Introduction
Malignancies remain a major challenge to global
public health (1). One of the most
common head and neck malignancies worldwide, especially in southern
China, Southeast Asia, and North Africa, is nasopharyngeal
carcinoma (NPC) (2). There are
three subtypes of NPC, classified by the World Health Organization,
that include squamous cell carcinoma, non-keratinizing carcinoma
and undifferentiated carcinoma (3).
Radiotherapy is the standard treatment for
early-stage NPC, but chemotherapy is also very important in the
standard treatment of locally advanced and metastatic NPC. The
common chemotherapies used to treat NPC are cisplatin, carboplatin,
5-fluorouracil, gemcitabine, paclitaxel, anthracyclines, bleomycin,
epirubicin and cetuximab (2).
However, one of the major obstacles in successfully treating
advanced NPC is the development of drug resistance, which can
result from a variety of factors, including increased DNA damage
tolerance (4). Because of this
major obstacle that drug resistance poses, new and better treatment
options for NPC are urgently needed.
In the last few decades, natural compounds have
gained increasing attention as a source of potential anticancer
agents. Xanthones are oxygen-containing heterocyclic compounds
widely distributed in various plants and microorganisms. It is well
known that xanthones have remarkable pharmacological effects, such
as anticancer, antioxidant, anti-inflammatory, antidiabetic,
antimicrobial, antithrombotic and hepatoprotective activities
(5–7). Among them, the anticancer potential of
xanthones has drawn an increasing amount of attention. For example,
some xanthones, such as gambogic acid (8–12) and
α-mangostin (13–16), have been shown to exhibit anticancer
properties, including antiproliferation, antiangiogenesis and
antimetastasis.
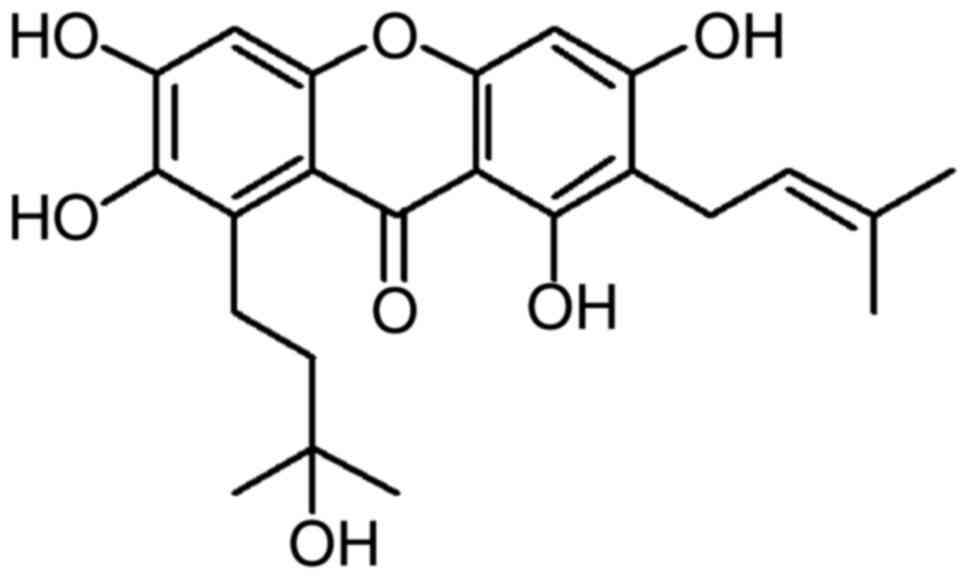
Garcinone C
(C23H26O7, MW 414.5, Fig. 1), a xanthone derivative, is a
natural compound extracted from Garcinia oblongifolia Champ.
(a traditional Chinese medicine) that is used as an
anti-inflammatory, analgesia, astringency and granulation-promoting
medicine. Recently, it was reported that garcinone C exhibits
cytotoxicity against MCF-7, A549, Hep-G2 and CNE human cancer cell
lines in vitro (17).
However, the function and molecular mechanism of this compound in
cell growth and cell cycle progression have not been yet
elucidated. In the present study, CNE1 (well-differentiated
squamous cell carcinoma), CNE2 (non-keratinizing carcinoma), HK1
(well-differentiated squamous cell carcinoma) and HONE1
(undifferentiated carcinoma) NPC cells were treated with different
concentrations of garcinone C and then subjected to biochemical,
microscopic, flow cytometric, and molecular analyses to determine
the efficacy and mechanism of action of garcinone C in NPC.
Materials and methods
Plant material
The bark of Garcinia oblongifolia Champ was
collected from Wuzhishan, Hainan, China in October 2013 and
identified by Dr Xilong Zheng, an expert from the Crops Research
Institute, Guangdong Academy of Agricultural Sciences, Guangzhou,
China. A voucher specimen was deposited in the South China
Botanical Garden, Chinese Academy of Sciences, Guangzhou,
China.
Extraction, isolation, and
identification of garcinone C
The air-dried bark (10.0 kg) was powdered and
extracted three times with 90% ethanol for 48 h per extraction. The
extract was concentrated under vacuum, suspended in 50% ethanol,
and then successively partitioned with petroleum ether, EtOAc. The
EtOAc soluble extract (320.0 g) was subjected to silica gel column
chromatography (100–200 mesh) and eluted with a gradient solvent
system of CCl3-MeOH (from 90:10 to 70:30) to yield 10
sub-fractions, A-J. Sub-fraction E was re-subjected to silica gel
column chromatography (CCl3-MeOH-H2O,
100:11:2) and then purified by Sephadex LH-20 column chromatography
eluted with methanol to yield garcinone C (28 mg).
The physiochemical characteristics of garcinone C
were identified by electron spray ionization mass spectrometry,
1H and 13C nuclear magnetic resonance (NMR)
spectra. Garcinone C (C23H26O7)
(Fig. 1): yellow needles, >98.0%
purity. ESIMS (m/z): 415 [M + H]+, 437 [M +
Na]+, 413 [M-H]−. 1H-NMR (500 MHz,
(CD3)2CO-d6): δ
13.92 (s, OH-1), 1.65 (3H, s, H-14), 1.32 (6H, d, H-19, 20), 1.79
(3H, s, H-15), 1.94–1.82 (2H, m, H-17), 3.35 (2H, d, J=7.2
Hz, H-11), 3.58–3.43 (2H, m, H-16), 5.29 (1H, t, J=7.2 Hz,
H-12), 6.39 (1H, s, H-4), 6.79 (1H, s, H-5). 13C-NMR
(125 MHz, (CD3)2CO-d6):
δ161.2 (C-1), 110.7 (C-2), 162.7 (C-3), 92.9 (C-4), 155.8 (C-4a),
101.0 (C-5), 153.9 (C-6), 141.3 (C-7), 131.0 (C-8), 111.8 (C-8a),
183.0 (C-9), 103.6 (C-9a), 153.2 (C-10a), 21.9 (C-11), 123.5
(C-12), 131.3 (C-13), 25.8 (C-14), 17.8 (C-15), 22.5 (C-16), 44.1
(C-17), 71.1 (C-18). Garcinone C was dissolved in dimethylsulfoxide
(DMSO) to prepare 20 mM garcinone C stock solution.
Cell culture
Human NPC cell lines CNE1, CNE2, HK1, and HONE1 were
a kind gift of Dr Chao-Nan Qian (Department of Nasopharyngeal
Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China).
Cells were cultured at 37°C, in a humidified atmosphere with 5%
CO2, in Dulbecco's modified Eagles medium supplemented
with 10% fetal bovine serum (FBS), 1% L-glutamine, 100 U/ml
penicillin, 100 µg/ml of streptomycin and 0.25 µg/ml of
Fungizone® (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The cells were harvested by trypsinization and
plated 24 h before treatment with garcinone C or paclitaxel.
MTS assay for cell viability
CNE1, CNE2, HK1 and HONE1 cells were plated in
96-well plates and 24 h later, they were treated with 1.25, 1.875,
2.5, 3.75, 5, 7.5, 10, 15 and 20 µM of garcinone C or 1/1,000 DMSO
for 24, 48 and 72 h. As a positive control, CNE1 and HONE1 cells
were treated with or without paclitaxel for 24, 48 and 72 h. Cell
viability was measured using Promega's CellTiter 96
AQueous One Solution Cell Proliferation Assay (Promega,
Madison, WI, USA) and read using a Multiskan GO Microplate
spectrophotometer (Thermo Fisher Scientific) at 490 nm. The cell
viability ratio was calculated according to the following equation:
cell viability (%) = treated group/control group × 100%. The
half-maximal inhibitory concentration (IC50) was
calculated using the Bliss method. The results were estimated from
the data of six technical replicates and three biological
replicates.
Colony formation assay
Cells were diluted and seeded at 500 cells per well
in 6-well plates. The following day, CNE1 and HK1 cells were
treated with 0.625 and 0.9375 µM garcinone C or DMSO, and CNE2 and
HONE1 cells were treated with 1.25 and 2.5 µM garcinone C or DMSO.
Medium was replaced with fresh medium containing garcinone C every
4 days. After incubation for 10 days, colonies were fixed with 4%
paraformaldehyde for 20 min, visualized with crystal violet, and
then scanned with Canon CanoScan 9000F Color Image Scanner. Visible
colonies were counted. Colony formation rate (%) = number of
colonies/number of seeded cells × 100%.
Flow cytometry analysis of
apoptosis
CNE1 and CNE2 cells were seeded in 25-cm2
flasks and treated with 5, 7.5 and 10 µM of garcinone C or 1/2,000
dimethyl sulfoxide (DMSO) for 24, 48 and 72 h. Harvested cells were
washed with Dulbecco's phosphate-buffered saline (DPBS) and stained
with Annexin V and propidium iodide (PI), according to the
instructions of the FITC Annexin V Apoptosis Detection kit I (BD
Biosciences, San Diego, CA, USA). Apoptotic cells were quantified
by BD Accuri C6 Flow Cytometer (BD Biosciences). Stained cell
populations were defined as: lower left quadrant, living cells
(Annexin V−/PI−); upper left quadrant,
primary necrotic cells (Annexin V−/PI+);
lower right quadrant, early apoptotic cells (Annexin
V+/PI−); upper right quadrant, late apoptotic
cells (Annexin V+/PI+). Approximately 50,000
events were collected per sample. Apoptotic cell percentage =
[upper right quadrant (UR) + lower right quadrant (LR)] %.
Cell cycle analysis
CNE1, CNE2, HK1 and HONE1 cells were treated with 5,
7.5 and 10 µM of garcinone C or 1/2,000 DMSO for 24, 48 and 72 h.
Harvested cells were washed with DPBS and stained with PI according
to the protocol of the Cycletest™ Plus DNA Reagent kit (BD
Biosciences). Cell cycle distribution was analyzed using the BD
Accuri C6 flow cytometer. Approximately 30,000 events were
collected per sample. The results were further analyzed using
ModFit LT software (Verity Software House Inc., Topsham, ME,
USA).
Transmission electron microscopy
(TEM)
To investigate and study the cell damage caused by
garcinone C, CNE1 and HONE1 cells were respectively cultured with
10 µM garcinone C or DMSO for 24, 48 and 72 h. After washing with
DPBS, the cells were fixed with 1 ml of 3% glutaraldehyde and
stored at 4°C. Cells were then washed three times with
phosphate-buffered saline (PBS) and further postfixed with 1%
osmium tetroxide for 1–2 h. After rinsing three times with PBS, the
cells were dehydrated in a graded series of 50, 70 and 90% ethanol
and then 90% ethanol:90% acetone (1:1), 90% acetone, and three
times with 100% acetone. After dehydration, the cells were embedded
in epoxy resin 618 for sectioning. Ultrathin sections were obtained
using a Leica EM UC7 ultramicrotome (Leica Microsystems, Buffalo
Grove, IL, USA). After staining with uranyl acetate and lead
citrate, the sections were examined and photographed with a Hitachi
H-7650 transmission electron microscope (Hitachi, Tokyo,
Japan).
Protein isolation and western blot
analysis
Cells were treated with 5, 7.5 and 10 µM of
garcinone C for 24, 48 and 72 h. Cell lysates were prepared in RIPA
lysis buffer supplemented with a protease inhibitor cocktail,
Phenylmethylsulfonyl fluoride, and Phosphatase Inhibitors Cocktail
2 and 3, and then stored at 4°C overnight. Total lysates were
centrifuged for 20 min, at 13,523 × g at 4°C, and the supernatant
was collected and stored at −80°C. The quantitative analysis of
protein was determined using the Pierce BCA Protein Assay Reagent
Kit (Thermo Fisher Scientific, Rockford, IL, USA) and 50 µg of
protein was used for SDS-PAGE electrophoresis. Antibodies against
cyclin B1 (#4138), cyclin D1 (#2926), cyclin E2 (#4132), cdc2
(#9112), CDK7 (#2916), GAPDH (#2118), Atg3 (#3415), AKT (#9272),
Stat5 (#9358), Stat3 (#9139), 4E-BP1 (#9644), mTOR (#2972), GSK-3β
(#9315), AMPKα (#2532), Histone H2B (#12364), anti-rabbit IgG
(#7074), and anti-mouse IgG (#7076) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Anti-DNA PKcs [Y393]
(ab32566) and anti-ATR antibodies (ab10312) were purchased from
Abcam (Cambridge, MA, USA). GAPDH was used as an equal loading
control. Thermo Scientific Pierce ECL Western Blotting Substrate
(Rockford, IL, USA) and Kodak X-OMAT BT Film (Kodak, Rochester, NY,
USA) were used to detect the bound immune complexes. The films were
then developed by a Kodak X-OMAT 2000 Processor and scanned with a
Canon CanoScan 9000F Color Image Scanner (Canon, Melville, NY,
USA).
Statistical analysis
All experiments were repeated at least three times.
Statistical significance of the differences between two samples was
evaluated by the unpaired two-tailed Student's t-test. Significance
was established at *P<0.05 or **P<0.01. Statistical
significance of the differences among samples was assessed by
one-way analysis of variance followed by the least significant
difference and Student-Neumann-Keuls post hoc comparison. The
analyses were performed with SPSS 13.0 software (SPSS Inc.,
Chicago, IL, USA). The threshold of significance was defined as
P<0.05.
Results
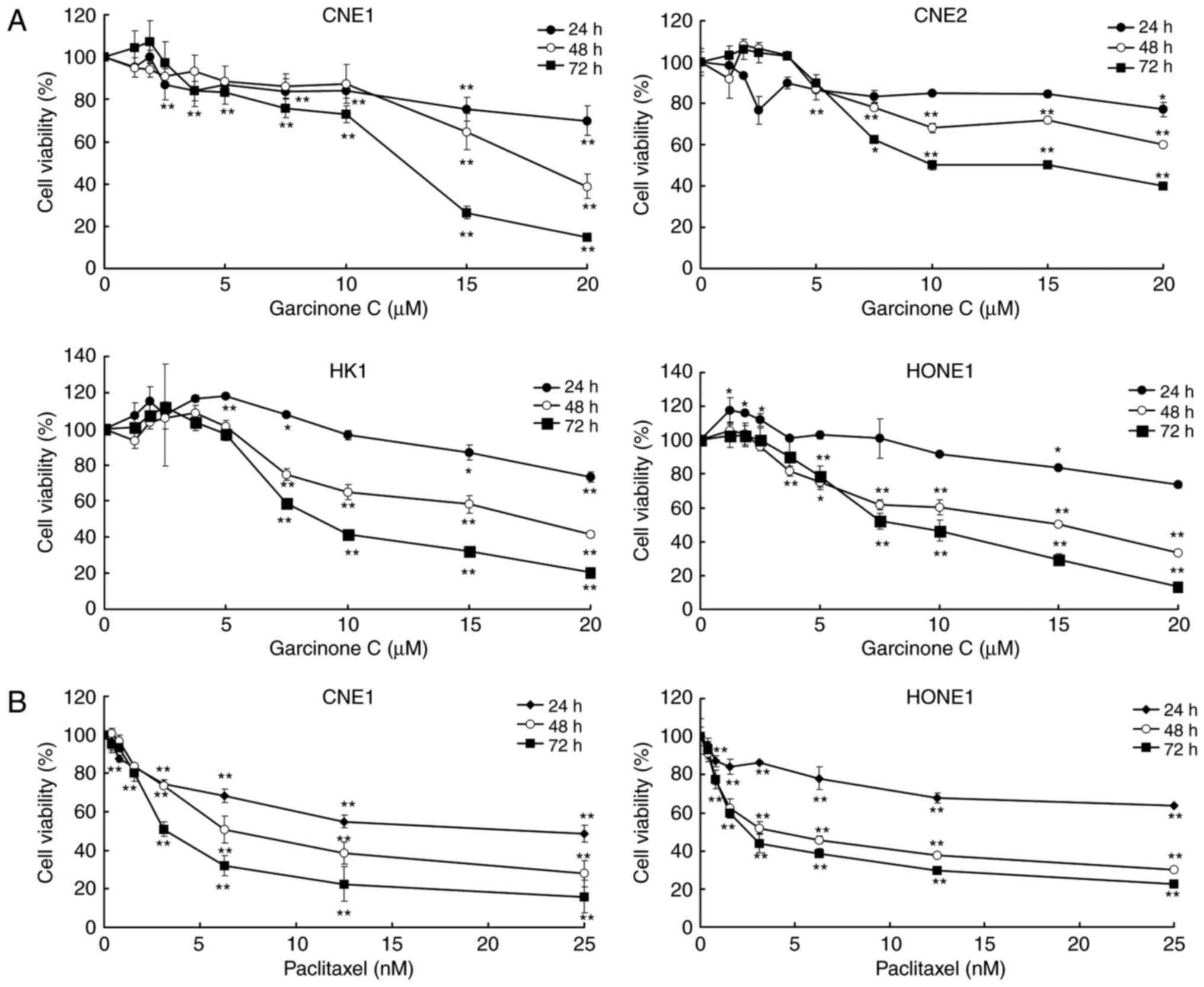
Garcinone C inhibits the cell
viability of human NPC cells in a time- and dose-dependent
manner
To investigate the effect of garcinone C on the
viability of the human NPC cell lines CNE1, CNE2, HK1 and HONE1,
the cells were treated with garcinone C at a concentration ranging
from 0 to 20 µM for 24, 48 and 72 h. After exposure to garcinone C,
cell viability was determined using an MTS assay. The results
showed that garcinone C treatment inhibited cell viability in these
four cell lines in a time- and dose-dependent manner (Fig. 2). The IC50 value of
garcinone C after 72 h of incubation was 10.68±0.89, 13.24±0.20,
9.71±1.34 and 8.99±1.15 µM for CNE1, CNE2, HK1 and HONE1 cells,
respectively. Paclitaxel, an antitumor drug that is known to
inhibit cell viability, was used as a positive control for the MTS
assay. As shown in Fig. 2,
paclitaxel robustly inhibited cell viability in the CNE1 and HONE1
cells, in a time- and dose-dependent manner, with a 72 h
IC50 of 5.72±1.43 and 3.5±0.80 nM.
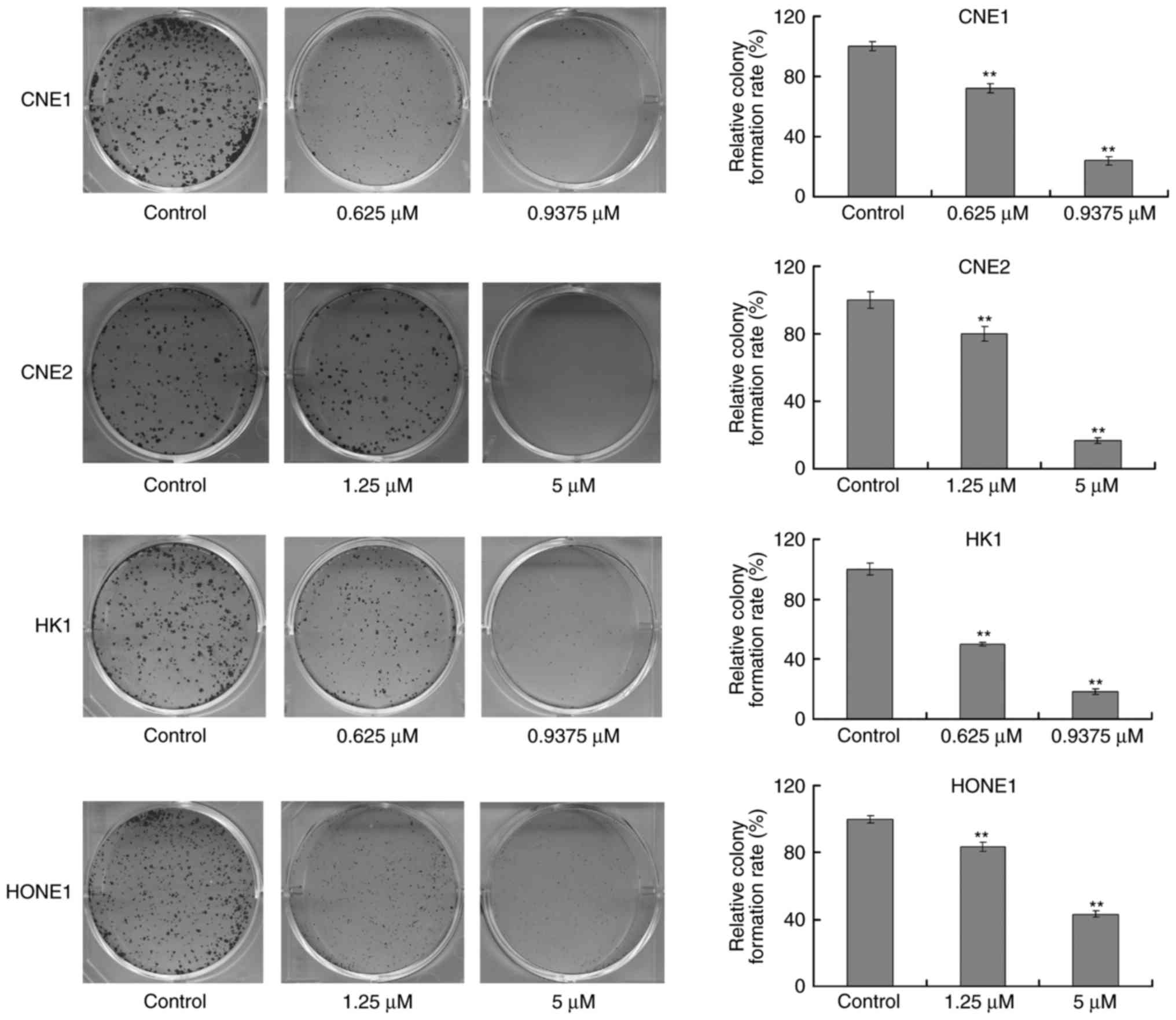
Garcinone C reduces the
colony-formation ability of human NPC cells
To further demonstrate the anticancer effects of
garcinone C, the colony formation ability of NPC cells after
treatment with garcinone C was assessed. CNE1 and HK1 cells were
treated with 0.625 or 0.9375 µM of garcinone C for 10 days, while
CNE2 and HONE1 cells were treated with 1.25 or 5 µM of garcinone C
for 10 days. The results demonstrated that garcinone C
significantly delayed the ability of NPC cells to form colonies
(P<0.01), in a dose-dependent manner (Fig. 3).
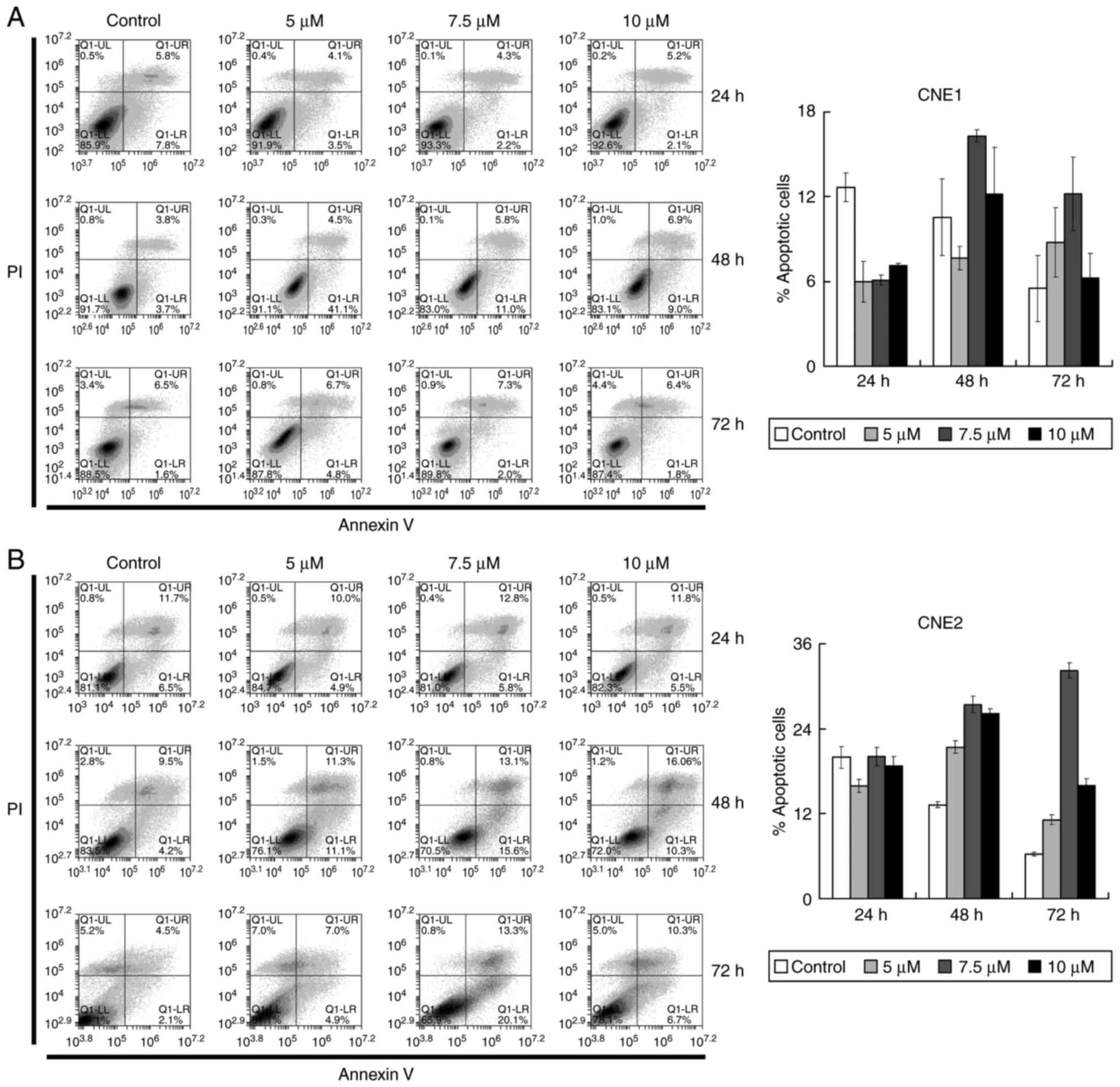
Garcinone C slightly induces the
apoptosis of NPC cells
Having found that garcinone C caused inhibition of
cell growth, we investigated whether this was due to the induction
of apoptosis in the CNE1 and CNE2 cells. Cells were treated with 5,
7.5 and 10 µM of garcinone C or DMSO (control) for 24, 48 and 72 h.
Subsequently, the cells were stained with Annexin V and PI, and
subjected to flow cytometry to quantify the amount of apoptotic
cells. The results revealed that garcinone C caused a slight
increase in the apoptotic cell population in CNE1 and CNE2 cells
(Fig. 4). However, the modest
increase in the number of apoptotic cells after garcinone C
treatment suggest that apoptosis may not be the major reason for
the cell growth inhibition.
Garcinone C induces S phase cell cycle
arrest
To assess whether garcinone C induces cell growth
inhibition via alterations in cell cycle progression, we evaluated
the effect of garcinone C on the cell cycle. As shown in Fig. 5, there was a significant decrease in
the proportion of cells in the G0/G1 phase,
and a significant increase in the proportion of cells in the S
phase after cells were treated with garcinone C, vs. the control.
For example (Fig. 5B), the majority
of control-treated CNE2 cells were in the
G0/G1 phase (53.38±0.55%), with a
G2/M-phase fraction of 18.45±0.38%, and the remaining
control cells (28.17±0.93%) were found to be in the S phase.
However, garcinone C treatment produced a shift in the DNA content
histogram. The G0/G1 phase fraction decreased
significantly to 47.23±0.93% after 24 h of 10 µM garcinone C
treatment, 14.33±0.25% after 48 h of 10 µM garcinone C treatment,
and 4.03±0.08% after 72 h of 10 µM garcinone C treatment. The
decrease in the G0/G1-phase fraction was
offset by a rise in the S-phase fraction, which increased from
28.17±0.93 to 42.99±0.88% after 24 h of treatment, 71.47±0.45%
after 48 h of treatment, and 71.73±0.15% after 72 h of treatment.
These results indicated that garcinone C efficiently inhibited the
cell division by arresting cells in the S phase, suggesting that
the inhibition of NPC cell proliferation by garcinone C is the
result of cell cycle blockade.
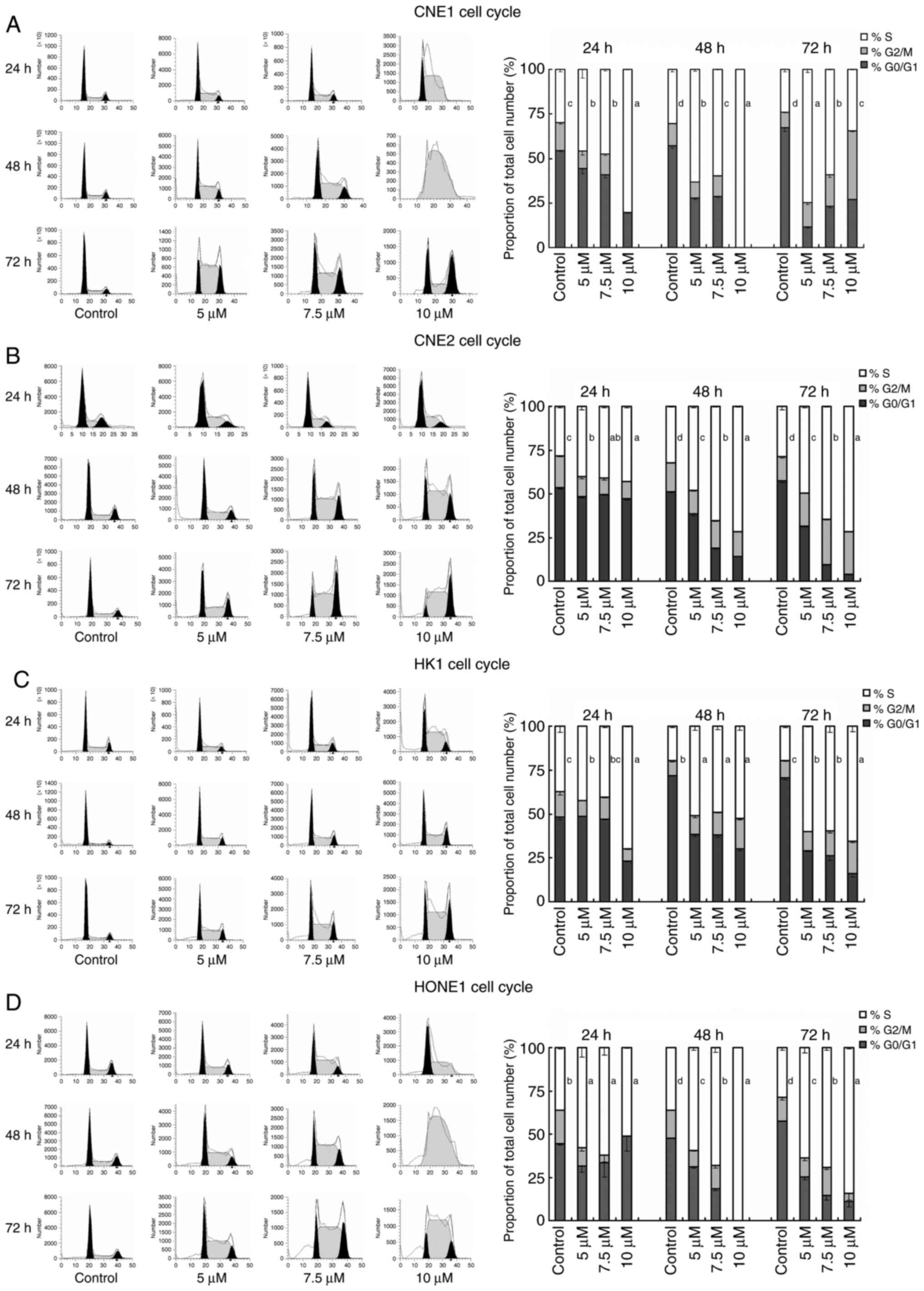 | Figure 5.Induction of CNE1, CNE2, HK1, and
HONE1 cell cycle arrest by garcinone C. The population distribution
of four cell lines was assessed by cell cycle analysis using PI
staining and flow cytometry. Right panel, the percentage of cells
in the G0/G1, S and G2/M phases of
the cell cycle 24, 48 and 72 h after 5, 7.5, and 10 µM garcinone C
treatment (n=3; error bars represent mean ± SD; a different letter
on the right of the S phase column indicates a significant
difference between different concentrations of garcinone C;
P<0.05, one-way analysis of variance, and least significant
difference/Student-Neumann-Keuls post hoc test). Left panel,
representative flow cytometry profiles from three independent
experiments for each condition. |
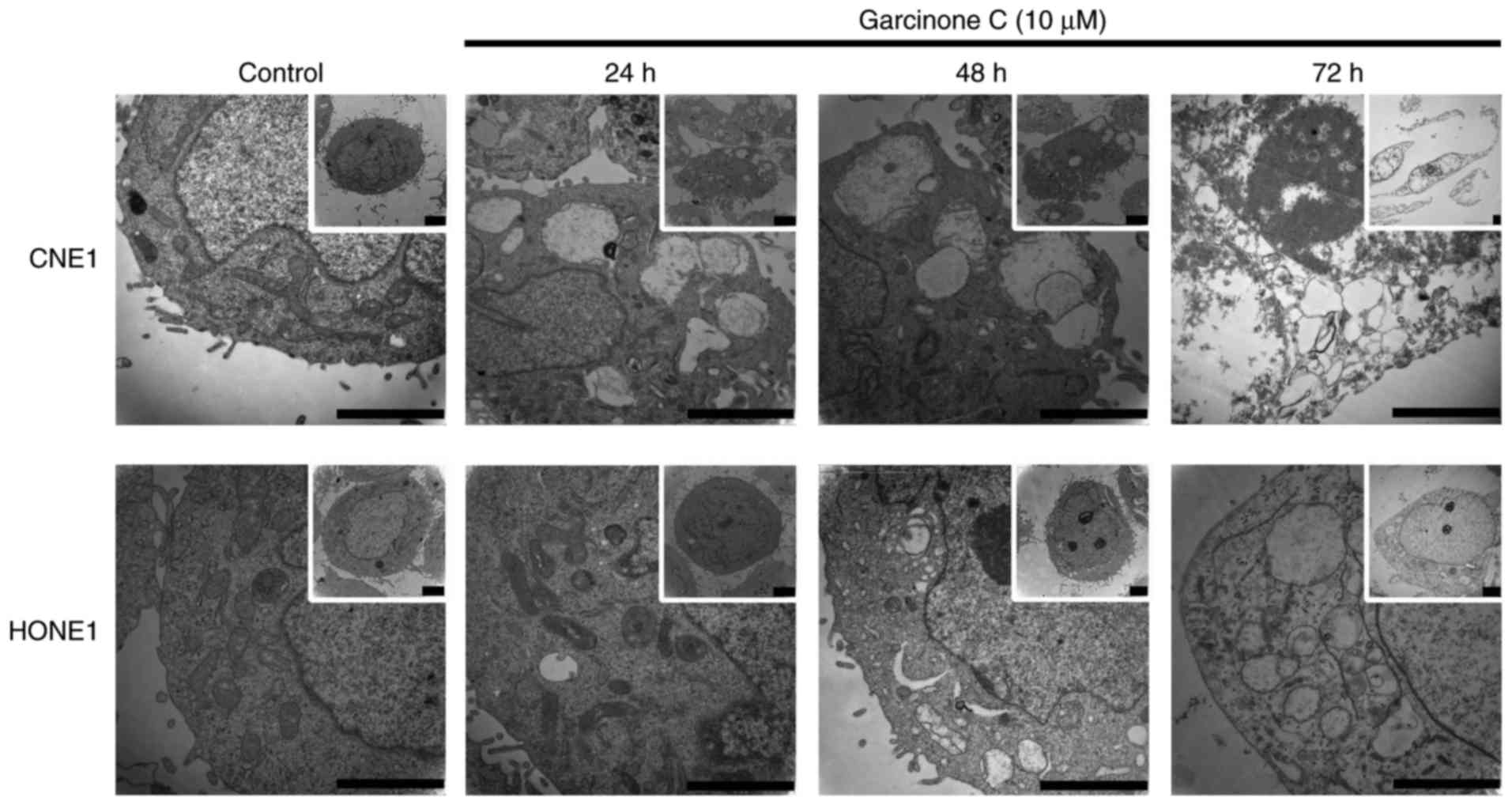
High-dose garcinone C induces cell
necrosis in a time-dependent manner
Next, we examined cells for ultrastructural
differences using TEM. As shown in Fig.
6, DMSO-treated control CNE1 and HONE1 cells exhibited normal
morphology, with microvilli on the cell surface, abundant
mitochondria and ribosomes. Upon exposure to 10 µM of garcinone C,
cells exhibited a time-dependent increase in necrotic morphology,
including cell swelling, rough endoplasmic reticulum degranulation,
endoplasmic reticulum dilatation, mitochondrial swelling, vacuolar
degeneration and loss of microvilli.
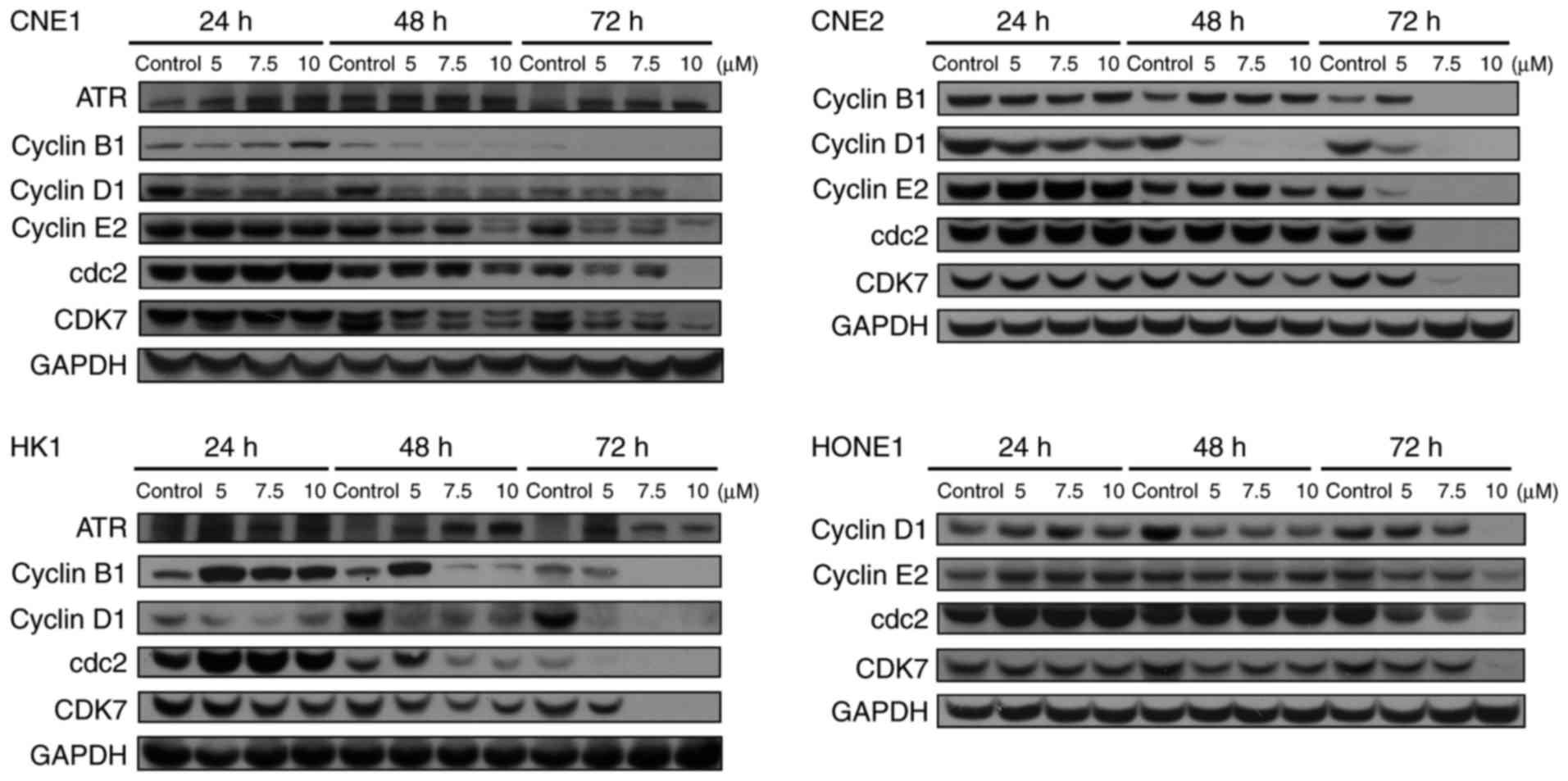
Garcinone C affects the expression
levels of cell cycle-related proteins
Having noted that garcinone C induces cell cycle
arrest in NPC cells, we investigated whether garcinone C could
regulate the expression levels of cell cycle-related proteins. In
agreement, a decrease in cyclin B1, cyclin D1, cyclin E2, cdc2 and
CDK7 expression was observed in a dose- and time-dependent manner
after treatments of 5, 7.5 and 10 µM garcinone C for 24, 48 and 72
h in CNE1, CNE2, HK1 and HONE1 cells (Fig. 7). Additionally, an increase in
ataxia telangiectasia and Rad3 related protein (ATR) expression was
observed in CNE1 and HK1 cells. Together, our data indicated that
garcinone C induced cell cycle arrest via the downregulation of
cyclin B1, cyclin D1, cyclin E2, cdc2 and CDK7, presumably through
ATR activation.
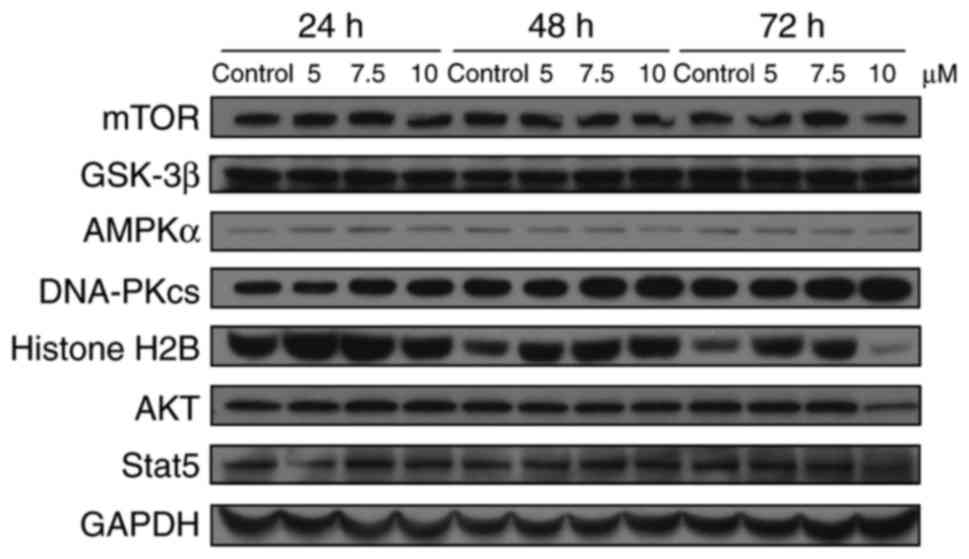
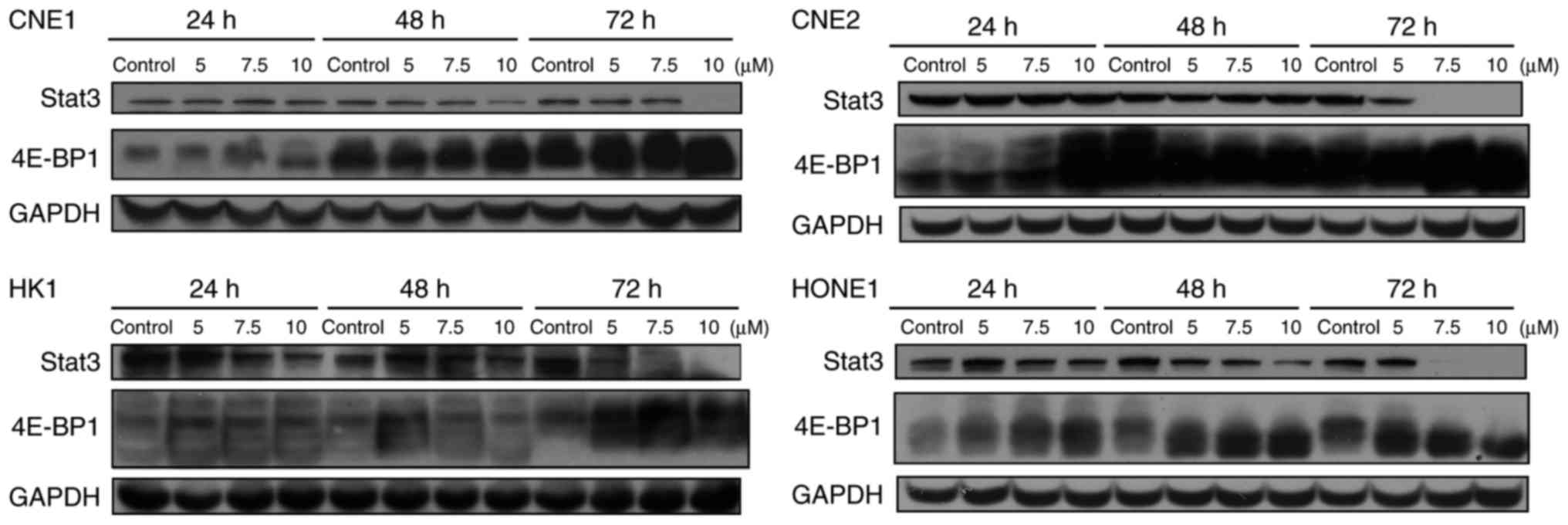
Garcinone C decreases the expression
level of Stat3 and increases the expression level of 4E-BP1
To further evaluate the molecular mechanisms
involved in the anticancer activity of garcinone C, we assessed the
involvement of several key signal transduction pathways implicated
in tumor progression by western blot analysis. As shown in Fig. 8, our results showed that treatment
of CNE1 cells with garcinone C did not significantly affect the
protein levels of mTOR, GSK-3β, AMPKα, DNA-dependent protein
kinase, catalytic subunit (DNA-PKcs), Histone H2B, AKT, or Stat5 in
a time- or dose-dependent manner. However, we noted a decrease in
the levels of Stat3 and an increase in the levels of 4E-BP1 in the
four NPC cell lines treated with garcinone C (Fig. 9).
Discussion
In the present study, we demonstrated that garcinone
C significantly inhibited the cell proliferation and
colony-formation ability of NPC cells in vitro, in a dose-
and time-dependent manner. Mechanistically, the inhibitory effect
of garcinone C on cell growth could be ascribed to the arrest of
the cell cycle in the S phase.
DNA damage checkpoints play an important role in
normal nasopharyngeal epithelial cells by protecting them from
genome instability and tumorigenesis. In NPC, targeting activation
of the DNA damage checkpoint is a major therapeutic strategy
(18). Moreover, increased DNA
damage tolerance is one of the reasons for the development of drug
resistance (19). In this study, we
detected the expression levels of DNA damage-related proteins, ATR
and DNA-PKcs after treatments with garcinone C. The results
revealed that garcinone C induced the expression level of ATR, but
did not effect the expression level of DNA-PKcs. ATR is a
phosphatidylinositol 3 kinase-related kinase (PIKK) family member
that is activated in response to sensing DNA damage/replication
blocks and triggers the DNA damage checkpoint, leading to the
arrest of cell cycle progression (20). Although cell growth and cell cycle
inhibition are complex processes, which involve many pathways,
activation of the DNA damage checkpoint by ATR accumulation could
be one of the mechanisms responsible for the ability of garcinone C
to inhibit cell growth and induce cell cycle arrest. Therefore,
garcinone C might be a new compound for targeting the DNA damage
checkpoint in NPC cells and could be a novel drug candidate to use
in combination with other agents to enhance the antitumor activity
of other chemotherapies.
Cells die through two main processes: necrosis,
defined as cell death due to unexpected and accidental cell damage,
or apoptosis. In this study, tumor cell necrosis, morphologically
characterized by a gain in cell volume, swelling of organelles, and
subsequent loss of intracellular contents, was detected within 72 h
of 10 µM high-dose garcinone C treatment. However, the presence of
necrosis can only indicate that a cell has died, but not
necessarily how death occurred (21). Moreover, necrotic pathways are
poorly defined and require further elucidation. Therefore, our data
indicate that garcinone C-induced NPC cell necrosis is associated
with S phase cell cycle arrest, but further investigation is
required.
The signal transducer and activator of transcription
(Stat) family, including Stat1-6, regulates many aspects of cell
growth, survival and differentiation. In particular, Stat3 is
constitutively activated in many different types of cancer, plays a
pivotal role in tumor growth, and possesses oncogenic potential
(22,23). In this study, we found that
garcinone C did not affect the expression level of Stat5, but
significantly inhibited the expression of Stat3 in four NPC cell
lines, in a time- and dose-dependent manner. Thus, our findings
suggested that garcinone C inhibits NPC cell growth through a
mechanism that likely involves Stat3 inhibition.
The eukaryotic translation initiation factor 4E
(elF4E)-binding protein 1 (4E-BP1), a translation repressor
protein, contributes to the inhibition of protein synthesis and
growth inhibition of tumor cells (24). Consistent with our results, it has
been reported that some natural compounds could enhance the
expression of 4E-BP1 and trigger tumor cell death (25). 4E-BP1 is also regulated by
AKT/GSK-3β/AMPKα/mTOR signaling pathways (26). However, our western blot results
showed that although garcinone C upregulated the expression of
4E-BP1, there was no effect on the expression level of AKT, GSK-3β,
AMPKα, or mTOR, which suggested that garcinone C might target
4E-BP1 via an AKT/GSK-3β/AMPKα/mTOR-independent pathway.
In conclusion, garcinone C treatment was able to
decrease the cell viability and colony-formation ability in CNE1
(well-differentiated squamous cell carcinoma), CNE2
(non-keratinizing carcinoma), HK1 (well-differentiated squamous
cell carcinoma) and HONE1 (undifferentiated carcinoma) NPC cells.
Furthermore, garcinone C was able to induce S phase cell cycle
arrest in all NPC cell lines, and downregulate the expression
levels of cell cycle-related proteins cyclin B1, cyclin D1, cyclin
E2, cdc2 and CDK7, presumably through ATR activation. Our findings
also demonstrated that garcinone C decreased the expression level
of Stat3 and increased the expression level of 4E-BP1. Moreover, a
high-dose of garcinone C induced necrosis in NPC cells. Taken
together, these results revealed that the potential use of
garcinone C as a chemotherapeutic agent in NPC warrants further
investigation.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China Key Project 81130046 (to J.Z.),
NSFC81171993 (to Yi Lu), NSFC81272415 (to Yi Lu), NSFC81660606 (to
X.L.), and NSFC81460397 (to X.L.); China Postdoctoral Science
Foundation 2014M552535XB (to X.L.); Guangxi Key Projects
2013GXNSFEA053004 (to J.Z.); Natural Science Foundation of Guangxi
1355004-5 (to J.Z.), 2012GXNSFCB053004 (to Yi Lu),
2014GXNSFBA118155 (to X.L.), and 2015GXNSFAA139151 (to X.L.);
Guangxi Ministry of Education 201202ZD022 (to Yi Lu) and
201201ZD004 (to J.Z.).
References
|
1
|
Zou M, Zhang X and Xu C: IL6-induced
metastasis modulators p-STAT3, MMP-2 and MMP-9 are targets of
3,3′-diindolylmethane in ovarian cancer cells. Cell Oncol (Dordr).
39:47–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guigay J, Temam S, Bourhis J, Pignon JP
and Armand JP: Nasopharyngeal carcinoma and therapeutic management:
The place of chemotherapy. Ann Oncol. 17 Suppl 10:x304–x307. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shanmugaratnam K: Histological typing of
nasopharyngeal carcinoma. IARC Sci Publ. 1–12. 1978.
|
|
4
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Downregulation of NEK11 is associated with drug resistance in
ovarian cancer. Int J Oncol. 45:1266–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Kuang L, Pan X, Liu J, Wang Q, Du
B, Li D, Luo J, Liu M, Hou A and Qian M: Isoalvaxanthone inhibits
colon cancer cell proliferation, migration and invasion through
inactivating Rac1 and AP-1. Int J Cancer. 127:1220–1229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aisha AF, Abu-Salah KM, Ismail Z and Majid
AM: In vitro and in vivo anti-colon cancer effects of Garcinia
mangostana xanthones extract. BMC Complement Altern Med.
12:1042012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panda SS, Chand M, Sakhuja R and Jain SC:
Xanthones as potential antioxidants. Curr Med Chem. 20:4481–4507.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi T, Yi Z, Cho SG, Luo J, Pandey MK,
Aggarwal BB and Liu M: Gambogic acid inhibits angiogenesis and
prostate tumor growth by suppressing vascular endothelial growth
factor receptor 2 signaling. Cancer Res. 68:1843–1850. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rong JJ, Hu R, Song XM, Ha J, Lu N, Qi Q,
Tao L, You QD and Guo QL: Gambogic acid triggers DNA damage
signaling that induces p53/p21Waf1/CIP1 activation
through the ATR-Chk1 pathway. Cancer Lett. 296:55–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X and Chen W: Gambogic acid is a
novel anti-cancer agent that inhibits cell proliferation,
angiogenesis and metastasis. Anticancer Agents Med Chem.
12:994–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi X, Chen X, Li X, Lan X, Zhao C, Liu S,
Huang H, Liu N, Liao S, Song W, et al: Gambogic acid induces
apoptosis in imatinib-resistant chronic myeloid leukemia cells via
inducing proteasome inhibition and caspase-dependent Bcr-Abl
downregulation. Clin Cancer Res. 20:151–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Lei Y, Yuan P, Li L, Luo C, Gao
R, Tian J, Feng Z, Nice EC and Sun J: ROS-mediated autophagy
induced by dysregulation of lipid metabolism plays a protective
role in colorectal cancer cells treated with gambogic acid. PLoS
One. 9:e964182014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata MA, Iinuma M, Morimoto J, Kurose
H, Akamatsu K, Okuno Y, Akao Y and Otsuki Y: α-Mangostin extracted
from the pericarp of the mangosteen (Garcinia mangostana Linn)
reduces tumor growth and lymph node metastasis in an
immunocompetent xenograft model of metastatic mammary cancer
carrying a p53 mutation. BMC Med. 9:692011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jittiporn K, Suwanpradid J, Patel C, Rojas
M, Thirawarapan S, Moongkarndi P, Suvitayavat W and Caldwell RB:
Anti-angiogenic actions of the mangosteen polyphenolic xanthone
derivative α-mangostin. Microvasc Res. 93:72–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, Li
X, Han L, Li W, Sun H, et al: α-Mangostin inhibits hypoxia-driven
ROS-induced PSC activation and pancreatic cancer cell invasion.
Cancer Lett. 347:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li P, Tian W and Ma X: Alpha-mangostin
inhibits intracellular fatty acid synthase and induces apoptosis in
breast cancer cells. Mol Cancer. 13:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu M, Qiu SX, Xu Y, Wu J, Chen Y, Yu Y and
Xiao G: A new xanthone from the pericarp of Garcinia mangostana.
Nat Prod Commun. 8:1733–1734. 2013.PubMed/NCBI
|
|
18
|
Poon RY: DNA damage checkpoints in
nasopharyngeal carcinoma. Oral Oncol. 50:339–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson SW, Ozols RF and Hamilton TC:
Mechanisms of drug resistance in ovarian cancer. Cancer. 71(2
Supp1): S644–S649. 1993.
|
|
20
|
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fink SL and Cookson BT: Apoptosis,
pyroptosis, and necrosis: Mechanistic description of dead and dying
eukaryotic cells. Infect Immun. 73:1907–1916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao Q, Zeng Z, Guo X, Li X, Wei F, Zhang
W, Li X, Chen P, Liang F, Xiang B, et al: LPLUNC1 suppresses
IL-6-induced nasopharyngeal carcinoma cell proliferation via
inhibiting the Stat3 activation. Oncogene. 33:2098–2109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Constantinou C, Elia A and Clemens MJ:
Activation of p53 stimulates proteasome-dependent truncation of
eIF4E-binding protein 1 (4E-BP1). Biol Cell. 100:279–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakravarthy R, Clemens MJ, Pirianov G,
Perdios N, Mudan S, Cartwright JE and Elia A: Role of the eIF4E
binding protein 4E-BP1 in regulation of the sensitivity of human
pancreatic cancer cells to TRAIL and celastrol-induced apoptosis.
Biol Cell. 105:414–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma BB, Lui VW, Hui CW, Lau CP, Wong CH,
Hui EP, Ng MH, Tsao SW, Li Y and Chan AT: Preclinical evaluation of
the AKT inhibitor MK-2206 in nasopharyngeal carcinoma cell lines.
Invest New Drugs. 31:567–575. 2013. View Article : Google Scholar : PubMed/NCBI
|