Introduction
Lung cancer is one of the common malignant tumors,
accounting for 12% of all primarily diagnosed cancer cases, and is
established as the main cause of cancer-associated mortality
worldwide (1,2). Non-small cell lung cancer (NSCLC) is a
prominent type of lung cancer, comprising 85% of all lung cancer
cases worldwide (3,4). Although therapeutic strategies
including targeted therapy, immunotherapy and traditional
chemotherapy have achieved considerable success in improving the
prognosis of patients with NSCLC over the past decades, the
majority of patients still suffer from local aggravation and/or
systemic metastasis, and do not survive >5 years following
diagnosis (5). Therefore, it is
essential to identify novel therapeutics that are capable of
significantly elevating the 5-year survival rate while causing
little side-effects in NSCLC patients.
With advances in research on Traditional Chinese
Medicine (TCM), many agents extracted from natural plants have
attracted increasing levels of public attention in recent years for
their apparent favorable pharmacokinetic characteristics and mild
side-effects. Gallic acid (3,4,5-trihydroxybenzoic acid; GA), a
natural phenolic compound, is one such plant extract that is
present in abundance in tea, grapes, gall-nuts and red wine
(6,7). It has been reported to possess various
pharmacological and biological properties, including antibacterial,
antiviral and antitumor activities. Recently, there has been an
increased research focus on the antitumor capacity of GA in
different cancer cell lines, including oral, lung, pancreatic and
cervical cancer cells (8,9), and it is thought that regulation of
apoptosis may be critically involved in the antitumor effects of
GA. However, understanding of how GA induces cell apoptosis is
still limited.
Signal transducer and activator of transcription 3
(STAT3) is a member of the STAT transcription factor family, which
has been associated with various biological processes, including
cell growth, survival and metastasis (10). STAT3 mainly exists in the cytoplasm,
where it can be phosphorylated at Tyr705 via Janus kinase
(JAK)-mediated tyrosine phosphorylation when stimulated by
cytokines. The phosphorylated STAT3 translocates into the nucleus,
combines with DNA sites, and regulates various cellular processes,
including cell apoptosis and proliferation (11,12).
Persistent activation of STAT3 has been observed in >70% of
solid and hematological tumors, which may be one of the most
notable differences between normal and malignant cells (13). Additionally, a previous study
reported that aberrant activation of STAT3 is present in the
majority of NSCLC cell lines and ~55% of NSCLC patients (14), indicating a potential association
between STAT3 activation and NSCLC development. However, further
investigation is required in order to fully illustrate how STAT3
expression is associated with NSCLC development.
Taking these findings into consideration, the
present study hypothesized and investigated whether GA exerts its
anticancer effects on NSCLC A549 cells by modulating the
phosphorylation of JAK1 and STAT3, and the expression of downstream
apoptotic molecules, including B-cell lymphoma 2 (Bcl-2) and
Bcl-2-associated X protein (Bax). Notably, it was also evaluated
and confirmed that GA facilitates the anticancer effects of
cisplatin in A549 cells by regulating the JAK/STAT3 signaling
pathway.
Materials and methods
Materials
The human NSCLC cell line A549 was purchased from
Shanghai Bioleaf Biotech Co., Ltd. (Shanghai, China). GA of purity
>98% was purchased from Shanghai Source Biological Technology
Co., Ltd. (Shanghai, China). Primary antibodies against JAK1,
STAT3, p-STAT3Tyr705, Bax, Bcl-2, β-actin and GAPDH, and
the secondary antibody anti-rabbit horseradish peroxidase
(HRP)-immunoglobulin (Ig) G were acquired from Wuhan Boster
Biological Technology Co., Ltd. (Wuhan, China). The antibody
against phosphorylated (p)-JAK1Y1022 was acquired from
Elabscience Biotechnology Co., Ltd (Chengdu, China). An MTT Cell
Proliferation and Cytotoxicity Assay kit, Annexin V-fluorescein
isothiocyanate (FITC) Apoptosis Detection kit, RPMI-1640 medium,
penicillin-streptomycin liquid, trypsin-EDTA solution (0.25%) with
phenol red and crystal violet were obtained from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China). Fetal bovine
serum (FBS) was purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Cell culture
The A549 cell line was maintained in RPMI-1640
medium containing 10% FBS, 100 U/ml penicillin and 0.1 mg/ml
streptomycin, in a humidified incubator at 37°C with 5%
CO2. Cells at logarithmic phase were used in the
following experiments.
Cell viability assay
An MTT assay was performed to evaluate the effects
of cisplatin (Jiangsu Haosen Pharmaceutical Group, Co., Ltd.,
Jiangsu, China), GA and their combination on cell viability. A549
cells were seeded in 96-well plates at a density of
2×104 cells/well. The cells were treated at 37°C with GA
[varied dose (0–52 µg/ml) of GA for 24 h, or 12, 20 and 28 µg/ml GA
for 6, 24 and 48 h], cisplatin [varied dose (0–32 µg/ml) of
cisplatin for 24 h] or the two compounds combined (2.5 µg/ml of
cisplatin, 28 µg/ml of GA or the two combined for 6, 12, 24 and 48
h) when the cells reached 80% confluency. The medium was removed
following 6–24 h of incubation, 10 µl MTT (5 mg/ml) was added to
each well, and the cells were incubated for a further 4 h. The
medium of each well was then removed and replaced by 110 µl
dimethylsulfoxide at the end of the incubation. Finally, the
absorbance of each well at 492 nm was measured with a
spectrophotometer (Thermo Fisher Scientific, Inc.) and cell
viability was evaluated by analyzing the absorbance of each
group.
Cell apoptosis assay
A549 cells were seeded into 6-well plates at a
density of 2×105/well and divided into the following
groups: i) Control group (Control), treated with normal medium; ii)
GA group (GA), treated with 12–28 µg/ml GA; iii) cisplatin group
(Pt 2.5), treated with 2.5 µg/ml cisplatin; and iv) GA + cisplatin
group (Pt 2.5 + GA28), treated with 2.5 µg/ml cisplatin + 28 µg/ml
GA. Cells from each group were treated at 37°C for 6 and 24 h,
respectively. Cells were collected via centrifuged at 300 × g at
37°C for 5 min and washed 3 times with cold phosphate-buffered
saline (PBS) and then suspended in binding buffer. The cells were
then stained with Annexin V-FITC according to the instructions of
the Apoptosis Detection kit (Beijing Solarbio Science &
Technology Co., Ltd.). Finally, a flow cytometer (Sysmex Partec
Gmbh, Görlitz, Germany) was used to determine the percentage of
apoptotic cells in each group.
Crystal violet staining assay
A549 cells were cultured in 96-well plates and
treated as aforementioned for 24 h when cells reached a confluence
of 70–80%. The medium was removed at the end of the treatment
period and cells were washed with cold PBS and fixed in 4%
paraformaldehyde for 30 min at room temperature. The cells were
then washed again with cold PBS and stained at room temperature
with crystal violet for a further 2 min. Finally, the cells were
washed with PBS and dried naturally prior to being observed under
an inverted phase contrast microscope (Olympus Corporation, Tokyo,
Japan).
Western blot analysis
Cells were treated with GA, cisplatin or the two
compounds combined for 24 h, as aforementioned. The total proteins
of cells in each group were then extracted using a Total Protein
Extraction kit (Nanjing KeyGen Biotech, Co., Ltd. Nanjing, China)
according to the manufacturer's instructions. The concentration of
proteins was quantified with a BCA Protein Assay kit (Beijing
Solarbio Science & Technology Co., Ltd.). The proteins were
then mixed with sample buffer, separated on 10% SDS-polyacrylamide
gels and transferred onto PVDF membranes. The membranes were
blocked at room temperature for 2 h with 5% dried skimmed milk or
5% bovine serum albumin (Beijing Solarbio Science & Technology
Co., Ltd.) dissolved in TBST (containing 0.02% Tween-20) and
incubated with the primary antibodies at 4°C overnight: JAK1 (cat.
no. BA1808), p-JAK1 (cat. no. ENP0154), STAT3 (cat. no. BA0621),
p-STAT3 (cat. no. P00007), Bax (cat. no. BA0315), Bcl-2 (cat. no.
BA0412), β-actin (cat. no. BM0627) or GAPDH (cat. no. BA2913) at
the dilution of 1:1,000-1:5,000. Subsequently, the membranes were
washed 3 times with TBST, incubated with anti-rabbit IgG secondary
antibody conjugated with HRP (1:5,000; cat. no. BA1056) for 1 h at
room temperature and then washed 3 times with TBST. Finally, the
signals indicating expression levels of target proteins were
detected using Chemiluminescent HRP Substrate (EMD Millipore,
Billerica, MA, USA) and ImageJ 1.8.0 software (National Institutes
of Health, Bethesda, MD, USA).
Immunofluorescent staining assay
Cells were cultured in 24-well plates on sterile
glass coverslips placed in each well and treated as aforementioned.
The cells were then fixed at 4°C with 4% paraformaldehyde for 30
min and washed twice with PBS. Triton X-100 solution (0.1%) was
used to disrupt the cytomembrane, then cells were blocked in 10%
normal goat serum (Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 1 h, and incubated with p-STAT3
(dilution, 1:1,000; cat. no. P00007) at 4°C overnight.
Subsequently, the cells were incubated with biotin-labeled
secondary antibody (dilution, 1:64; cat. no. BA1090; Wuhan Boster
Biological Technology Co., Ltd.) for 40 min at 37°C. Finally,
nuclei were stained with DAPI at room temperature for 1 min and the
slides were observed with a fluorescent microscope (Olympus
Corporation; magnification, ×200).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of cells treated with the various
doses of GA (12–28 µg/ml) were isolated with TRIzol reagent (Thermo
Fisher Scientific, Inc.), and then a NanoDrop ND-1000
spectrophotometer was used to measure the concentration and purity
of RNA samples. Subsequently, 2 µg total RNA was reverse
transcribed (37°C for 15 min and 85°C for 5 sec) into cDNA using a
SuperScript III First-Strand Synthesis System for RT-PCR
(Invitrogen; Thermo Fisher Scientific, Inc.). PCR was performed
with a 25 µl reaction mixture including 2 µl cDNA. RT-qPCR was
performed using SYBR Premix Ex Taq II supplied (Takara Bio, Inc.,
Otsu, Japan). Primers used for the RT-qPCR were as follows: GAPDH
forward, 5′-ACTTTGGTATCGTGGAAGGACTCAT-3′ and reverse,
5′-GTTTTTCTAGACGGCAGGTCAGG-3′; Bax forward,
5′-TTTTGCTTCAGGGTTTCATCCA-3′ and reverse,
5′-TGCCACTCGGAAAAAGACCTC-3′; Bcl-2 forward,
5′-ATCGCCTGTGGATGACTGA-3′ and reverse,
5′-GAGACAGCCAGGAGAAATCAAAC-3′; STAT3 forward,
5′-ACCAAGCGAGGACTGAGCATC-3′ and reverse,
5′-CAGCCAGACCCAGAAGGAGAA-3′; and JAK1 forward,
5′-ACCAGGATGCGGATAAATAATG-3′ and reverse,
5′-GTTTCCAAGGTAGCCAAGTATTT-3′. qPCR amplification was performed in
two steps: An initial step at 95°C for 30 sec, and then 40 cycles
of 95°C for 5 sec and 60°C for 30 sec. Finally, the expression
levels of target mRNAs were calculated according to
2−ΔΔCq method (15).
Statistical analysis
All quantitative data were presented as the mean ±
standard deviation, and statistical analysis was performed with
SPSS 19.0 (IBM Corp., Armonk, NY, USA). One-way analysis of
variance followed by the Least Significant Difference post hoc test
was applied to analyze the differences among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
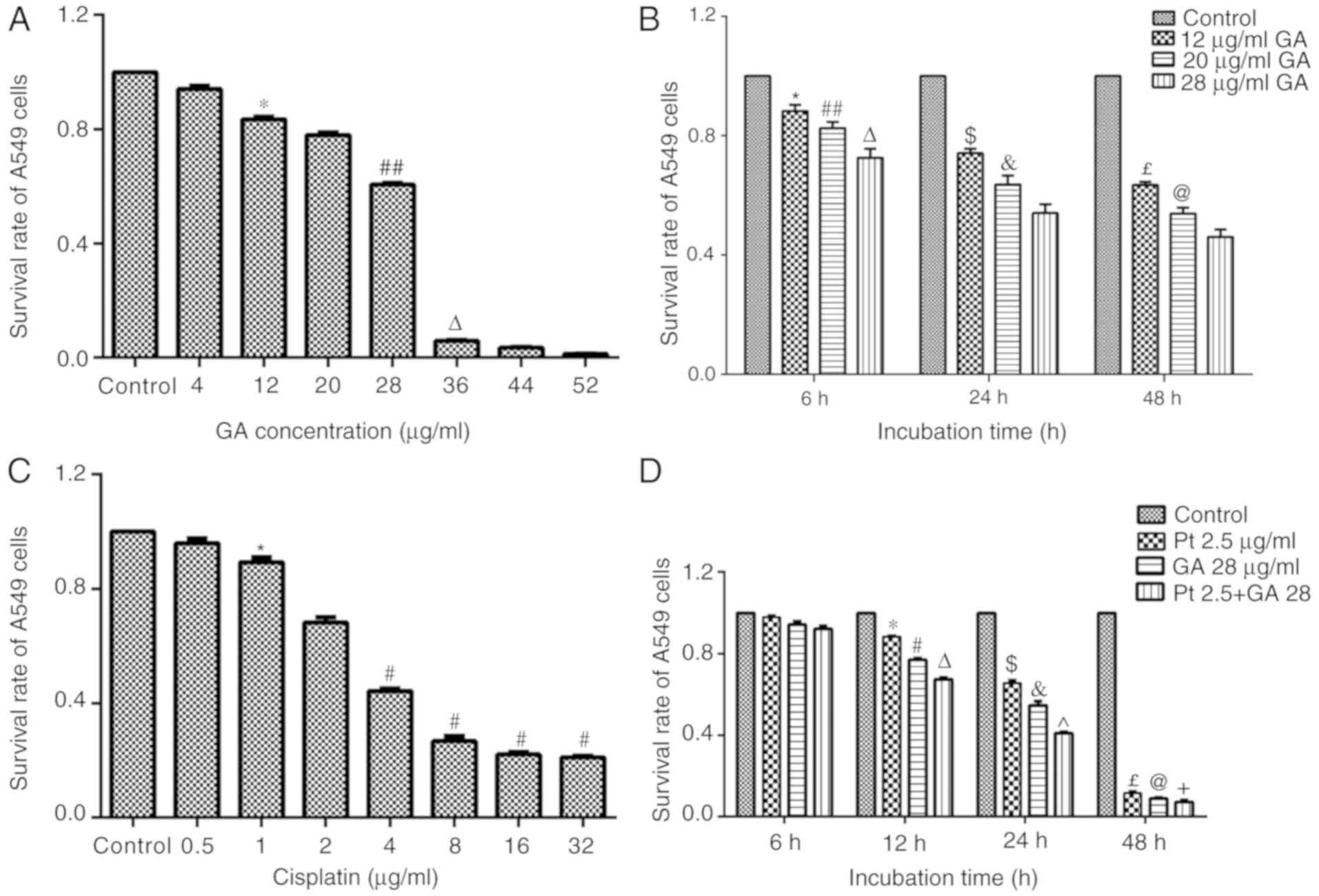
GA decreases the viability of A549
cells
A549 cells were treated with GA (0–52 µg/ml) for 24
h and cell viability was detected by MTT assay. It was observed
that GA decreased cell viability in a dose-dependent manner
(Fig. 1A). Specifically, 12 µg/ml
GA significantly decreased cell viability when compared with that
of control cells (P<0.05); the greater concentration of 28 µg/ml
in turn caused a greater inhibition of cell viability when compared
with 12 µg/ml GA (P<0.05); cell viability was <10% when A549
cells were treated with GA at a dose of 36 µg/ml GA; and cell
viability was further inhibited when cells were treated with 52
µg/ml GA. Based on these findings, 12–28 µg/ml GA was adopted for
subsequent studies. It was further identified that the viability of
cells was significantly inhibited by GA in dose- and time-dependent
manners (Fig. 1B).
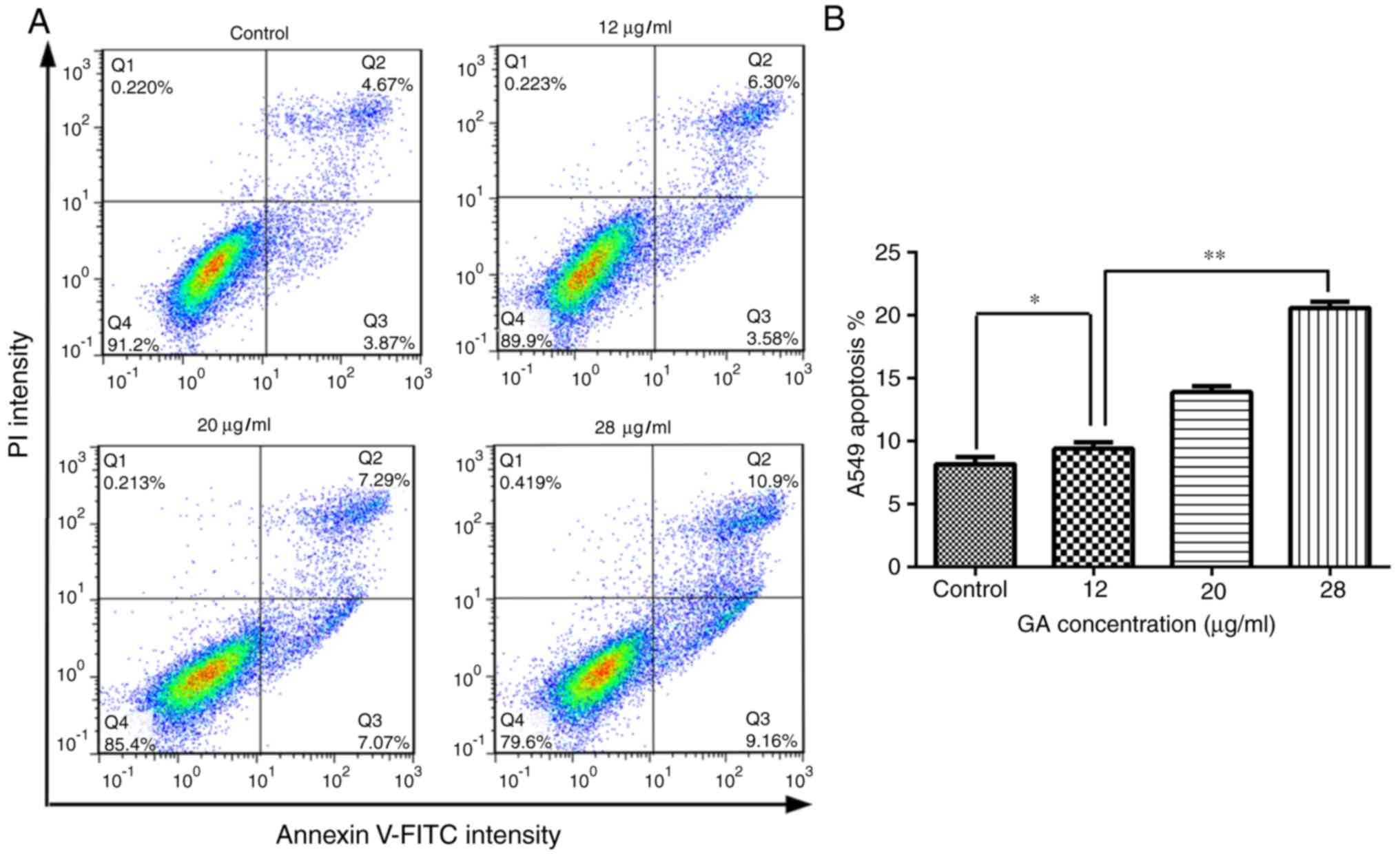
GA induces the apoptosis of A549
cells
To investigate the influence of GA on apoptosis,
A549 cells were treated with 12, 20 or 28 µg/ml GA for 24 h and the
number of apoptotic cells was calculated by flow cytometry. The
results demonstrated that 12 µg/ml GA significantly increased the
percentage of early and total apoptotic cells when compared with
the control group following 24 h of incubation (P<0.05).
Notably, treatment with 28 µg/ml GA led to a more significant
increase in apoptosis in A549 cells compared with 12 µg/ml GA
(P<0.01), which indicated that GA induced A549 cell apoptosis in
a dose-dependent manner (Fig.
2).
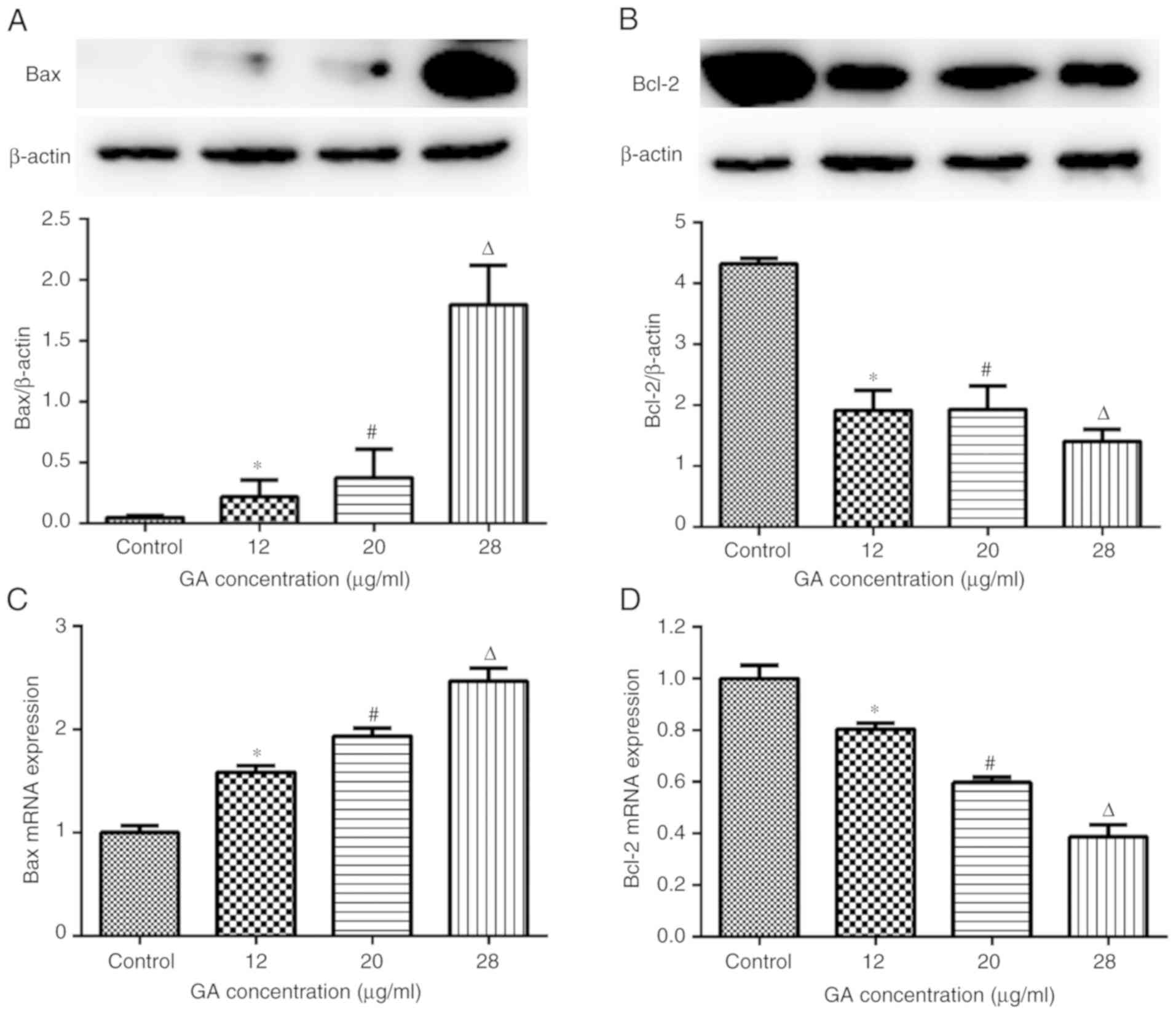
GA interferes with the expression of
Bax and Bcl-2 in A549 cells
To further ascertain the antitumor effects of GA in
A549 cells, the expression of Bax and Bcl-2 in cells from each
group was measured by RT-qPCR and western blot analysis. The
results revealed that GA upregulated Bax and downregulated Bcl-2 at
the gene and protein levels (Fig.
3). Specifically, 12 µg/ml GA enhanced Bax protein (Fig. 3A) and gene (Fig. 3C) expression relative to the levels
in control cells (P<0.05); and increased concentrations of GA
(20 and 28 µg/ml) further enhanced Bax expression at the gene and
protein levels when compared with 12 µg/ml GA (P<0.05). By
contrast, 12 µg/ml GA decreased the expression of Bcl-2 protein
(Fig. 3B) and mRNA (Fig. 3D) compared with control treatment
(P<0.05), and further inhibition of Bcl-2 expression was
observed when A549 cells were treated with 20 or 28 µg/ml GA
(P<0.05 vs. 12 µg/ml GA; Fig. 3B and
D).
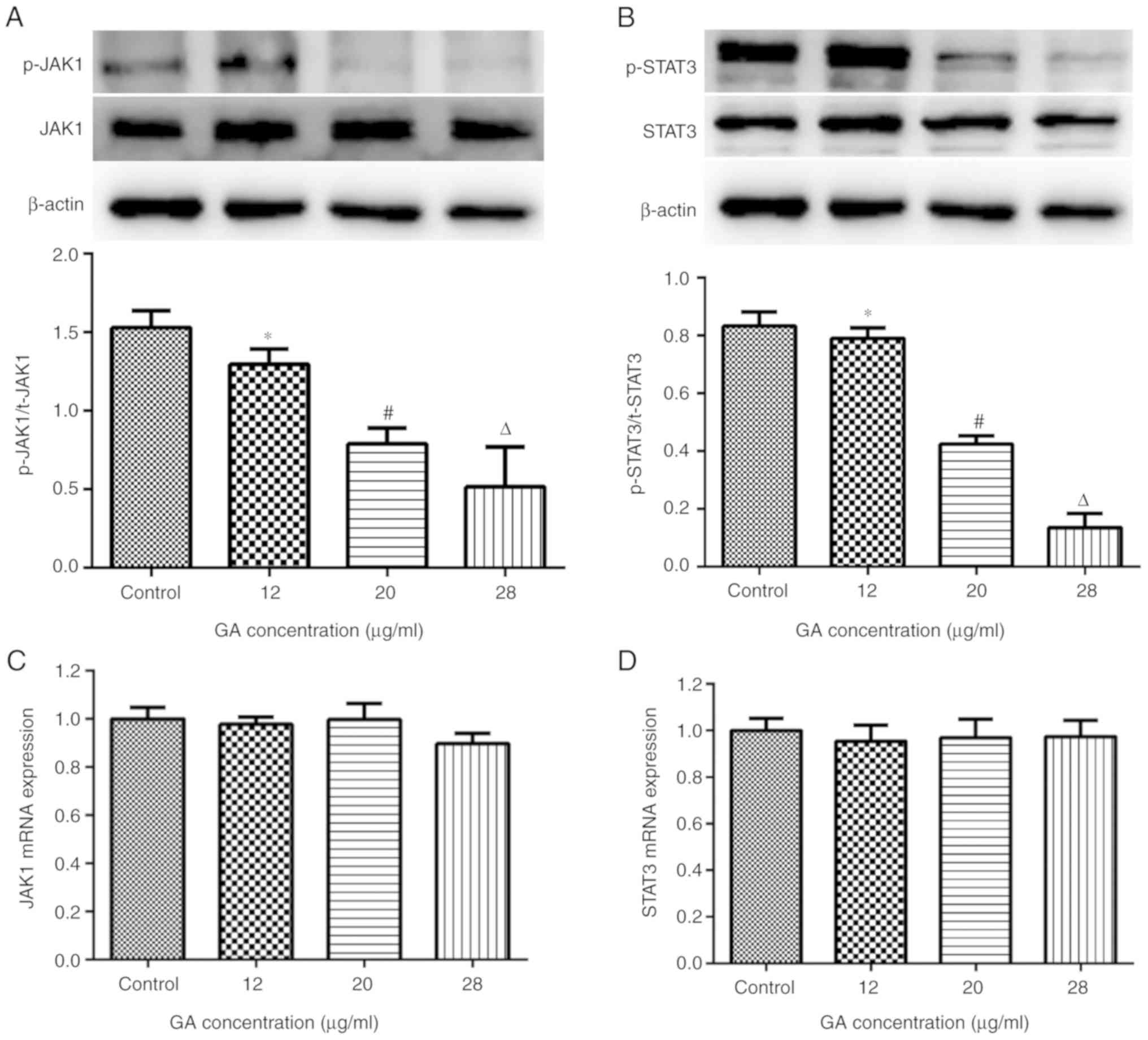
GA inhibits the JAK/STAT3 signaling
pathway in A549 cells
It is widely accepted that the JAK/STAT3 signaling
pathway is involved in various biological processes, including cell
proliferation, survival and development (14). To determine whether the JAK/STAT3
signaling pathway was associated with the anticancer effects of GA,
the expression of JAK1 and STAT3 was examined in A549 cells from
each group by RT-qPCR and western blotting. The results revealed
that 12 µg/ml GA reduced the levels of p-JAK1Y1022 and
p-STAT3Tyr705 when compared with control treatment
(P<0.05); greater reductions in p-JAK1Y1022 and
p-STAT3Tyr705 expression were observed when cells were
treated with 20 or 28 µg/ml GA (P<0.05 vs. 12 µg/ml; Fig. 4A and B), which indicated that GA
reduced p-JAK1Y1022 and p-STAT3Tyr705 in a
dose-dependent manner. By contrast, varied doses of GA exerted
little influence on the gene expression of JAK1 and STAT3 (Fig. 4C and D), which indicated that GA
interfered with the phosphorylation of JAK1 and STAT3, rather than
expression.
GA enhances the effects of cisplatin
on the proliferation of A549 cells
To confirm the optimum concentration of cisplatin
for subsequent investigations, an MTT assay was performed. As
presented in Fig. 1C, treatment
with cisplatin (0–32 µg/ml) for 24 h significantly decreased the
viability of A549 cells in a dose-dependent manner (P<0.05). It
was determined that the half-maximal inhibitory concentration of
cisplatin was between 2 and 4 µg/ml, and thus 2.5 µg/ml cisplatin
was adopted for subsequent assays.
To evaluate the effects of combined treatment with
GA and cisplatin (Pt 2.5 + GA28 group), cells were treated with the
established doses of GA and/or cisplatin for 6–48 h and evaluated
by MTT assay. The results demonstrated that GA, cisplatin and their
combined treatment decreased cell viability in a time-dependent
manner (P<0.01). Furthermore, cotreatment with GA markedly
strengthened the effects of cisplatin at different time points
(P<0.05; Fig. 1D).
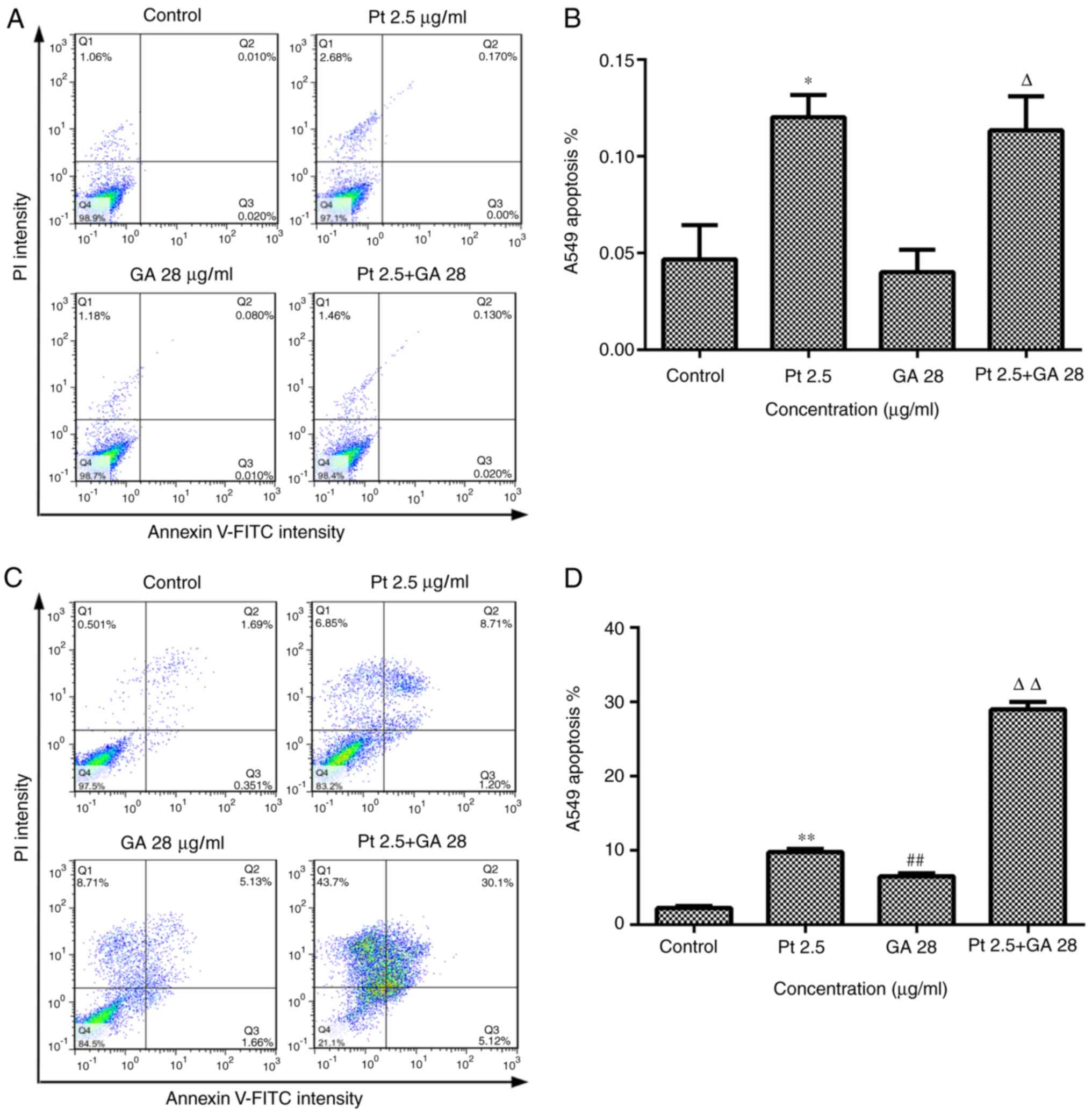
GA enhances the effects of cisplatin
on the apoptosis of A549 cells
To confirm whether GA influenced the stimulatory
effects of cisplatin on apoptosis, A549 cells were treated with GA,
cisplatin or the two combined for 6 or 24 h, and cell apoptosis was
measured by cytometry. The results indicated that cisplatin
treatment for 6 or 24 h increased the apoptosis of A549 cells by
varying extents (P<0.05 vs. Control), while GA significantly
increased the percentage of apoptotic cells at 24 h but not at 6 h
following incubation (P<0.05 vs. Control at 24 h). Notably, no
combined effect was observed when A549 cells were treated with
cisplatin and GA for 6 h, which may be due to 28 µg/ml GA not
exhibiting any apoptosis-inducing effects on A549 cells following 6
h of incubation. However, combined treatment with GA and cisplatin
induced a significant increase in apoptosis when compared with
single treatment with either of the two agents following 24 h of
incubation (P<0.01 vs. single treatments), which indicated that
GA increased the apoptosis-inducing effects of cisplatin on A549
cells (Fig. 5C and D).
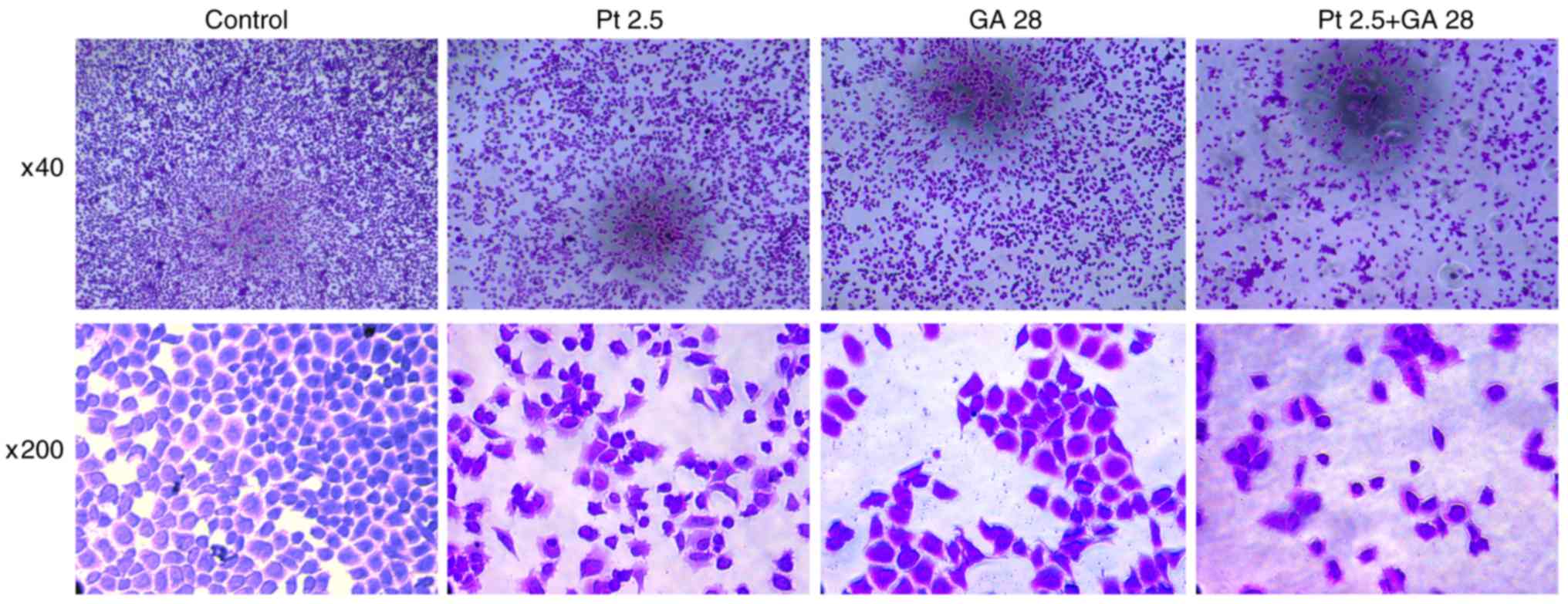
GA enhances the effects of cisplatin
on the morphological changes of A549 cells
The morphological changes of cells from different
groups were observed by crystal violet staining assay (Fig. 6). Cellular structure was intact in
the control group, while cells exhibited apparent apoptotic changes
such as cell shrinkage, nuclear chromatin condensation and
fragmentation when treated with GA, cisplatin, or a combination of
the two agents for 24 h. Additionally, cells in the experimental
groups exhibited an evident decrease in the number of cells;
notably, combined treatment with GA and cisplatin together lead to
the greatest decrease in cell number. These results revealed that
GA or cisplatin treatment alone resulted in morphological changes
in A549 cells, though their combination could lead to more evident
changes.
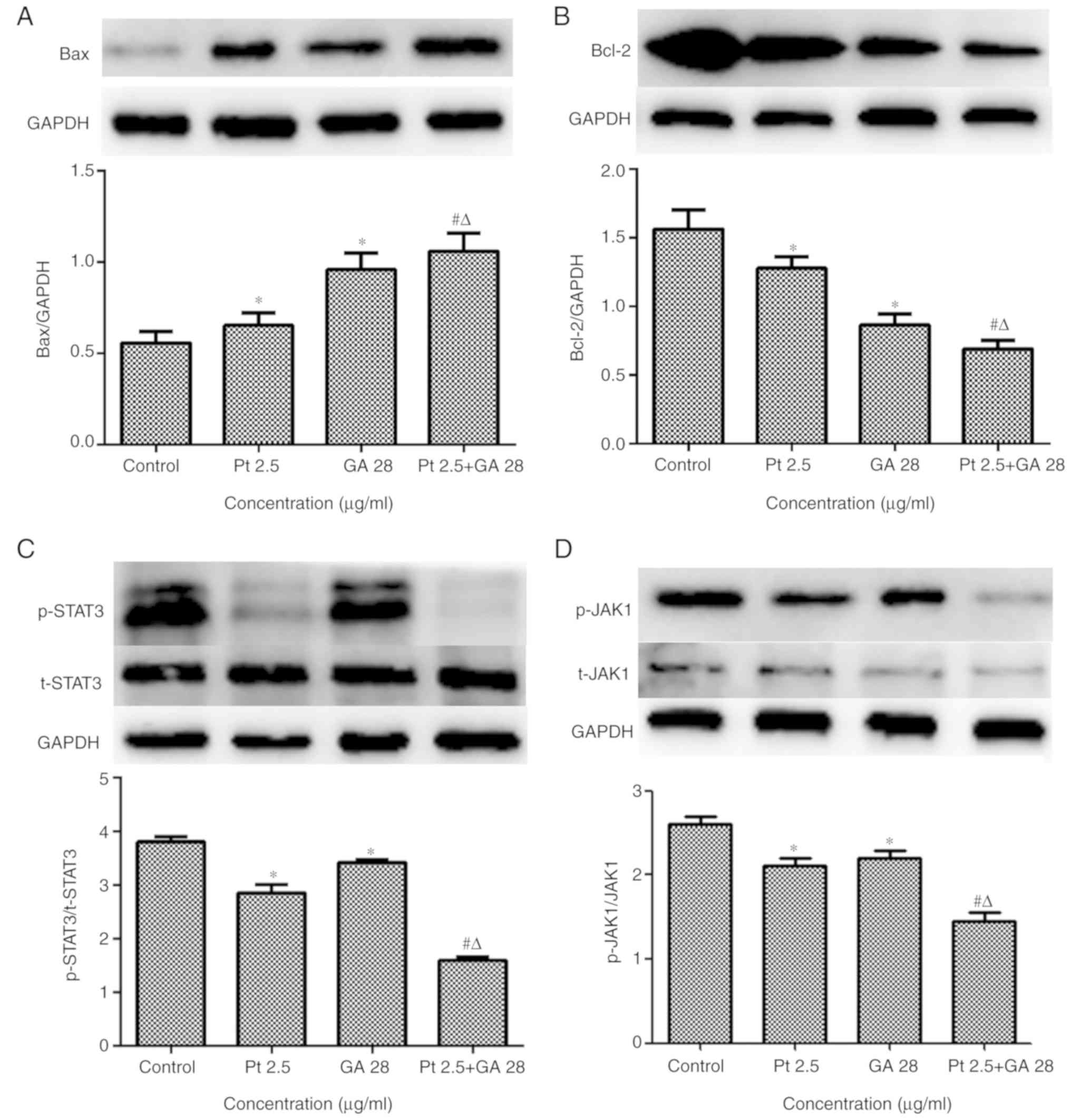
GA strengthens the effects of
cisplatin on the JAK/STAT3 signaling pathway
To determine whether GA could enhance the effects of
cisplatin on the regulation of the JAK/STAT3 signaling pathway,
several major molecules associated with apoptosis, anti-apoptosis
and proliferation in cells were examined by western blot and
immunofluorescent staining assays. The results demonstrated that GA
or cisplatin treatment alone increased Bax protein expression and
decreased Bcl-2 protein expression (P<0.05 vs. Control); while
combined treatment with the two agents significantly enhanced these
effects on the expression of Bax and Bcl-2 (P<0.05 vs. single
treatments; Fig. 7A and B).
The expression of the JAK/STAT3 signaling pathway in
cells treated with GA, cisplatin or the two agents combined was
also evaluated. The results demonstrated that the levels of
p-STAT3Tyr705 and p-JAK1Y1022 were
significantly decreased in cells treated with GA or cisplatin alone
(P<0.05 vs. Control); however, more marked decreases were
observed in the combined treatment group (P<0.05 vs. single
treatments; Fig. 7C and D).
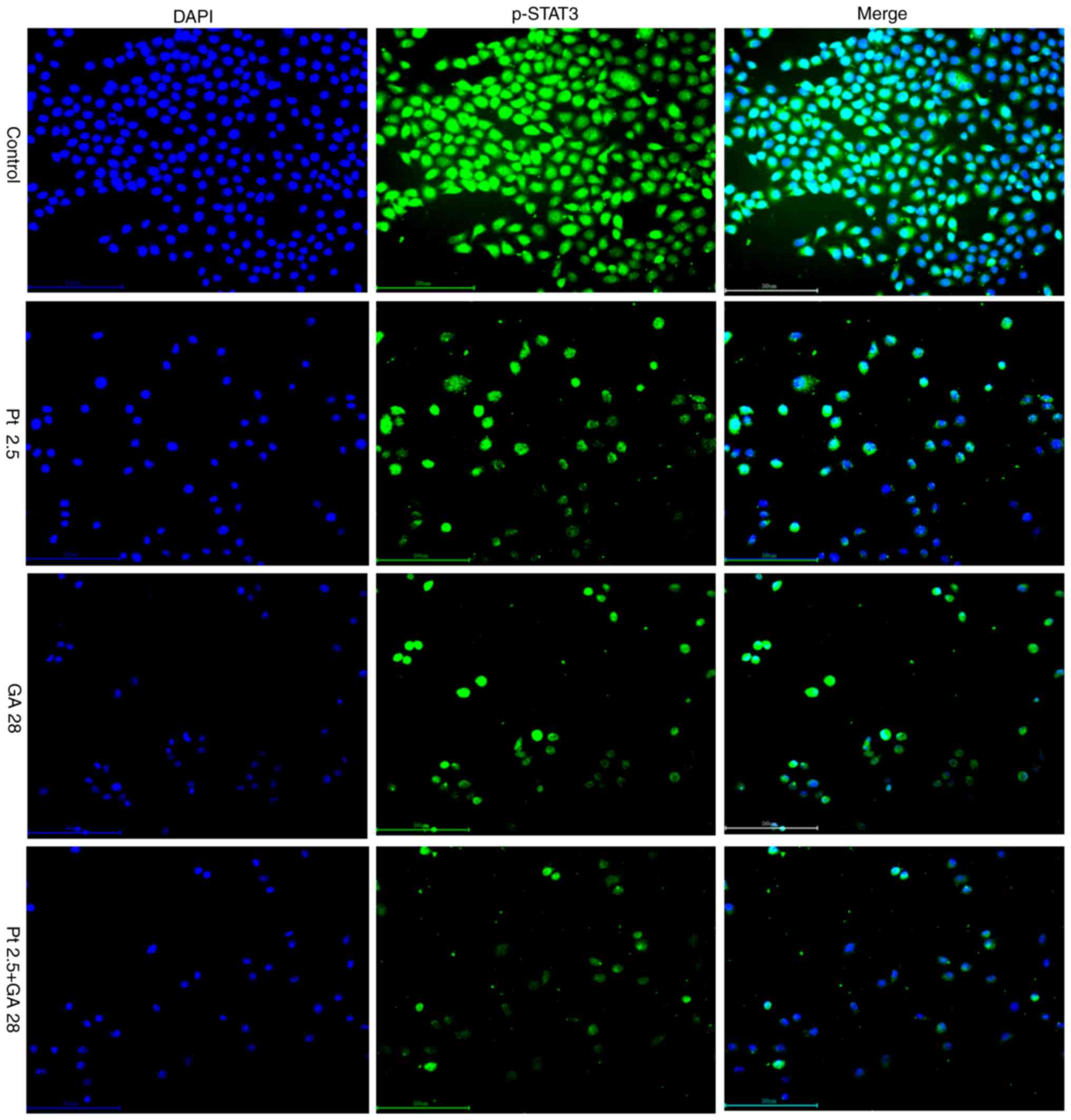
Additionally, the results of immunofluorescent staining revealed
that GA, cisplatin or the combination of the two agents suppressed
the phosphorylation of STAT3 and the translocation of p-STAT3 from
the cytoplasm to the nucleus (Fig.
8), which was consistent with the results of western blotting.
Notably, these results also revealed that combined treatment with
GA and cisplatin lead to markedly stronger suppression of the
phosphorylation of STAT3 and translocation of p-STAT3.
Discussion
Lung cancer is one of the most common types of
cancers and is characterized by a high mortality rate and
resistance to chemo- and/or radiation therapy is easily acquired
(16). For the majority of patients
with NSCLC, there is difficulty in selecting the optimum
therapeutic regimens. Cisplatin-based chemotherapy has achieved
considerable success in improving the prognosis and 5-year survival
rate of patients compared with non-cisplatin regimens. However, the
use of cisplatin is markedly limited by its side-effects, including
nephrotoxicity, severe nausea and vomiting (17). There is an urgent requirement to
identify novel drugs with little or no side-effects. Recently,
plant-derived compounds have attracted increasing levels of public
attention for their potential anticancer activities and low
toxicity. GA is one such product that exists in various plants and
may possess anticancer activity in various cancer cells including
those of lung cancers (8). A
previous study has reported that GA could enhance the effects of
chemotherapeutic agents in lung cancer (7). However, the underlying mechanisms are
still not fully understood.
It is well known that apoptosis is a strictly
programmed cell death process, which serves a critical role in
maintaining the balance between cell survival and death (18). Normally, apoptosis is a critically
regulated physiological process; however, abnormal cellular
proliferation and accumulation of genetic defects may occur in
instances of impaired apoptotic mechanisms, which could further
lead to tumorigenesis and resistance to treatment (19). Therefore, abnormal cellular
proliferation and evasion of apoptosis are considered to be
hallmarks of cancer, and the majority of antitumor drugs exert
their effects by inhibiting cellular proliferation and inducing
cell apoptosis. In the present study, the anticancer capacity of GA
and its auxiliary effects on cisplatin were evaluated, and the
results demonstrated that GA and cisplatin had marked effects on
decreasing A549 cell viability in dose- and time-dependent manners.
Notably, combined treatment with GA significantly enhanced the
effects of cisplatin. The present study has also identified that
individual GA or cisplatin treatment induced apoptosis in A549
cells, and furthermore, cotreatment with GA enhanced the
apoptosis-inducing effects of cisplatin. These results were
consistent with previous studies reporting that GA inhibited the
growth and induced the apoptosis of hepatic stellate (6), prostate cancer (8) and ovarian cancer cells (9).
Apoptotic pathways are known for their functions in
modulating the balance between cell proliferation and apoptosis by
regulating the expression of a series of growth factors, cytokines
and vasoactive substances (20). An
imbalance between cell proliferation and apoptosis is one of the
main causes of tumorigenesis (21).
Among the key factors involved, the JAK/STAT3 signaling pathway has
recently gained increased research focus. Transient activation of
the JAK/STAT3 signaling pathway in normal tissue is involved in
numerous fundamental biological processes, including cell
proliferation and apoptosis, and the development of organs
(22). However, persistent
activation of the JAK/STAT3 signaling pathway has been observed in
several types of cancers including NSCLC (14). Inhibition of the JAK/STAT3 signaling
pathway has therefore been recognized as a promising therapeutic
strategy for NSCLC. In addition, the JAK/STAT3 pathway may regulate
many gene products associated with apoptosis and anti-apoptosis,
including Bax and Bcl-2 (23).
Based on this knowledge, the expression levels of
JAK1, p-JAK1Y1022, STAT3 and p-STAT3Tyr705
were determined in A549 cells treated with GA, cisplatin or a
combination of the two agents in the present study. The results
demonstrated that GA and cisplatin had little effect on the
expression of total (t)-JAK1 or t-STAT3, while the phosphorylation
of JAK1 and STAT3 was suppressed by GA and cisplatin in a
dose-dependent manner. Furthermore, it was also identified that GA
markedly enhanced the effects of cisplatin on blocking the
phosphorylation of JAK1 and STAT3, and that the changes in
p-JAK1Y1022 and p-STAT3Tyr705 levels were
consistent with the changes in cell viability, and contrary to the
rate of cell apoptosis. These findings were consistent with
previous studies indicating that decreased activation of the
JAK/STAT3 signaling pathway could inhibit the growth of ovarian
(24) and prostate cancer (10), and renal cell carcinoma (25). To explore the underlying mechanisms
by which GA exerted its anticancer effects and auxiliary effects on
cisplatin, the major molecules associated with apoptosis, namely
Bcl-2 and Bax, were assessed, and the results revealed that the
expression of Bcl-2 was downregulated while that of Bax was
upregulated in A549 cells treated with GA. Furthermore, GA enhanced
the effects of cisplatin on the expression of Bcl-2 and Bax. These
results indicated that GA inhibited proliferation and induced
apoptosis in A549 cells by regulating apoptotic signaling pathways.
However, further studies are still required in order to elucidate
how GA affected the downstream JAK/STAT3 signaling pathway and the
role of GA.
In conclusion, the present study confirmed, to the
best of our knowledge for the first time, that GA suppressed
proliferation and induced apoptosis in NSCLC A549 cells in dose-
and time-dependent manners, potentially by modulating the
JAK1/STAT3 signaling pathway. Notably, the results of the present
study suggested that GA exerted an auxiliary effect on cisplatin
anticancer activity by blocking the phosphorylation of JAK1 and
STAT3, and modulating the expression of downstream apoptotic
molecules. However, the further studies are still required to
illustrate the role of GA in other potential apoptosis pathway
associations. In addition, animal studies and clinical trials will
be necessary in order to confirm the anticancer effects of GA on
NSCLC.
Acknowledgements
The authors would like to thank Dr Longfu Zhou, Mr.
Yaolei Zhang and Mr. Yaxing Feng (Central Laboratory, The General
Hospital of Western Theater Command, Sichuan, China) for their
excellent technical support during the present study.
Funding
The present study was supported by Innovation
Project of Sichuan Medical Association (grant no. Q17005) and
Scientific Research Project of Sichuan Health and Family Planning
Commission (grant no. 18PJ020).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZX and XF were the major contributors in designing
the research. TZ and LM were the major contributors in conducting
the experiments, interpreting the data and drafting the manuscript.
PW and WL assisted with the MTT and immunofluorescent staining
assays. TL and RG were involved in the cell apoptosis assay and
RT-qPCR. XD and ZL performed western blotting. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu F, Dai C, Fu Y, Loo JF, Xia D, Gao SP,
Ma Z and Chen Z: Physalin A exerts antitumor activity in non-small
cell lung cancer cell lines by suppressing JAK/STAT3 signaling.
Oncotarget. 7:9462–9476. 2016.PubMed/NCBI
|
|
2
|
Kubo H, Suzuki T, Matsushima T, Ishihara
H, Uchino K, Suzuki S, Tada S, Yoshimura M and Kondo T:
Cyclin-dependent kinase-specific activity predicts the prognosis of
stage I and stage II non-small cell lung cancer. Bmc Cancer.
14:7552014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cromie MM and Gao W:
Epigallocatechin-3-gallate enhances the therapeutic effects of
leptomycin B on human lung cancer a549 cells. Oxid Med Cell Longev
2015. 2173042015.
|
|
4
|
Zheng XJ, Yang ZX, Dong YJ, Zhang GY, Sun
MF, An XK, Pan LH and Zhang SL: Downregulation of leptin inhibits
growth and induces apoptosis of lung cancer cells via the Notch and
JAK/STAT3 signaling pathways. Biol Open. 5:794–800. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbone DP, Gandara DR, Antonia SJ,
Zielinski C and Paz-Ares L: Non-small-cell lung cancer: Role of the
immune system and potential for immunotherapy. J Thorac Oncol.
10:974–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang YJ, Hsu SL, Liu YT, Lin YH, Lin MH,
Huang SJ, Ho JA and Wu LC: Gallic acid induces necroptosis via
TNF-α signaling pathway in activated hepatic stellate cells. PLoS
One. 10:e1207132015.
|
|
7
|
Wang R, Ma L, Weng D, Yao J, Liu X and Jin
F: Gallic acid induces apoptosis and enhances the anticancer
effects of cisplatin in human small cell lung cancer H446 cell line
via the ROS-dependent mitochondrial apoptotic pathway. Oncol Rep.
35:3075–3083. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaur M, Velmurugan B, Rajamanickam S,
Agarwal R and Agarwal C: Gallic acid, an active constituent of
grape seed extract, exhibits anti-proliferative, pro-apoptotic and
antitumorigenic effects against prostate carcinoma xenograft growth
in nude mice. Pharm Res. 26:2133–2140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Z, Chen AY, Rojanasakul Y, Rankin GO
and Chen YC: Gallic acid, a phenolic compound, exerts
anti-angiogenic effects via the PTEN/AKT/HIF-1alpha/VEGF signaling
pathway in ovarian cancer cells. Oncol Rep. 35:291–297. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Kim C, Baek SH, Ko JH, Lee SG,
Yang WM, Um JY, Sethi G and Ahn KS: Capsazepine inhibits JAK/STAT3
signaling, tumor growth, and cell survival in prostate cancer.
Oncotarget. 8:17700–17711. 2017.PubMed/NCBI
|
|
11
|
Wen W, Liang W, Wu J, Kowolik CM, Buettner
R, Scuto A, Hsieh MY, Hong H, Brown CE, Forman SJ, et al: Targeting
JAK1/STAT3 signaling suppresses tumor progression and metastasis in
a peritoneal model of human ovarian cancer. Mol Cancer Ther.
13:3037–3048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou F, Cheng L, Qiu LX, Wang MY, Li J,
Sun MH, Yang YJ, Wang JC, Jin L, Wang YN and Wei QY: Associations
of potentially functional variants in IL-6, JAKs, STAT3 with
gastric cancer risk in an eastern Chinese population. Oncotarget.
7:28112–28123. 2016.PubMed/NCBI
|
|
13
|
Gritsina G, Xiao F, O'Brien SW, Gabbasov
R, Maglaty MA, Xu RH, Thapa RJ, Zhou Y, Nicolas E, Litwin S, et al:
Targeted blockade of JAK/STAT3 signaling inhibits ovarian carcinoma
growth. Mol Cancer Ther. 14:1035–1047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang CL, Liu YY, Ma YG, Xue YX, Liu DG,
Ren Y, Liu XB, Li Y and Li Z: Curcumin blocks small cell lung
cancer cells migration, invasion, angiogenesis, cell cycle and
neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One.
7:e379602012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee CY, Sher HF, Chen HW, Liu CC, Chen CH,
Lin CS, Yang PC, Tsay HS and Chen JJ: Anticancer effects of
tanshinone I in human non-small cell lung cancer. Mol Cancer Ther.
7:3527–3538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Xiao J, Yang Y and Cao B:
Oxaliplatin-based doublets versus cisplatin or carboplatin-based
doublets in the first-line treatment of advanced nonsmall cell lung
cancer. Medicine. 94:e10722015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian HR, Shi ZQ, Zhu HP, Gu LH, Wang XF
and Yang Y: Interplay between apoptosis and autophagy in colorectal
cancer. Oncotarget. 8:62759–62768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu G, Pei F, Yang F, Li L, Amin AD, Liu
S, Buchan JR and Cho WC: Role of autophagy and apoptosis in
non-small-cell lung cancer. Int J Mol Sci. 18(pii): E3672017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin G, Zhao J, Yang YI, Liu K, Jiang Y,
Zhang X, Zhang Y, Huang Y, Lu J and Dong Z: JAK/STAT3 signaling
pathway mediates endothelial-like differentiation of immature
dendritic cells. Oncol Lett. 10:3471–3477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang J, Lau V, Wu PC, Lai CL and Lau J:
Imbalance between cell proliferation and programmed cell dealth
*apoptosis* in hepatocellular carcinoma. Gastroenterology. 108 (4
Suppl 3):A10631995. View Article : Google Scholar
|
|
22
|
Wei W, Wu J, Liu L, Tian Y, Buettner R,
Hsieh MY, Horne H, Dellinger TH, Han ES, Jove R, et al: Synergistic
antitumor effect of combined inhibition of EGFR and JAK/STAT3
pathways in human ovarian cancer. Mol Cancer. 14:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang SN, Fu J, Shankar S and Srivastava
RK: EGCG enhances the therapeutic potential of gemcitabine and
CP690550 by inhibiting STAT3 signaling pathway in human pancreatic
cancer. PLoS One. 7:e310672012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen W, Wu J, Liu L, Tian Y, Buettner R,
Hsieh MY, Horne D, Dellinger TH, Han ES, Jove R, et al: Synergistic
anti-tumor effect of combined inhibition of EGFR and JAK/STAT3
pathways in human ovarian cancer. Mol Cancer. 14:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Priceman SJ, Xin H, Zhang W, Deng J,
Liu Y, Huang J, Zhu W, Chen M, Hu W, et al: Icaritin inhibits
JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One.
8:e816572013. View Article : Google Scholar : PubMed/NCBI
|