Introduction
Prostate cancer (PCa) is the second most prevalent
cancer and the fifth leading cause of cancer-related death
affecting men worldwide (1). Recent
genomic and oncogenetic study involved in PCa onset and progression
highlighted several genomic alterations. Among these, gene fusion
of the androgen-regulated trans-membrane-serine protease gene
(TMPRSS2) and erythroblast transformation-specific (ETS) family
members are considered as hallmarks, and the fusion of ETS-related
gene (ERG) with TMPRSS2 is the most frequent (2). TMPRSS2-ERG fusion gene leads to
overexpression of ERG, and plays an important role in PCa
progression and invasiveness (2).
The function of TMPRSS2-ERG in PCa has been explored
using PCa cell lines, human tissues and mouse models. Some research
demonstrated that aberrant TMPRSS2-ERG expression in mouse prostate
can induce prostatic intraepithelial neoplasia (PIN), and a study
showed that patients with ERG-positive high-grade PIN are much more
likely to progress to PCa (3,4). While
others have highlighted that TMPRSS2-ERG expression cooperates with
or induces other genomic alterations, such as PDE4D7 or EZH2
overexpression, PTEN deletion, PI3K pathway, or androgen receptor
(AR) signaling to promote PCa genesis, progression, migration and
invasion (5–8). These studies provide functional
insight into the role of TMPRSS2-ERG in PCa.
Matrix metalloproteinases (MMPs), especially MMP-9,
have well-recognized roles in cancer metastasis and invasion.
Plexins (PLXNs) are a family of transmembrane receptors for
semaphorins, there were investigations showing that overexpression
of PLXNB1 can activate ErbB2, hinder Rac and R-Ras binding, inhibit
R-Ras GAP activity, or promote AR translocation to the nucleus to
increase the motility and invasion of PCa (9,10).
Given the potential ability of TMPRSS2-ERG fusion gene in
invasiveness, we hypothesized that MMP-9 and/or PLXNB1 might be the
target gene of TMPRSS2-ERG to promote PCa invasion and
metastasis.
In this study, we therefore aimed to characterize
the relation of TMPRSS2-ERG and MMP-9 as well as PLXNB1 in
regulation of PCa aggressiveness. Additionally, the expression and
clinicopathological association of the three genes of Chinese PCa
patients were also investigated.
Materials and methods
Clinical samples
Our study consisted of 55 needle biopsy tissues of
bone metastatic PCa, 50 radical resection tissues from localized
PCa, and 30 electrosection tissues of benign prostatic hyperplasia
(BPH). None of the patient received preoperative radiation or
androgen deprivation therapy. All the cases were collected from
2009–2015 at Northern Jiangsu People's Hospital (Jiangsu, China),
and approved by the local ethics committee of Northern Jiangsu
People's Hospital, in accordance with the guidelines of the 1975
Declaration of Hesinki. Patients' clinicopathological variables are
shown in Table I. Tissues were
collected directly from the operating room, part of the tissue from
each case was immersed in formalin immediately, and the rest stored
in liquid nitrogen.
 | Table I.Clinicopathological variables of
patient sample (mean ± standard deviation). |
Table I.
Clinicopathological variables of
patient sample (mean ± standard deviation).
|
| Metastatic PCa
(n=55) | Localized PCa
(n=50) | BPH (n=30) |
|---|
| Age (years) | 70.8±5.8 | 70.2±5.8 | 68.1±7.7 |
| PSA (ng/ml) | 36.3±34.4 | 27.5±27.1 |
6.7±6.6 |
| Volume
(cm3) | 56.3±29.5 | 44.1±21.6 | 66.4±57.8 |
| Gleason score |
|
|
|
| ≤6 | 3
(5.5%) | 10 (26.0%) | – |
| 7 | 16 (29.1%) | 20 (44.0%) | – |
| ≥8 | 36 (65.4%) | 20 (30.0%) | – |
| Pathological tumor
stage |
|
|
|
| 2 | – | 33 (66%) | – |
| 3 | – | 12 (24%) | – |
| 4 | – | 5
(10%) | – |
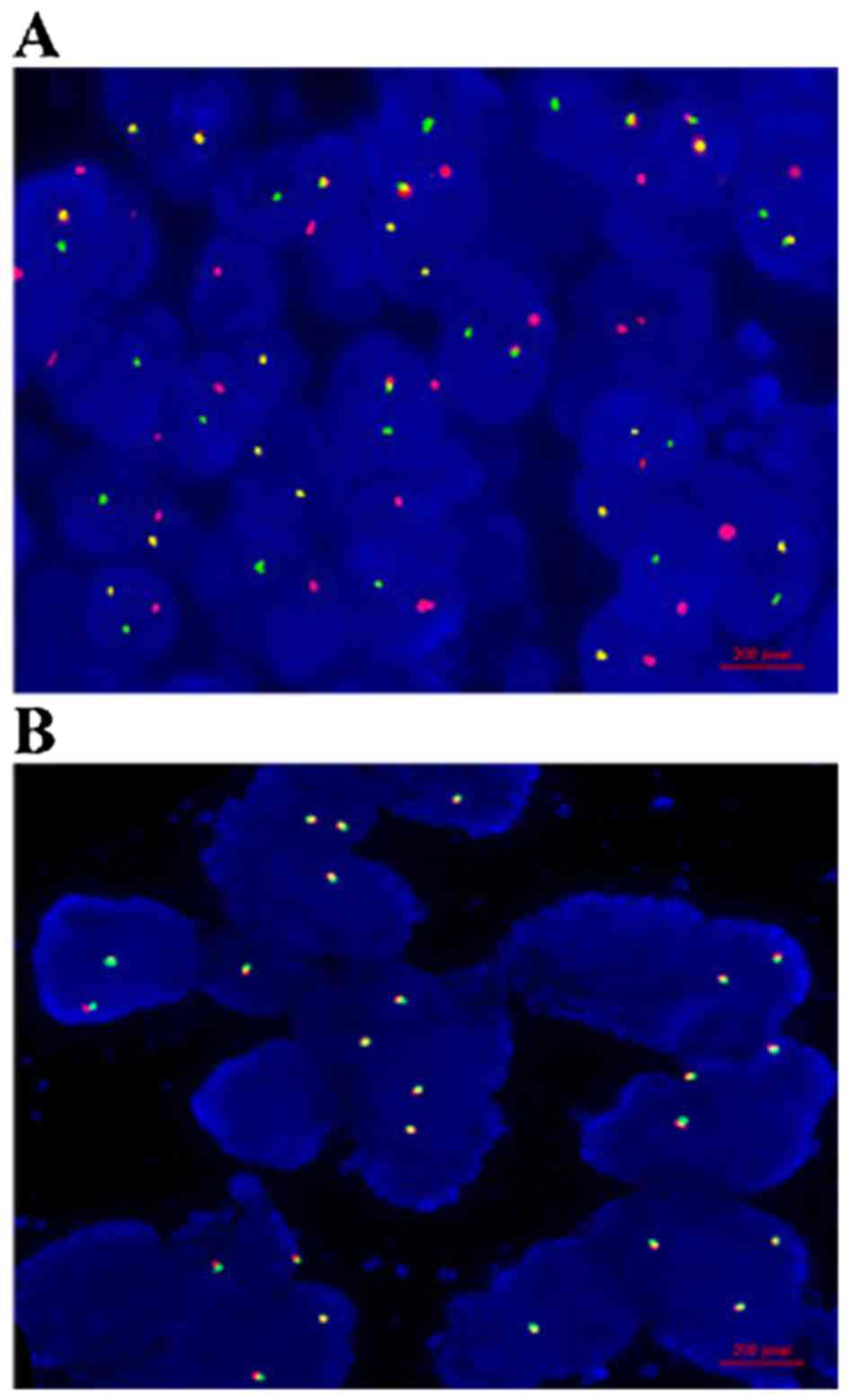
Fluorescence in situ hybridization
(FISH) analysis
Four-micrometer (4-µm) sections of the
formalin-fixed, paraffin-embedded tissues were cut for FISH
analysis. TMPRSS2-ERG fusion was tested by a dual-color dual-fusion
model probes (Beijing GP Medical Technologies, Ltd., Beijing,
China) according to the manufacturer's protocol. Slides were
examined using ImagingZ1 microscope (Carl Zeiss, Oberkochen,
Germany). FISH signals were scored manually (100X oil immersion) in
morphologically intact and non-overlapping nuclei. The positive
signal patterns are one yellow/one green in a cell, which revealed
a deletion pattern of fusion, or one yellow/one green/one red in a
cell, which illustrated an insertion pattern. Additionally, the
negative signal pattern is two yellow signals in a cell. The
criteria to determine a PCa case TMPRSS2-ERG fusion-positive was
that ≥20 cells with the positive signal pattern in a random count
of 100 cells.
Cell culture
Human PCa cell lines VCaP (TMPRSS2-ERG
fusion-positive) and PC-3 (TMPRSS2-ERG fusion-negative) were
obtained from Chinese Academy of Sciences Typical Culture
Preservation Committee Cell Bank and maintained in RPMI-1640 and
Ham's/F12 medium (Hyclone Laboratories Inc., Logan, UT, USA)
respectively, and supplemented with 10% fetal bovine serum (Clark
Bioscience, USA) at 37°C in a humidified 5% CO2
incubator.
siRNA-mediated knockdown
VcaP and PC-3 cells were seeded at a density of
5×105 cells/well into 6-well plates and cultured
overnight at 37°C with 5% CO2 until the cells reached
70–80% confluency. Small interfering RNA (siRNA) transfection on
two cell lines was carried out using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
protocol. Specific siRNA sequences targeting the human ERG, MMP-9,
and PLXNB1 were all designed and synthesized respectively
(GenePharma Pharmaceutical Co., Shanghai, China), and the sequences
are provided in Table II. The NC
group was defined as negative control, and the mock group was the
ones supplemented with the transfection reagent only.
 | Table II.The sequences of primers and
siRNA. |
Table II.
The sequences of primers and
siRNA.
| Gene | Forward primer | Reverse primer |
|---|
| ERG | 5′-GTG CCA AAC ATC
CTA TTT CC-3′ | 5′-CAT TTA TAC ACT
ACG AGT TG-3′ |
| MMP-9 | 5′-CCT TCT ACG GCC
ACT ACT GT-3′ | 5′-CAC TTG TCG GCG
ATA AGG AA-3′ |
| PLXNB1 | 5′-TCT GCT CAG TGA
CCT GGT TG-3′ | 5′-CTA CGG AGT CCC
TCA CGA AG-3′ |
| β-actin | 5′-GGG ACC TGA CTG
ACT ACC TC −3′ | 5′-TCA TAC TCC TGC
TTG CTG AT −3′ |
| ERG siRNA | 5′-CGA CAU CCU UCU
CUC ACA UAU-3′ | 5′-UGA UGU UGA UAA
AGC CUU A-3′ |
| MMP-9 siRNA | 5′-CUA UGG UCC UCG
CCC UGA ATT-3′ | 5′-UUC AGG GCG AGG
ACC AUA GAG-3′ |
| PLXNB1 siRNA | 5′-AAG GUA UAC AGA
CAG AUG GAC AUC C-3′ | 5′-AAG CUC GAA AUA
UCU CCU-3′ |
| Negative siRNA | 5′-UUC UCC GAA CGU
GUC ACG UTT-3′ | 5′-ACG UGA CAA GUU
CGG AGA ATT-3′ |
Cell proliferation, migration and
invasion assays
Cell proliferation was determined using MTT assay in
96-well plate (1×104 cells/well). After transfection for
12, 24, 48 or 72 h, at 10 µl/well MTT solution (5 mg/ml) was added
and incubated for 4 h. The supernatant was removed and DMSO (100
µl/well) added. The absorbance [estimated by optical density (OD)]
was measured at 492 nm using Epoch Microplate Spectrophotometer
(BioTek, VT, USA).
Cell migration assays were performed with
1×105 cells in a serum-free medium and seeded on the
upper chamber of Transwell inserts with a pore size of 8 µm. While
the cell invasion assays were performed on Matrigel-coated upper
chamber with 1×105 cells, the culture medium containing
10% FBS as a chemoattractant was added to the lower chamber. After
24-h incubation, cells were fixed and labeled with crystal violet,
and the migrated or invaded cells were counted in five randomly
selected microscopic fields under ×200 microscopy (Olympus, Tokyo,
Japan).
RNA extraction and quantitative
real-time PCR (qRT-PCR)
Total RNA isolated from human prostate tissues and
cells was extracted using TRIzol reagent according to the
manufacturer's instructions (Invitrogen). cDNA was reverse
transcribed from 1 µg total RNA using PrimeScript™ RT Master Mix
kit (Takara, Dalian, China) according to the manufacturer's
protocol. qRT-PCR was performed with the SYBR Premix Ex Taq
(Takara). All primer sequences are listed in Table II. Relative mRNA expression levels
were calculated by the comparative 2−∆∆Ct method.
Western blot analysis
We randomly selected 10 cases from each group of
patients to detect the protein expression. Total protein of human
prostate tissues and PCa cell lines was extracted using RIPA lysate
buffer (Beyotime, Shanghai, China) and the concentration was
quantified by BCA protein assay kit (Beyotime). Protein lysates (20
µg per lane) were separated by sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes. After blocking with 5% fat-free milk, the
membranes were incubated with primary antibodies at 4°C overnight,
followed by horseradish peroxidase-conjugated secondary antibodies.
Immunoreactive bands were visualized using FluorChem FC2 (Alpha
Innotech, CA, USA). The primary antibodies to ERG, MMP-9 and PLXNB1
were all purchased from Abcam (Cambridge, MA, USA), and were
diluted 1,000, 5,000 and 5,000 times respectively. The internal
reference GaPDH was from KangChen Biotech (Shanghai, China) and
diluted 5,000 times.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (IBM Corp., NY, USA), with a significance level of 0.05
(two-tailed probability). Data were expressed as percentage or
means ± standard deviation. The distributions of continuous and
categorical variables between cases and controls were compared
using Student's t-test and one-way ANOVA analysis. Correlations
between the clinicopathological parameters and gene expression were
determined by Chi-square test and rank correlation analysis.
Results
MMP-9 and PLXNB1 expression were
associated with TMPRSS2-ERG expression in human PCa tissues
To investigate whether MMP-9 and PLXNB1 are target
genes of TMPRSS2-ERG in PCa, we first examined their expression in
human samples. A total of 135 cases were successfully analyzed by
FISH, qRT-PCR, and 30 randomly selected cases by western blot
analysis. Overall, TMPRSS2-ERG fusion was positive in 47.3% (26/55)
of metastatic PCa, 28.0% (14/50) of localized PCa, and 0.0% (0/30)
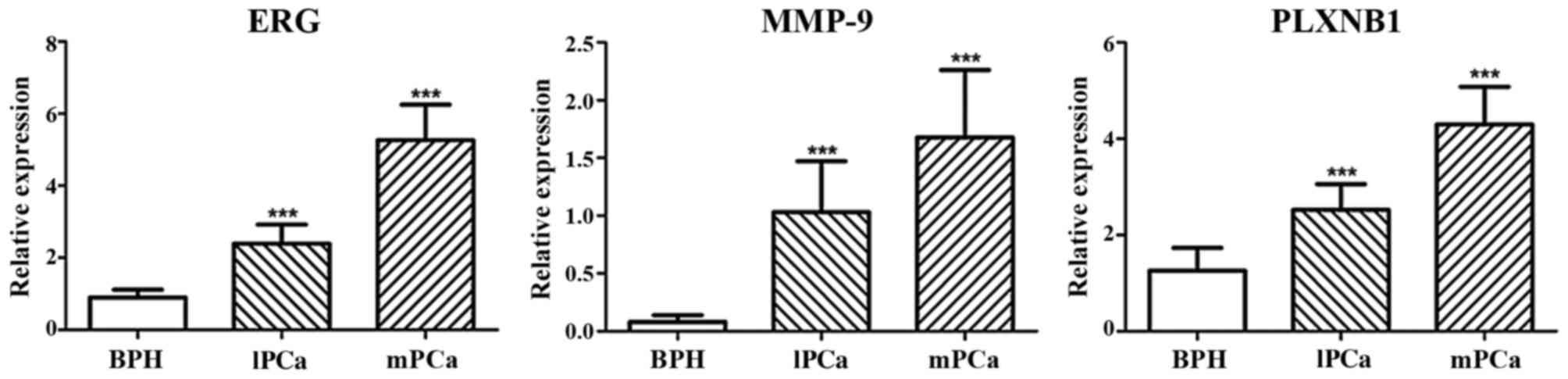
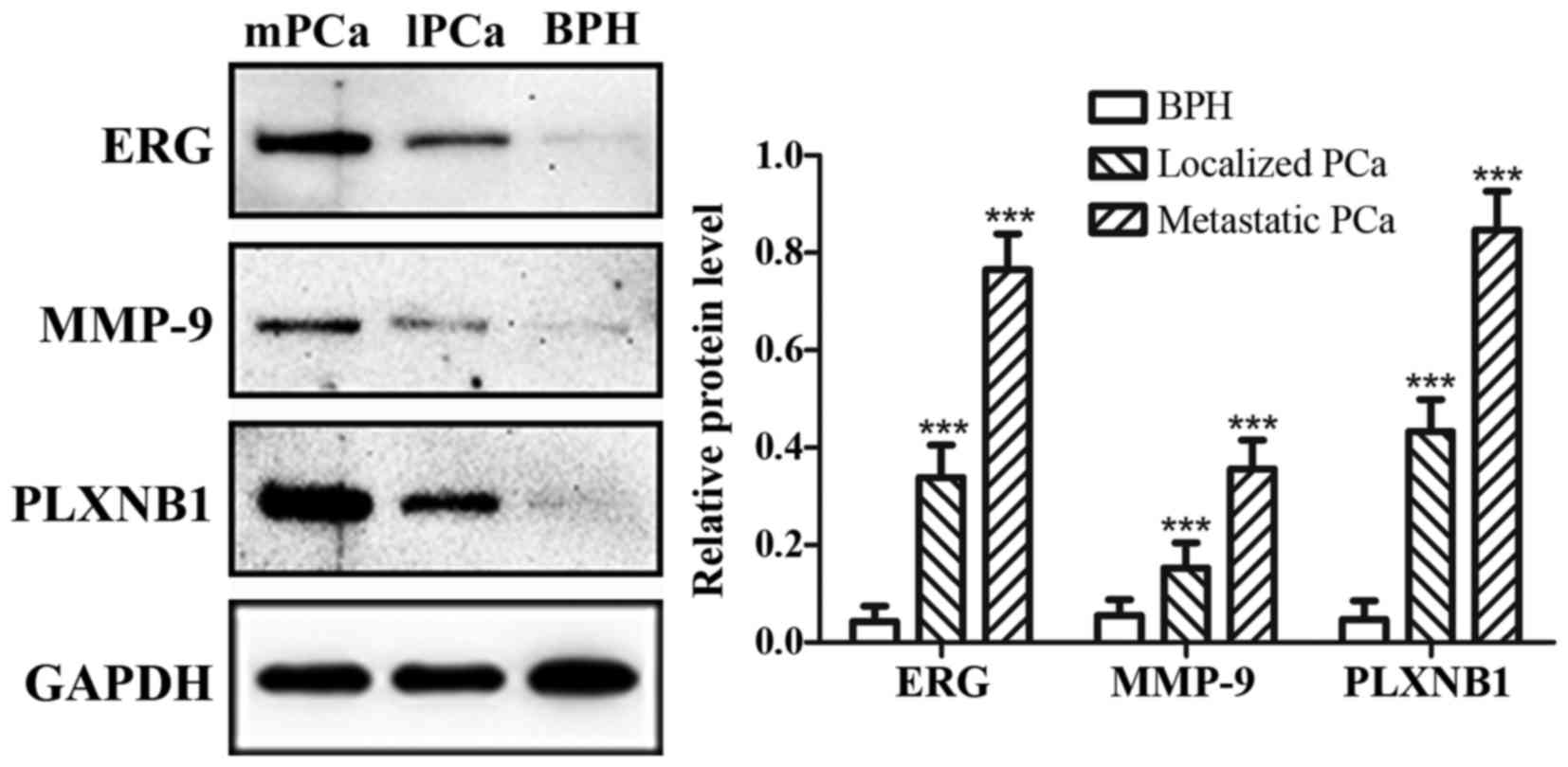
of BPH (Fig. 1). The mRNA
expression of ERG, MMP-9 and PLXNB1 were increasing gradually from
BPH to metastatic PCa, as well as protein expression (Figs. 2 and 3). Additionally, in metastatic PCa cohort,
localized PCa cohort and total PCa cohort, the mRNA expression
levels of ERG, MMP-9 and PLXNB1 were all significantly higher in
TMPRSS2-ERG-positive PCa samples than in TMPRSS2-ERG-negative
samples (P-values were all <0.0001).
The mRNA levels of ERG, MMP-9 and PLXNB1 were all
positively correlated with TMPRSS2-ERG fusion (P<0.0001), in
metastatic PCa group the spearman values were 0.757, 0.833 and
0.865 respectively, in the localized PCa group were 0.778, 0.781
and 0.889, respectively, and in total PCa group were 0.549, 0.806
and 0.626, respectively. Moreover, the mRNA expression of MMP-9 and
PLXNB1 were positively correlated with ERG mRNA level
(P<0.0001), in metastatic PCa group the Spearman values were
0.705 and 0.751, in localized PCa group were 0.640 and 0.710, and
in total PCa group were 0.762 and 0.919. While there was no
relationship between MMP-9 and PLXNB1 in each of the PCa groups.
Furthermore, there was no relationship among the genes with each
other in BPH samples.
Chi-square test demonstrated that high Gleason score
patients tend to the fusion of TMPRSS2 and ERG gene in metastatic
PCa group (P=0.007) and total PCa group (P=0.039). The relationship
between gene expression and clinicopathological parameters is shown
in Table III. Obviously, there
was no relationship in metastatic PCa samples. While in localized
PCa group, TMPRSS2-ERG fusion gene positive rate was positively
correlated with serum PSA level, Gleason score and pathological
tumor stage, additionally, the Gleason score and pathological tumor
stage were positively correlated with the expression of MMP-9 and
PLXNB1. In total PCa samples, the Gleason score was positively
correlated with the expression of each gene, and serum PSA level
was positively correlated with TMPRSS2-ERG fusion gene positive
rate.
 | Table III.The relation between the expression
of T-E fusion gene and clinicopathological parameters in metastatic
and localized PCa, respectively. |
Table III.
The relation between the expression
of T-E fusion gene and clinicopathological parameters in metastatic
and localized PCa, respectively.
|
| Metastatic PCa | Localized PCa | Total PCa |
|---|
|
|
|
|
|
|---|
| Parameters | T-Ea | ERG | MMP-9 | PLXNB1 | T-E | ERG | MMP-9 | PLXNB1 | T-E | ERG | MMP-9 | PLXNB1 |
|---|
| Age/year |
|
|
|
|
|
|
|
|
|
|
|
|
|
Spearman value | 0.055 | −0.060 | 0.055 | −0.155 | −0.107 | −0.073 | −0.038 | −0.173 | −0.030 | 0.000 | 0.031 | −0.068 |
|
P-value | 0.855 | 0.663 | 0.690 | 0.258 | 0.461 | 0.615 | 0.791 | 0.229 | 0.763 | 0.998 | 0.756 | 0.490 |
| PSA/(ng/ml) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Spearman value | 0.147 | 0.063 | 0.048 | 0.050 | 0.284 | 0.078 | 0.179 | 0.158 | 0.217 | 0.159 | 0.180 | 0.177 |
|
P-value | 0.285 | 0.646 | 0.725 | 0.717 |
0.046 | 0.592 | 0.214 | 0.273 |
0.026 | 0.106 | 0.066 | 0.071 |
|
Volume/(cm3) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Spearman value | −0.015 | −0.066 | −0.107 | −0.023 | 0.136 | 0.102 | 0.098 | 0.063 | 0.092 | 0.182 | 0.123 | 0.172 |
|
P-value | 0.914 | 0.632 | 0.438 | 0.870 | 0.347 | 0.480 | 0.500 | 0.666 | 0.349 | 0.063 | 0.212 | 0.079 |
| Gleason score |
|
|
|
|
|
|
|
|
|
|
|
|
|
Spearman value | 0.255 | 0.100 | 0.055 | 0.242 | 0.382 | 0.259 | 0.335 | 0.304 | 0.352 | 0.312 | 0.328 | 0.368 |
|
P-value | 0.060 | 0.467 | 0.691 | 0.075 |
0.006 | 0.069 |
0.017 | 0.032 |
<0.0001 | 0.001 | 0.001 |
<0.0001 |
| Pathological tumor
stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
Spearman value | – | – | – | – | 0.818 | 0.671 | 0.660 | 0.692 | – | – | – | – |
|
P-value | – | – | – | – |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 | – | – | – | – |
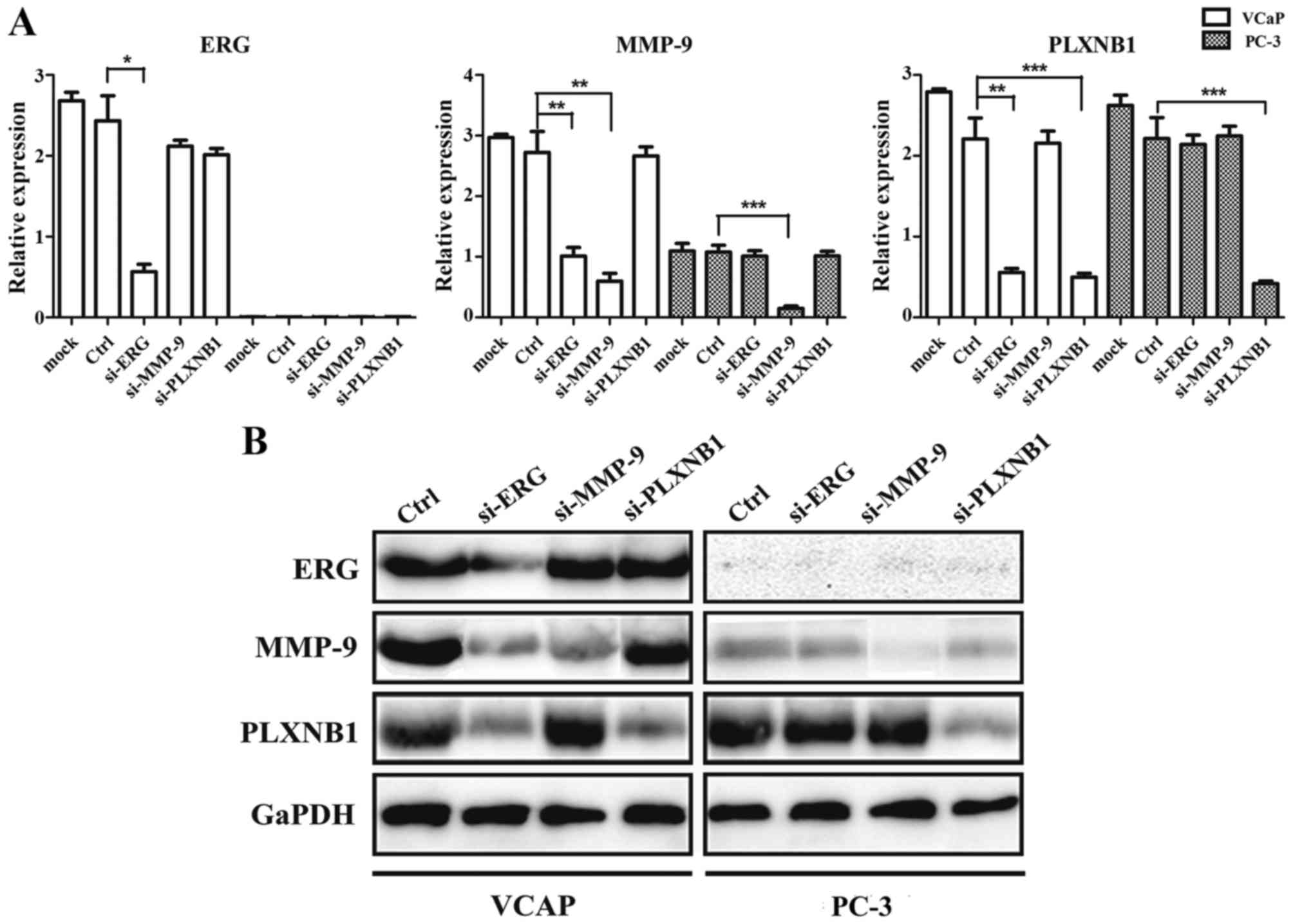
Successful transformation of siRNA to
PCa cells and inhibition of the expression of target genes
To determine the role of TMPRSS2-ERG fusion gene,
MMP-9 and PLXNB1 in the invasiveness of PCa cells, specific siRNA
targeting of ERG, MMP-9 and PLXNB1 were transiently transfected
into PCa cells, and, the mRNA and protein of the target genes were
detected. qRT-PCR and western blot analysis confirmed the
downregulation of ERG, MMP-9 and PLXNB1 in VCaP cells, and
downregulation of MMP-9 and PLXNB1 in PC-3 cells, compared to the
cells transfected with negative siRNA (Fig. 4). We also discovered that
downregulated ERG expression can significantly decrease the mRNA
and protein expression of MMP-9 and PLXNB1 in VCaP cells.
The effects of siRNA on proliferation,
migration and invasion in PCa cells
Consistent with previous findings, the mRNA and
protein expression level of ERG were high in PCa cell line VCaP,
but almost no expression was observed in PC-3 cells (Fig. 4). Using MTT assay, we found that
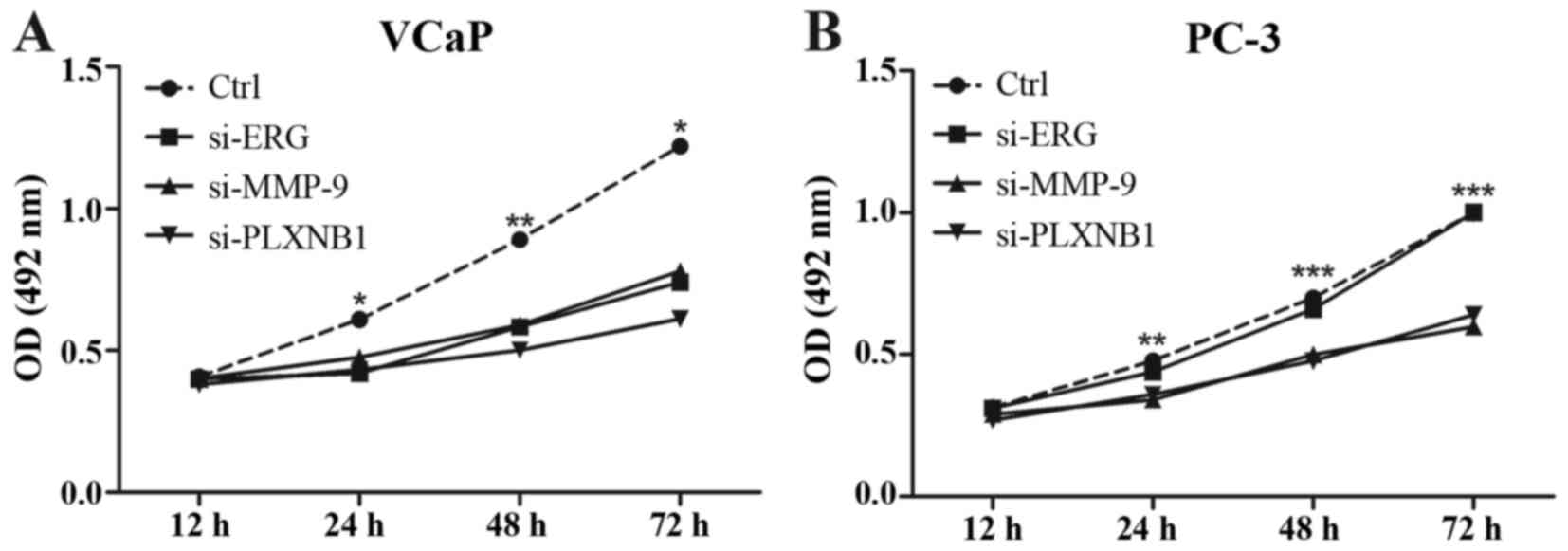
ERG, MMP-9 and PLXNB1 knockdown VCaP cells have higher apoptosis
rate (Fig. 5A); while only MMP-9
and PLXNB1 knockdown have higher apoptosis rate in PC-3 cells
(Fig. 5B).
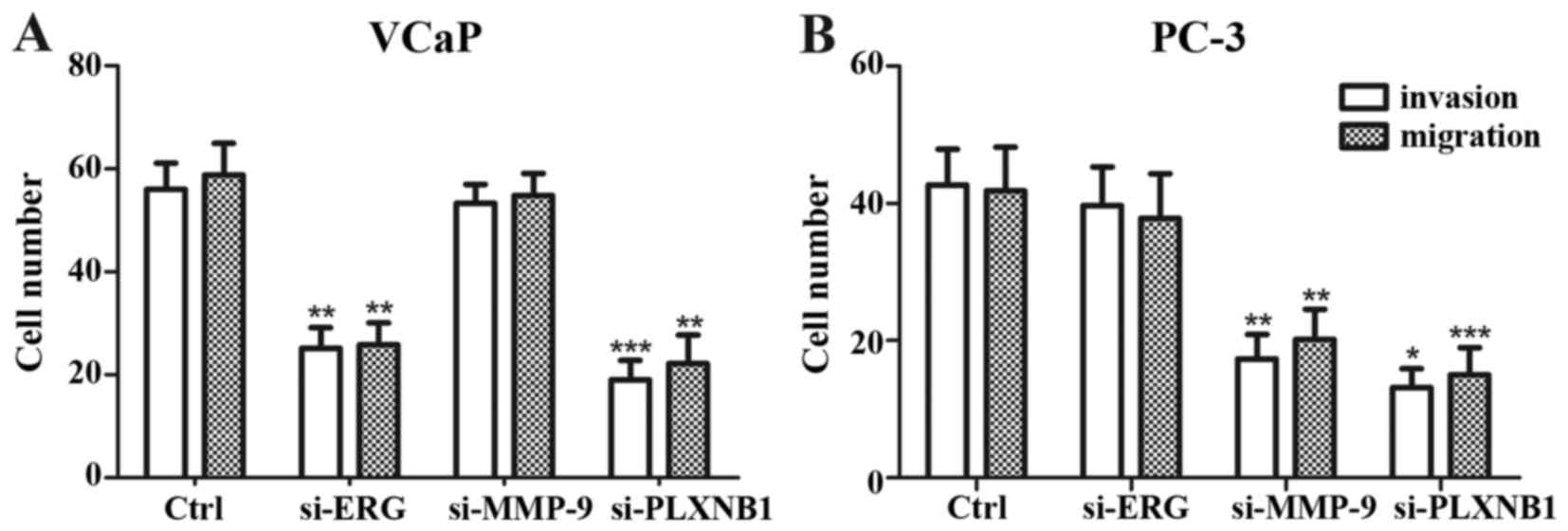
In VCaP cells, siRNA knockdown of ERG significantly
decreased migration and invasive capacity (P=0.002 and 0.008
respectively, Fig. 6A), similarly,
knockdown of PLXNB1 also reduced migration and invasive capacity
(P=0.005 and <0.0001 respectively, Fig. 6A). In PC-3 cells, knockdown of MMP-9
downregulated the migration and invasive capacity significantly
(P=0.002 and 0.007 respectively, Fig.
6B); and knockdown of PLXNB1 observably decreased the migration
and invasive ability (P≤0.0001 and 0.015 respectively, Fig. 6B).
Discussion
The mechanism and treatment of metastatic PCa have
been the focus of targeted therapy as a new treatment with great
application prospects. Santoni et al indicated that fully
understanding the role of TMPRSS2-ERG is important for
individualised therapy in PCa patients (11). Urbinati et al designed a kind
of siRNA anti-TMPRSS2-ERG and successfully downregulated the
expression of this fusion gene, and observably inhibited the
proliferation of PCa cells (12).
The above showed the potential and importance of TMPRSS2-ERG in PCa
targeting treatment.
Here, using human samples, we showed that, as
expected, the positive rate of TMPRSS2-ERG fusion in human prostate
tissues was increasing with the degree of invasiveness.
Additionally, this fusion can obviously upregulate the mRNA and
protein expression of ERG, as well as MMP-9 and PLXNB1. These
findings are in agreement with the role of TMPRSS2-ERG as revealed
using various PCa cell models (2,10,13).
The positive rate of TMPRSS2-ERG fusion detected by FISH in our
study was 38.1% (40/105). While, Magi-Galluzzi et al
reported that TMPRSS2-ERG fusion was present in 50.0% (21/42) of
Caucasian, 31.3% (20/64) of African-American, and 15.9% (7/44) of
Japanese, and Dong et al demonstrated the incidence was
14.3% (13/91) by biopsy specimens or 11.1% (2/18) by radical
prostatectomy samples (14,15). The reasons why the frequency of gene
fusion varies among different studies is complex, probably owing to
race, methods of obtaining specimens, the sensitivity of the
technique used, the number of samples included in the study, the
criteria used to determined a positive signal and the patients from
different areas.
MMP-9 has been confirmed to be correlated with
increased invasion and metastasis in various tumor types, including
PCa (16). In fact, several
transcription factors, such as twist-related protein 1 (Twist1),
high-mobility group protein A2 (HMGA2), mitogen-activated protein
kinase 1 (MAPK1), and signal transducer and activator of
transcription 3 (STAT3), have been shown to control MMP-9
expression in numerous cancer types (17–20).
Here, we showed that MMP-9 expression was correlated with
TMPRSS2-ERG as well as ERG in PCa samples, and was positively
regulated by ERG which is upregulated by TMPRSS2-ERG fusion in VCaP
cells. Additionally, it has been reported that ETS-1 promotes the
invasiveness of paclitaxel-resistant and hormone-refractory PCa
cells by increasing MMP-9 expression, moreover, a recent study
demonstrated that overexpression of ETV4 can upregulate the
invasiveness of PCa through increasing MMP-9 expression (21,22).
ERG is not only a transcription factor, but also a member of the
ETS family, so these findings emphasized the importance of ETS in
controlling MMP-9 expression in PCa. However, knockdown of the
expression of MMP-9 did not weaken TMPRSS2-ERG-induced cell
migration or invasion in VCaP cells. This result was similar to
that treating TMPRSS2-ERG expressing PCa cells with MMP-9 inhibitor
(23). Therefore, future studies
are required to investigate the role of MMP-9 in
TMPRSS2-ERG-positive PCa cells using in vivo models.
PLXNs activate many characteristics of the invasive
phenotype seen in cancer progression, inducing changes in
extracellular matrix (ECM) adhesion, motility, scatter, and
branching morphogenesis (24). It
was revealed that PLXNA2 expression was higher in more aggressive
breast cancer cell types, and another study showed that TMPRSS2-ERG
positively and directly regulates PLXNA2 expression in PC3c cells
(13,25). Ye et al found that PLXNB1 was
significantly higher expressed in serous ovarian carcinomas
(26). Our results showed that,
similar to MMP-9, PLXNB1 was not only expressed higher in
metastatic PCa tissues, but positively and directly regulated by
TMPRSS2-ERG fusion in VCaP cells. Together with the finding that
downregulation of PLXNB1 in VCaP cells can decrease the migration
and invasion capacity, we first discovered that PLXNB1 contributes,
at least in part, to TMPRSS2-ERG-induced VCaP cell migration and
invasion. This finding supports the potential of PLXNB1 in PCa
metastasis.
The association between TMPRSS2-ERG fusion and PCa
clinical outcome has not yet been clearly established. Some studies
have shown that this gene fusion is not significantly associated
with PCa clinicopathological parameters (27,28).
However, other studies have demonstrated that it is associated with
higher clinical tumor stages and Gleason scores (29,30).
Our results showed that TMPRSS2-ERG is positively associated with
Gleason scores and serum PSA level in localized PCa samples and
total PCa samples, while the correlation was weak. Additionally,
the expression of MMP-9 and PLXNB1 were positively associated with
Gleason scores with a weak correlation. Compared to others
research, these uncertain results still need support of larger
sample numbers.
In conclusion, our data confirmed the important role
of TMPRSS2-ERG in PCa invasiveness, and demonstrated that PLXNB1,
but not MMP-9, was the target gene directly related to TMPRSS2-ERG
in PCa cell migration and invasion, providing novel insights into
the role of TMPRSS2-ERG in PCa invasiveness.
Acknowledgements
This study was supported by the generous funding of
the Yangzhou Science and Technology Natural Science Foundation
(YZ2014052), and Project of Jiangsu Province Health and Family
Planning Commission (H2015550).
References
|
1
|
International Agency for Research on
Cancer, . Prostate cancer: estimated incidence, mortality, and
prevalence worldwide. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxAccessed
March 20, 2014.
|
|
2
|
Tomlins SA, Rhodes DR, Perner S,
Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J,
Kuefer R, et al: Recurrent fusion of TMPRSS2 and ETS transcription
factor genes in prostate cancer. Science. 310:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klezovitch O, Risk M, Coleman I, Lucas JM,
Null M, True LD, Nelson PS and Vasioukhin V: A causal role for ERG
in neoplastic transformation of prostate epithelium. Proc Natl Acad
Sci USA. 105:2105–2110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park K, Dalton JT, Narayanan R, Barbieri
CE, Hancock ML, Bostwick DG, Steiner MS and Rubin MA: TMPRSS2:ERG
gene fusion predicts subsequent detection of prostate cancer in
patients with high-grade prostatic intraepithelial neoplasia. J
Clin Oncol. 32:206–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Böttcher R, Henderson DJ, Dulla K, van
Strijp D, Waanders LF, Tevz G, Lehman ML, Merkle D, van Leenders
GJ, Baillie GS, et al: Human phosphodiesterase 4D7 (PDE4D7)
expression is increased in TMPRSS2-ERG-positive primary prostate
cancer and independently adds to a reduced risk of post-surgical
disease progression. Br J Cancer. 113:1502–1511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fallahabadi ZR, Daloii MR Noori, Mahdian
R, Behjati F, Shokrgozar MA, Abolhasani M, Asgari M and Shahrokh H:
Frequency of PTEN alterations, TMPRSS2-ERG fusion and their
association in prostate cancer. Gene. 575:755–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
King JC, Xu J, Wongvipat J, Hieronymus H,
Carver BS, Leung DH, Taylor BS, Sander C, Cardiff RD, Couto SS, et
al: Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation
in prostate oncogenesis. Nat Genet. 41:524–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Chi P, Rockowitz S, Iaquinta PJ,
Shamu T, Shukla S, Gao D, Sirota I, Carver BS, Wongvipat J, et al:
ETS factors reprogram the androgen receptor cistrome and prime
prostate tumorigenesis in response to PTEN loss. Nat Med.
19:1023–1029. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Damola A, Legendre A, Ball S, Masters JR
and Williamson M: Function of mutant and wild-type plexinb1 in
prostate cancer cells. Prostate. 73:1326–1335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williamson M, de Winter P and Masters JR:
Plexin-B1 signalling promotes androgen receptor translocation to
the nucleus. Oncogene. 35:1066–1072. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santoni M, Scarpelli M, Mazzucchelli R,
Lopez-Beltran A, Cheng L, Epstein JI, Cascinu S, Briganti A, Catto
JW, Montorsi F, et al: Current histopathologic and molecular
characterisations of prostate cancer: towards individualised
prognosis and therapies. Eur Urol. 69:186–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urbinati G, Ali HM, Rousseau Q, Chapuis H,
Desmaële D, Couvreur P and Massaad-Massade L: Antineoplastic
effects of siRNA against TMPRSS2-ERG junction oncogene in prostate
cancer. PLoS One. 10:e01252772015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian TV, Tomavo N, Huot L, Flourens A,
Bonnelye E, Flajollet S, Hot D, Leroy X, de Launoit Y and
Duterque-Coquillaud M: Identification of novel TMPRSS2:ERG
mechanisms in prostate cancer metastasis: involvement of MMP9 and
PLXNA2. Oncogene. 33:2204–2214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magi-Galluzzi C, Tsusuki T, Elson P,
Simmerman K, LaFargue C, Esgueva R, Klein E, Rubin MA and Zhou M:
TMPRSS2-ERG gene fusion prevalence and class are significantly
different in prostate cancer of Caucasian, African-American and
Japanese patients. Prostate. 71:489–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong J, Xiao L, Sheng L, Xu J and Sun ZQ:
TMPRSS2:ETS fusions and clinicopathologic characteristics of
prostate cancer patients from Eastern China. Asian Pac J Cancer
Prev. 15:3099–3103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Li Q, Li K, Xiao P and Yin R:
Twist-related protein 1-mediated regulation of mesenchymal change
contributes to the migration and invasion of cervical cancer cells.
Oncol Lett. 10:3107–3112. 2015.PubMed/NCBI
|
|
18
|
Shi Z, Li X, Wu D, Tang R, Chen R, Xue S
and Sun X: Silencing of HMGA2 suppresses cellular proliferation,
migration, invasion, and epithelial-mesenchymal transition in
bladder cancer. Tumour Biol. 37:7515–7523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XW, Tuergan M and Abulizi G: Expression
of MAPK1 in cervical cancer and effect of MAPK1 gene silencing on
epithelial-mesenchymal transition, invasion and metastasis. Asian
Pac J Trop Med. 8:937–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato T, Fujita Y, Nakane K, Kojima T,
Nozawa Y, Deguchi T and Ito M: ETS1 promotes chemoresistance and
invasion of paclitaxel-resistant, hormone-refractory PC3 prostate
cancer cells by up-regulating MDR1 and MMP9 expression. Biochem
Biophys Res Commun. 417:966–971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi M, Liu Z, Shen C, Wang L, Zeng J, Wang
C, Li C, Fu W, Sun Y and Han B: Overexpression of ETV4 is
associated with poor prognosis in prostate cancer: Involvement of
uPA/uPAR and MMPs. Tumour Biol. 36:3565–3572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomlins SA, Laxman B, Varambally S, Cao X,
Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, et al:
Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia.
10:177–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: Cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gabrovska PN, Smith RA, Tiang T, Weinstein
SR, Haupt LM and Griffiths LR: Semaphorin-plexin signalling genes
associated with human breast tumourigenesis. Gene. 489:63–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye S, Hao X, Zhou T, Wu M, Wei J, Wang Y,
Zhou L, Jiang X, Ji L, Chen Y, et al: Plexin-B1 silencing inhibits
ovarian cancer cell migration and invasion. BMC Cancer. 10:6112010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun QP, Li LY, Chen Z, Pang J, Yang WJ,
Zhou XF, Qiu JG, Su ZL, He D and Gao X: Detection of TMPRSS2-ETS
fusions by a multiprobe fluorescence in situ hybridization assay
for the early diagnosis of prostate cancer: A pilot study. J Mol
Diagn. 12:718–724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perner S, Mosquera JM, Demichelis F, Hofer
MD, Paris PL, Simko J, Collins C, Bismar TA, Chinnaiyan AM, De
Marzo AM, et al: TMPRSS2-ERG fusion prostate cancer: An early
molecular event associated with invasion. Am J Surg Pathol.
31:882–888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mehra R, Tomlins SA, Shen R, Nadeem O,
Wang L, Wei JT, Pienta KJ, Ghosh D, Rubin MA, Chinnaiyan AM, et al:
Comprehensive assessment of TMPRSS2 and ETS family gene aberrations
in clinically localized prostate cancer. Mod Pathol. 20:538–544.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Font-Tello A, Juanpere N, de Muga S,
Lorenzo M, Lorente JA, Fumado L, Serrano L, Serrano S, Lloreta J
and Hernández S: Association of ERG and TMPRSS2-ERG with grade,
stage, and prognosis of prostate cancer is dependent on their
expression levels. Prostate. 75:1216–1226. 2015. View Article : Google Scholar : PubMed/NCBI
|