Introduction
Osteosarcoma (OS) is the most frequent primary
malignant bone tumor in children and young adults and has a high
tendency for local invasion and early metastasis (1,2).
Conventional treatments such as surgery, chemotherapy and
radiotherapy still result in a poor prognosis for OS due to early
lung metastasis. Recently, with the use of neoadjuvant chemotherapy
and improvements in surgical techniques, the 5-year survival rate
has improved for some patients with localized OS (3), but for patients with metastatic
disease, the survival rate (~10–20%) remains unchanged (4,5).
Furthermore, chemoresistance and adverse side-effects induced by
chemotherapeutic agents cannot be neglected (6). Therefore, new effective therapeutic
agents for OS are urgently needed.
Since plant-derived compounds promote apoptosis,
autophagy, programmed cell death, mitotic catastrophe and
senescence in cancer cells (7),
many different natural compounds from various plants have been
suggested to not only prevent, but also treat cancers. Several
studies have reported that compounds extracted from natural plants
exert inhibitory effects on OS (8,9).
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), the most
abundant and pungent component in a variety of hot peppers, has
been used extensively as a food additive in countries worldwide
(10). As a medicinal compound,
capsaicin is currently used to treat pain and inflammation caused
by various diseases, including rheumatoid arthritis, herpes zoster
and cluster headaches (11). More
recently, the anticancer effects of capsaicin have garnered
increasing attention. The literature states that capsaicin
suppresses several malignant human cell lines, including prostate
cancer (12), leukemia (13), gastric cancer (14), hepatocarcinoma (15), glioma (16) and breast cancer (17). However, only a few studies have
reported the effects of capsaicin on OS (18). Furthermore, since the molecular
mechanisms involved in the anticancer effects of capsaicin are
complicated, a lack of consensus regarding its mechanisms for
inducing OS cell apoptosis exists in the literature. For instance,
Ying et al (19)
demonstrated that capsaicin possesses strong efficacy in inducing
human OS cell apoptosis via activation of the AMPK signaling
pathway and c-Jun NH2-terminal kinases. Cho et al (20) found that capsaicin could induce
apoptosis in the OS MG63 cell line and further demonstrated that
the caspase cascade and antioxidant enzymes were the underlying
regulatory signaling pathways involved in capsaicin-induced
apoptosis. In addition, Jin et al revealed that capsaicin
could induce immunogenic cell death in human OS MG63 cells in
vitro (21). However, these
results were predominantly obtained with relatively high
concentrations of capsaicin. Other than apoptosis induction in OS
cells, mechanisms that may explain the anti-OS activities at low
concentrations of capsaicin remain unclear. Therefore, we evaluated
the effects of capsaicin on proliferation, cell cycle arrest and
apoptosis induction using 3 OS cell lines (MG63, 143B and HOS) and
explored the underlying mechanisms with the goal of obtaining
comprehensive results that describe the effect of capsaicin on OS
cells.
Materials and methods
Reagents
Capsaicin
(trans-8-methyl-N-vanillyl-6-nonenamide) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) and
Dulbecco's modified Eagle's medium (DMEM) were purchased from
HyClone (Logan, UT, USA). Cell viability and cytotoxicity test kit,
Cell Counting Κit-8 (CCK-8), was purchased from Dojindo Molecular
Technologies (Kimamoto, Japan). An Annexin V-FITC/propidium iodide
(PI) double staining test kit was purchased from KeyGen Biotech
(Nanjing, China). A 5-ethynyl-2′-deoxyuridine (EdU) cell
proliferation assay kit was purchased from RiboBio (Guangzhou,
China). RIPA lysis buffer, phenylmethanesulfonyl fluoride (PMSF), a
bicinchoninic acid (BCA) protein assay kit, bovine serum albumin
(BSA), a JC-1 mitochondrial membrane potential assay kit, a
caspase-3 activity assay kit, and mouse anti-human actin (cat. no.
AA128) and tubulin (cat. no. AT819) were purchased from Beyotime
Biotech (Shanghai, China). Mouse anti-human proliferating cell
nuclear antigen (PCNA) (cat. no. sc-53407) were purchased from
Santa Cruz Biotechnology (San Francisco, CA, USA). Rabbit
anti-human p21 (cat. YT3497) was purchased from ImmunoWay
Biotechnology (Newark, DE, USA). Rabbit anti-human Bax (cat. no.
5023), Bcl-2 (cat. no. 4223), p-ERK1/2 (cat. no. 4370), ERK1/2
(cat. no. 4695), p-p38 (cat. no. 4511), p38 (cat. no. 8690), p-JNK
(cat. no. 4668), JNK (cat. no. 9252) and Ki67 (cat. no. 9027) were
purchased from Cell Signaling Technology (Boston, MA, USA). Goat
anti-rabbit (cat. no. 111-035-003) and goat anti-mouse secondary
antibodies (cat. no. 115-035-003) were purchased from Jackson
ImmunoResearch Laboratories (West Grove, PA, USA).
Cell and cell culture
The OS cell lines MG63, 143B and HOS were purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and maintained in DMEM containing 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C in an atmosphere containing 5%
CO2.
Cell viability assay
Cells were seeded into 96-well plates at a density
of 5,000 cells/well and incubated at 37°C for 24 h with 6
replicates. The cells were treated with various concentrations of
capsaicin (0, 50, 100, 150, 200, 250 or 300 µM) for 24 h.
Additionally, 2-wells containing capsaicin alone were established
to register the background absorbance at each concentration. Then,
10 µl of CCK-8 was added to each well, and the plates were
incubated for an additional hour. The cells were subsequently
placed in a microplate reader to detect absorbance at 450 nm. Cell
viability was calculated using the following formula: Cell
viability (%) = (test absorbance - background absorbance)/(control
absorbance - background absorbance) × 100%.
Assessment of apoptosis using flow
cytometry
Cellular apoptosis was detected via flow cytometry
using an Annexin V-FITC/PI kit. Briefly, the cells were seeded into
10-cm dishes (106 cells/dish) for 24 h and then treated
with various concentrations of capsaicin (0, 100, 150, 200 or 250
µM) for 24 h. The cells were then collected and washed twice with
ice-cold phosphate-buffered saline (PBS) and stained with Annexin
V-FITC and PI according to the manufacturer's guidelines, after
which the samples were read on a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). The distribution of viable
(FITC−/PI−), early apoptotic
(FITC+/PI−), late apoptotic
(FITC+/PI+) and necrotic
(PI+/FITC−) cells was calculated and
analyzed. Both early and late apoptotic cells were recorded as
apoptotic cells, and the results were expressed as the percentage
of total cells.
Caspase-3 activity assessment
The activity levels of caspase-3 were detected using
a caspase-3 assay kit according to the manufacturer's instructions.
In brief, after cells were treated with various concentrations of
capsaicin (0, 100, 150, 200 or 250 µM), they were collected, lysed
and centrifuged at 16,000 × g for 20 min at 4°C. The supernatant
containing protein was collected, and the protein concentrations
were measured using BCA methods. Then, ~50 µg of protein was
incubated with buffer containing Ac-DEVD-pNA (2 mM) at 37°C
overnight, and the absorbance of yellow pNA (the cleavage product)
was measured using a microplate reader at a wavelength of 405 nm.
In addition, caspase-3 activity was calculated as a fold of the
optical density (OD) of the different capsaicin concentrations
relative to the OD of the control group.
Mitochondrial membrane potential
assessment
The mitochondrial membrane potential (ΔΨm) was
detected using a JC-1 mitochondrial membrane potential assay kit.
Cells were seeded in 10-cm dishes at a density of 106
cells/dish for 24 h, and then treated with various concentrations
of capsaicin for an additional 24 h. Cells were collected and
suspended in 0.5 ml of medium and incubated with 0.5 ml of JC-1 at
37°C for 20 min, after which the samples were washed 2 times with
ice-cold JC-1 buffer and measured using flow cytometry.
EdU proliferation assay
The effects of capsaicin on OS cell proliferation
were determined using an EdU cell proliferation assay kit according
to the manufacturer's protocol. Briefly, cells were seeded into
24-well plates at an initial density of 5×104 cells/well
and incubated for 12 h to allow adherence. Afterwards, they were
treated with various concentrations of capsaicin (0, 100, 150, 200
or 250 µM) for another 24 h. The cells were then incubated with 50
µM EdU for 2 h before they were fixed with 4% paraformaldehyde.
After the cells were permeabilized with 0.3% Triton X-100, the
incorporated EdU was stained with Apollo 488 (green fluorescence),
and the nuclei were stained with Hoechst 33342 (blue fluorescence).
Finally, the percentage of EdU-positive cells was determined using
fluorescence microscopy.
Colony formation assay
Cells were seeded into 6-well plates at a density of
500 cells/well and incubated for 12 h in medium supplemented with
10% FBS. Next, the cells were treated with various concentrations
of capsaicin (0, 100, 150, 200 or 250 µM) for 24 h, after which
they were incubated with complete medium for another 10 days until
colonies began to form. The medium was discarded, and the cells
were washed twice with ice-cold PBS. After the cells were fixed
with 4% paraformaldehyde for 20 min, they were stained with 0.1%
crystal violet for 10 min. The number of clones containing at least
50 cells in 4 different visual fields was counted under a
microscope.
Cell cycle analysis
Cells were seeded in 10-cm dishes at a density of
106 cells/dish and treated with various concentrations
of capsaicin (0, 100, 150, 200 or 250 µM) for 24 h. Cells were the
collected and fixed in 70% ice-cold ethanol at −20°C overnight.
Then, the cells were incubated with 10 mg/ml RNase and 50 µg/ml PI
for 30 min. The cell cycle distribution was assessed using flow
cytometry.
Western blot analysis
After cells were treated with capsaicin at the
indicated concentrations (0, 100, 150, 200 or 250 µM) for 24 h,
they were lysed in RIPA lysis buffer containing PMSF and
phosphatase inhibitors to extract the total intracellular proteins.
Protein samples (30–50 µg/lane) were separated on a 8–12% gel using
SDS-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride (PVDF) membranes, which were blocked with
either 5% skim milk or 5% BSA at room temperature for 1 h and then
incubated with the corresponding primary antibodies (1:800)
overnight at 4°C. After the membranes were washed with
Tris-buffered saline with Tween-20 (TBST), they were incubated with
secondary antibody for 1 h at 37°C. The membranes were washed, and
the reactive protein bands were detected with an enhanced
chemiluminescence (ECL) detection system and developed on film.
Xenograft tumor model
Male nude mice (4 weeks old) were supplied by the
Experimental Animal Center of Chongqing Medical University. All
animal studies were approved by the Ethics Committee at Chongqing
Medical University. The mice were housed with free access to a
commercial diet and water under specific pathogen-free conditions.
After the mice were acclimated for one week prior to study
initiation, they were then subcutaneously injected with 100 µl of
an HOS cell suspension in sterile PBS at a density of
2×106 cells/ml. After the tumor volume reached 100
mm3, capsaicin treatment was initiated. Ten mice were
randomized into 2 groups (5/group), The capsaicin group was
administered capsaicin 50 mg/kg body weight in 100 µl of PBS
containing 0.2% ethanol, and the control group received 100 of PBS
containing 0.2% ethanol; the treatments were administered via oral
gavage. The groups received their respective treatments every 3
days for a total of 4 weeks until the mice were sacrificed. The
tumor volume was measured every 3 days after treatment according to
the following formula: 1/2 × a2b (a is the short axis
and b is the long axis of the tumor). Mice were sacrificed under
anesthesia on day 28, and the xenograft tumors from each animal
were removed, measured, and then embedded in paraffin for
immunohistochemistry (IHC).
Proliferation index in the xenograft
tumor
IHC was performed to evaluate PCNA and Ki67
expression in xenograft tumor tissues. Briefly, paraffin-embedded
xenograft tumor tissue sections were dewaxed in xylene and
subsequently rehydrated in a graded series of ethanol. Antigen
retrieval on the deparaffinized sections was performed by immersing
the samples in 0.1 M citrate buffer (pH 6.0), boiling the sections
in the microwave for 10 min, and then cooling the sections to room
temperature. Endogenous peroxidase activity was then blocked by
immersing the sections in methanol containing 3% hydrogen peroxide
for 10 min. After the sections were blocked in goat serum for 10
min at room temperature, they were incubated with the antibodies
targeting Ki67 (1:200) and PCNA (1:200) overnight at 4°C. Then, the
sections were incubated with the secondary antibody at 37°C for 30
min. Streptavidin-conjugated peroxidase was added to the sections,
which were incubated for another 10 min at room temperature.
Diaminobenzidine (DAB) substrate was added for 5 min to visualize
the bound antibodies. PCNA and Ki67 expression was evaluated by
counting the number of positive cells from 5 randomly fields under
a light microscope at a magnification of ×400. Data are presented
as the percentage of the positive cells.
Statistical analysis
All the data are presented as the mean ± standard
deviation (SD) of 3 independent experiments. Analysis of variance
(ANOVA) and Student's t-test were used to determine significant
differences between groups. A two-tailed P-value <0.05 was
considered to indicate a significant difference.
Results
Capsaicin decreases the viability of
OS cells in a dose-dependent manner
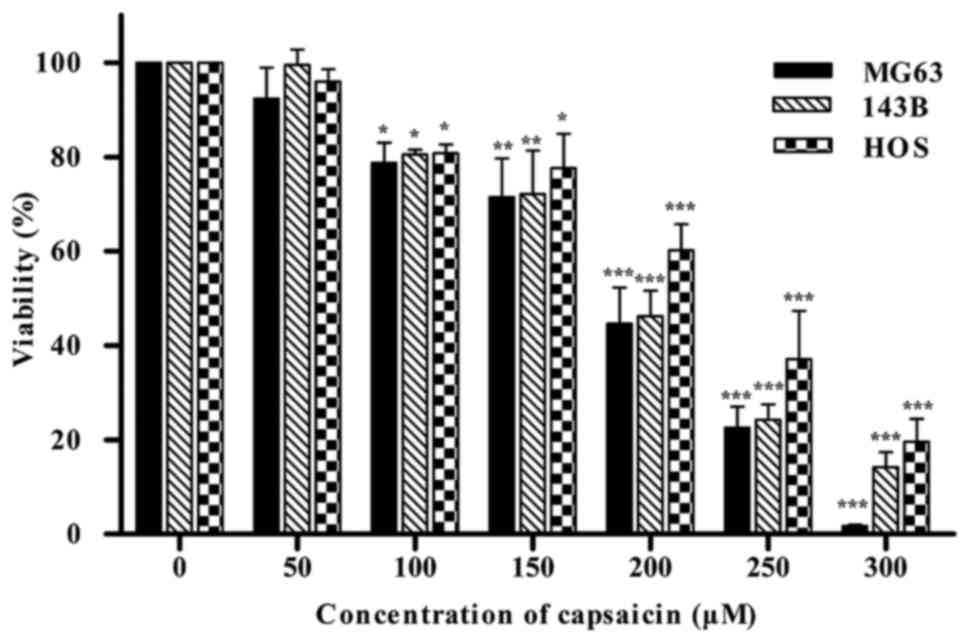
The CCK-8 assay was used to examine the effects of
capsaicin on the viability of OS cells in vitro. Three OS
cell lines (MG63, 143B and HOS) were treated with a wide range of
concentrations of capsaicin (0, 50, 100, 150, 200, 250 or 300 µM)
for 24 h. As shown in Fig. 1,
starting at 100 µM capsaicin, the cell viability progressively
decreased with elevated concentrations of capsaicin in all the 3 OS
cell lines with a IC50 value of ~200 µM. These results
indicated that capsaicin could decrease the viability of OS cells
in a dose-dependent manner, with 100 µM determined as the minimal
effective concentration in all 3 OS cell lines.
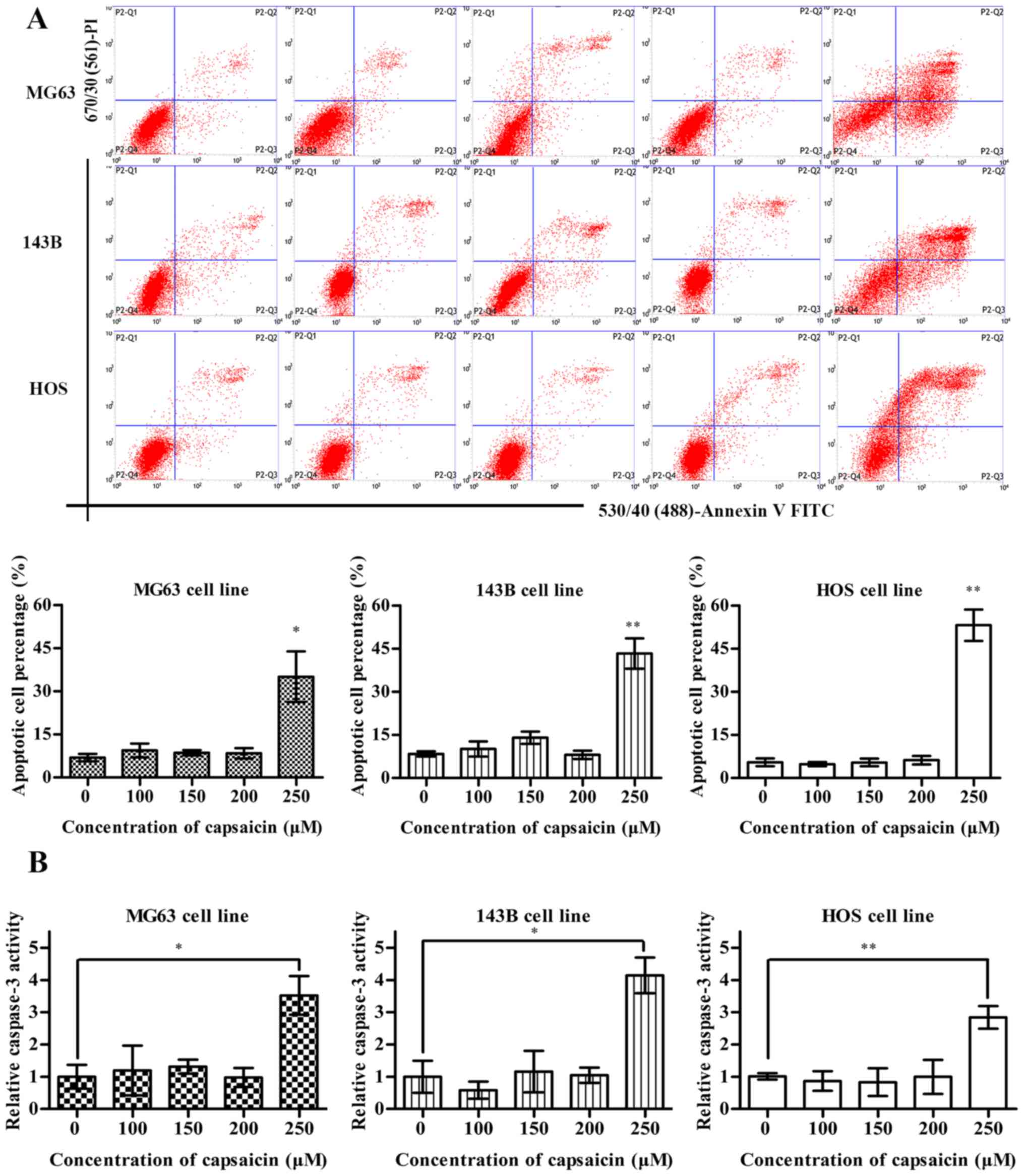
Capsaicin induces apoptosis in OS
cells at a relatively high concentration
To elucidate whether the decrease in OS cell
viability caused by capsaicin was associated with apoptosis
induction, the number of apoptotic OS cells that were treated with
various concentrations of capsaicin (0, 100, 150, 200 or 250 µM)
was determined via flow cytometry using Annexin V-FITC/PI double
staining. Notably, OS cell apoptosis was only observed at
relatively high concentrations of capsaicin (starting at 250 µM) in
all 3 OS cell lines. In addition, no apoptotic effects were
observed at capsaicin concentrations between 0 and 200 µM (Fig. 2A). As caspases are proteolytic
enzymes that critically mediate apoptosis, the increased caspase
activity results in cell apoptosis, with caspase-3 activation as an
ultimate executioner of apoptotic pathways. As shown in Fig. 2B, only treatment with the highest
tested concentration of capsaicin (250 µM) for 24 h resulted in
elevated caspase-3 activities in the OS cell lines, which were
3.5-, 4- and 3-fold higher compared to the control treatment (0 µM)
in MG63, 143B and HOS cells, respectively. Meanwhile, when cells
were treated with low concentrations of capsaicin (between 100 and
200 µM) for 24 h, no obvious increase in activated caspase-3 was
observed in all 3 OS cell lines. In conclusion, these results
suggested that high concentrations of capsaicin could modulate the
activation of caspase-dependent apoptotic signaling pathways in OS
cells.
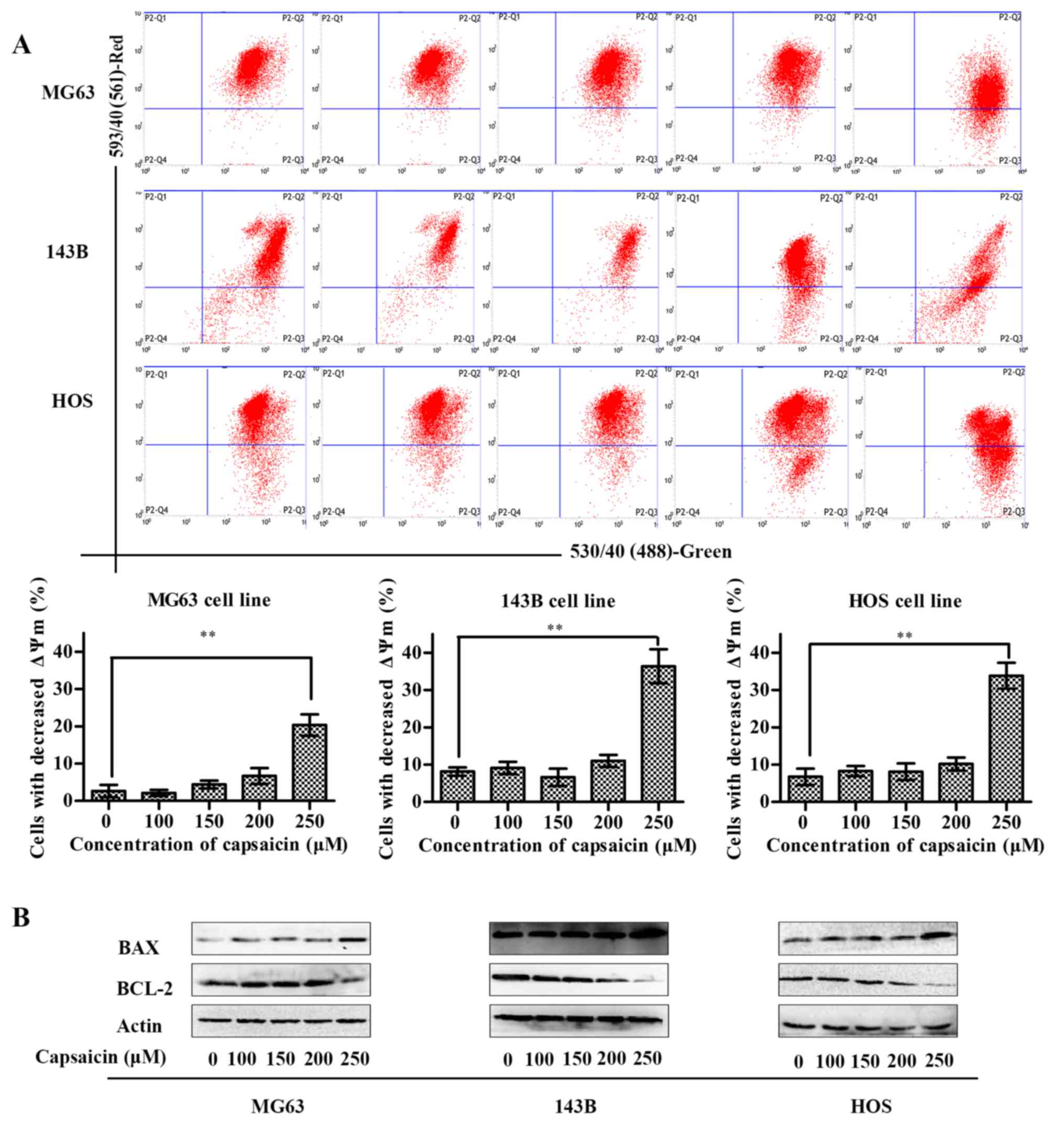
The mitochondrial apoptosis pathway is
involved in capsaicin-induced apoptosis in OS cells
Caspase-3 can be activated in apoptotic cells both
by death receptor-mediated (extrinsic) and mitochondrial-mediated
(intrinsic) pathways (22). To
further determine whether capsaicin-induced apoptosis in OS cells
is mediated by mitochondrial dysfunction, we measured the
mitochondrial membrane potential (ΔΨm) by adding JC-1 dye to the
cells and measuring the fluorescence using flow cytometry. An
intact mitochondrial membrane allows for JC-1 accumulation in the
mitochondria, which may emit red fluorescence. Conversely, the loss
of the ΔΨm prevents this accumulation, and JC-1 may remain as a
monomer in the cytosol and emit green fluorescence (23). Thus, an increase in green
fluorescence is indicative of a decrease in ΔΨm. As shown in
Fig. 3A, when cells were treated
with low concentrations of capsaicin (between 100 and 200 µM) for
24 h, no obvious variations in ΔΨm were observed compared to the
control cells (0 µM capsaicin) in all 3 OS cell lines. However,
compared to control cells, all 3 OS cell lines treated with 250 µM
capsaicin exhibited significant decreases in the ΔΨm. Since the ΔΨm
was reduced in capsaicin-treated cells, we further assessed the Bax
and Bcl-2 protein expression, both of which are involved in the
mitochondrial apoptotic pathway. As shown in Fig. 3B, compared to the control (0 µM
capsaicin), treatment with 250 µM capsaicin for 24 h induced the
upregulation of Bax protein and downregulation of Bcl-2 protein in
all 3 OS cell lines. However, there were no differences in Bax and
Bcl-2 protein expression among the OS cell lines treated with
capsaicin at relative low concentrations which were from 0 to 200
µM. Taken together, these data indicated that capsaicin induced OS
cell apoptosis starting at the concentration of 250 µM via the
mitochondrial apoptotic pathway.
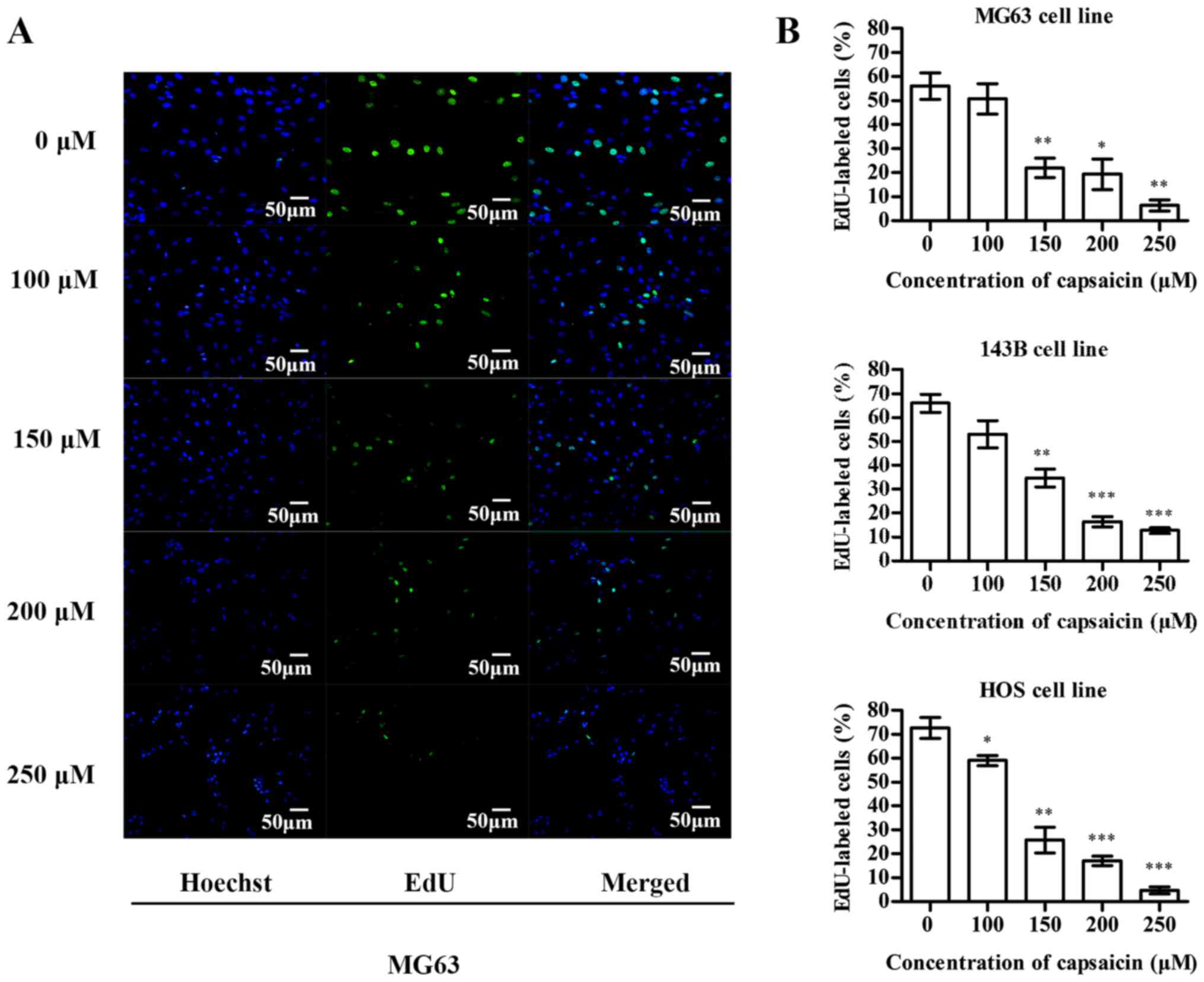
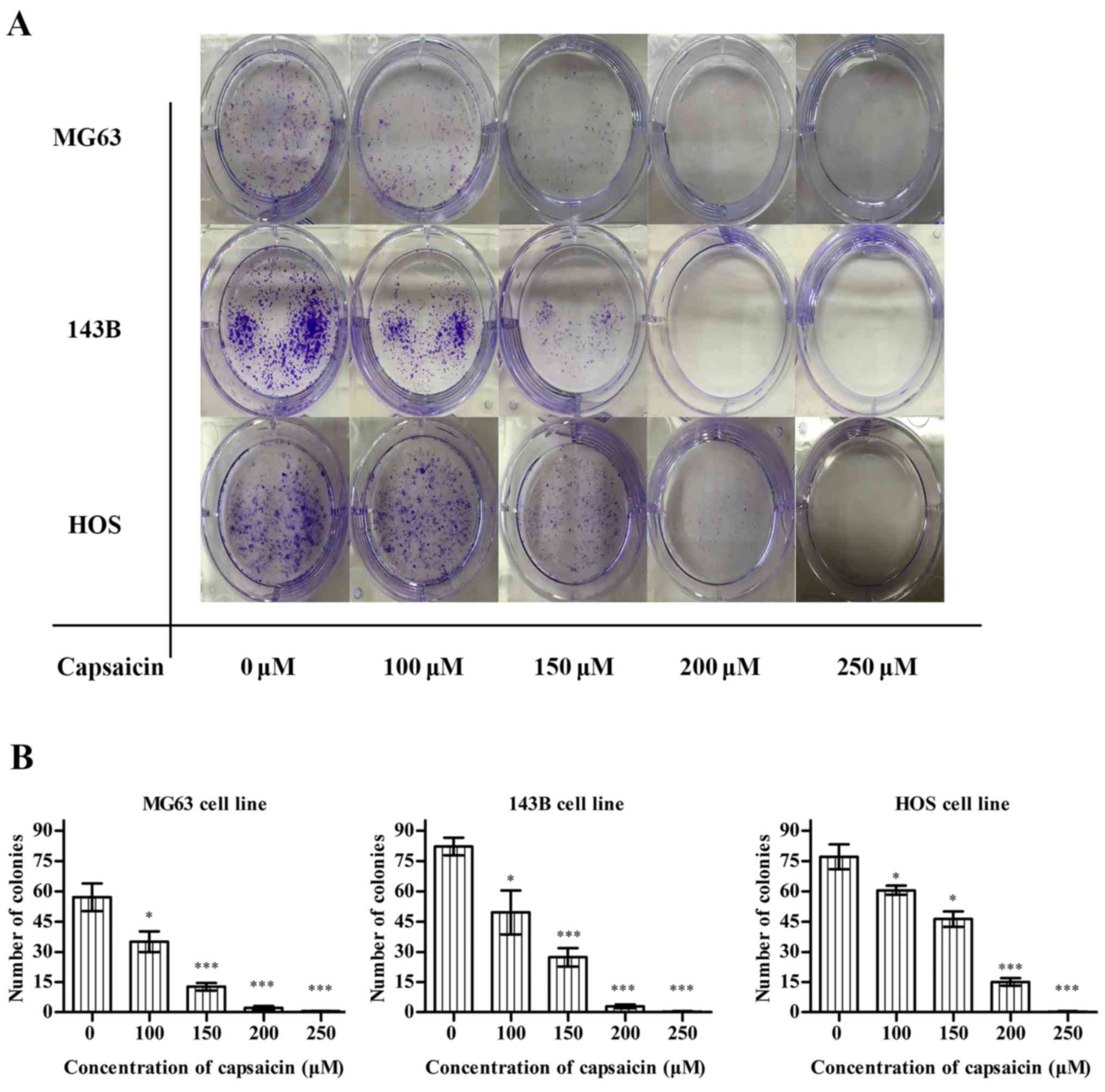
Capsaicin inhibits the proliferation
and decreases the colony formation ability of OS cells
The inhibitory effects of capsaicin on OS cell
proliferation was detected using the EdU incorporation assay. In
MG63 cells (Fig. 4A), compared to
the untreated groups (0 µM), the percentage of EdU-labeled cells
decreased after treatment with multiple concentrations of capsaicin
(between 100 and 250 µM) for 24 h. Similarly, the EdU-labeled 143B
and HOS cells showed a dose-dependent decrease after a 24-h
treatment with capsaicin (Fig. 4B).
These observations indicated that capsaicin exerted significant
inhibitory effects on OS cell proliferation. Furthermore, we
determined the tumorigenicity of OS cells using the colony
formation assay. After a 24-h treatment with various concentrations
of capsaicin, OS cells were cultured for another 10 days, and the
number of colonies was counted. As shown in Fig. 5A and B, capsaicin decreased the
number of colonies in a dose-dependent manner starting at 100 µM in
all the 3 OS cell lines.
Capsaicin induces cell cycle arrest at
the G0/G1 phase in OS cells
Cell proliferation is a complicated process closely
related to cell cycle progression. To further investigate whether
modulating the cell cycle is responsible for capsaicin-mediated
cell growth inhibition, we analyzed the number of cells at the
G0/G1, S and G2/M phases using flow cytometry. As shown in Fig. 6A and B, after treatment with various
concentrations of capsaicin for 24 h, the number of cells in the
G0/G1 phase was increased in accordance with a decrease in cell
number in the S phase in all 3 OS cell lines. In MG63 cells, the
percentage of control cells (0 µM) in G0/G1 was 38.32±2.03%, which
increased to 44.9±2.16, 54.12±0.90, 59.93±2.52 and 68.56±2.30%
after treatment with 100, 150, 200 and 250 µM capsaicin,
respectively, for 24 h. Meanwhile, the percentage of cells in the S
phase showed a corresponding decrease from 52.38±2.01 to
47.95±1.21, 33.29±0.99, 32.81±1.80 and 27.44±2.32% after treatment
with 100, 150, 200 and 250 µM capsaicin, respectively. Capsaicin
treatments in the other 2 OS cell lines (143B and HOS) showed
similar outcomes. PCNA and P21 have been demonstrated as important
cell cycle regulatory factors. P21 is a cofactor that interacts
with PCNA to influence cell cycle regulation (24). To investigate whether the observed
capsaicin-induced G0/G1 arrest was caused by any interaction with
PCNA and/or P21, western blotting was performed to detect changes
in PCNA and P21 expression. In the OS cell lines, the expression of
PCNA was decreased with the increasing capsaicin concentrations
compared to the control cells. Conversely, P21 expression was
slightly increased in cells treated with 100 µM capsaicin and
showed further significant increases with higher capsaicin
concentrations. Furthermore, similar changes in P21 and PCNA
expression were also observed in the other 2 OS cell lines
(Fig. 6C and D). Taken together,
these data indicated that capsaicin inhibited OS cell proliferation
by inducing cycle arrest at the G0/G1 phase, which may involve the
regulation of P21 and PCNA.
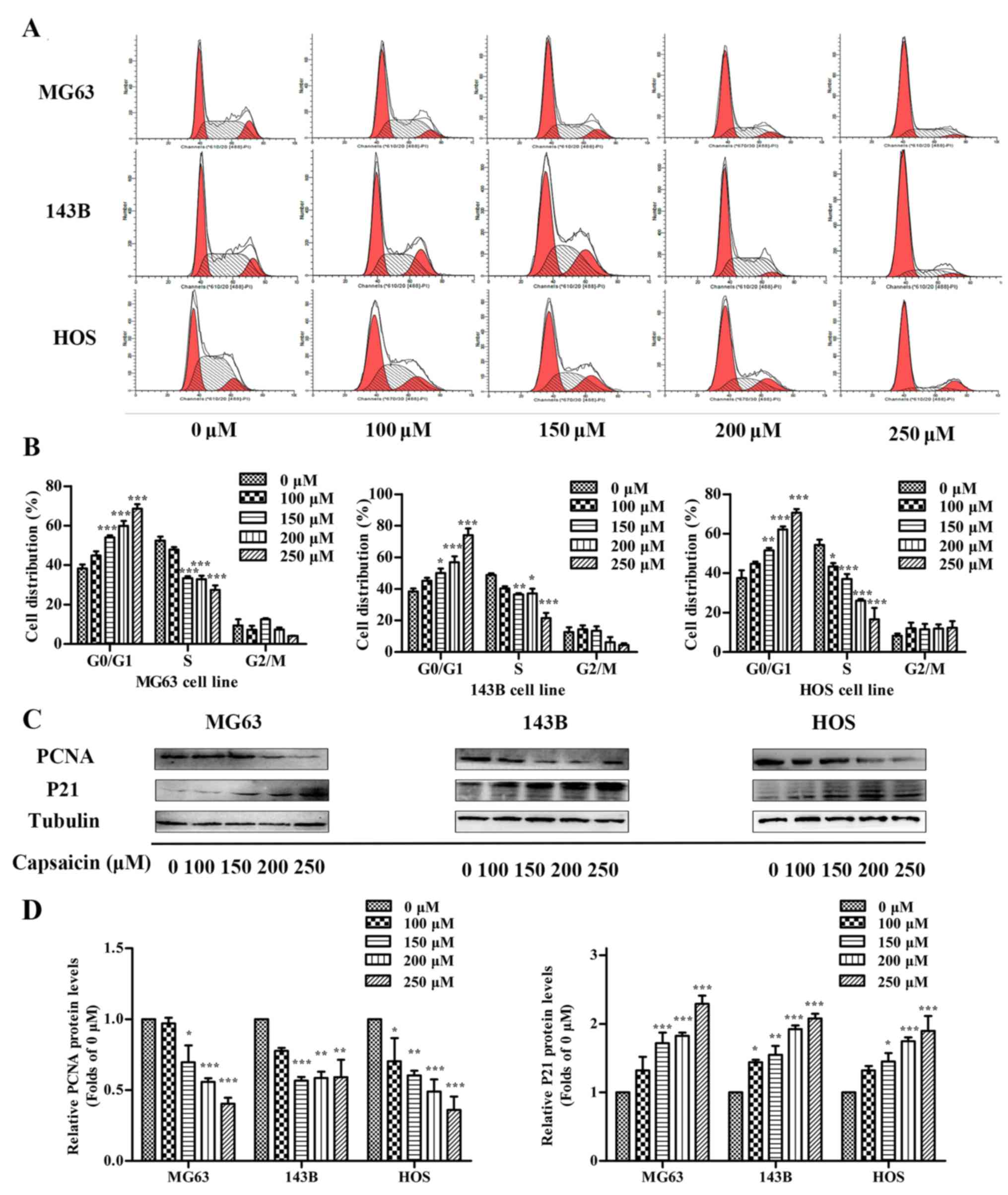 | Figure 6.Effects of capsaicin on cell cycle
distribution in OS cells. (A) Three OS cell lines (MG63, 143B and
HOS) were treated with various concentrations of capsaicin (0, 100,
150, 200 or 250 µM) for 24 h, stained with PI and analyzed using
flow cytometry. (B) The percentage of cells in each phase is
presented as the mean ± SD of 3 independent experiments;
*p<0.05, **p<0.01 and ***p<0.001 vs. the control (0 µM).
(C) OS cells were treated with various concentrations of capsaicin
(0, 100, 150, 200 or 250 µM) for 24 h. Cell lysates were prepared
to assess PCNA, P21 and tubulin (loading control) protein
expression levels via western blotting. (D) The results are shown
as the fold changes in the levels of PCNA and P21 normalized to the
level of actin. The data are presented as the mean ± SD of 3
independent experiments; *p<0.05, **p<0.01 and ***p<0.001
vs. the control (0 µM). |
MAPK signaling pathways are involved
in the capsaicin-induced toxic effect on OS cells
MAPK signaling pathways are important for tumor
development, and regulating these pathways has been previously
demonstrated to affect capsaicin-induced anticancer activity
(25). Thus, we determined whether
MAPKs were implicated in the inhibitory effects of capsaicin in OS
cells (Fig. 7A and B). In the 3 OS
cell lines, the levels of total ERK1/2, p38 and JNK were not
altered in cells subjected to capsaicin treatment compared to
untreated cells. However, treatment with various concentrations of
capsaicin resulted in significant decrease in p-ERK1/2 and p-p38 in
a dose-dependent manner, which corresponded to the inhibitory
effects of capsaicin on proliferation observed in the present
study. In contrast, the p-JNK levels remained at basal levels in
the presence of up to 200 µM capsaicin, but these levels
significantly increased at a concentration of 250 µM in all 3 OS
cell lines, which corresponded to the capsaicin-induced apoptotic
effects observed in the present study. Taken together, these
results demonstrated that the capsaicin-induced effects on OS cells
may involve the regulation of MAPK pathways.
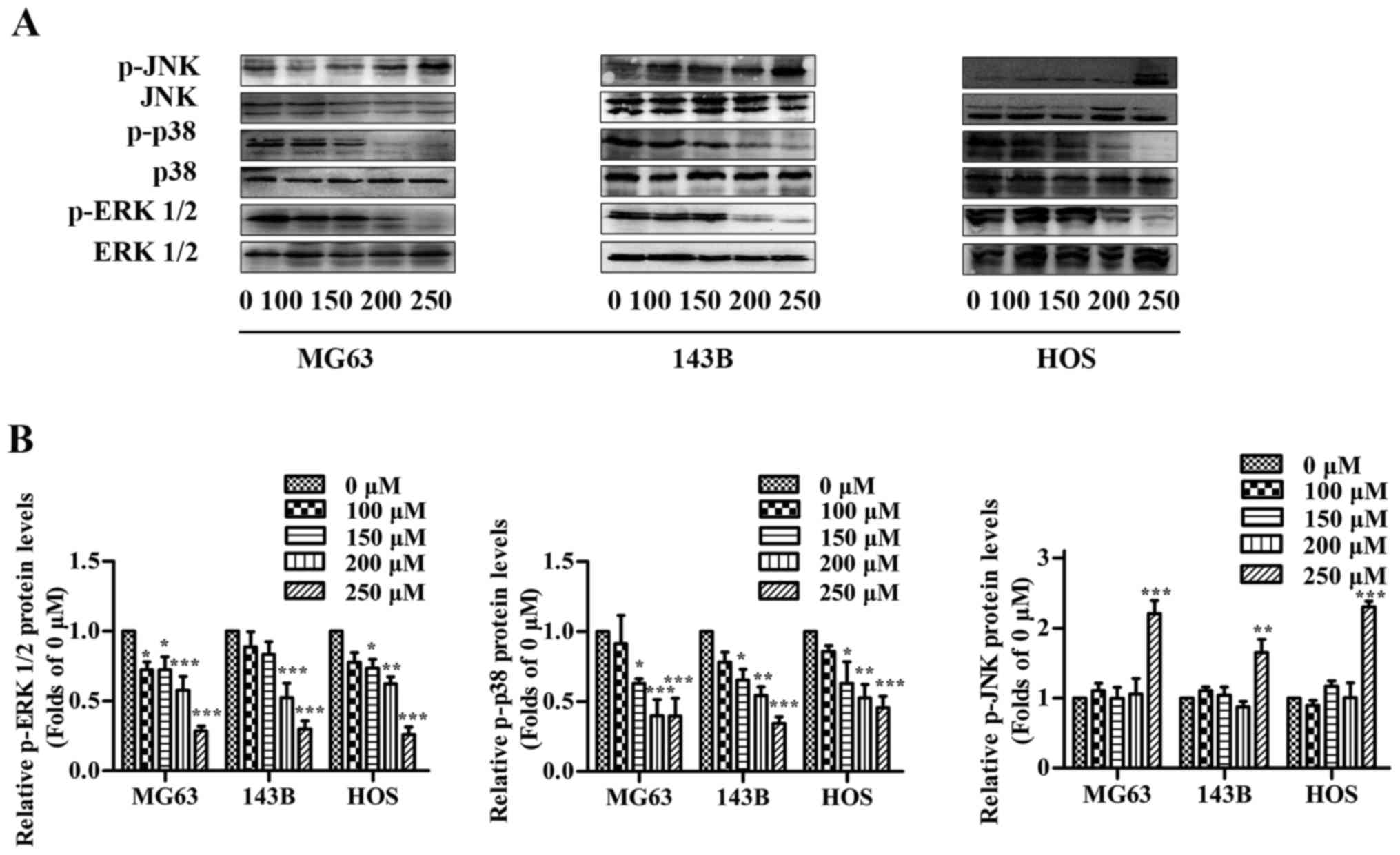 | Figure 7.Effects of capsaicin on MAPK
signaling pathways in OS cells. OS cells were treated with various
concentrations of capsaicin (0, 100, 150, 200 or 250 µM) for 24 h.
(A) Cell lysates were prepared to assess the expression levels of
p-ERK1/2, ERK1/2, p-p38, p38, p-JNK and JNK using western blotting.
(B) The results are shown as the relative levels of p-ERK1/2, p-p38
and p-JNK to ERK1/2, p38 and JNK, respectively. The data are
presented as the mean ± SD of 3 independent experiments;
*p<0.05, **p<0.01 and ***p<0.001 vs. the control (0
µM). |
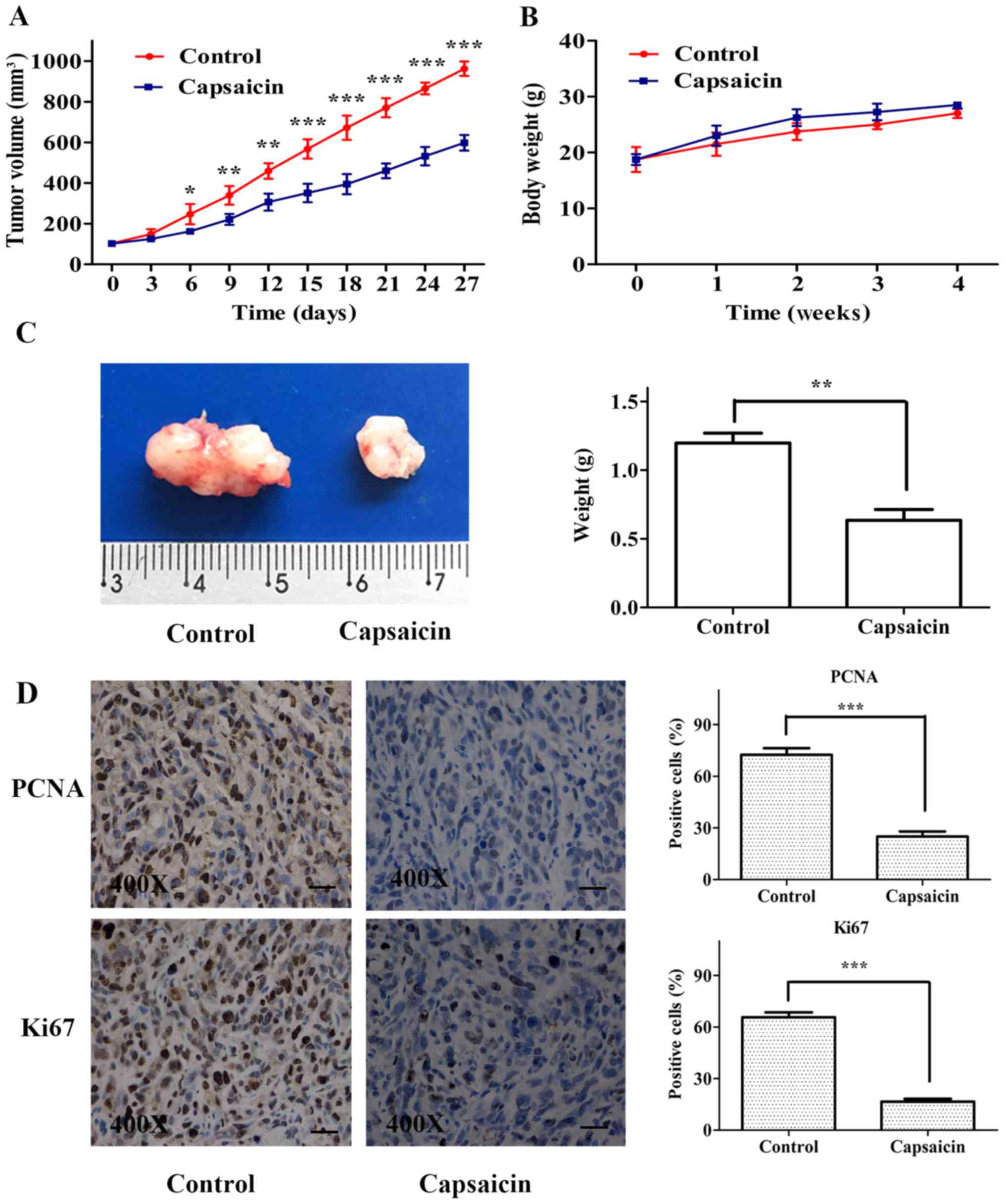
Capsaicin inhibits tumor growth in a
xenograft model of human OS
We evaluated the in vivo antitumor potential
of capsaicin using an OS xenograft model. HOS cells were
subcutaneously implanted in nude mice, and the tumor volumes were
measured every 3 days. As shown in Fig.
8A, the capsaicin-treated group exhibited significantly smaller
OS tumors than the control group. No significant difference in body
weight was observed during the experimental period between the
control and capsaicin-treated groups (Fig. 8B). At the end of the experiment, the
tumor weight measurements indicated that capsaicin significantly
decreased the tumor weight compared to that in tumors from the
control group (Fig. 8C).
Furthermore, the proliferation indices (as indicated by PCNA and
Ki67 expression) were lower in tumor specimens from the
capsaicin-treated group than those from the control group (Fig. 8D). These findings indicated that
capsaicin efficiently suppressed OS tumor growth in
vivo.
Discussion
It is widely accepted that eliminating cancer cells
by inducing cell apoptosis is a crucial element in chemotherapy
(26,27). Capsaicin is an active molecular
component of hot chili peppers, and significant evidence has shown
that capsaicin can trigger apoptosis in numerous cancer cell lines.
In addition, the significant concentration of capsaicin that could
trigger apoptotic cell death for most cancer cells varied between
200 and 300 µM (28). Lee et
al (29,30) reported that the prominent apoptotic
effect of capsaicin on A172 human glioblastoma cells and HepG2
human hepatoma cells were initially observed at concentrations of
200 and 250 µM, respectively. In the present study, we investigated
capsaicin-induced apoptosis in osteosarcoma (OS) cells using 2
independent methods: detection of phosphatidylserine (PS)
translocation through Annexin V/PI double staining and measurement
of caspase-3 activation. Our results showed that capsaicin-induced
apoptosis was observed at a concentration of 250 µM in all 3 tested
OS cell lines; these data were in accordance with previous results
in other human cancer cells. Furthermore, the ΔΨm of OS cells was
decreased after treatment with 250 µM capsaicin, which were
coincident with the apoptosis results. Together with the observed
upregulation of Bax and simultaneous downregulation of Bcl-2 in OS
cells after treatment with 250 µM capsaicin, our results indicated
that capsaicin could induce apoptosis in OS cells through the
intrinsic pathway starting at a concentration of 250 µM.
Most studies exploring the toxicity of capsaicin in
OS cells have focused on the mechanisms underlying
capsaicin-induced apoptosis (18,20).
In addition, numerous studies have reported that the
capsaicin-induced anticancer effects are primarily dependent on
apoptotic machinery. Nevertheless, apoptosis induction by capsaicin
cannot be considered as a default pathway, particularly since
defective apoptosis is considered a major hallmark of cancer cells
(31). Moreover, the apoptotic
effects induced by capsaicin were usually observed at high
concentrations. Thus, it is likely that capsaicin may work through
other pathways to exert its anticancer effects on cancerous cells.
Based on our results, capsaicin-associated toxicity in OS cells was
not completely coincident with apoptotic effects, which began to
manifest at a concentration of 250 µM. Indeed, the results of the
CCK assay indicated that capsaicin decreased the viability of OS
cells in a dose-dependent manner from 0 to 300 µM with an
IC50 value of ~200 µM in all the 3 OS cell lines.
Specifically, the ability of capsaicin to reduce the CCK-8 value in
the cells may merely reflect its negative effects on mitochondrial
metabolic activity in the cell, which can be influenced by many
factors such as cell number, viability, proliferation and
apoptosis. Therefore, the decrease in OS cell viability in the
absence of apoptosis induction upon treatment with relatively low
concentrations of capsaicin may involve some other mechanisms.
Along with the extensive research available on the
anticancer effects of capsaicin, recent literature has indicated
that cell survival mechanisms may be activatde in cancer cell lines
subject to capsaicin treatment. Lewinska et al (32) reported that capsaicin did not
trigger apoptosis in prostate carcinoma cells (DU145) and lung
carcinoma cells (A549) at doses up to 250 µM. In addition, they
demonstrated that the DNA damage response may be involved in the
resistance of DU145 and A549 cancer cells to apoptosis.
Furthermore, Choi et al (17) reported that capsaicin did not induce
apoptosis in the breast cancer cell lines MCF7 and MDA-MB-231, and
they also confirmed that capsaicin-induced autophagy associated
with endoplasmic reticulum stress may play a critical role in
impeding cell death. Chien et al (18) suggested that an anti-apoptotic
pathway was activated in parallel with pro-apoptotic pathway and
was mediated by autophagy in G292 OS cells. In conclusion,
capsaicin may exert dual effects on cancer cells in a
dose-dependent manner. The explicit protective mechanisms involved
in promoting OS cell survival in response to low concentrations of
capsaicin need to be elucidated in future studies. In addition to
these above mentioned protective mechanisms (which have yet to be
proven), the results from the present study showed that the
decrease in OS cell viability at low concentrations of capsaicin
was not the result of apoptotic cell death. Thus, we speculated
that a capsaicin-mediated decrease in metabolic activity at lower
concentrations (as estimated using the CCK-8 assay) may reflect a
cytostatic function of capsaicin rather than its ability to
stimulate apoptosis. We further investigated whether capsaicin
could inhibit the growth of OS cells. Since inhibition of DNA
synthesis in cancer cells can be quantified by EdU incorporation
into cancer cells, which correlates with cell proliferation
(33), we conducted the EdU label
assay to determine the proliferation status of OS cells. After
treatment with 100, 150, 200 or 250 µM of capsaicin for 24 h, the
number of EdU-labeled cells in the treated groups decreased
significantly in a dose-dependent manner. Unlike the
capsaicin-induced apoptotic effects in OS cells, which only
occurred at high concentrations of capsaicin, these results
indicated that the capsaicin-induced loss of OS cell viability was
primarily due to the inhibition of proliferation rather than cell
apoptosis, particularly in cells treated with a relatively low
concentration of capsaicin.
The ability of cancer cells to form colonies is
essential for the metastasis of a malignant tumor to distant organs
(34). Therefore, we investigated
the colony forming ability of the 3 OS cells and observed that
capsaicin treatment significantly reduced the number of colonies in
a dose-dependent manner compared with the untreated cells; these
data highlight the great antiproliferative effects of capsaicin on
OS cells.
Cells proliferate through the cell cycle, which is
divided into the G0/G1, S and G2/M phases. Throughout the cell
cycle, DNA checkpoints ensure the integrity of DNA replication.
Increasing evidence has indicated that capsaicin leads to DNA
damage, resulting in cell cycle modulation (35). To the best of our knowledge, there
are few studies concerning capsaicin-induced cell cycle arrest in
OS cells. Jin et al (36)
reported that capsaicin exerted G0/G1 cell cycle arrest in human
colon cancer cells. In contrast, Lin et al (37) observed that capsaicin increased the
number of human KB cancer cells in G2/M phase. These inconsistent
findings on capsaicin-induced cell cycle arrest in cancer cells
suggested that the mode of this activity depends on the phenotypic
and genotypic diversity of different cancer cells. To address the
OS cell cycle arrest that may account for capsaicin-induced
proliferation inhibition, we investigated the cell cycle
distribution of OS cells treated with capsaicin. Our results
demonstrated for the first time that capsaicin induced G0/G1 arrest
in 3 human OS cell lines in a dose-dependent manner, which was
consistent with the inhibitory effects on proliferation.
Furthermore, previous studies have also shown that
capsaicin-induced G0/G1 arrest in other cancer cells occurs via
induction of the cyclin-dependent kinase inhibitor P21 (38,39).
Tong et al (40) reported
that transfection of an antisense RNA targeting PCNA resulted in
growth inhibitory effects and G0/G1 phase arrest in bladder cancer.
Next, we performed western blotting to determine whether capsaicin
modulated the OS cell cycle by regulating P21 and PCNA.
Congruously, our results showed that P21 expression was increased
and associated with a decrease in PCNA expression in all 3 OS cell
lines in a dose-dependent manner, which may be related to
capsaicin-induced cell cycle arrest.
The MAPK family plays a critical role in OS cell
survival, proliferation and angiogenesis. In addition, some other
studies showed that capsaicin could alter the MAPK pathways in
cancer cells (25,41). Based on previous studies, we
hypothesized that capsaicin may mediate its anti-OS effects by
regulating MAPK signaling pathways. In the present study, all 3
cell lines exhibited p-ERK1/2 and p-p38 downregulation after
capsaicin treatment in a dose-dependent manner, whereas p-JNK was
increased significantly only at a concentration of 250 µM. The
ERK1/2 signaling pathway is known to contribute to cell
proliferation, which is also partially involved in G0/G1 cell cycle
arrest (42,43). Previous studies have reported that
capsaicin induced anticancer effects on fibrosarcoma cells
partially by suppressing ERK1/2 phosphorylation in human
fibrosarcoma cells (44).
Consistent with previous findings, our results indicated that
capsaicin significantly inhibited ERK1/2 phosphorylation, which was
correlated with a decrease in cell viability, inhibition of
proliferation and induction of G0/G1 cycle arrest. In contrast,
regulation of p38 phosphorylation was diverse among different
cancer cell lines treated with capsaicin. Park et al
(25) reported that capsaicin
suppressed the phosphorylation of p38 in human AGS gastric cancer
cells. However, Liu et al (41) demonstrated that capsaicin mediated
its anticancer effects by activating p38 in human renal carcinoma.
In the present study, capsaicin significantly inhibited the
phosphorylation of p38 in all 3 OS cell lines, which was also
correlated with the capsaicin-induced antiproliferative effects on
OS cells. Furthermore, the JNK signaling pathway has been
associated with apoptosis in tumor cells, including OS (45,46).
In the present study, the p-JNK levels were significantly elevated
after treatment with 250 µM capsaicin, which was correlated with
the capsaicin-induced apoptotic effects in all 3 OS cell lines;
these data suggest that capsaicin-induced OS cell apoptosis is at
least partially involved in activating the JNK signaling pathway.
Our results are consistent with those of previous findings that
capsaicin could increase JNK phosphorylation and thus promote
apoptosis in cancer cells (41,47).
Taken together, the findings in the present study strongly suggest
that inactivation of ERK1/2 and p38 plays an important role in
capsaicin-induced inhibition of proliferation and cell cycle arrest
in OS cells, whereas JNK activation participated in
capsaicin-induced apoptosis.
Finally, the xenograft tumor model was used to
investigate the inhibitory effects of capsaicin on OS cells in
vivo. Our results demonstrated that capsaicin possessed potent
antiproliferative effects on HOS xenograft tumors. Moreover,
capsaicin administration did not significantly affect the body
weight of mice, which indicated that capsaicin may be a relatively
effective and safe agent against OS.
In conclusion, our results showed that capsaicin has
profound in vitro and in vivo antiproliferative
effects against human OS cells. Moreover, MAPK signaling pathways
were involved in the anti-OS effects induced by capsaicin. The
inactivation of ERK1/2 and p38 may participate in the
capsaicin-induced inhibition of proliferation in OS cells starting
at a concentration of 100 µM. However, capsaicin-induced apoptosis
in OS cells, which was observed at a dose of 250 µM, may be
involved in JNK activation. These results may enrich our
understanding of the mechanisms that mediate the capsaicin-induced
anti-OS effects. In addition, the capsaicin-induced apoptotic
effects in OS cells were only observed upon treatment with a
relatively high concentration of capsaicin. Nevertheless, the
inhibition of proliferation and cell cycle arrest induced by
capsaicin could be observed at lower concentrations. Thus, these
results strongly indicate that capsaicin may exert more therapeutic
benefits as an adjunct to current cancer therapies rather than as
an independent anticancer agent.
Acknowledgements
This study was supported by the Chongqing Science
and Technology Committee foundation (cstc2017jcyjB0312).
References
|
1
|
Murphey MD, Robbin MR, McRae GA, Flemming
DJ, Temple HT and Kransdorf MJ: The many faces of osteosarcoma.
Radiographics. 17:1205–1231. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: Where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kempf-Bielack B, Bielack SS, Jürgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuijjer ML, Hogendoorn PC and
Cleton-Jansen AM: Genome-wide analyses on high-grade osteosarcoma:
Making sense of a genomically most unstable tumor. Int J Cancer.
133:2512–2521. 2013.PubMed/NCBI
|
|
5
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen KG and Sikic BI: Molecular pathways:
Regulation and therapeutic implications of multidrug resistance.
Clin Cancer Res. 18:1863–1869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R
and Darwiche N: Cell death mechanisms of plant-derived anticancer
drugs: Beyond apoptosis. Apoptosis. 20:1531–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin S, Shen JN, Wang J, Huang G and Zhou
JG: Oridonin induced apoptosis through Akt and MAPKs signaling
pathways in human osteosarcoma cells. Cancer Biol Ther. 6:261–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim S, Park M, Yeom SI, Kim YM, Lee JM,
Lee HA, Seo E, Choi J, Cheong K, Kim KT, et al: Genome sequence of
the hot pepper provides insights into the evolution of pungency in
Capsicum species. Nat Genet. 46:270–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fattori V, Hohmann MS, Rossaneis AC,
Pinho-Ribeiro FA and Verri WA: Capsaicin: Current understanding of
its mechanisms and therapy of pain and other pre-clinical and
clinical uses. Molecules. 21:8442016. View Article : Google Scholar
|
|
12
|
Mori A, Lehmann S, O'Kelly J, Kumagai T,
Desmond JC, Pervan M, McBride WH, Kizaki M and Koeffler HP:
Capsaicin, a component of red peppers, inhibits the growth of
androgen-independent, p53 mutant prostate cancer cells. Cancer Res.
66:3222–3229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito K, Nakazato T, Yamato K, Miyakawa Y,
Yamada T, Hozumi N, Segawa K, Ikeda Y and Kizaki M: Induction of
apoptosis in leukemic cells by homovanillic acid derivative,
capsaicin, through oxidative stress: Implication of phosphorylation
of p53 at Ser-15 residue by reactive oxygen species. Cancer Res.
64:1071–1078. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JD, Kim JM, Pyo JO, Kim SY, Kim BS, Yu
R and Han IS: Capsaicin can alter the expression of tumor
forming-related genes which may be followed by induction of
apoptosis of a Korean stomach cancer cell line, SNU-1. Cancer Lett.
120:235–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung MY, Kang HJ and Moon A:
Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells
involves Bcl-2 downregulation and caspase-3 activation. Cancer
Lett. 165:139–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao S, Li W, Tsubouchi R, Haneda M,
Murakami K and Yoshino M: Involvement of peroxynitrite in
capsaicin-induced apoptosis of C6 glioma cells. Neurosci Res.
51:175–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi CH, Jung YK and Oh SH: Autophagy
induction by capsaicin in malignant human breast cells is modulated
by p38 and extracellular signal-regulated mitogen-activated protein
kinases and retards cell death by suppressing endoplasmic reticulum
stress-mediated apoptosis. Mol Pharmacol. 78:114–125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chien CS, Ma KH, Lee HS, Liu PS, Li YH,
Huang YS and Chueh SH: Dual effect of capsaicin on cell death in
human osteosarcoma G292 cells. Eur J Pharmacol. 718:350–360. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ying H, Wang Z, Zhang Y, Yang TY, Ding ZH,
Liu SY, Shao J, Liu Y and Fan XB: Capsaicin induces apoptosis in
human osteosarcoma cells through AMPK-dependent and
AMPK-independent signaling pathways. Mol Cell Biochem. 384:229–237.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho WH, Lee HJ, Choi YJ, Oh JH, Kim HS and
Cho HS: Capsaicin induces apoptosis in MG63 human osteosarcoma
cells via the caspase cascade and the antioxidant enzyme system.
Mol Med Rep. 8:1655–1662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin T, Wu H, Wang Y and Peng H: Capsaicin
induces immunogenic cell death in human osteosarcoma cells. Exp
Ther Med. 12:765–770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hensley P, Mishra M and Kyprianou N:
Targeting caspases in cancer therapeutics. Biol Chem. 394:831–843.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smiley ST, Reers M, Mottola-Hartshorn C,
Lin M, Chen A, Smith TW, Steele GD Jr and Chen LB: Intracellular
heterogeneity in mitochondrial membrane potentials revealed by a
J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA.
88:pp. 3671–3675. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cazzalini O, Scovassi AI, Savio M, Stivala
LA and Prosperi E: Multiple roles of the cell cycle inhibitor
p21(CDKN1A) in the DNA damage response. Mutat Res. 704:12–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SY, Kim JY, Lee SM, Jun CH, Cho SB,
Park CH, Joo YE, Kim HS, Choi SK and Rew JS: Capsaicin induces
apoptosis and modulates MAPK signaling in human gastric cancer
cells. Mol Med Rep. 9:499–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ledgerwood EC and Morison IM: Targeting
the apoptosome for cancer therapy. Clin Cancer Res. 15:420–424.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giménez-Bonafé P, Tortosa A and
Pérez-Tomás R: Overcoming drug resistance by enhancing apoptosis of
tumor cells. Curr Cancer Drug Targets. 9:320–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bley K, Boorman G, Mohammad B, McKenzie D
and Babbar S: A comprehensive review of the carcinogenic and
anticarcinogenic potential of capsaicin. Toxicol Pathol.
40:847–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YS, Kang YS, Lee JS, Nicolova S and
Kim JA: Involvement of NADPH oxidase-mediated generation of
reactive oxygen species in the apototic cell death by capsaicin in
HepG2 human hepatoma cells. Free Radic Res. 38:405–412. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee YS, Nam DH and Kim JA: Induction of
apoptosis by capsaicin in A172 human glioblastoma cells. Cancer
Lett. 161:121–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okada H and Mak TW: Pathways of apoptotic
and non-apoptotic death in tumour cells. Nat Rev Cancer. 4:592–603.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lewinska A, Jarosz P, Czech J, Rzeszutek
I, Bielak-Zmijewska A, Grabowska W and Wnuk M: Capsaicin-induced
genotoxic stress does not promote apoptosis in A549 human lung and
DU145 prostate cancer cells. Mutat Res Genet Toxicol Environ
Mutagen. 779:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salic A and Mitchison TJ: A chemical
method for fast and sensitive detection of DNA synthesis in vivo.
Proc Natl Acad Sci USA. 105:pp. 2415–2420. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin J, Lin G, Huang H, Xu D, Yu H, Ma X,
Zhu L, Ma D and Jiang H: Capsaicin mediates cell cycle arrest and
apoptosis in human colon cancer cells via stabilizing and
activating p53. Int J Biol Sci. 10:285–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin CH, Lu WC, Wang CW, Chan YC and Chen
MK: Capsaicin induces cell cycle arrest and apoptosis in human KB
cancer cells. BMC Complement Altern Med. 13:46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lehen'kyi V, Flourakis M, Skryma R and
Prevarskaya N: TRPV6 channel controls prostate cancer cell
proliferation via Ca2+/NFAT-dependent pathways.
Oncogene. 26:7380–7385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thoennissen NH, O'Kelly J, Lu D, Iwanski
GB, La DT, Abbassi S, Leiter A, Karlan B, Mehta R and Koeffler HP:
Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and
-negative breast cancer cells by modulating the EGFR/HER-2 pathway.
Oncogene. 29:285–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tong Q, Zeng F, Lin C, Zhao J and Lu G:
Growth inhibiting effects of antisense eukaryotic expression vector
of proliferating cell nuclear antigen gene on human bladder cancer
cells. Chin Med J. 116:1203–1206. 2003.PubMed/NCBI
|
|
41
|
Liu T, Wang G, Tao H, Yang Z, Wang Y, Meng
Z, Cao R, Xiao Y, Wang X and Zhou J: Capsaicin mediates caspases
activation and induces apoptosis through P38 and JNK MAPK pathways
in human renal carcinoma. BMC Cancer. 16:7902016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chambard JC, Lefloch R, Pouysségur J and
Lenormand P: ERK implication in cell cycle regulation. Biochim
Biophys Acta. 1773:1299–1310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1- to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG,
Song GY, Kwon KI, Jeong TC and Jeong HG: Suppression of EGF-induced
tumor cell migration and matrix metalloproteinase-9 expression by
capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK,
p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 55:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kuo WH, Chen JH, Lin HH, Chen BC, Hsu JD
and Wang CJ: Induction of apoptosis in the lung tissue from rats
exposed to cigarette smoke involves p38/JNK MAPK pathway. Chem Biol
Interact. 155:31–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie
T, Zhang N and Ye ZM: Celastrol induces apoptosis and autophagy via
the ROS/JNK signaling pathway in human osteosarcoma cells: An in
vitro and in vivo study. Cell Death Dis. 6:e16042015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sánchez AM, Malagarie-Cazenave S, Olea N,
Vara D, Chiloeches A and Díaz-Laviada I: Apoptosis induced by
capsaicin in prostate PC-3 cells involves ceramide accumulation,
neutral sphingomyelinase, and JNK activation. Apoptosis.
12:2013–2024. 2007. View Article : Google Scholar : PubMed/NCBI
|