).
In fact, nearly 40% of cancer patients in the US may use at least
one type of CAM approach (2).
Physicians are particularly interested in using various foods as
CAMs, and are trying to discover novel food-based CAMs owing to
their pharmacologic safety and familiarity for use. Among many CAM
agents, curcumin, a natural polyphenol derived from the rhizome of
the plant Curcuma longa, has shown promise in the treatment
of cancer patients (3). Curcumin
has been shown to significantly inhibit the growth of many types of
cancer cells by regulating various molecules associated with cancer
cell proliferation (4). It also
potentiates the anticancer effects of chemotherapeutic agents in
vitro and in vivo (5–7).
However, the clinical efficacy of curcumin is still
limited, which is most likely due to its low bioavailability
(8). To address this drawback,
Theracurmin® (nanocurcumin), a novel form of curcumin,
was developed using a microparticle and surface-controlled drug
delivery system (9).
Theracurmin® shows improved bioavailability and water
solubility compared to curcumin (9). Additionally, Theracurmin®
improves the feasibility of in vitro testing and eventual
in vivo administration (10,11).
Despite these biological advantages, there have been no studies on
the effects of Theracurmin® in urological cancer. Here,
we investigated the anticancer properties of
Theracurmin® on human prostate cancer and bladder cancer
cells in vitro and compared them to those of curcumin.
Moreover, we examined the relevant molecular mechanisms of the
potential anticancer effects of Theracurmin® in these
urological malignancies.
Materials and methods
Cell lines and reagents
Human prostate cancer cell lines (LNCaP, PC3, and
DU145) were obtained from the American Type Culture Collection
(ATCC; Rockville, MD, USA). We used RPMI-1640 medium (Welgene,
Daegu, Korea) for LNCaP and PC3, and Dulbecco's modified Eagle's
medium (DMEM; Welgene) for DU145 cells as the basal culture medium.
Human bladder cancer cell lines (T24, 253J, and HTB9) were also
purchased from ATCC. To establish a cisplatin-resistant cell line
(T24R2), we performed serial desensitization of T24 cells as
previously described (12). The
human bladder cancer cell lines were cultured in RPMI-1640 medium.
All media were supplemented with 10% fetal bovine serum (Welgene),
and 1% penicillin-streptomycin, and 1% non-essential amino acids
(both from Invitrogen, Carlsbad, CA, USA). Curcumin (Sigma-Aldrich,
St. Louis, MO, USA) and Theracurmin® (Handok
Pharmaceuticals Co., Ltd., Korea) were prepared in dimethyl
sulfoxide (DMSO) and diluted into the growth medium such that the
final concentration of DMSO did not exceed 0.1% (v/v). Medium
containing 0.1% DMSO was used as the negative control.
Cell proliferation assay
To examine the cell proliferation, we used the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Gaithersburg, MD, USA). Cells (2×103 cells/well) were
seeded onto 96-well plates and incubated for 24 h. Cells were
treated with either curcumin or Theracurmin® for 24, 48,
and 72 h, and then 10 μl of CCK-8 solution was added into
the 96-well plates. After 4 h of incubation, the absorbance at 450
nm was determined using a plate reader (Molecular Devices,
Sunnyvale, CA, USA). Cell viability results are reported as fold
change compared to data derived from the first day of cell
seeding.
Clonogenic assay
Cells (1×103 cells/well) were seeded onto
35-mm2 dishes and treated with either curcumin or
Theracurmin® for 48 h. To form visible colonies, the
cells were cultured for an additional 14 day in either curcumin- or
Theracurmin®-free culture condition. After fixation with
10% neutral-buffered formalin solution, the samples were stained
with 0.1% crystal violet solution (both from Sigma-Aldrich).
Finally, the samples were photographed, and the number of visible
colonies comprising more than 50 individual cells was determined by
using a SZX7 stereomicroscope (Olympus, Tokyo, Japan).
Flow cytometry for cell cycle
analysis
Cells (3×105 cells/well) were plated on
60-mm2 dishes and incubated with either curcumin or
Theracurmin® for 48 h. The cells were fixed in 70%
ethanol and reacted with RNase A (10 μg/ml) for 1 h at 37°C.
Then, cells were stained with propidium iodide solution (10
μg/ml) for 30 min at 4°C in a dark room. The cell cycle
distribution was analyzed using a BD FACSCalibur cytometer (BD
Biosciences, San Jose, CA, USA).
Western blot analysis
To extract total proteins, the cells were lysed with
radio-immunoprecipitation assay buffer [50 mM Tris-HCl (pH 8.0),
150 mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1%
sodium dodecyl sulfate (SDS) and 1 mM phenylmethylsulfonyl
fluoride]. After measuring protein concentrations of each sample
using the BCA protein assay kit (Pierce, Rockford, IL, USA),
samples were prepared with equal amounts of protein in 1X SDS
buffer. Protein samples were electrophoresed on SDS-PAGE gels and
transferred onto poly-vinylidene difluoride membranes (Millipore,
Billerica, MA, USA). After the samples were blocked with 5% (w/v)
non-fat dry milk at room temperature for 1 h, the blots were
incubated with primary antibodies overnight at 4°C. Primary
antibodies used in the present study were anti-cleaved caspase-3,
-8, -9, poly(adenosine diphosphate-ribose) polymerase (PARP),
cytochrome c, cyclin D1, cyclin E1, p-Akt, Akt, p-Erk, and
Erk (1:1,000; Cell Signaling Technology, Inc., Beverly, MA, USA).
The blots were incubated with horseradish peroxidase-conjugated
secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA,
USA) for 45 min at room temperature. Finally, protein expression
levels were detected using an Enhanced Chemiluminescence Western
Blot Substrate kit (Pierce).
Statistical analysis
Three independent experiments were performed in
triplicate, and the data are presented as mean ± standard error of
the mean (SEM). The statistical analysis was conducted using
GraphPad Prism software (GraphPad Software Inc., San Diego, CA,
USA). We considered data with p-values <0.05 as significant, as
determined by Turkey's multiple range tests.
Results
Theracurmin® significantly
inhibits the proliferation of human prostate cancer cells
To examine the cytotoxic effects of
Theracurmin® on human prostate cancer cells, we treated
cells with this agent for 24, 48 and 72 h and examined the cell
viability using the CCK-8 assay. Cell viability of the PC3, DU145
and LNCaP cells was significantly decreased in a dose- and
time-dependent manner after Theracurmin® treatment
(Fig. 1A). As shown in Fig. 1B, the cytotoxic effects of curcumin
were similar to those of Theracurmin®. Moreover, we
found that Theracurmin® markedly impaired the clonogenic
proliferation of human prostate cancer cells in a dose-dependent
manner (Fig. 1Ca and Da). We also
confirmed that the effects of curcumin on clonogenic proliferation
were similar to those of Theracurmin® (Fig. 1Cb and Db).
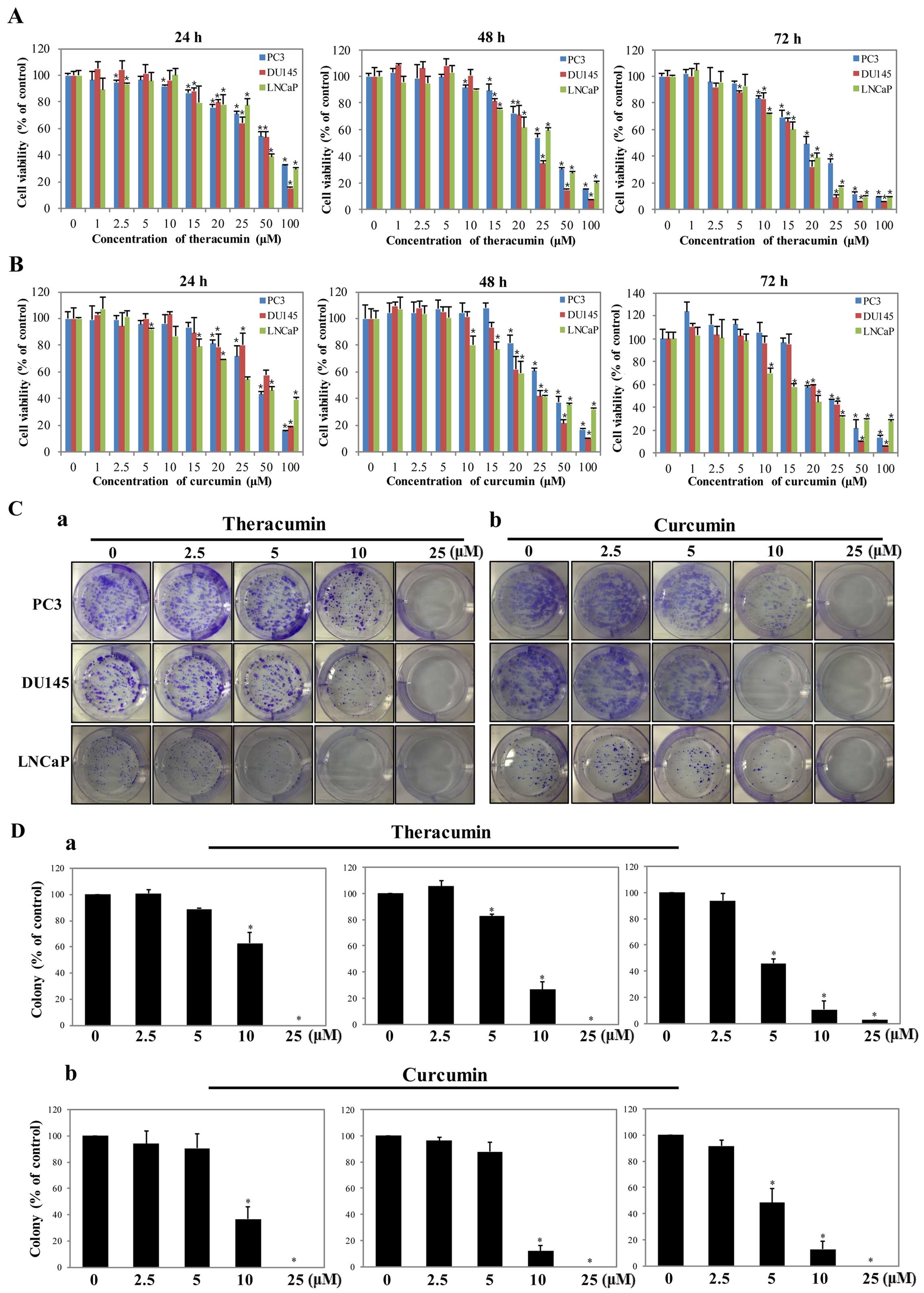 | Figure 1Anticancer effects of
Theracurmin® and curcumin treatment in human prostate
cancer cells. Cell viability assessments for determining cytotoxic
effects of (A) Theracurmin® or (B) curcumin treatment in
human prostate cancer cell lines (PC3, DU145, and LNCaP). The cells
were treated with different concentrations of each agent, ranging
from 1 to 100 μM, for 24, 48, and 72 h. Mock (0) was used as
a negative control. The rates of cell viability are shown as the
mean percentage of control ± standard error of the mean (n=3,
*P<0.05). Clonogenic assay (C and D) for examining
colony formation after 14 days of (a) Theracurmin® or
(b) curcumin treatment in human prostate cancer cell lines (PC3,
DU145 and LNCaP). Cells were treated with different concentrations
of Theracurmin® (0, 2.5, 5, 10 and 25 μM) for 48
h. (C) Crystal violet staining was used for visualizing significant
colonies consisting of more than 50 individual cells in (a)
Theracurmin®- and (b) curcumin-treated groups. (D) The
numbers of colonies are shown as representative bar graphs with the
mean percentage of control ± standard error of the mean (n=3,
*P<0.05). |
These results indicate that Theracurmin®
and curcumin have similar anticancer effects on human prostate
cancer cells in both androgen-dependent and -independent cells in a
dose- and time-dependent manner.
Theracurmin® exerts anticancer
effects by inducing apoptotic cell death and cell cycle disturbance
in human prostate cancer cells
To further examine the molecular mechanisms of the
anticancer activities of Theracurmin® in human prostate
cancer cells, we investigated the expression patterns of molecules
associated with apoptosis, as well as the cell cycle distribution
patterns after Theracurmin® treatment. Western blot
analysis showed that pro-apoptotic proteins (cleaved PARP,
caspase-3, -8, and -9, cytochrome c, and Bad) were
upregulated, whereas total PARP and anti-apoptotic molecule (Bcl-2)
were downregulated after treatment with Theracurmin® in
the PC3, DU145 and LNCaP cells, respectively (Fig. 2A). We also noted that the expression
patterns of apoptosis-related proteins after curcumin treatment did
not differ from those after Theracurmin® treatment
(Fig. 2A). Additionally,
Theracurmin® treatment resulted in cell cycle
disturbances, such as G2/M arrest, remarkably in PC3 cells, but
subtly in the DU145 and LNCaP cells (Fig. 2B). Consistent with these results,
aberrant expression of regulatory molecules at cell cycle
checkpoints (cyclin B1, D1 and E1) was observed with higher doses
of Theracurmin® (Fig.
2A). Similar to these findings, curcumin treatment also induced
cell cycle dysregulation, particularly G2/M arrest (Fig. 2C), with irregular changes in the
expression of relevant checkpoint molecules (Fig. 2A).
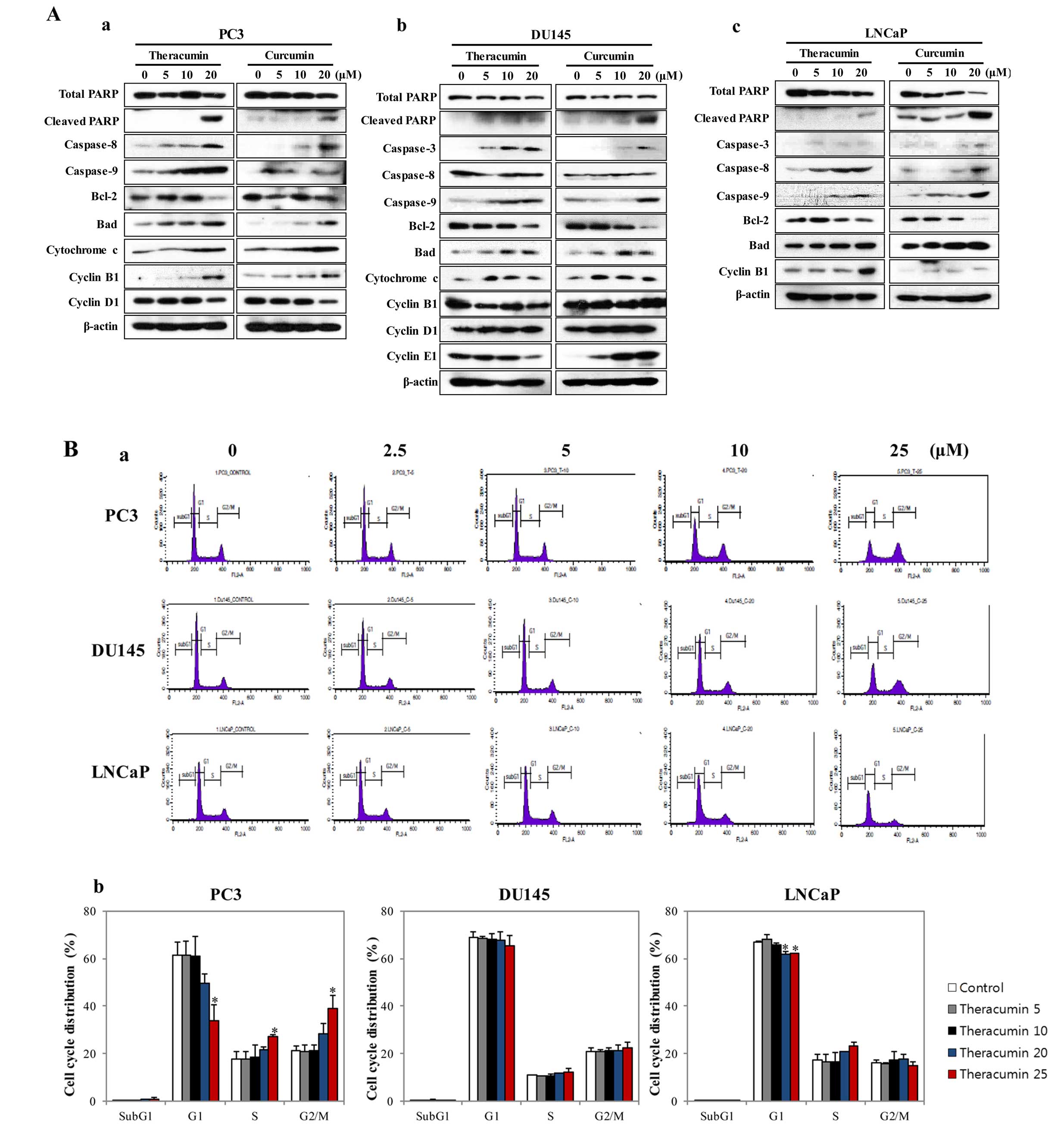 | Figure 2Theracurmin® and curcumin
treatment induce apoptotic cell death and cell cycle dysregulation
in human prostate cancer cells. (A) Western blot analysis for the
expression of molecules associated with apoptosis (total PARP,
cleaved PARP, caspase-3, caspase-8, caspase-9, Bcl-2, Bad, and
cytochrome c) and cell cycle regulation (cyclin B1, cyclin
D, and cyclin E) in human prostate cancer cells (a) PC3, (b) DU145,
and (c) LNCaP after treatment with various concentrations (0, 5, 10
and 20 μM) of Theracurmin® and curcumin for 48 h.
β-actin was used as a loading control. (B) Cell cycle analysis by
flow cytometry with propidium iodide staining in human prostate
cancer cells (PC3, DU145, and LNCaP) after treatment with various
concentrations (0, 5, 10 and 20 μM) of
Theracurmin® for 48 h: (a) the representative results
and (b) the proportion of cells in cell cycle phases (G1, S, and
G2/M) are presented as a percentage of the control.
Theracurmin® and curcumin treatment induce apoptotic
cell death and cell cycle dysregulation in human prostate cancer
cells. (C) Cell cycle analysis by flow cytometry with propidium
iodide staining in human prostate cancer cells (PC3, DU145, and
LNCaP) after treatment with various concentrations (0, 5, 10 and 20
μM) of curcumin for 48 h: (a) the representative results and
(b) the proportion of cells in cell cycle phases (G1, S, and G2/M)
are presented as a percentage of the control. |
These findings suggest that Theracurmin®
and curcumin treatment led to the reduction in cell viability and
clonogenic potential via the induction of apoptotic cell death in
human prostate cancer cells. Partly, cell cycle disturbance, such
as G2/M arrest, played an important role in the cytotoxicity of
Theracurmin® treatment in both androgen-dependent and
-independent cells.
Theracurmin® treatment
efficiently suppresses the growth of human bladder cancer
cells
We next examined the cytotoxic effects of
Theracurmin® on human bladder cancer cell lines (T24,
293J, and HTB9) as well as on the cisplatin-resistant cell line,
T24R2. Similar to the results using prostate cancer cells,
Theracurmin® treatment markedly reduced the viability of
the bladder cancer cell lines in a dose- and time-dependent manner
(Fig. 3A). Despite the subtle
difference in responsiveness, the overall patterns of cytotoxic
effects were similar between the cells treated with
Theracurmin® and curcumin (Fig. 3B). Of note, these specific agents
exerted a marked anticancer effect on the cisplatin-resistant T24R2
cells.
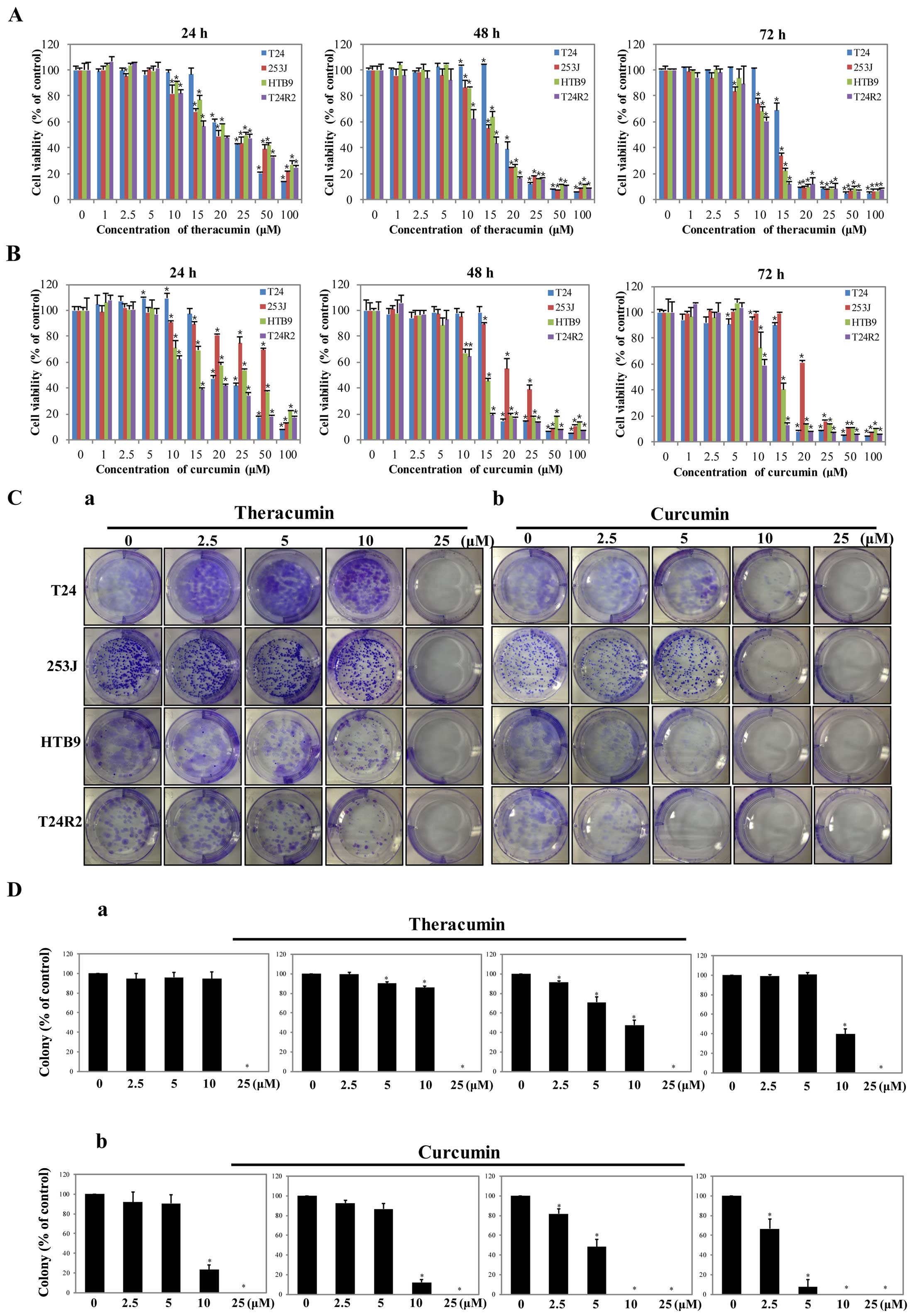 | Figure 3Anticancer effects of
Theracurmin® and curcumin treatment in human bladder
cancer cells. Cell viability assessments for determining the
cytotoxic effects of (A) Theracurmin® or (B) curcumin
treatment in human prostate cancer cell lines (T24, 253J, HTB9, and
cisplatin-resistant T24R2). The cells were treated with different
concentrations of each agent, ranging from 1 to 100 μM, for
24, 48 and 72 h. Mock (0) was used as a negative control. The rates
of cell viability are shown as the mean percentage of control ±
standard error of the mean (n=3, *P<0.05). (C and D)
Clonogenic assay for examining the colony formation after 14 days
of (a) Theracurmin® or (b) curcumin treatment in human
prostate cancer cell lines (T24, 253J, HTB9, and
cisplatin-resistant T24R2). Cells were treated with different
concentrations of Theracurmin® (0, 2.5, 5, 10 and 25
μM) for 48 h. (C) Crystal violet staining was used for
visualizing significant colonies consisting of more than 50
individual cells in (a) Theracurmin®- or (b)
curcumin-treated groups. (D) The numbers of colonies are shown as
representative bar graphs with the mean percentage of control ±
standard error of the mean (n=3, *P<0.05). |
We revealed that Theracurmin® treatment
also significantly diminished the clonogenic proliferation of human
bladder cancer cells in a dose-dependent manner (Fig. 3Ca and Da). Although the
anti-clonogenic effect of Theracurmin® was relatively
lower than that observed with curcumin treatment (Fig. 3Cb and Db), our results indicate that
Theracurmin® and curcumin have similar anticancer
effects on human bladder cancer cells, even in cisplatin-resistant
cells.
Anticancer effects of
Theracurmin® are induced by apoptotic cell death and
cell cycle dysregulation in human bladder cancer cells
To explore the molecular aspects of the anticancer
effects of Theracurmin® in human bladder cancer cells,
we examined the expression patterns of apoptosis- and cell
cycle-regulating molecules after adding Theracurmin®.
Similar to the findings in prostate cancer cells, the pro-apoptotic
proteins (cleaved PARP, caspase-3, -8, and -9, cytochrome c,
and Bad) were highly expressed, whereas total PARP and the
expression of anti-apoptotic molecule Bcl-2 reduced in a
dose-dependent manner after treatment with Theracurmin®
(Fig. 4A). Additionally, cell cycle
analysis revealed that this agent induced sub-G1 arrest, indicating
apoptotic cell death (Fig. 4B).
Theracumin also led to cell cycle dysregulation, including S and/or
G2/M phase arrest (Fig. 4B). In
western blot analysis, the amounts of checkpoint molecules (cyclin
B1 and cyclin D) reduced at higher doses of Theracurmin®
in bladder cancer cells, even in cisplatin-resistant T24R2 cells
(Fig. 4A). We also found that
curcumin induced similar outcomes of apoptotic cell death and cell
cycle dysregulation to those with Theracurmin® (Fig. 4A and C). Our findings indicate that
Theracurmin® and curcumin have comparable anticancer
effects by reducing cell viability through the induction of
apoptotic cell death and cell cycle arrest in the various types of
human bladder cancer cells.
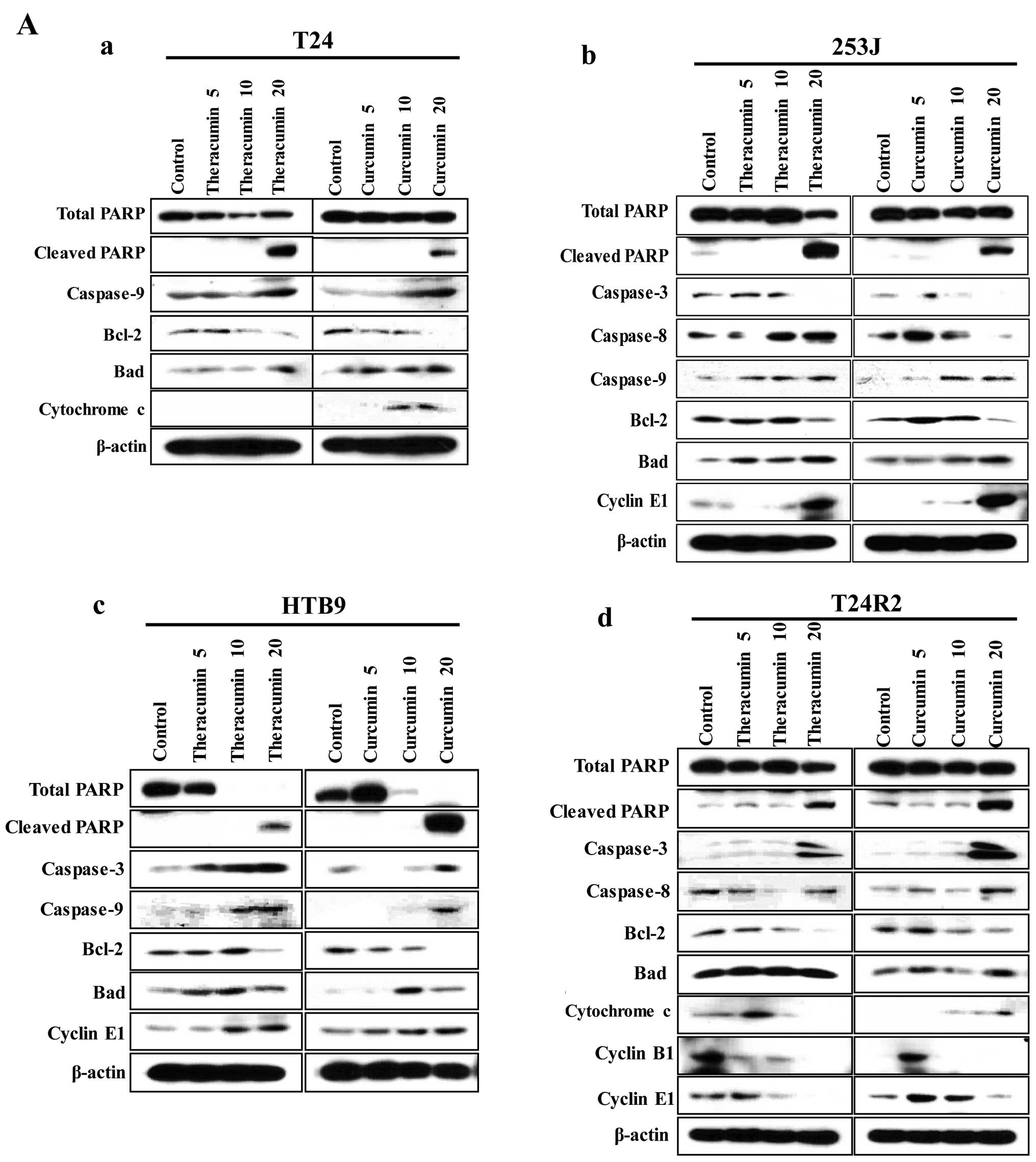 | Figure 4Theracurmin® and curcumin
treatments induce apoptotic cell death and cell cycle dysregulation
in human bladder cancer cells. (A) Western blot analysis for the
expression of molecules associated with apoptosis (total PARP,
cleaved PARP, caspase-3, caspase-8, caspase-9, Bcl-2, Bad, and
cytochrome c) and cell cycle regulation (cyclin B1, cyclin
D, cyclin E) in human bladder cancer cells (a) T24, (b) 253J, (c)
HTB9, and (d) cisplatin-resistant T24R2 after treatments with
various concentrations (0, 5, 10 and 20 μM) of
Theracurmin® or curcumin for 48 h. β-actin was used as a
loading control. Continued. Theracurmin® treatment
induces apoptotic cell death and cell cycle dysregulation in human
bladder cancer cells. (B) Cell cycle analysis by flow cytometry
with propidium iodide staining in human bladder cancer cells (T24,
253J, HTB9, and cisplatin-resistant T24R2) after treatment with
various concentrations (0, 5, 10 and 20 μM) of
Theracurmin®. (a) The representative results and (b) the
proportion of cell in cell cycle phases (G1, S, and G2/M) are
presented as a percentage of the control. Continued. Curcumin
treatment induces apoptotic cell death and cell cycle dysregulation
in human bladder cancer cells. (C) Cell cycle analysis by flow
cytometry with propidium iodide staining in human bladder cancer
cells (T24, 253J, HTB9, and cisplatin-resistant T24R2) after
treatment with various concentrations (0, 5, 10 and 20 μM)
of curcumin for 48 h. (a) The representative results and (b) the
proportion of cells in cell cycle phases (G1, S, and G2/M) are
presented as a percentage of the control. |
Discussion
Prostate and bladder cancer are usually diagnosed in
older men, who may be vulnerable to the side effects from
first-line chemotherapeutic agents, such as docetaxel and
cisplatin. In this context, natural compounds can be beneficial as
an alternative therapeutic approach for treating these urological
cancers (13). Many in vitro
and in vivo studies suggest that curcumin, a yellow curry
pigment, can significantly inhibit the growth, proliferation,
invasive capabilities, and metastatic potential of prostate and
bladder cancer cells (14–16).
Although many preclinical studies have shown
curcumin to be a promising anticancer agent in urological cancer,
its poor bioavailability is a major drawback to its clinical
application for cancer patients (13). The limited oral bioavailability of
curcumin may be attributed to its poor solubility in water and
extensive systemic metabolism after oral intake. For example, a
dose-escalation study carried out by Lao et al (17) demonstrated that no curcumin was
detected in the blood of 24 individuals after oral administration
of curcumin at various doses ranging from 500 to 8,000 mg. To
overcome this obstacle, an innovative form of curcumin as a
nanoparticle colloidal dispersion (Theracurmin®) has
been developed with higher stability and improved bioavailability
(9). Several clinical trials have
shown that Theracurmin® has no significant adverse
effects in human subjects, indicating that this novel agent is well
tolerated (9,18–22).
Moreover, Theracurmin® significantly inhibited the
proliferation of esophageal adenocarcinoma cells (OE33 and OE19),
whereas it did not impair the proliferation of normal esophageal
cells (HET-1A) in a recent study by Milano et al (21).
Despite these promising results showing
Theracurmin® to be a safe and effective anticancer
agent, no proof-of-concept studies have been carried out in
urological cancer. In this study, we first revealed that
Theracurmin® significantly reduced the cell viability in
human prostate cancer and bladder cancer cells, particularly
showing comparable anticancer efficacy with curcumin. Notably, the
anticancer effects of Theracurmin® were exerted by
inducing apoptotic cell death and cell cycle disturbance in these
urological cancer cells. Similar to our findings, Kamat et
al (5) revealed that curcumin
suppressed the proliferation of bladder cancer cells (RT4V6 and
KU-7) in a dose-dependent manner by inducing cell cycle arrest and
potentiating the apoptotic effects of chemotherapeutic drugs. Park
et al also showed that curcumin treatment induced growth
inhibition of T24 bladder cancer cells driven by cell cycle
disturbance, specifically G2/M phase arrest (22). In a study by Aggarwal et al,
curcumin enhanced the anticancer effects of chemotherapeutic agents
by degradation of cyclin E expression via upregulation of p21 and
p27, resulting in G1 phase arrest in LNCaP prostate cancer cells
(23).
Apoptosis and cell cycle arrest, such as in the S or
G2/M phases, are regarded as the cellular response pathways
following diverse stimuli, including DNA damage (24). In this regard, these distinct
processes are intimately related and together play a crucial role
in the responsiveness of cancer cells to anticancer agents
(24). The complex interplay of
various regulators is fundamental in processes involved in the
progression of apoptotic cell death and the cell cycle. For
apoptosis, there are two classifications of regulatory molecules:
those that modulate mitochondrial function (e.g., Bcl-2, Bcl-xL,
and Bax) and those that regulate the apoptotic cascades (e.g.,
caspase-3, -8 and -9) (25). For
cell cycle regulation, there are two key classes of regulatory
proteins, cyclins and cyclin-dependent kinases (CDKs). For example,
cyclin E and CDK2 play an important role in the progression of G1
through S phase, as well as cyclin B and CDK1 are key regulatory
molecules for the G2/M phase (26).
Mechanistically, curcumin has been shown to modulate
many molecular targets, including transcription factors, oncogenic
growth factors, and inflammatory cytokines with relevant multiple
signaling pathways, resulting in inhibition of cell proliferation,
invasion and metastasis (27). The
activity of NF-κB, which is constitutively activated in several
malignancies, can be inhibited by curcumin treatment in several
cancer cells. The expression of Wnt/β-catenin signaling pathway,
frequently upregulated during initiation and progression of several
tumors, can also be blocked by curcumin (28,29).
For instance, curcumin reduces the accumulation of nuclear
β-catenin and its target protein Tcf-4 in colon cancer cells,
leading to growth arrest and apoptotic cell death (30). Curcumin can regulate other
well-known oncogenic signaling pathways, such as STAT3 and
PI3K/AKT, by suppressing their phosphorylation (31,32).
Additionally, curcumin is a potent inhibitor of several
pro-inflammatory cytokines, such as IL-1, IL-5, IL-8 and IL-12,
involved in tumor angiogenesis and metastasis (33).
However, our study did not provide a more
comprehensive data for the molecular mechanisms of the anticancer
activities of Theracurmin® in human prostate and bladder
cancer cells. Our data only demonstrated the phenotypic changes of
human prostate cancer and bladder cancer cells after
Theracurmin® and curcumin treatment, which is the major
limitation of our study. In order to provide more concrete
evidence, we should explore the relevant signaling pathways. More
importantly, this study only provided in vitro results for
the anticancer effects of Theracurmin® in both prostate
cancer and bladder cancer cells. Thus, an in vivo efficacy
study using xenograft models is required to consolidate our results
and show that Theracurmin® treatment can be a useful CAM
approach for both prostate cancer and bladder cancers.
In summary, we provides in vitro evidence
that Theracurmin®, an innovative nano-form of curcumin,
exhibits anticancer effects by inducing apoptotic cell death and
cell cycle dysregulation in human prostate and bladder cancer
cells. Further mechanistic studies are required to fully appreciate
the clinical potential of Theracurmin® as a promising
alternative medicine agent in these urological cancers.
Acknowledgments
The present study was supported by a grant from
Handok Pharmaceuticals in Korea. The funders had no role in study
design, experiments, data collection and analysis, preparation of
the manuscript, or the decision to publish.
References
|
1
|
Rossi E, Vita A, Baccetti S, Di Stefano M,
Voller F and Zanobini A: Complementary and alternative medicine for
cancer patients: Results of the EPAAC survey on integrative
oncology centres in Europe. Support Care Cancer. 23:1795–1806.
2015. View Article : Google Scholar
|
|
2
|
Ventola CL: Current issues regarding
complementary and alternative medicine (CAM) in the United States:
Part 1: The widespread use of CAM and the need for better-informed
health care professionals to provide patient counseling. P T.
35:461–468. 2010.PubMed/NCBI
|
|
3
|
Tuorkey MJ: Curcumin a potent cancer
preventive agent: Mechanisms of cancer cell killing. Interv Med
Appl Sci. 6:139–146. 2014. View Article : Google Scholar
|
|
4
|
Duvoix A, Blasius R, Delhalle S,
Schnekenburger M, Morceau F, Henry E, Dicato M and Diederich M:
Chemopreventive and therapeutic effects of curcumin. Cancer Lett.
223:181–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamat AM, Sethi G and Aggarwal BB:
Curcumin potentiates the apoptotic effects of chemotherapeutic
agents and cytokines through down-regulation of nuclear
factor-kappaB and nuclear factor-kappaB-regulated gene products in
IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer
cells. Mol Cancer Ther. 6:1022–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian F, Fan T, Zhang Y, Jiang Y and Zhang
X: Curcumin potentiates the antitumor effects of 5-FU in treatment
of esophageal squamous carcinoma cells through downregulating the
activation of NF-κB signaling pathway in vitro and in vivo. Acta
Biochim Biophys Sin (Shanghai). 44:847–855. 2012. View Article : Google Scholar
|
|
8
|
Li L, Braiteh FS and Kurzrock R:
Liposome-encapsulated curcumin: In vitro and in vivo effects on
proliferation, apoptosis, signaling, and angiogenesis. Cancer.
104:1322–1331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki H, Sunagawa Y, Takahashi K,
Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H,
Fujita M, et al: Innovative preparation of curcumin for improved
oral bioavailability. Biol Pharm Bull. 34:660–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bisht S, Feldmann G, Soni S, Ravi R,
Karikar C and Maitra A and Maitra A: Polymeric
nanoparticle-encapsulated curcumin ('nanocurcumin'): A novel
strategy for human cancer therapy. J Nanobiotechnology. 5:32007.
View Article : Google Scholar
|
|
11
|
Anand P, Nair HB, Sung B, Kunnumakkara AB,
Yadav VR, Tekmal RR and Aggarwal BB: Design of curcumin-loaded PLGA
nanoparticles formulation with enhanced cellular uptake, and
increased bioactivity in vitro and superior bioavailability in
vivo. Biochem Pharmacol. 79:330–338. 2010. View Article : Google Scholar
|
|
12
|
Ho JN, Byun SS, Lee S, Oh JJ, Hong SK, Lee
SE and Yeon JS: Synergistic antitumor effect of triptolide and
cisplatin in cisplatin resistant human bladder cancer cells. J
Urol. 193:1016–1022. 2015. View Article : Google Scholar
|
|
13
|
Philippou Y, Hadjipavlou M, Khan S and
Rane A: Complementary and alternative medicine (CAM) in prostate
and bladder cancer. BJU Int. 112:1073–1079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klempner SJ and Bubley G: Complementary
and alternative medicines in prostate cancer: From bench to
bedside? Oncologist. 17:830–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamat AM, Tharakan ST, Sung B and Aggarwal
BB: Curcumin potentiates the antitumor effects of Bacillus
Calmette-Guerin against bladder cancer through the downregulation
of NF-kappaB and upregulation of TRAIL receptors. Cancer Res.
69:8958–8966. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian B, Wang Z, Zhao Y, Wang D, Li Y, Ma
L, Li X, Li J, Xiao N, Tian J, et al: Effects of curcumin on
bladder cancer cells and development of urothelial tumors in a rat
bladder carcinogenesis model. Cancer Lett. 264:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lao CD, Ruffin MT IV, Normolle D, Heath
DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL and Brenner
DE: Dose escalation of a curcuminoid formulation. BMC Complement
Altern Med. 6:102006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanai M, Imaizumi A, Otsuka Y, Sasaki H,
Hashiguchi M, Tsujiko K, Matsumoto S, Ishiguro H and Chiba T:
Dose-escalation and pharmacokinetic study of nanoparticle curcumin,
a potential anticancer agent with improved bioavailability, in
healthy human volunteers. Cancer Chemother Pharmacol. 69:65–70.
2012. View Article : Google Scholar
|
|
19
|
Kanai M, Otsuka Y, Otsuka K, Sato M,
Nishimura T, Mori Y, Kawaguchi M, Hatano E, Kodama Y, Matsumoto S,
et al: A phase I study investigating the safety and
pharmacokinetics of highly bioavailable curcumin (Theracurmin) in
cancer patients. Cancer Chemother Pharmacol. 71:1521–1530. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morimoto T, Sunagawa Y, Katanasaka Y,
Hirano S, Namiki M, Watanabe Y, Suzuki H, Doi O, Suzuki K, Yamauchi
M, et al: Drinkable preparation of Theracurmin exhibits high
absorption efficiency - a single-dose, double-blind, 4-way
crossover study. Biol Pharm Bull. 36:1708–1714. 2013. View Article : Google Scholar
|
|
21
|
Milano F, Mari L, van de Luijtgaarden W,
Parikh K, Calpe S and Krishnadath KK: Nano-curcumin inhibits
proliferation of esophageal adenocarcinoma cells and enhances the T
cell mediated immune response. Front Oncol. 3:1372013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park C, Kim GY, Kim GD, Choi BT, Park YM
and Choi YH: Induction of G2/M arrest and inhibition of
cyclooxygenase-2 activity by curcumin in human bladder cancer T24
cells. Oncol Rep. 15:1225–1231. 2006.PubMed/NCBI
|
|
23
|
Aggarwal BB, Banerjee S, Bharadwaj U, Sung
B, Shishodia S and Sethi G: Curcumin induces the degradation of
cyclin E expression through ubiquitin-dependent pathway and
up-regulates cyclin-dependent kinase inhibitors p21 and p27 in
multiple human tumor cell lines. Biochem Pharmacol. 73:1024–1032.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nowsheen S and Yang ES: The intersection
between DNA damage response and cell death pathways. Exp Oncol.
34:243–254. 2012.PubMed/NCBI
|
|
25
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He M, Li Y, Zhang L, Li L, Shen Y, Lin L,
Zheng W, Chen L, Bian X, Ng HK, et al: Curcumin suppresses cell
proliferation through inhibition of the Wnt/β-catenin signaling
pathway in medulloblastoma. Oncol Rep. 32:173–180. 2014.PubMed/NCBI
|
|
29
|
Lu Y, Wei C and Xi Z: Curcumin suppresses
proliferation and invasion in non-small cell lung cancer by
modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitro Cell
Dev Biol Anim. 50:840–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park CH, Hahm ER, Park S, Kim HK and Yang
CH: The inhibitory mechanism of curcumin and its derivative against
beta-catenin/Tcf signaling. FEBS Lett. 579:2965–2971. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alexandrow MG, Song LJ, Altiok S, Gray J,
Haura EB and Kumar NB: Curcumin: A novel Stat3 pathway inhibitor
for chemoprevention of lung cancer. Eur J Cancer Prev. 21:407–412.
2012. View Article : Google Scholar :
|
|
32
|
Xu X, Qin J and Liu W: Curcumin inhibits
the invasion of thyroid cancer cells via down-regulation of
PI3K/Akt signaling pathway. Gene. 546:226–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou H, Beevers CS and Huang S: The
targets of curcumin. Curr Drug Targets. 12:332–347. 2011.
View Article : Google Scholar :
|


















