Introduction
Esophageal cancer is the eighth most common cancer
worldwide (1). Despite the latest
evolutions in treatment, the overall mortality rate of esophageal
cancer patients remains high, with a 5-year survival of only 9.8%
in Europe (2,3). Therefore, the need for the development
of new therapies is high and preclinical research plays herein a
crucial role.
The majority of preclinical research in esophageal
carcinoma has been performed in heterotopic models (subcutaneous
xenograft tumors) (4). However,
orthotopic tumor models, where tumors are grown at their primary
site, are preferred, since they more closely resemble tumor
development in patients (5).
Furthermore, it has been proven that interaction between the tumor
and its microenvironment plays a crucial role during carcinogenesis
(6). This tumor microenvironment is
considerably different when esophageal tumors are grown
subcutaneous (heterotopic), i.e. different blood supplies leading
to different metastatic routes.
Various preclinical research in esophageal carcinoma
has been performed using orthotopic models. Tumor cells are
injected either directly in the esophageal wall, or subcutaneously
in donor animals to transplant tumor fragments onto the surgically
injured esophageal wall. The surgical procedures to induce
orthotopic esophageal tumors are technically challenging due to the
location and size of the esophagus in laboratory animals (mostly
mice). Five surgical approaches to the esophagus have been
described: (i) median laparotomy (7–12),
(ii) median laparotomy combined with transgastric approach
(13), (iii) subcostal laparotomy
(14), (iv) transoral (15) and (v) cervical approach (16). Tumor take varies between 0 and 100%
(mean, 80.06%), and seems to depend more on the aggressiveness of
the tumor cell line, than on the surgical technique. A total of 9
different esophageal squamous cell carcinoma (ESSC) cell lines
(81-T, KYSE30, KYSE150, SLMT-1, TE1, TE8, TE4, TE10 and T.Tn) and 3
esophageal adenocarcinoma (EAC) cell lines [(OE19) (9,11,17,18),
PT1590 (10,19) and OE33 (9)] have been described for orthotopic use.
Since EAC has become the main subtype in patients in the US and
Northern and Western Europe (20),
the present study focused on EAC. Overall, there is a lack of
preclinical orthotopic EAC models. Of the 3 EAC cell lines,
previously described, for orthotopic use, OE33 represents locally
advanced EAC. This cell line was used by Habibollahi et al
for diagnostic properties (9), but
only in 5 mice. They described orthotopic OE33 tumors of 2–3 mm in
diameter at 4 weeks after injection. OE19 and PT1590, in contrast,
are representative cell lines for aggressive metastatic EAC.
Moreover, OE19 overexpresses Her2, which is found in only a
minority of EAC patients [17–32% of gastroesophageal junction (GEJ)
tumors (21)].
The aim of the present study was to establish an
orthotopic EAC model in the mouse based on two generally available
human EAC cell lines, OE33 and OACM5 1.C. In vivo tumor take
and growth were evaluated (orthotopic as well as subcutaneous) and
in vitro cell line characterization was performed.
Materials and methods
In vitro
Cell lines
The human EAC cell lines OE33 and OACM5 1.C were
obtained from Dr W. Dinjens (Department of Pathology, Erasmus MC,
Rotterdam, The Netherlands) and are available at the European
Collection of Authenticated Cell Cultures (ECACC) (nos. 96070808
and 11012006, respectively). MDA-MB-231 GFP Luc, human mammary
carcinoma cell lines (ATCC, HTB-26) and HCT8/E11, human colon
adenocarcinoma cell line (ATCC no. CCL-244), were controls for the
in vitro experiments. OE33, HCT-8/E11 and MDA-MB-231 GFP Luc
were cultured at 37°C in a 10% CO2 humidified atmosphere in
Dulbecco's modified Eagle's medium (DMEM) (Life Technologies,
Ghent, Belgium), supplemented with 10% fetal bovine serum (FBS),
penicillin-streptomycin and fungizone. Doxycycline (50 µg/100 ml
medium) was added to the medium of the MDA-MB-231 GFP Luc cell line
to express GFP. OACM5 1.C and the in vivo selected cell line
OACM5 1.C SC1 (described below) were cultured at 37°C in 5% CO2
humidified atmosphere in RPMI-1640 medium supplemented with
GlutaMAX™-I (both from Life Technologies), 10% FBS,
penicillin-streptomycin and fungizone. EAC cell lines and the in
vivo selected cell line OACM5 1.C SC1 were authenticated by STR
DNA profiling. Microscopic images were captured using a phase
contrast microscope (Leica DMI3000B; Leica, Diegem, Belgium).
Sphere formation assay
One million single cells were diluted in 6 ml
culture medium in an Erlenmeyer flask (50 ml). They were incubated
for 72 h on a Gyrotory shaker at 37°C and 70 rpm in 5 or 10% CO2.
Aggregation was analyzed with a phase contrast microscope and was
scored on at least 50 aggregates. They were scored as compacted
(individual cells not visible) or loose (individual cells still
visible) (n=2). HCT8/E11 and MDA-MB-231 GFP Luc cells were used as
a control for a respectively compacted and loose sphere
formation.
Collagen invasion assay
The assay was performed as described in a previous
study (22). Briefly, 1×105 cells
were seeded as a single-cell suspension on a 0.1% type I collagen
gel (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After 24
h of incubation at 37°C and 5 or 10% CO2, invasiveness was scored
(n=2×2) and expressed as a mean. HCT8/E11 and MDA-MB-231 GFP Luc
cells were used as a control for a respectively high and low
invasive cell line.
Colony formation assay
Single cells (1,000)
were seeded in T75 falcons (15 ml culture medium) and cultured for
14 days at 37°C. Colonies were stained with 0.5% crystal violet,
scanned and counted using ImageJ software (NIH, Bethesda, MA, USA).
Results are expressed as the mean percentage of colonies formed
from 1,000 cells [colony formation index (CFI)] (n=2×5). HCT8/E11
and MDA-MB-231 GFP Luc cells were used as a control for a
respectively positive and negative colony formation cell line.
Western blotting
Cells were lysed and sonicated for 10 sec on ice.
Lysates were diluted to a protein concentration of 1 µg/µl and
boiled for 5 min at 95°C. Equal amounts of proteins were separated
on 8 and 10% gels and transferred to nitrocellulose membranes.
Membranes were blocked [phosphate-buffered saline (PBS), 5% non-fat
milk, 0.5% Tween] and immunostained with primary antibodies:
E-cadherin M106 (Takara, The Netherlands), P-cadherin 610228 (BD
Biosciences, Erembodegem, Belgium), vimentin V6389, α-catenin
C2081, β-catenin C2206 and cytokeratin C2931, recognizing subtype
(4, 5, 6, 8, 10, 13 and 18) (Sigma-Aldrich, St. Louis, MO, USA).
Then, the secondary antibodies were applied, either ECL™ anti-mouse
IgG or ECL™ anti-rabbit IgG (GE Healthcare UK Ltd.,
Buckinghamshire, UK). Immunodetection was performed with Pierce ECL
Western Blotting Substrate (Thermo Scientific, Rockford, IL, USA)
and imaged with ProXima 2850 (Isogen Life Science, De Meern, The
Netherlands). HCT8/E11 was used as positive control for E-cadherin,
P-cadherin and cytokeratin. MDA-MB-231 GFP Luc cells were used as a
positive control for vimentin. Both cell lines were positive
controls for α-catenin and β-catenin.
In vivo
Animals
Animal experiments were approved by the Animal
Ethics Committee of Ghent University, Belgium (ECD 14/82). Athymic
mice (Foxn1nu male) were obtained from Envigo (The Netherlands),
and were kept under environmentally controlled conditions (12-h
normal light/dark cycle, 20–23°C and 50% relative humidity) with
food and water ad libitum. At 8 weeks of age, tumor cells were
implanted (subcutaneous or orthotopic) under general anesthesia
(Isoflurane, Abbott, Belgium). At the end of the experiments, or
when humane endpoints were reached, mice were euthanized by
cervical dislocation.
Subcutaneous tumor model
Subcutaneous tumors were grown to evaluate overall
growth behavior of the cell lines in mice and to provide tumors for
in vivo selection of cancer cells. Under general anesthesia,
tumor cells suspended in 100 µl of Matrigel/injection site were
injected SC in both hind legs. Tumor nodules were measured biweekly
with calipers and volumes were calculated according to the
following formula: V = (length × width)3/2 × π/6.
Orthotopic tumor model
Mice were supine positioned on a heating pad. Under
general anesthesia and analgesia (ketoprofen, 5 mg/kg, SC) a
vertical skin incision of 10 mm was medially performed in the upper
abdomen. Abdominal muscles were split and the peritoneum was opened
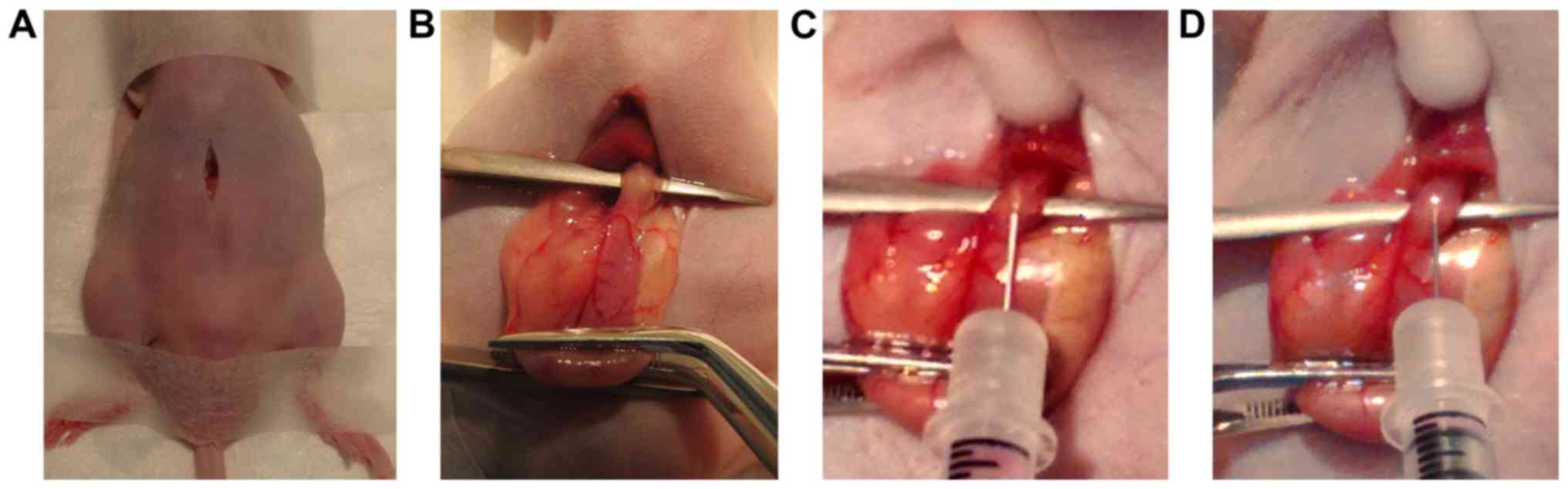
through sharp dissection (Fig. 1A).
The liver was gently elevated with a moist Q-tip to give access to
the abdominal esophagus. The stomach was lifted extra-corporeally
by traction on the greater curvature with a forceps. A
micro-forceps was positioned underneath the distal esophagus to
lift it (Fig. 1B). While the
esophagus was stretched by gentle tension on the stomach by an
assistant, a 30-gauge needle was inserted in the distal part of the
esophageal wall and tunneled approximally for ~3 mm (Fig. 1C). Tumor cells, suspended in 20 µl
Matrigel/animal were injected slowly, resulting in local bulging
(Fig. 1D). At body temperature,
Matrigel solidifies within seconds, minimalizing the risk of
intra-abdominal spilling of tumor cells. The stomach was cautiously
repositioned and the abdominal wall and skin were closed with a
running PDS 6–0 suture. Hartmann solution (500 µl) was administered
SC to prevent dehydration. Animals were followed up daily, and
weighed 2 times/week.
Subcutaneous (TTSC) and orthotopic tumor take
(TTorth) were defined as the percentage of macroscopic tumor
nodules (confirmed on histology) over the total number of
injections. At 7 weeks, mice were euthanized and tumors were
excised for histopathology.
Magnetic resonance imaging
A subpopulation of mice with orthotopic tumors (OE33
tumor nodules, n=5) were evaluated by magnetic resonance imaging
(MRI) at 1, 2, 3, 5, 8 and 12 weeks after tumor injection to follow
tumor progression. MR images were acquired on a 7T system (Bruker
PharmaScan 70/16, Ettlingen, Germany) with a mouse body volume
coil. Mice were anesthetized with isoflurane (5% induction, 1.5%
maintenance, 0.3 l/min) and warmed with a water-based heating
blanket. Respiration was monitored using a respiration pad
underneath the mouse. Anatomical information was obtained with a
T2-weighted sequence (TurboRARE) with the following parameters: TR,
3,661 and TE, 37.1 msec, 109 µm in-plane resolution, 30 contiguous
transverse slices of 600 µm, and acquisition time 9′1′′. Mice were
euthanized 15 weeks after tumor induction.
In vivo selection of cancer cells
To obtain subcultures of cell lines that grow well
in mice, tumors (SC and orthotopic) were excised under sterile
conditions and divided into small pieces. Tumor fragments were
dissociated (gentleMACS Dissociator; Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany) along with a collagenase 1 mg/ml (Sigma-Aldrich)
in PBSD+ mixture to disrupt tissue structures. The suspension was
filtered through a cell strainer (70 µm), and centrifuged. Cells
were seeded in T75 falcons and incubated. After 24 h, non-adherent
cells were cleared and replaced by fresh culture medium.
Tumor samples and histology
Tumors were excised, fixed with 4% formaldehyde,
processed and embedded in paraffin. Tumor sections of 5 µm were cut
with a microtome (Microm HM355S; Thermo Scientific). Hematoxylin
and eosin (H&E) and Ki67 stainings [ready-to-use DAKO
EnVision™+ System-HRP kit (K4011)] were performed according to the
standard protocols. Slides were scanned on magnifications of ×100
and ×200, and proliferation indices were determined by an overall
visual scoring system. Tumors were categorized as lowly, moderately
or highly proliferative. Microscopic images were captured with a
light microscope (ColorView I, BX43F; Olympus, Tokyo, Japan).
Statistical methods
Statistical analysis was performed using GraphPad
Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Mann-Whitney
test was used to compare the in vitro results of the
parental and in vivo selected cell line. Fisher's exact test
was used to compare tumor take rates. Results are summarized as
mean with standard deviation (SD), and were considered
statistically significant when the probability of a type I error
was ≤0.05.
Results
Cell characterization of OE33
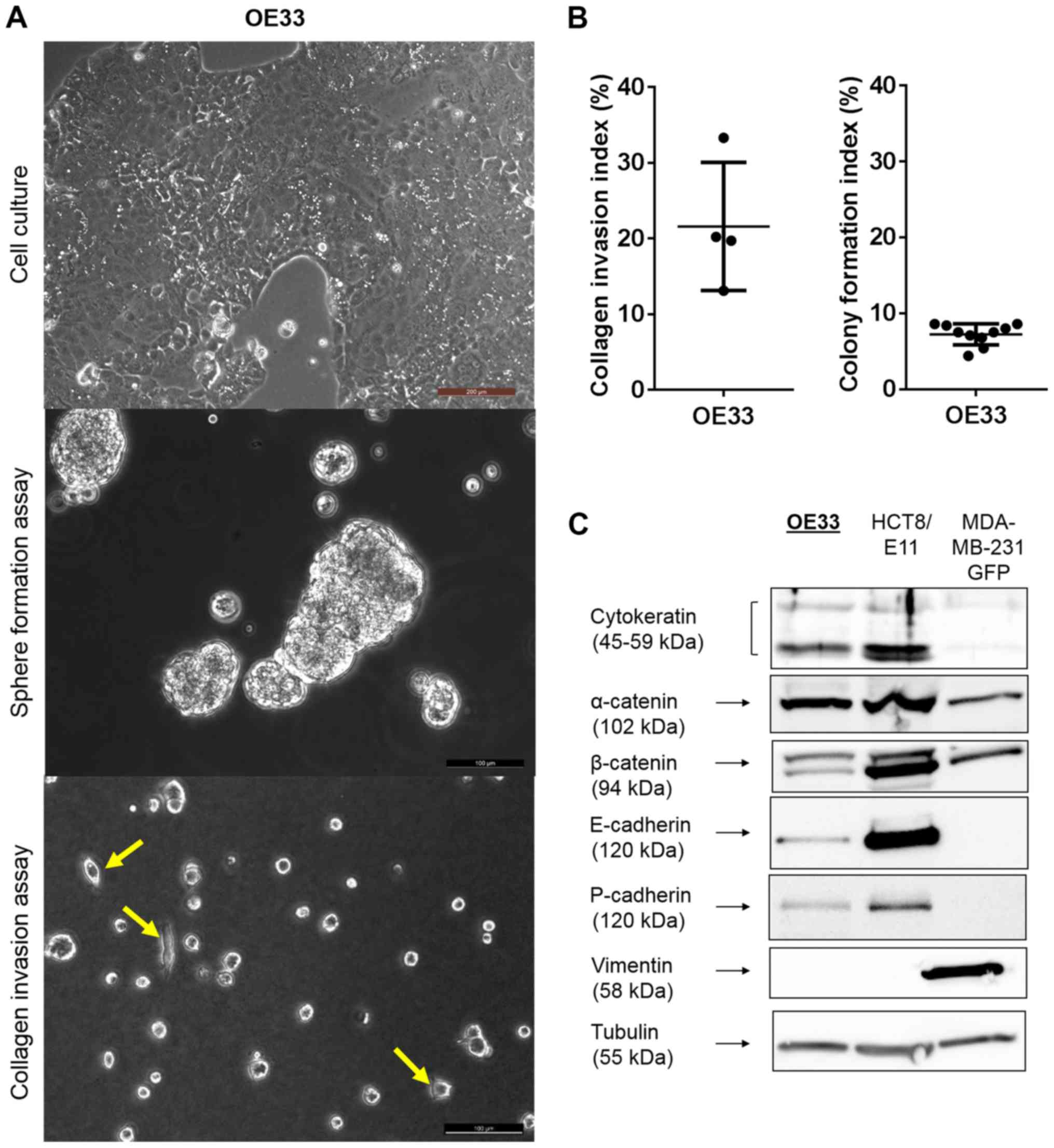
OE33 cells had an epithelial morphology,
characterized by adherent cells, cell-cell contacts and a typical
formation of islands (Fig. 2A,
upper panel). These cell-cell contacts resulted in the ability to
form compact spheres under Gyrotory shaking (Fig. 2A, middle panel). On type I collagen
gels, 21.6% [95% confidence interval (CI), 8.12 and 35.04%)] of the
OE33 cells showed cellular extensions invading the matrix (Fig. 2A, lower panel; and B, left). When
seeded at a low density on tissue culture substrate, only a limited
number of these cells were able to form a colony [mean CFI, 7.23%;
95% CI, 6.24 and 8.23%)] (Fig. 2B,
right). Additionally, western blotting was performed (Fig. 2C). OE33 cells expressed cytokeratin,
an intermediate filament supporting the epithelial origin of the
cancer cell line. Furthermore, the cells showed expression of
α-catenin, β-catenin, E-cadherin and P-cadherin, proteins important
for cell-cell adhesion and tissue organization. They did not
express vimentin, a major cytoskeletal component in mesenchymal
cells.
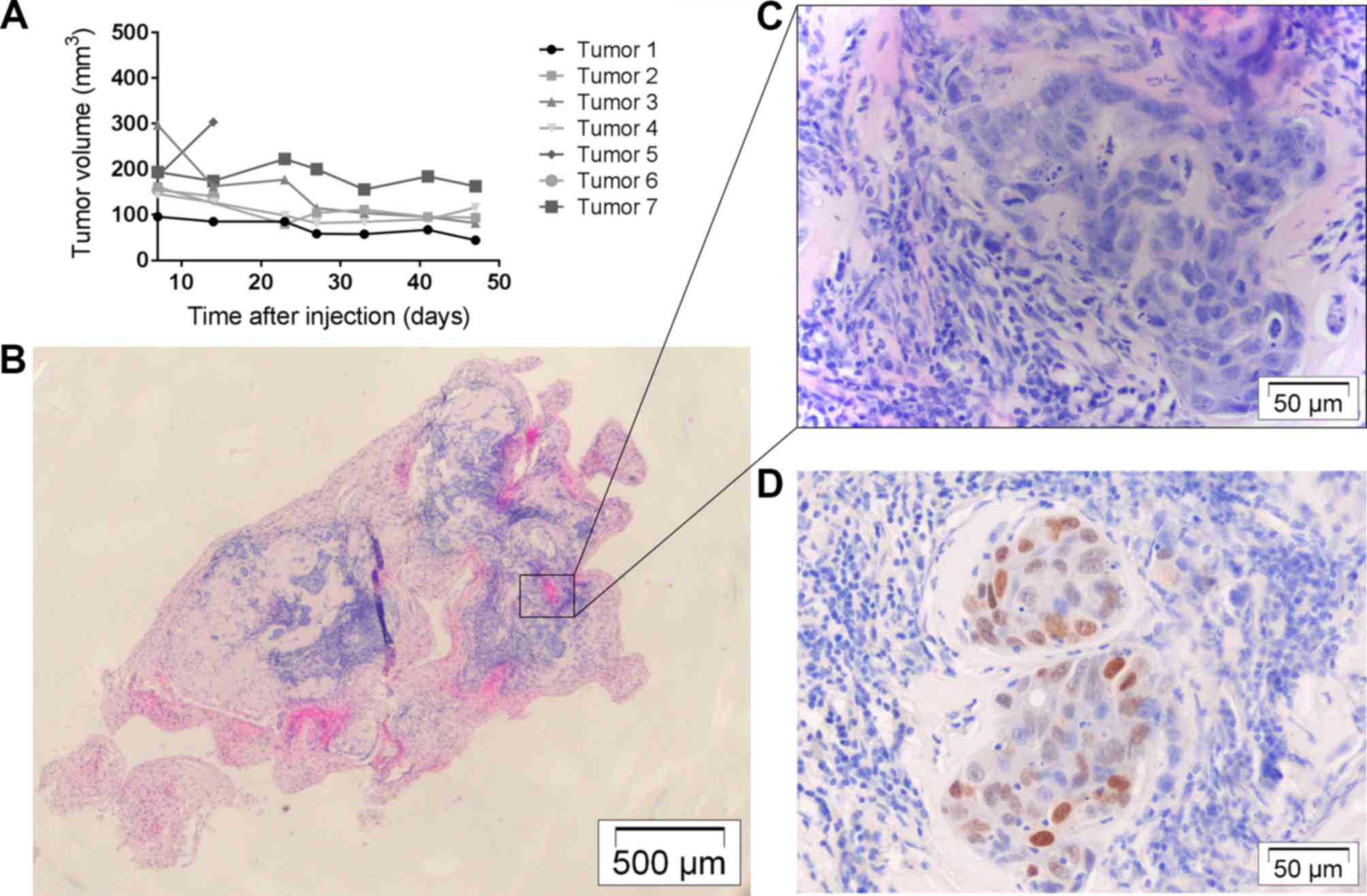
Tumor development with OE33
Four mice were subcutaneously injected bilaterally
with OE33 cells (Table I). These
all resulted in similar small tumor nodules, but volumes seemed to
decrease, progressively (Fig. 3A).
Histologically, nodules consisted of well-differentiated tumor
cells organized in islands and surrounded by infiltrating stromal
cell connective tissue (Fig. 3B and
C). They were not invasive into surrounding tissues and Ki67
indices were low to moderate (Fig.
3D). Two nodules were used for in vivo selection and
were confirmed to contain tumor cells through that means.
 | Table I.Summary of in vivo
experiments. |
Table I.
Summary of in vivo
experiments.
|
| OE33 | OACM5 1.C | OACM5 1.C SC1 |
|---|
|
|
|
|
|
|---|
|
| SC | Orthotopic | SC | Orthotopic | SC | Orthotopic |
|---|
|
|
|
|
|
|
|
|
|---|
| Injected tumor
cells (x106) | 1 | 5 | 0.5 | 1 | 1.5 | 1 | 1.5 | 1 |
| Number of tumor
cell implantations | n=2 | n=5 | n=5 | n=7 | n=8 | n=6 | n=10 | n=6 |
| Macroscopic tumor
nodule | 2/2 | 5/5 | 3/4a | 4/7 | 4/8 | 0/4b | 10/10 | 2/6 |
| Microscopic tumor
cells | 2/2 | 5/5 | 3/4 | 4/7 | 7/8 | 0/4 | 10/10 | 3/6 |
| Tumor take (TT)
(%) | 100 | 63.6 | 50 | 0 | 100 | 33.3 |
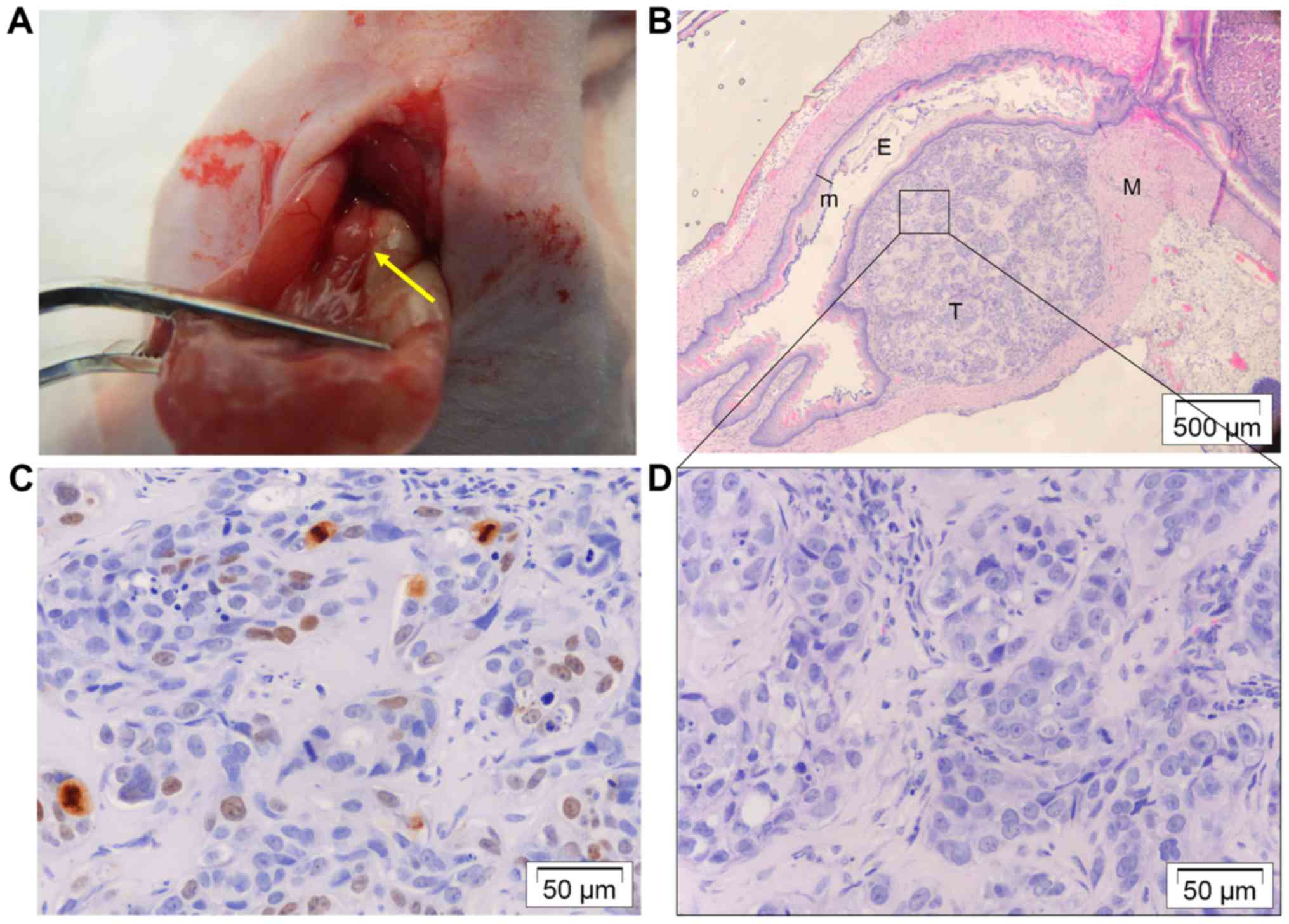
Twelve mice were orthotopically injected with OE33
cells (Table I). Seven animals
developed tumor nodules at the distal site of the esophagus without
evidence of metastasis (liver, diaphragm, peritoneum and omentum
were free of lesions) (Fig. 4A).
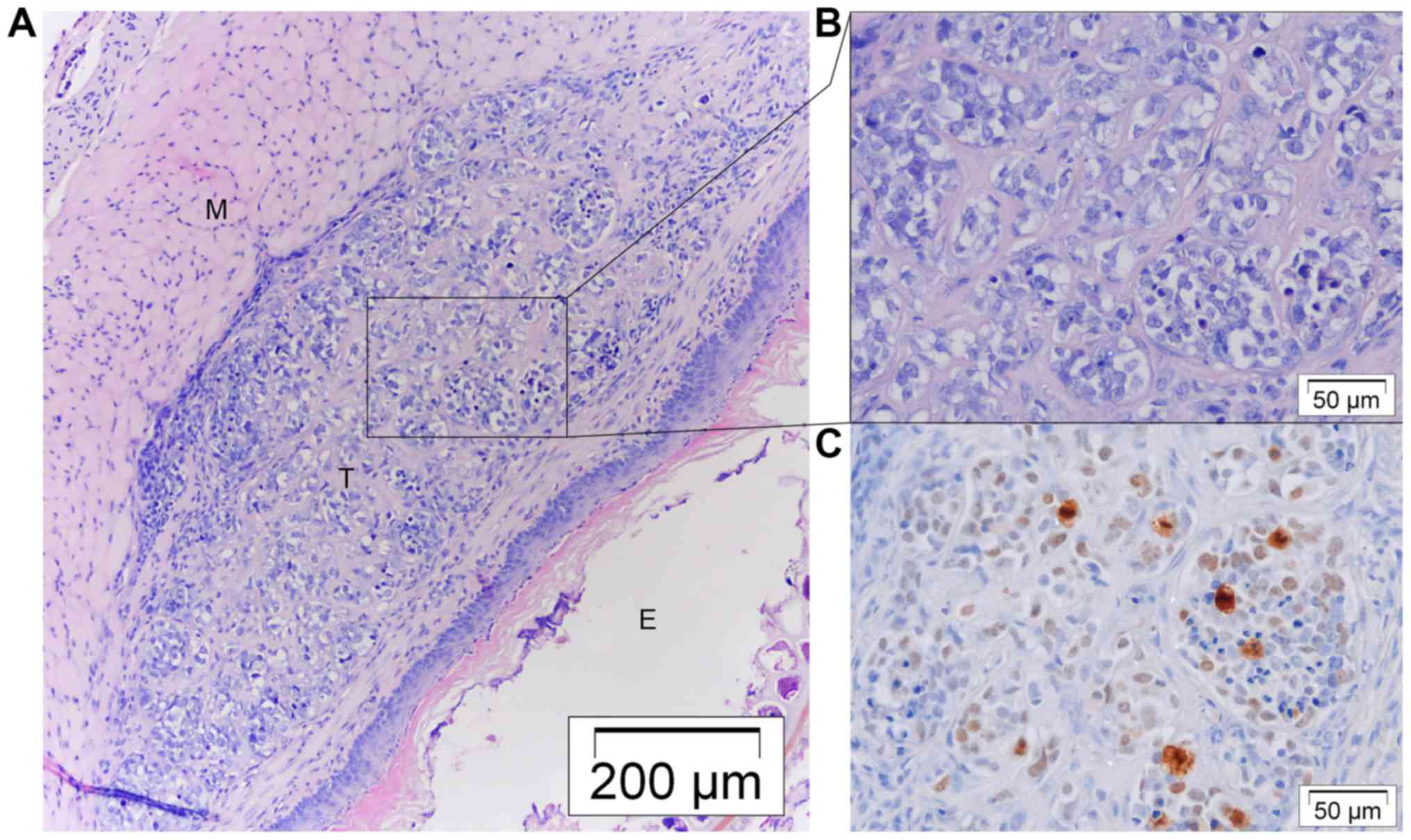
Tumors were located at the submucosal space and were not invasive
into surrounding tissue (Fig. 4B and
D). They were well differentiated and had a low proliferation
index (Fig. 4C). Three nodules were
used for in vivo selection and were confirmed to contain
tumor cells through that means.
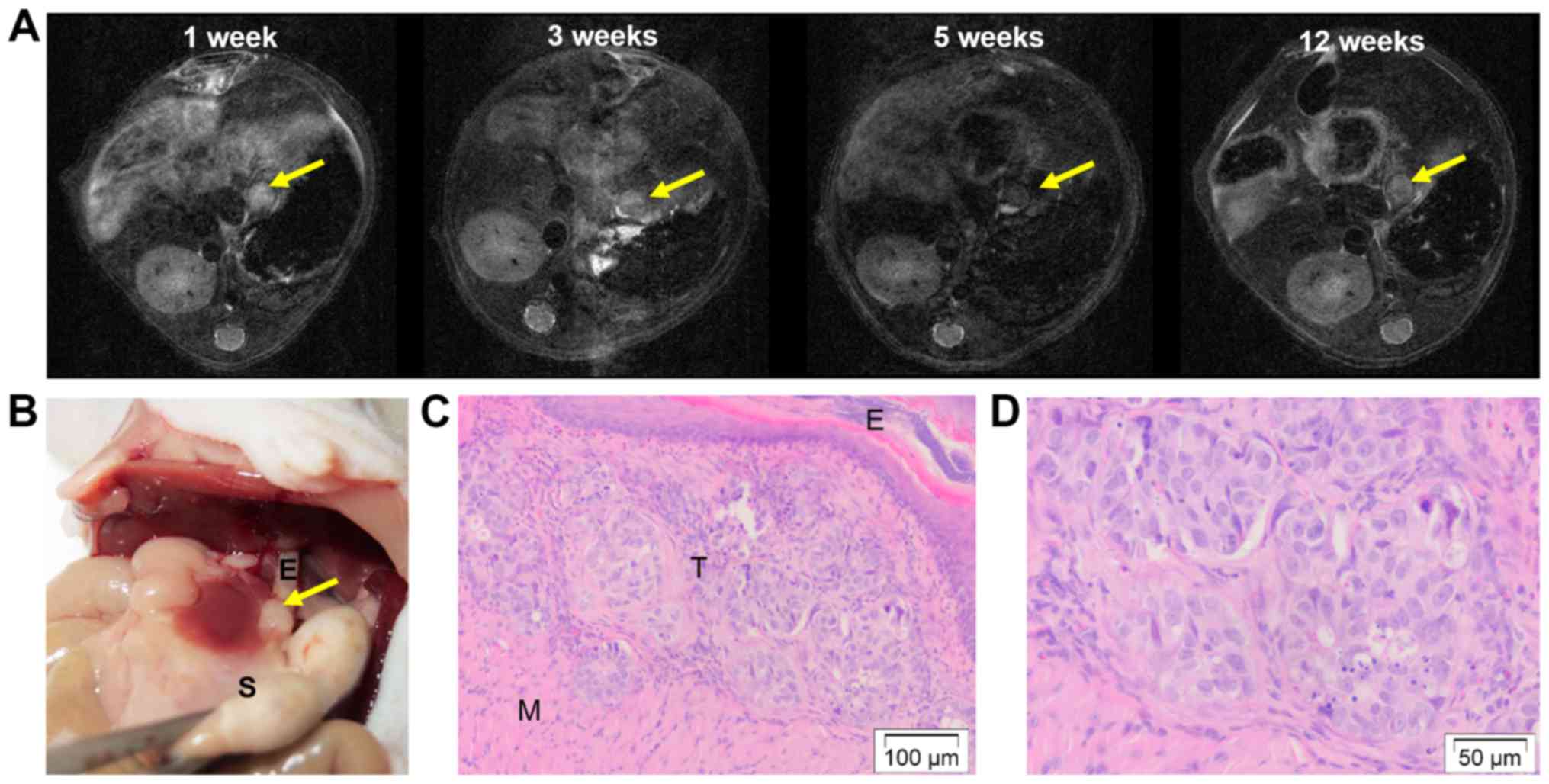
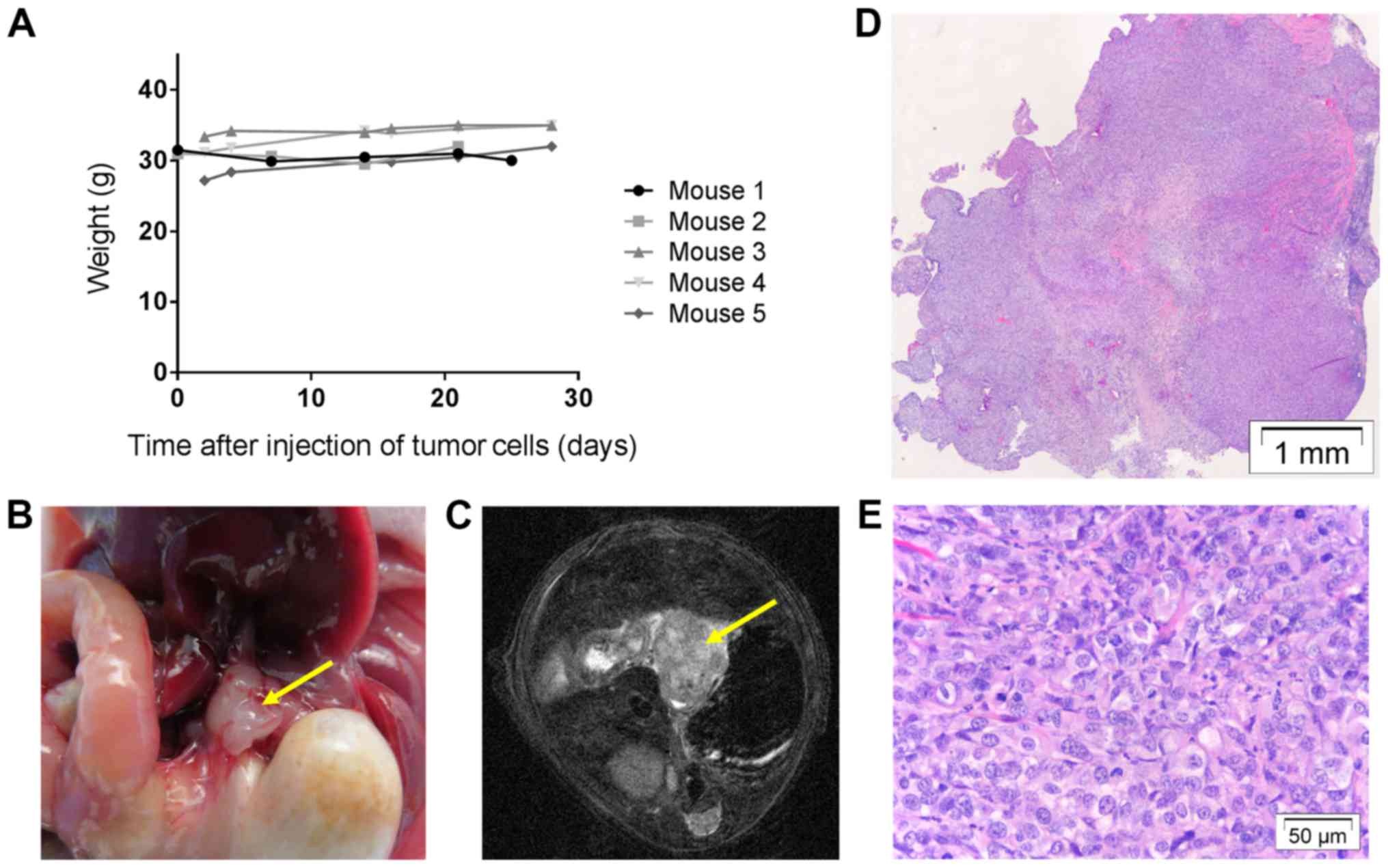
MRI scans were performed in a subset of mice (n=5)
to follow tumor development (Fig.
5A). At the initial MRI scan 1 week post-tumor induction, all
of them showed a clear tumor-like nodule at the distal site of the
esophagus. During follow-up, volumes remained the same and at the
end 4 out of 5 animals showed a tumor-like nodule on MRI. These
were confirmed to contain tumor cells microscopically (Fig. 5B-D).
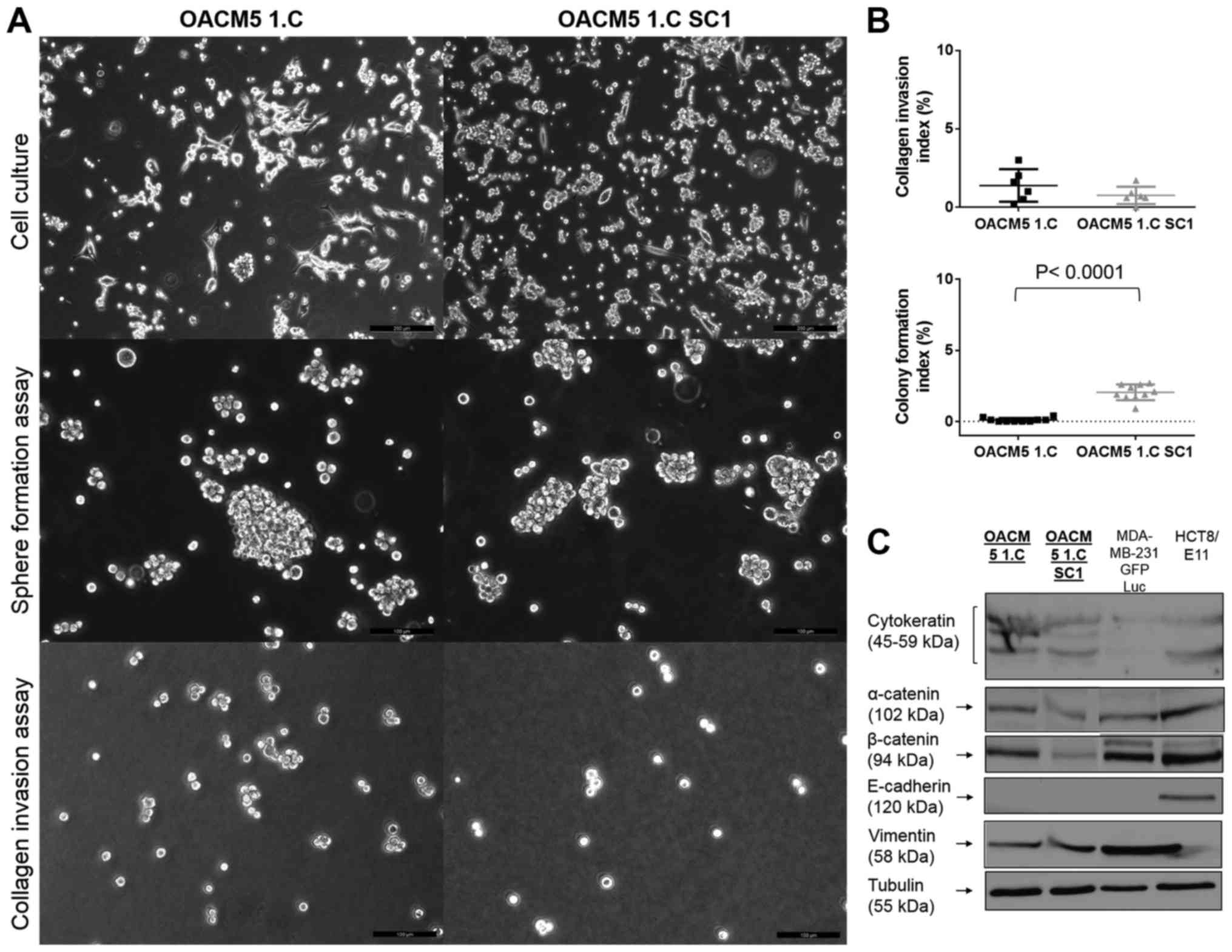
Cell characterization of OACM5
1.C
OACM5 1.C cells had two morphological subtypes: a
majority of multicellular floating cell clusters, and some adherent
cells with a fibroblast-like appearance, growing as single cells
(Fig. 6A, upper panel). These did
not form cell-cell contacts, and only very few cells were adherent
to plastic. OACM5 1.C cells were not able to form compact spheres
under Gyrotory shaking, but formed loose cell clusters with
recognition of individual cells (Fig.
6A, middle panel). Furthermore, they were non-invasive into
collagen gels [mean 1.38%, 95% CI, −0.30 and 2.47%)] (Fig. 6A lower panel, and B, upper graph),
and were not clonogenic [CFI=0.10%, 95% CI, −0.001 and 0.201%)]
(Fig. 6B, lower graph). OACM5 1.C
cells expressed cytokeratin as determined by western blotting
supporting the epithelial origin of the cancer cell line. They
expressed β-catenin and poorly expressed α-catenin, but lacked
expression of E-cadherin to consolidate cell-cell contacts. OACM5
1.C expressed vimentin representing the mesenchymal characteristics
of the cell line (Fig. 6C).
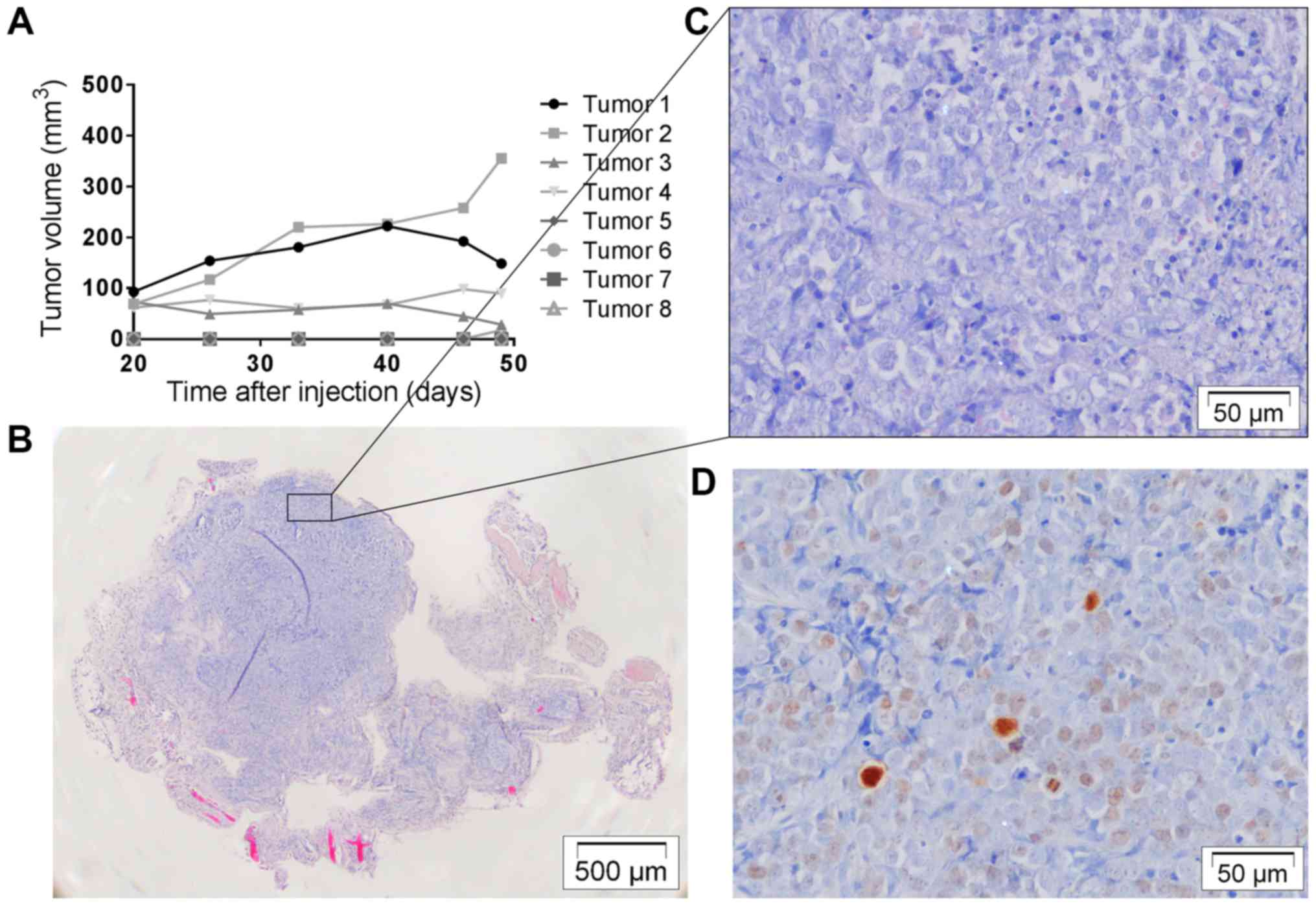
Tumor development with OACM5 1.C
Four mice were subcutaneously injected bilaterally
with OACM5 1.C cells (Table I).
Four out of 8 injections resulted in macroscopic tumor nodules. One
nodule had an exponential growth curve, while the others remained
stable (Fig. 7A). Histology showed
nodules packed with tumor cells with little infiltrating stromal
cells. They were not invasive into surrounding tissues and Ki67
staining was overall low to moderate (Fig. 7B-D). Injection sites that did not
develop macroscopic nodules (4/8) resulted in palpable fibrous
remnants in which some loose tumor cell islands could be identified
on histology. One nodule was used for in vivo selection, and
was confirmed to contain tumor cells through that means. An
additional 6 mice were orthotopically injected with OACM5 1.C cells
(Table I). Of 4 mice evaluable, no
tumor nodules, metastasis or involved lymph nodes were
macroscopically visible and histology was negative for tumor
cells.
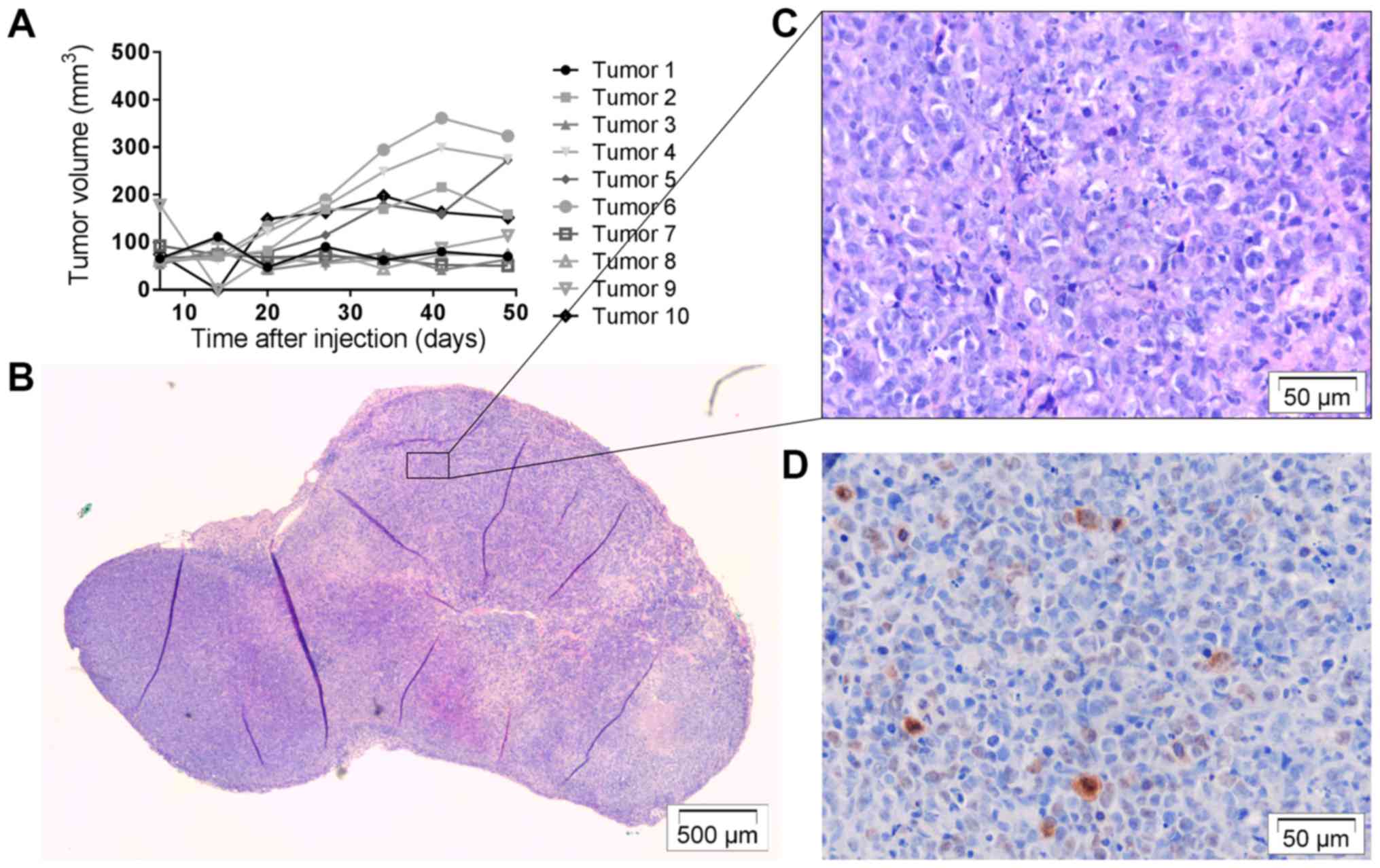
Establishment of new in vivo selected
cell line OACM5 1.C SC1
OACM5 1.C cells harvested from a SC tumor nodule,
were stable through different in vitro passages and were
re-injected into mice according to the above protocols. Five mice
were subcutaneously injected bilaterally with OACM5 1.C SC1 cells,
resulting in 10 macroscopically visible tumors (Table I). Five out of 10 were fast growing
(Fig. 8A). Histology showed the
presence of tumor cells in all nodules (Fig. 8B and C) and Ki67 staining was low to
moderate (Fig. 8D). An additional 6
mice were orthotopically injected with OACM5 1.C SC1 cells, leading
to 2 small macroscopic tumor nodules (Table I). No metastasis was observed.
Histology confirmed the presence of tumor cells, and nodules did
not invade surrounding tissues (Fig.
9A-C). In vivo selection of OE33 cells was not
successful (n=5). Tumor cells were microscopically present, but did
not survive different in vitro passages.
Comparison of OACM5 1.C and OACM5 1.C
SC1
Both cell lines had the same morphological
appearance in vitro (Fig.
6A, upper panel) and in vivo (Figs. 7 and 8). Furthermore, they had the same cell
line characteristics concerning sphere formation and collagen
invasion (Fig. 6A and B). Moreover,
cell-cell adhesion and cytoskeletal protein expression were similar
(Fig. 6C). However, the in
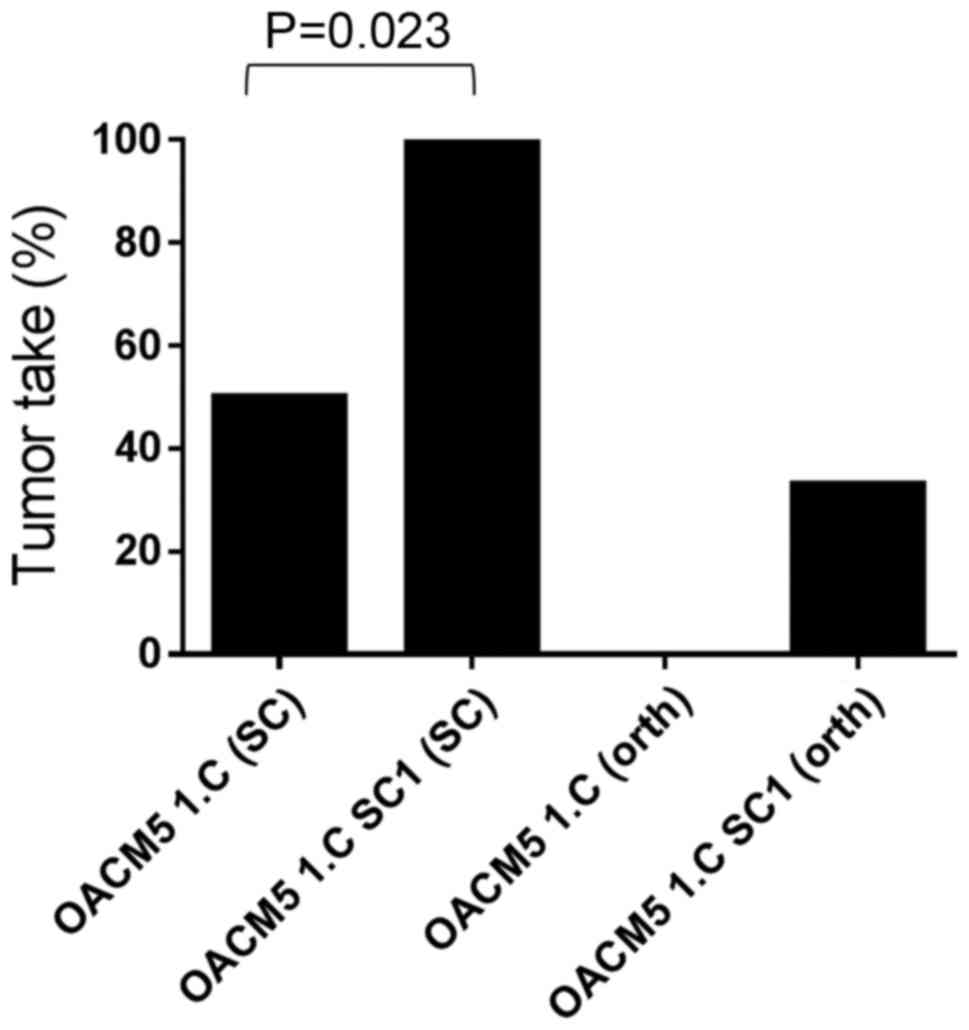
vivo selected cell line had higher subcutaneous tumor take
rates when compared with the parental cell line [TTsc=100 vs. 50%
(P<0.023) (Fig. 10)]. This may
be related to the significant higher clonogenicity (P<0.0001) of
the in vivo selected cell line compared to the parental cell
line in vitro.
Discussion
The present study investigated the orthotopic growth
potential of two generally available EAC cell lines, OE33 and OACM5
1.C, and a third cell line obtained through in vivo
selection, OACM5 1.C SC1. Additionally, in vitro experiments
were performed to better understand the functional characteristics
in relationship with in vivo growth behavior.
OE33 showed successful orthotopic xenografts in
63.6% (n=12) of the cases. Nevertheless, the volumes remained
stable during follow-up, as noted on the serial MRI scans.
Subcutaneous tumor take was higher (TTsc=100%, n=7), but resulted
in similar small tumor nodules with stable to decreasing volumes.
To the best of our knowledge, only one previous study used OE33
cells for orthotopic use. The study was diagnostic and had similar
results to ours. Small tumors of 2–3 mm in diameter at 4 weeks
after injection (n=5) were observed (9). OE33 seems to be a low aggressive cell
line with a high subcutaneous and orthotopic tumor take in nude
mice, but extremely slow growth pattern. The decreasing
subcutaneous volumes may be explained by clearance of Matrigel with
slow replacement of tumor cells.
In contrast to the OE33 cell line, OACM5 1.C cells
were not able to develop orthotopic tumor nodules (TTorth=0%, n=6).
In addition, subcutaneous tumor take was low (TTsc=50%, n=8). To
improve these poor tumor take rates, a technique of in vivo
selection of tumor cells was applied. As such, the new cell line
OACM5 1.C SC1 was established, and successfully led to a
significant higher subcutaneous tumor take than the parental cell
line [100% (n=10) vs. 50% (n=8); P<0.023]. Orthotopic tumor take
did not significantly differ [33.3% (n=6) vs. 0% (n=6); P=0.467].
Cell lines had similar in vitro characteristics, except for
the significant increased ability of the in vivo selected
cell line to form colonies (P<0.0001). The latter may partially
explain the increased tumor take rate.
Another correlation between the in vitro and
in vivo results was seen in the invasiveness of the cell
lines. The investigated EAC cell lines were almost non-invasive in
collagen type I gels in vitro and none of the xenografts in
the mouse experiments invaded the surrounding tissues.
In addition to in vivo selection, improved
tumor take rates may be achieved by simply increasing the amount of
injected tumor cells. Unfortunately, the injection volume in the
esophageal wall is limited. As such, the amount of injectable tumor
cells is also limited to ~1.5×106 cells/injection. This can be
bypassed by transplanting a subcutaneous tumor fragment on the
esophageal wall according to the technique of Gros et al
(10). An additional experiment was
performed with transplantation of 1 mm3 tumor fragments of a
subcutaneous OE33 tumor on the esophageal wall of 7 mice. Due to
postoperative complications, 3 animals died within the first week
postoperative. The remaining 4 did not show any vital tumor on the
esophageal wall up to 70 days of follow up. We believe this is a
technically more difficult procedure, with a low success rate when
fragments of slow growing tumor nodules are used and we conclude
that this is not beneficial for the investigated cell lines.
It needs to be mentioned that the development of EAC
in this tumor model differs from the situation in patients. While
the pathogenesis is not yet fully understood, it is believed that
chronic inflammation of the esophageal mucosa can develop
dysplasia, and eventually evolves to EAC. As such, gastroesophageal
reflux disease (GERD) is one of the major risk factors for
developing EAC, besides obesity (1). In literature, several other models
have been described, that reflect the clinical situation more
closely (4). On one hand, different
reflux models have been used: surgical esphagojejunostomy (23) or drinking of caustic substances
(24). These reflux models lead to
<50% cancer development in a time period of 6 months making it
unreliable for therapeutic studies (4). On the other hand, the use of
genetically engineered mouse models (GEMMs) has been investigated.
Transgenic mice with IL-1β overexpression were shown to develop
moderate inflammation by 6 months, with a small percentage of mice
developing high grade dysplasia or EAC after 20–22 months (24). Best results with GEMMs were obtained
in combination with the caustic substance deoxycholate (DCA), where
45% of mice developed EAC after a long follow-up period of 15
months (24). The technique of
injecting tumor cells in the esophageal wall is considered to be
the best option available for the development of a relative rapid
and reliable orthotopic mouse model.
Surprisingly, the three investigated EAC cell lines
grew more efficient subcutaneously than orthotopically. To rule out
technical issues with the orthotopic injection method, the
technique was checked with a highly aggressive ovarian carcinoma
cell line, SK-OV-3 Luc IP1 cells, that is known to be 100%
tumorigenic in Foxn1nu mice, according to previous experiments in
our research group (25). Injection
of 5×105 SK-OV-3 Luc IP1 cells in the esophageal wall resulted in
100% tumor take and 100% exponential tumor growth (n=5), confirmed
on IVIS, MRI and histology (Fig.
11). After 4 weeks, exophytic tumors of ~8 mm diameter were
observed. We believe the low orthotopic tumor take rates with the
investigated EAC cells is due to a combination of low aggressive
cells and the limited amount of injectable cells.
The fact that the OACM5 1.C SC1 experiments are
based on cells originating from one tumor nodule, could be a point
of discussion. Nevertheless, the in vivo selection technique
is a validated technique to improve cell line characteristics [such
as metastatic potential or take rates (25,26)].
Our aim was not to validate the technique, but to use it to improve
tumor take rates and to show it can be of use for esophageal cancer
models. The OACM5 1.C SC1 cell line was authenticated by STR assay,
was stable through different passages and led to increased tumor
take rates. The unsuccessful in vivo selection of OE33 was
probably due to the small amount of tumor cells in the excised
tumors and the low clonogenic potential of the cells. Repetitions
may most probably lead to the same results.
Finally, the follow-up of esophageal tumor growth in
mice is challenging (i.e. due to its location). Performing a
laparotomy at different time points is easy, fast and does not
require specialized tools or knowledge. However, this causes
intra-abdominal adhesions, making esophageal exposure more
difficult after every laparotomy, and may cause an inflammatory
reaction influencing tumor development. MRI imaging was already
confirmed to be feasible and accurate for the follow-up of
esophageal cancer in mice (10,17,18).
In the present study, a dedicated small animal MRI scanner was
used, leading to detailed images. Tumor nodules could be defined
precisely as hyper-intense nodular structures, at a fixed location,
slightly proximal of the gastroesophageal junction. In addition,
the volumes of nodules could be measured accurately. However, MRI
is not able to differentiate tumor tissue from inflammatory scar
tissue or residual Matrigel. If volumes increase, viable tumor
cells are plausible. If not, the presence of tumor cells cannot be
assured. It would be interesting to transfect the investigated EAC
cell lines with luciferase, such as shown by Gros et al
(10), to perform in vivo
fluorescence imaging in case of stable nodules, and be able to
differentiate viable tumor cells from scar tissue and Matrigel.
The present study is of interest for future
experiments. Particularly the OE33 cell line is appropriate for
orthotopic injection for diagnostic studies on EAC. Yet, various
limitations, such as low aggressive cells, slow growth pattern and
different etiology in patients should be kept in mind. It must be
mentioned that this is the first study to describe the growth
behavior of OACM5 1.C in mice. OACM5 1.C had a poor tumor take rate
at an orthotopic and ectopic site. The in vivo selected cell
line OACM5 1.C SC1 showed higher subcutaneous take rates. The use
of a more immunodeficient mouse strain (NOD SCID mice) could
improve tumor take and should be considered for future research
with these low aggressive cell lines.
In conclusion, little research is available
concerning esophageal cancer, particularly the EAC subtype, which
is the more prevalent type in the Western world. The present study
provides orthotopic and subcutaneous xenograft EAC models in mice,
which may hopefully contribute to further preclinical research on
EAC.
Acknowledgements
The authors would like to thank Natacha Rosseel for
the assistance with laboratory and animal procedures, and Glenn
Wagemans for the assistance with in vitro procedures.
References
|
1
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stahl M, Mariette C, Haustermans K,
Cervantes A and Arnold D: ESMO Guidelines Working Group:
Oesophageal cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 Suppl
6:vi51–vi56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gavin AT, Francisci S, Foschi R, Donnelly
DW, Lemmens V, Brenner H and Anderson LA: EUROCARE-4 Working Group:
Oesophageal cancer survival in Europe: A EUROCARE-4 study. Cancer
Epidemiol. 36:505–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tétreault MP: Esophageal cancer: Insights
from mouse models. Cancer Growth Metastasis. 8 Suppl 1:S37–S46.
2015. View Article : Google Scholar
|
|
5
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: Advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuroda S, Kubota T, Aoyama K, Kikuchi S,
Tazawa H, Nishizaki M, Kagawa S and Fujiwara T: Establishment of a
non-invasive semi-quantitative bioluminescent imaging method for
monitoring of an orthotopic esophageal cancer mouse model. PLoS
One. 9:e1145622014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song S, Chang D, Cui Y, Hu J, Gong M, Ma
K, Ding F, Liu ZH and Wang TY: New orthotopic implantation model of
human esophageal squamous cell carcinoma in athymic nude mice.
Thorac Cancer. 5:417–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Habibollahi P, Figueiredo JL, Heidari P,
Dulak AM, Imamura Y, Bass AJ, Ogino S, Chan AT and Mahmood U:
Optical imaging with a cathepsin B activated probe for the enhanced
detection of esophageal adenocarcinoma by dual channel fluorescent
upper GI endoscopy. Theranostics. 2:227–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gros SJ, Dohrmann T, Peldschus K, Schurr
PG, Kaifi JT, Kalinina T, Reichelt U, Mann O, Strate TG, Adam G, et
al: Complementary use of fluorescence and magnetic resonance
imaging of metastatic esophageal cancer in a novel orthotopic mouse
model. Int J Cancer. 126:2671–2681. 2010.PubMed/NCBI
|
|
11
|
Drenckhan A, Kurschat N, Dohrmann T, Raabe
N, Koenig AM, Reichelt U, Kaifi JT, Izbicki JR and Gros SJ:
Effective inhibition of metastases and primary tumor growth with
CTCE-9908 in esophageal cancer. J Surg Res. 182:250–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroda S, Fujiwara T, Shirakawa Y,
Yamasaki Y, Yano S, Uno F, Tazawa H, Hashimoto Y, Watanabe Y, Noma
K, et al: Telomerase-dependent oncolytic adenovirus sensitizes
human cancer cells to ionizing radiation via inhibition of DNA
repair machinery. Cancer Res. 70:9339–9348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furihata T, Sakai T, Kawamata H, Omotehara
F, Shinagawa Y, Imura J, Ueda Y, Kubota K and Fujimori T: A new in
vivo model for studying invasion and metastasis of esophageal
squamous cell carcinoma. Int J Oncol. 19:903–907. 2001.PubMed/NCBI
|
|
14
|
Ip JC, Ko JM, Yu VZ, Chan KW, Lam AK, Law
S, Tong DK and Lung ML: A versatile orthotopic nude mouse model for
study of esophageal squamous cell carcinoma. Biomed Res Int 910715.
2015. View Article : Google Scholar
|
|
15
|
Ohara T, Takaoka M, Sakurama K, Nagaishi
K, Takeda H, Shirakawa Y, Yamatsuji T, Nagasaka T, Matsuoka J,
Tanaka N, et al: The establishment of a new mouse model with
orthotopic esophageal cancer showing the esophageal stricture.
Cancer Lett. 293:207–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hori T, Yamashita Y, Ohira M, Matsumura Y,
Muguruma K and Hirakawa K: A novel orthotopic implantation model of
human esophageal carcinoma in nude rats: CD44H mediates cancer cell
invasion in vitro and in vivo. Int J Cancer. 92:489–496. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gros SJ, Kurschat N, Drenckhan A, Dohrmann
T, Forberich E, Effenberger K, Reichelt U, Hoffman RM, Pantel K,
Kaifi JT, et al: Involvement of CXCR4 chemokine receptor in
metastastic HER2-positive esophageal cancer. PLoS One.
7:e472872012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gros SJ, Kurschat N, Dohrmann T, Reichelt
U, Dancau AM, Peldschus K, Adam G, Hoffman RM, Izbicki JR and Kaifi
JT: Effective therapeutic targeting of the overexpressed HER-2
receptor in a highly metastatic orthotopic model of esophageal
carcinoma. Mol Cancer Ther. 9:2037–2045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gros SJ, Dohrmann T, Rawnaq T, Kurschat N,
Bouvet M, Wessels J, Hoffmann RM, Izbicki JR and Kaifi JT:
Orthotopic fluorescent peritoneal carcinomatosis model of
esophageal cancer. Anticancer Res. 30:3933–3938. 2010.PubMed/NCBI
|
|
20
|
Castro C, Bosetti C, Malvezzi M, Bertuccio
P, Levi F, Negri E, La Vecchia C and Lunet N: Patterns and trends
in esophageal cancer mortality and incidence in Europe (1980–2011)
and predictions to 2015. Ann Oncol. 25:283–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Almhanna K, Meredith KL, Hoffe SE,
Shridhar R and Coppola D: Targeting the human epidermal growth
factor receptor 2 in esophageal cancer. Cancer Control. 20:111–116.
2013.PubMed/NCBI
|
|
22
|
De Wever O, Hendrix A, De Boeck A,
Westbroek W, Braems G, Emami S, Sabbah M, Gespach C and Bracke M:
Modeling and quantification of cancer cell invasion through
collagen type I matrices. Int J Dev Biol. 54:887–896. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raggi M, Langer R, Feith M, Friess H,
Schauer M and Theisen J: Successful evaluation of a new animal
model using mice for esophageal adenocarcinoma. Langenbecks Arch
Surg. 395:347–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quante M, Bhagat G, Abrams JA, Marache F,
Good P, Lee MD, Lee Y, Friedman R, Asfaha S, Dubeykovskaya Z, et
al: Bile acid and inflammation activate gastric cardia stem cells
in a mouse model of Barrett-like metaplasia. Cancer Cell. 21:36–51.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Vlieghere E, Carlier C, Ceelen W,
Bracke M and De Wever O: Data on in vivo selection of SK-OV-3 Luc
ovarian cancer cells and intraperitoneal tumor formation with low
inoculation numbers. Data Brief. 6:542–549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|