Introduction
As the leading cause of cancer-associated
mortalities globally, lung cancer is frequently diagnosed in males
and females with an incidence ratio of ~2.1:1 in 2008 (1,2). In
2008, ~1.4 million people globally succumbed to lung cancer, which
represented 18% of all cancer-associated mortalities (3). Generally, lung cancer is classified
into two main types: Small cell lung cancer (SCLC); or non-SCLC
(NSCLC) (4,5). Accumulating evidence has revealed that
tobacco smoking is a major cause of lung cancer, as it is
associated with ~90% of all lung cancer diagnoses (6–9).
Furthermore, smokers have a 10-fold increased probability of
developing lung cancer, compared with nonsmokers (10).
The Wnt/β-catenin pathway has been indicated to
serve important roles in a number of cancer types. For example, it
has been reported that the metastatic behavior of lung cancer cell
lines is increased by increased Wnt/β-catenin signaling in
vitro (11). A previous study
demonstrated that Wnt family genes are frequently upregulated in
multiple human cancer types, including NSCLC (12,13).
Through β-catenin, oncogenic Wnt signaling is transduced. Wnt
signaling promotes the accumulation of β-catenin, and elevated
β-catenin translocates to the nucleus where it forms complexes with
transcription factors (14). This
in turn stimulates the expression of Wnt target molecules,
including the oncogenes Cyclin D1 and c-Myc (15,16).
Tripartite motif-containing (TRIM) family proteins,
with >80 members, contain three conserved domains, RING, B-box
and a coiled-coil region, and are regarded as E3 ubiquitin ligases
that are associated with human diseases, including intracellular
immunity and cancer (17–20). It has been reported that TRIM
proteins regulate multiple biological processes, including cell
proliferation and invasion (21–23).
Studies demonstrated an association between TRIM24 and TRIM29, and
the progression of solid tumors (24,25).
Elevated TRIM65 has also been observed in lung cancer, where it
facilitates the growth of tumors (26,27);
whereas, TRIM31 was reported to be downregulated in NSCLC, which
indicates that it may function as a tumor suppressor (28). TRIM52 is a novel TRIM protein that
contains only a unique expanded RING domain and a B-box2 domain
(29). Previous studies
demonstrated that TRIM52 could promote cell proliferation,
migration and invasion in hepatocellular carcinoma through
ubiquitination (30,31). Another study indicated that TRIM52
acts as an oncogene in ovarian cancer, where it is associated with
the nuclear factor-κB pathway (32). However, the effect of TRIM52 in lung
cancer remains largely unknown.
In the present study, a high expression of TRIM52
was observed in tumor tissues of patients with lung cancer and in
lung cancer cell lines. The downregulation of TRIM52 in lung cancer
cell lines significantly inhibited cell proliferation by blocking
cell cycle progression, which occurred concurrently with decreases
in β-catenin, proliferating cell nuclear antigen (PCNA), c-Myc and
Cyclin D1 expression. Furthermore, TRIM52-induced cell
proliferation and invasion were completely counteracted by the
Wnt/β-catenin inhibitor XAV939. These results indicated that TRIM52
downregulation inhibits lung cancer progression, possibly through
inactivation of the Wnt/β-catenin signaling pathway.
Materials and methods
Tumor and adjacent normal tissues of
patients with lung cancer
Following informed consent being obtained, 43 pairs
of tumor and paracancer tissues from 43 patients with lung cancer
treated at Longhua Hospital (Shanghai, China) were collected and
immediately frozen in liquid nitrogen at −196°C. After the tissues
were sectioned at 5 µm, the expression of TRIM52 was detected by
immunohistochemistry, according to the subsequent protocol. All
experiments in the present study were approved by the Ethics
Committee of Shanghai University of Traditional Chinese Medicine
(Shanghai, China).
Cell culture
A total of 5 cell lines derived from human lung
cancer (H1975, H466, A549, H358 and H1299), and a cell line derived
from the pulmonary epithelium (16HBE) were purchased from the Cell
Bank of the Chinese Academy of Science (Shanghai, China). These
cells were cultured in a 5% CO2 humidified-incubator at
37°C (Thermo Forma 3111; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with RPMI-1640 medium (cat. no. SH30809.01B; HyClone; GE
Healthcare Life Sciences; Logan, UT, USA) supplemented with 10%
fetal bovine serum (cat. no. 16000-044; Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotic (×100; a mixture of penicillin
and streptomycin; cat. no. P1400-100; Beijing Solarbio Science
& Technology Co., Ltd., Shanghai, China). During incubation,
the medium was replaced with fresh RPMI-1640 medium every two days
according to cellular demand until the experiments. After
selecting, three cell lines, H358, H1299 and H1975, were used for
subsequent experiments.
Lentiviral construction
Short hairpin RNA (shRNA) sequences targeted to the
TRIM52 gene (NM_032765.3) were synthesized and double
strand-annealed to form the shRNA construct. The shRNA construct
was inserted into Agel I/Ecol I restriction sites of a pLKO.1-puro
vector (Addgene, Inc., Cambridge, MA, USA). Subsequently, the 894
bp full-length coding DNA sequence region of TRIM52 containing the
EcoR I/BamH I restriction sites was synthesized by Genewiz, Inc.
(Shanghai, China) and was then inserted into EcoRI/BamHI
restriction sites of a pLVX-Puro vector (Clontech Laboratories,
Inc., Mountainview, CA, USA). pLKO.1-shTRIM52 and pLVX-Puro-TRIM52
were confirmed by DNA sequencing (Shanghai Meiji Biomedical
Technology Co., Ltd., Shanghai, China). Subsequently, 0.5 µg core
plasmid of pLKO.1-shTRIM52 or pLVX-Puro-TRIM52 and 1.5 µg mixed
viral packaging plasmids psPAX2 and pMD2G (Addgene, Inc.) were
added to 250 µl serum-free RPMI-1640 medium, and 9 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was added into a serum-free RPMI-1640 medium with
a total volume of 250 µl, and then the two were mixed and
transfected into 293T cells. The virus particles were obtained
after 48 h of transfection.
Experimental grouping
In vitro, to regulate the expression of TRIM52 in
lung cancer cell lines, lentivirus-mediated RNA interference or
overexpression was used. H358 or H1299 cells were infected with
RPMI-1640 medium (control), pLKO.1-puro vector (negative control
lentivirus; shNC), or shTRIM52 lentivirus (shTRIM52-1, shTRIM52-2,
shTRIM52-3 and shTRIM52-4), while H1975 cells were infected with
RPMI-1640 medium (control), pLVX-Puro vector (Clontech
Laboratories, Inc.) or TRIM52 recombinant lentivirus (oeTRIM52).
After 48 h, the efficiency of knockdown or overexpression was
detected by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting, according to the
subsequent protocols. shTRIM52-1, shTRIM52-4 and oeTRIM52
lentiviruses were used for subsequent experiments.
Furthermore, H358 or H1299 cells were infected with
RPMI-1640 medium, shNC, shTRIM52-1 or shTRIM52-4, and H1975 cells
were treated with vector, oeTRIM52, Vector + 20 µM XAV939
(Wnt/β-catenin inhibitor; S1180; Selleck Chemicals, Shanghai,
China) or oeTRIM52 + 20 µM XAV939. Assays to determine
proliferation and cell cycle, and western blot analysis were then
performed.
Immunohistochemistry
Following paraffin embedding, the tissue slides were
fixed for 48 h in 10% formalin at 4°C and then cut into 5 µm thick
sections, which were baked at a constant temperature in an oven at
65°C for 30 min, and then deparaffinized in two changes of xylenes
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) for 15 min
each. The deparaffinized sections were then rehydrated in 100, 95,
85 and 75% ethanol solutions for 5 min each, which was followed by
washing once in tap water for 10 min. Following antigen retrieval
with 0.01 M sodium citrate buffer (pH 6.0) at ~95°C for 15 min, the
slides were incubated with 0.3% H2O2 for 10
min at room temperature in a humidified chamber and washed with
0.02 M phosphate-buffered saline (PBS). Subsequently, the slides
were incubated with a rabbit antibody against TRIM52 (dilution
1:500; cat. no. NBP2-31651; Novus Biologicals, LLC, Littleton, CO,
USA) at room temperature for 1 h in a humidified chamber. The
slides were then incubated with a horseradish peroxidase-labeled
broad-spectrum secondary antibody (dilution 1:1,000; cat. no.
D-3004; Shanghai Long Island Biotechnology Co., Ltd., Shanghai,
China) at room temperature for 25 min. At room temperature,
following DAB staining for 5 min, a washing with tap water, (cat.
no. FL-6001; Shanghai Long Island Biotechnology Co., Ltd.), the
sections were stained with hematoxylin (cat. no. 714094; Zhuhai
BASO Biotechnology Co., Ltd., Zhuhai, China) for 3 min at room
temperature, exposed to 1% hydrochloric acid-alcohol at room
temperature for 3 sec for differentiation and flushed with tap
water once for 10 min. After drying, mounting and cover-slipping,
the slides were imaged by an upright light microscope at ×200
magnification (ECLIPSE Ni; Nikon Corporation, Tokyo, Japan) and
were analyzed by an IMS image analysis system (DS-Ri2; Nikon
Corporation).
RT-qPCR
RT-qPCR was performed to detect the TRIM52 mRNA
level in cells. The total RNA in cells (H1975, H466, A549, H358 and
H1299) that were or were not treated with lentiviruses was
extracted by TRIzol® reagent (cat. no. 1596-026;
Invitrogen; Thermo Fisher Scientific, Inc.), and following
quantification, the integrity of the RNA was confirmed by 1%
agarose gel electrophoresis. The extracted RNA was reversed
transcribed into cDNA using a Reverse Transcription kit (cat. no.
K1622; Fermentas; Thermo Fisher Scientific, Inc.). With cDNA used
as a template and a SYBR®-Green PCR kit (cat. no. K0223;
Thermo Fisher Scientific, Inc.), RT-qPCR reactions were performed
in an ABI 7300 Real-Time PCR system (ABI-7300; Applied Biosystems;
Thermo Fisher Scientific, Inc.). The expression of TRIM52 mRNA
normalized to GAPDH was analyzed by ABI Prism 7300 SDS software
1.4v (Applied Biosystems; Thermo Fisher Scientific, Inc.) and was
calculated using the 2−∆∆Cq method (33). The primer sequences were as follows:
TRIM52, forward, 5′-GTGCCATCTGCTTGGATTAC-3′, and reverse,
5′-TCATCTTCCTCCTCGTTCTG-3′, and GAPDH, forward,
5′-AATCCCATCACCATCTTC-3′, and reverse, 5′-AGGCTGTTGTCATACTTC-3′.
The RT-qPCR reaction conditions were as follows: 95°C for 10 min;
95°C for 15 sec and 60°C for 45 sec for 40 cycles; 95°C for 15 sec;
60°C for 1 min; 95°C for 15 sec; and 60°C for 15 sec (34).
Western blot analysis
Total proteins were extracted from
lentivirus-treated H358, H1299 or H1975 cells by
radioimmunoprecipitation assay buffer (Beijing Solarbio Science
& Technology Co., Ltd.; R0010), which contained protease and
phosphatase inhibitors, and were quantified by a Bicinchoninic
Assay quantification kit (Thermo Fisher Scientific, Inc.;
PICPI23223). Approximately 25 µg of proteins was separated by 10%
SDS-PAGE (JRDUN Biotechnology Co., Ltd, Shanghai), followed by a
semi-dry transfer onto polyvinylidene fluoride membranes (cat. no.
HATF00010; EMD Millipore, Billerica, MA, USA) by electroblotting.
After 1 h of blocking in 5% skim milk at room temperature (cat. no.
BYL40422; BD Biosciences; Becton, Dickinson and Company, Franklin
Lakes, NJ, USA), the blots were incubated with primary antibodies
against TRIM52 (1:200; cat. no. sc-135589; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), β-catenin (1:5,000; cat. no.
ab32572; Abcam, Cambridge, UK), PCNA (1:1,000; cat. no. ab29;
Abcam), c-Myc (1:1,000; cat. no. ab32072; Abcam), Cyclin D1
(1:5,000; cat. no. ab134175; Abcam) and β-actin (1:1,000; cat. no.
4970; Cell Signaling Technology, Inc., Danvers, MA, USA) with
gentle shaking at 4°C overnight. Following the incubation with the
horseradish peroxidase-conjugated secondary antibodies goat
anti-rabbit (cat. no. A0208), donkey anti-goat (cat. no. A0181),
and goat anti-mouse (cat. no. A0216) (1:1,000; Beyotime Institute
of Biotechnology, Shanghai, China) for 1 h at 37°C, the blots were
incubated with chemiluminescent detection reagent (cat. no.
WBKLS0100; EMD Millipore) for 5 min in the dark. Bound proteins
were then visualized using an Enhanced Chemiluminescent imaging
system (Tanon-5200; Tanon Science and Technology Co., Ltd.,
Shanghai, China). Finally, using β-actin as the loading control,
the relative protein levels were calculated by ImageJ software
1.47v (National Institutes of Health, Bethesda, MD, USA).
Proliferation assay
H358, H1299 or H1975 cells in a logarithmic growth
phase were digested by 0.25% trypsin (Beijing Solarbio Science
& Technology Co., Ltd.; P1300-100) and counted under a optical
microscope at ×10 magnification (cat. no. XDS-500C; Shanghai Cai
Kang Optical Instrument Co., Ltd., Shanghai, China) to prepare a
cell suspension of 3×104 cells/ml. Subsequently, 100 µl
of each cell suspension was inoculated in 96-well culture plates
(cat. no. TR4001; TrueLine, Romeoville, IL, USA) in triplicate and
cultured overnight at 37°C in a humidified 5% CO2
incubator. After 0, 24, 48 and 72 h of treatment according to the
experimental grouping, Cell Counting Kit-8 (CCK-8; cat. no. CP002;
Signalway Antibody, College Park, MD, USA) reagent and serum-free
RPMI-1640 medium were mixed at a volume ratio of 1:10, and 100 µl
of the mixture was added to each well. The plates were incubated
for 1 h at 37°C in a 5% CO2 incubator. Using a
microplate reader (cat. no. DNM-9602; Beijing Pulang New Technology
Co., Ltd., Beijing, China), the absorbance value (optical density)
at 450 nm was measured.
Cell cycle detection
Following treatment, according to the experimental
grouping, H358, H1299 or H1975 cells were collected and centrifuged
for 5 min at 1,000 × g at room temperature and were then
resuspended in 300 µl of PBS supplemented with 10% fetal bovine
serum. Subsequently, 700 µl absolute ethanol pre-cooled at −20°C
was added to fix the cells for 24 h at 4°C. The next day, following
centrifugation at 1,000 × g at room temperature for 5 min, the
fixed cells were washed with 1 ml pre-cooled PBS once.
Subsequently, the cell pellets were slowly and fully resuspended in
100 µl 1 mg/ml RNase A solution (cat. no. R8020-25; Beijing
Solarbio Science & Technology Co., Ltd.) and incubated in the
dark for 30 min at 37°C. Finally, the cells were incubated with 400
µl 50 µg/ml propidium iodide solution in the cell cycle and
apoptosis detection kit (cat. no. C001-200; Shanghai Qibao Xintai
Biological Technology Co., Ltd., Shanghai, China), which was added
to stain the nucleus, for 10 min in the dark at room temperature.
Following staining, the cell cycle status of these cells was
detected with a flow cytometer (BD Biosciences; Becton, Dickinson
and Company; Accuri C6) and analyzed by FlowJo software 7.6.1v
(Tree Star, Inc., Ashland, OR, USA).
Matrigel assay
Prior to inoculation, the 24-well plates and
Transwell chambers (cat. no. 3422; Costar; Corning, Inc., Corning,
NY, USA) were soaked in PBS for 5 min, and then the chambers were
coated with 80 µl Matrigel and clotted for 30 min in an incubator
at 37°C. Following overnight nutrient starvation in serum-free
RPMI-1640 medium, the treated cells (H1975 and A549) were
trypsinized and inoculated in the upper chamber (5×104
cells/well). RPMI-1640 medium with 10% fetal bovine serum was added
to the lower chamber. After 24 h of incubation, the non-invading
cells in the upper chamber were carefully scraped, and the cells
that had invaded into the lower chamber were fixed in 4%
formaldehyde (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) for 10 min at room temperature, followed by a 30 min
incubation in 0.5% crystal violet (Beijing Solarbio Science &
Technology Co., Ltd.; C8470) at room temperature. Subsequently,
using an upright optical microscope (cat. no. XDS-500C; Shanghai
Cai Kang Optical Instrument Co., Ltd.), the invading cells were
counted in 3 random fields at a magnification of ×200.
Statistical analysis
The statistical analyses of all data in the present
study were performed using GraphPad Prism 7.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). Student's t-test was used to
evaluate the differences between two groups, while one-way analysis
of variance followed by Tukey's multiple comparison was performed
to evaluate the comparisons among ≥3 groups. Based on at least
three independent experiments, quantitative data are shown as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
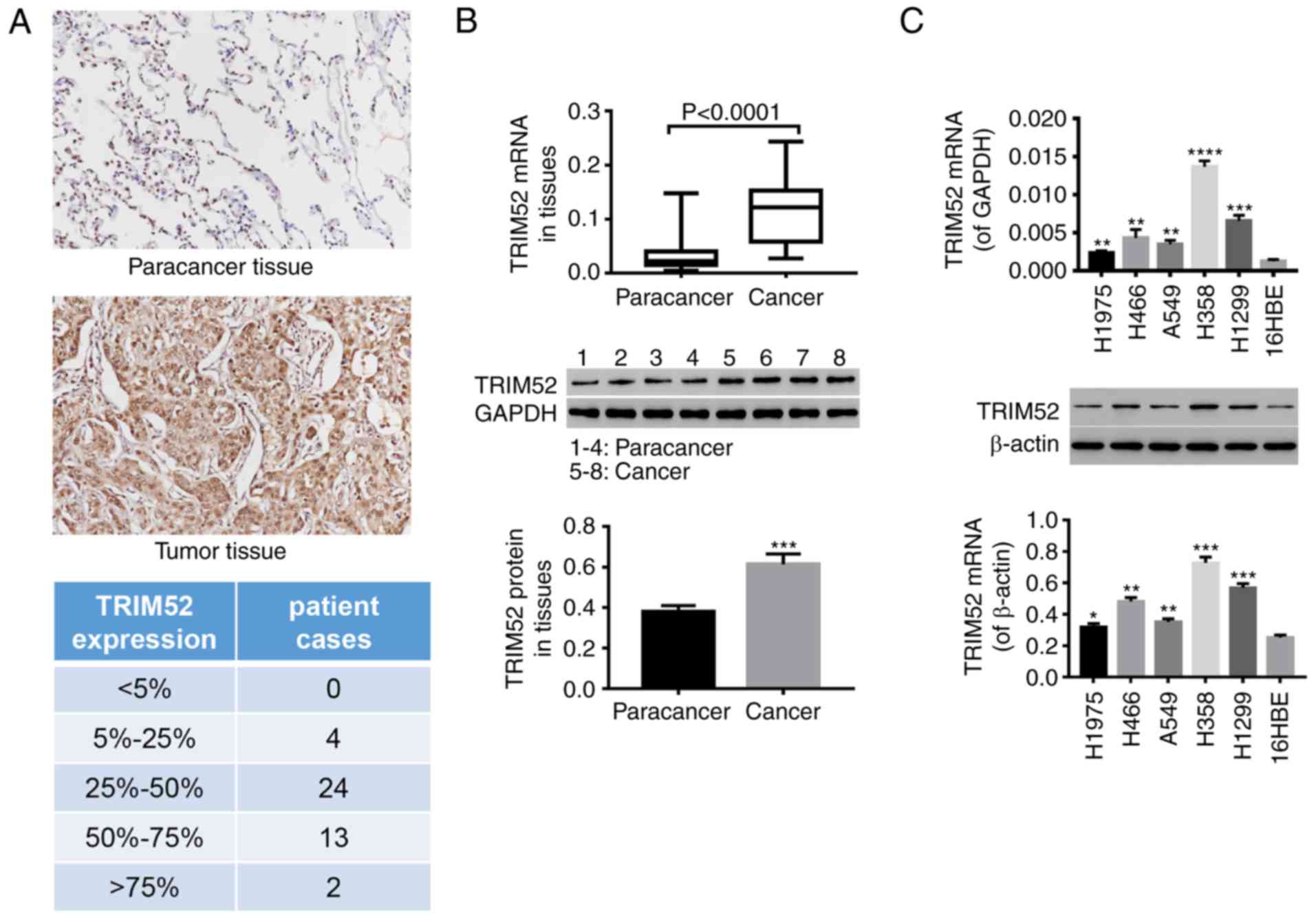
TRIM52 expression is elevated in the
tumor tissues of patients with lung cancer and in lung cancer cell
lines
After 43 pairs of lung tumor and paracancer tissues
were collected, immunohistochemistry (Fig. 1A) indicated that compared with the
paracancer tissues, the TRIM52 level was notably increased in
tumors. All cases were grouped according to the overall level of
TRIM52 expression in tissues: <5% positivity; 5≤n<25;
25≤n<50; 50≤n<75; and ≥75%. The increased expression of
TRIM52 was further demonstrated by RT-qPCR and western blot
analysis (Fig. 1B and C).
Statistical analysis of the immunohistochemical results
demonstrated that high expression of TRIM52 was observed in 97.7%
of tumor tissues (data not shown). Additionally, the TRIM52 mRNA
and protein levels in lung cancer cell lines (H1975, H466, A549,
H358 and H1299) were significantly increased, compared with
pulmonary epithelial cells (16HBE). The TRIM52 levels were
increased in H358 and H1299 cells, compared with the other cell
lines, while the levels were reduced in H1975 cells (Fig. 1C). These observations indicated that
TRIM52 may be involved in the development and progression of lung
cancer. A total of 3 lung cancer cell lines (H358, H1299 and H1975)
were therefore selected for the following experiments.
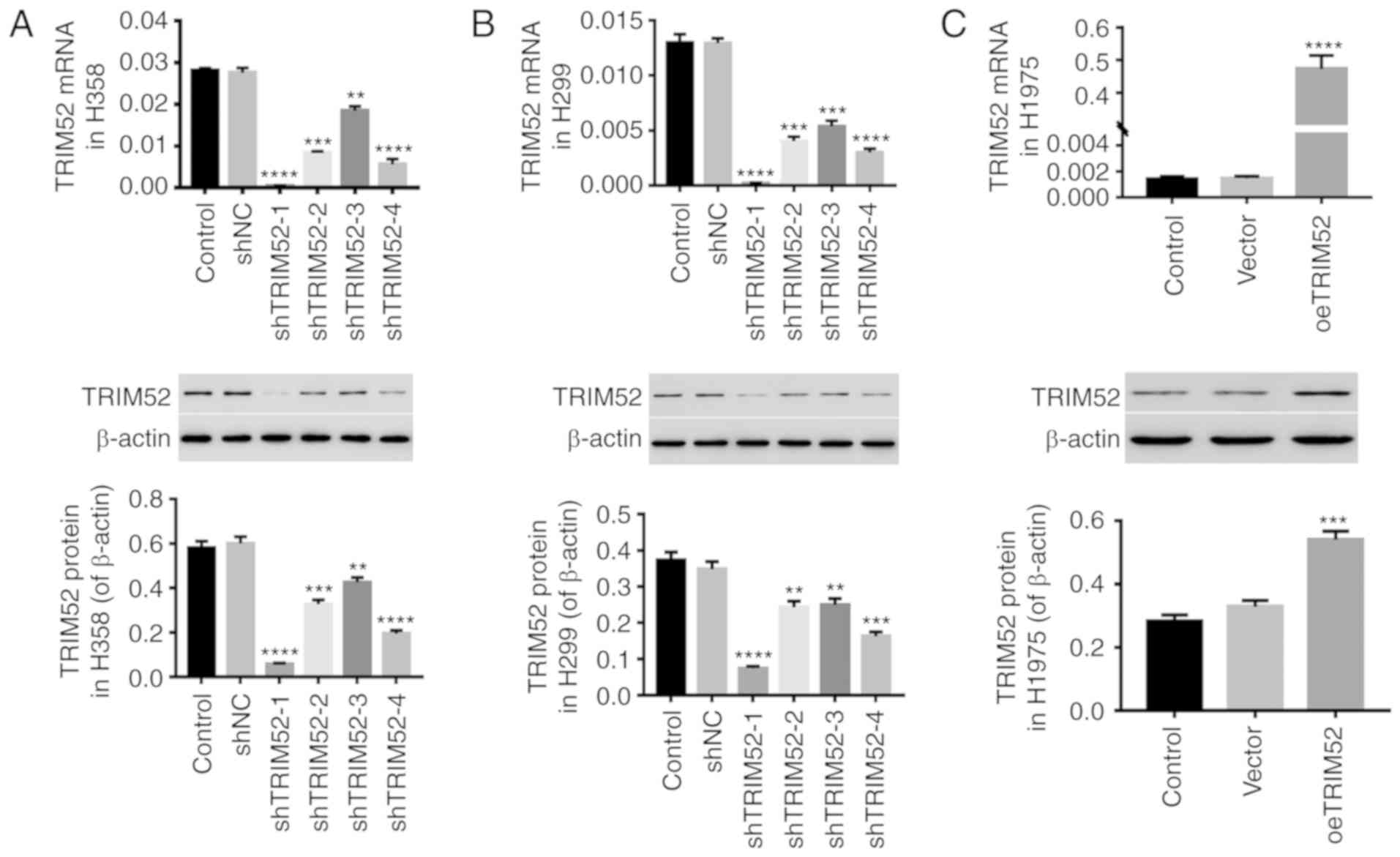
Down- and upregulation of TRIM52 in
lung cancer cell lines
To investigate the effect of TRIM52, shTRIM52 and
oeTRIM52 lentiviral vectors were used to regulate the TRIM52 level
in lung cancer cell lines. As depicted in Fig. 2, the levels of TRIM52 mRNA and
protein were significantly downregulated by shTRIM52 infection in
H358 (Fig. 2A) and H1299 (Fig. 2B) cells, and the effects of
shTRIM52-1 and shTRIM52-4 were more notable. Furthermore, the
TRIM52 level in H1975 cells was significantly upregulated by
oeTRIM52 (Fig. 2C). Therefore, the
shTRIM52-1, shTRIM52-4 and oeTRIM52 lentiviral vectors were
selected for further study due to their more effective regulation
of TRIM52 expression.
Downregulation of TRIM52 inhibits lung
cancer cell proliferation by cell cycle arrest
Following downregulation of the TRIM52 level in H358
and H1299 cells, cell proliferation and the cell cycle were
evaluated. As depicted in Fig. 3,
the proliferation of H358 and H1299 cells was notably inhibited
when TRIM52 was downregulated (Fig.
3A). Furthermore, downregulation of TRIM52 significantly
arrested the cell cycle at G1 phase in lung cancer cells, which
reduced the proportion of cells in S/G2 phase (Fig. 3B). Additionally, the protein levels
of β-catenin, PCNA, c-Myc and Cyclin D1 were significantly
decreased in TRIM52-silenced H358 and H1299 cells (Fig. 3C). All results indicated that TRIM52
downregulation exerted an inhibitory effect on the proliferation of
lung cancer cells by blocking cell cycle progression possibly via
Wnt/β-catenin signaling.
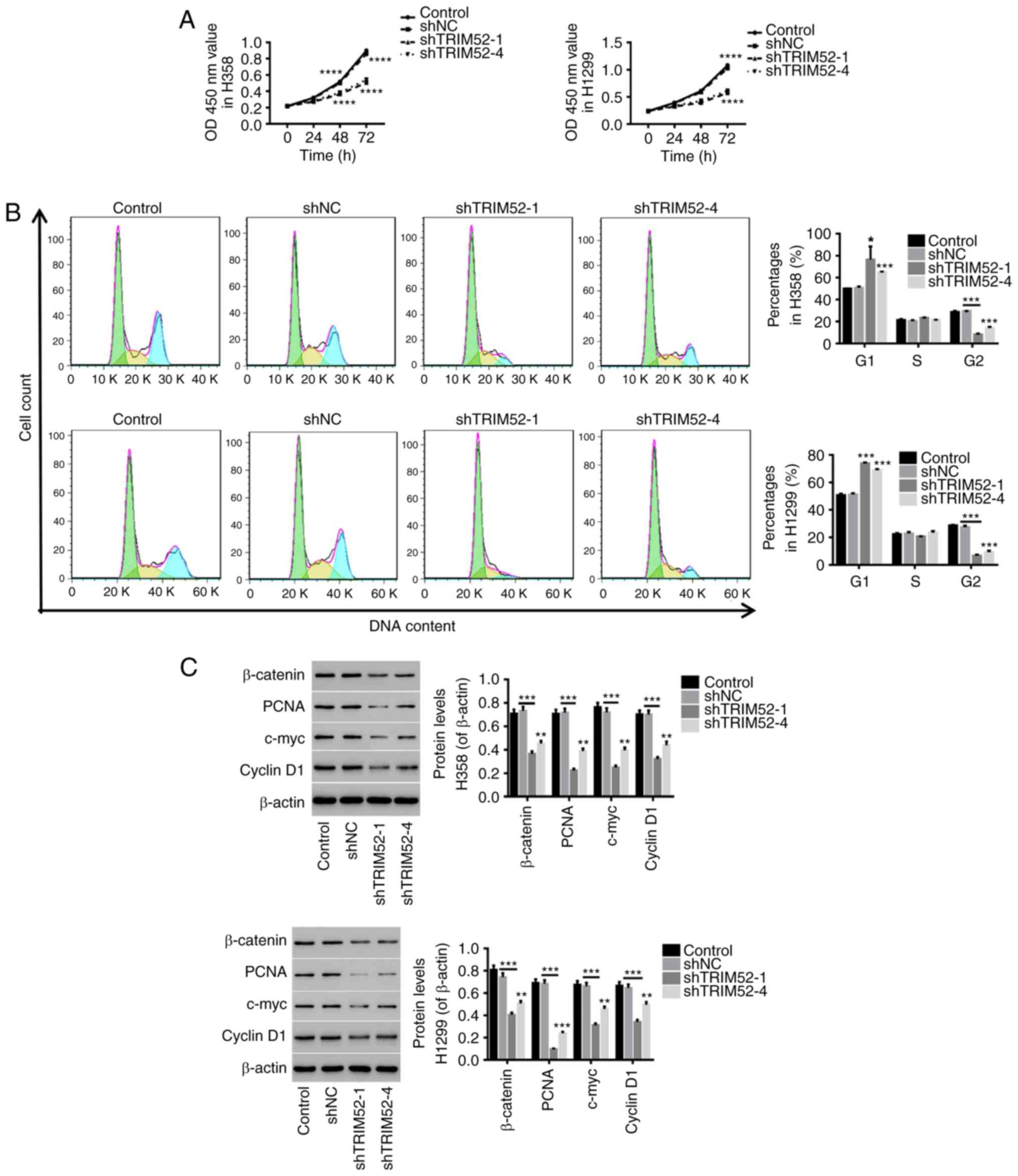 | Figure 3.Downregulation of TRIM52 inhibits
lung cancer cell proliferation via cell cycle arrest. Lung cancer
cells (H358 and H1299) were infected with shNC/shTRIM52
lentiviruses, while the cells treated with RPMI-1640 medium served
as controls. (A) The proliferation of TRIM52-slienced H358 and
H1299 cells was determined at 0, 24, 48 and 72 h with a Cell
Counting Kit-8 assay. (B) Subsequently, 48 h after infection, the
cell cycle was detected by flow cytometry. (C) The protein levels
of β-catenin, PCNA, c-Myc and Cyclin D1 were quantified by western
blotting. Data are presented as the mean ± standard deviation.
**P<0.01, ***P<0.001 and ****P<0.0001, compared with shNC.
TRIM52, tripartite motif 52; NC, control; sh, short hairpin; PCNA,
proliferating cell nuclear antigen. |
TRIM52 regulates cell proliferation,
cell cycle progression and invasion through the Wnt/β-catenin
pathway
Wnt/β-catenin signaling activation has been reported
to be a critical oncogenic event in the initiation and progression
of tumors, and c-Myc and Cyclin D1 are two downstream Wnt/β-catenin
signaling molecules (35). PCNA, a
non-histone nuclear protein that functions in DNA synthesis, is a
marker of cell proliferative activity in lung cancer (36) and has important prognostic value
(37,38). The application of the Wnt/β-catenin
inhibitor XAV939 has been reported in numerous studies (39,40).
In the present study, the Wnt/β-catenin inhibitor XAV-939 was
applied for further study. As depicted in Fig. 4, the upregulation of TRIM52
significantly promoted cell proliferation (Fig. 4A) and facilitated the entry of cells
into the S phase from the G1 phase (Fig. 4B), which was concurrent with
increases in β-catenin, PCNA, c-Myc and Cyclin D1 expression
(Fig. 4C). The upregulation of
TRIM52 in normal epithelial 16HBE cells also induced cell
proliferation and S-phase progression. Furthermore, the
invasiveness of H1975 and A549 cells was significantly increased by
TRIM52 upregulation (Fig. 4D). In
contrast, treatment with XAV-939 completely counteracted the effect
of TRIM52 upregulation in lung cancer cells, and a rescue effect of
TRIM52 upregulation was observed upon Wnt/β-catenin inhibition. It
has been reported that the activation of Wnt/β-catenin signaling is
frequently observed in lung cancer and that it promotes the
proliferation of lung cancer cells (11,41),
which is consistent with the present results. These results further
demonstrated that TRIM52 regulates the proliferation and
invasiveness of lung cancer cells possibly through regulation of
Wnt/β-catenin pathway activation.
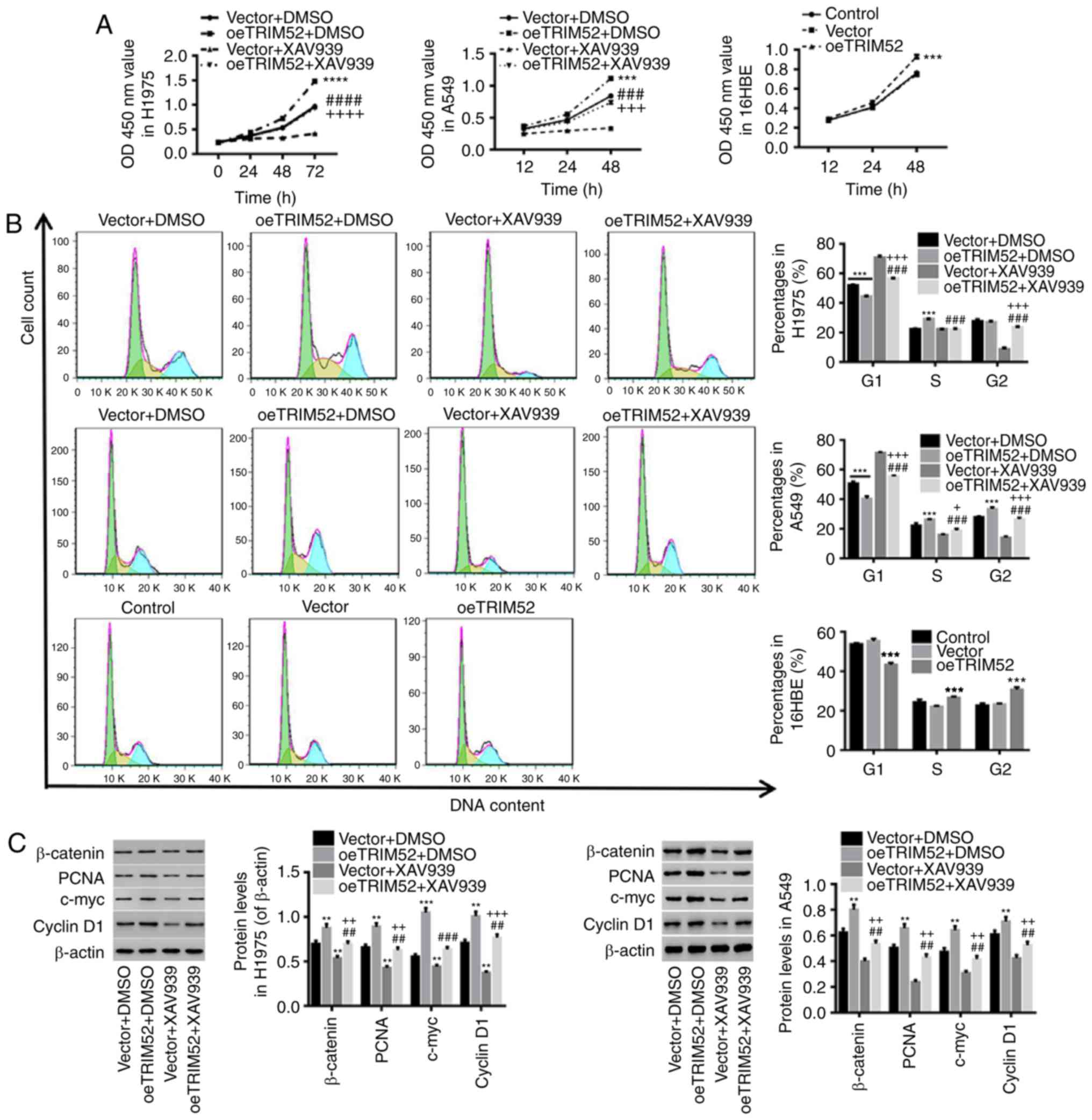 | Figure 4.TRIM52 regulates proliferation, cell
cycle and invasion through the Wnt/β-catenin pathway. H1975 and
A549 cells were treated with vector/oeTRIM52 lentiviruses and 20 µM
XAV939 (Wnt/β-catenin inhibitor), and 16HBE cells were treated with
vector/oeTRIM52 lentiviruses. RPMI-1640 medium-treated cells served
as controls. (A) The proliferation of treated-H1975, A549 and 16HBE
cells was assessed with a Cell Counting Kit-8 assay. (B) Using flow
cytometry, the cell cycle was evaluated after 48 h. (C) The levels
of β-catenin, PCNA, c-Myc and Cyclin D1 proteins were also
detected. (D) Invasiveness of H1975 and A549 cells was determined
with a Matrigel assay. Data are expressed as the mean ± standard
deviation. **P<0.01, ***P<0.001 and ****P<0.0001, oeTRIM52
or oeTRIM52 + DMSO, compared with vector or Vector + DMSO.
##P<0.01, ###P<0.001 and
####P<0.0001, oeTRIM52 + XAV939, compared with
oeTRIM52 + DMSO. +P<0.05, ++P<0.01,
+++P<0.001 and ++++P<0.0001, oeTRIM52 +
XAV939, compared with vector + XAV939. TRIM52, tripartite motif 52;
DMSO, dimethyl sulfoxide; PCNA, proliferating cell nuclear
antigen. |
Discussion
Increasing evidence demonstrates that TRIM proteins,
including TRIM29 (42), TRIM16
(43) and TRIM15 (44), are of great importance in the
development and progression of cancer. TRIM proteins were revealed
to be involved in the regulation of various cellular processes,
including cell proliferation, in cancer (45). A previous study indicated that
TRIM59 is overexpressed in NSCLC, and promotes the proliferation
and migration of NSCLC cells (46).
In the present study, it was determined that TRIM52 was elevated in
tumor tissues of patients with lung cancer and in tumor cell lines,
which indicates that TRIM52 may act as an oncogene in lung cancer.
The downregulation of TRIM52 in lung cancer cells significantly
inhibited cell proliferation by arresting cell cycle progression,
and TRIM52 upregulation promoted proliferation and invasion.
Notably, recent genomic analysis indicated that in certain genetic
cancer cell backgrounds, an appropriate expression of TRIM52 may be
essential for efficient proliferation and survival of certain
cancer cell lines (47,48), which are in agreement with the
present data. This indicates that the inhibitory effect of TRIM52
downregulation on the proliferation of lung cancer cells may
contribute to novel treatments for lung cancer.
Furthermore, it was also investigated the mechanism
that underlies TRIM52 in the regulation of lung cancer cell
proliferation and invasion. It has been reported that aberrant
activation of the Wnt/β-catenin pathway is associated with the
development and progression of cancer (49–51).
Control of Wnt/β-catenin signaling by disheveled binding antagonist
of β-catenin 3 has potential as a therapeutic strategy for
colorectal cancer (52). The
proto-oncogene c-Myc has been reported to serve a primary role in
the biological processes of tumors, including growth and apoptosis
(53). When it forms a complex with
its partner kinases, including cyclin dependent kinase 4 (CDK4) and
CDK6, Cyclin D1, which is overexpressed in a variety of human
cancer types, including breast and colon carcinoma cancer (16,54,55),
allows cells to proceed into the S phase (56). Compared with Cyclin D1, PCNA has
been reported to be elevated in the late G1 and S phases of the
cell cycle (57–59). Additionally, in the present study,
TRIM52-induced cell proliferation and S-phase cell cycle
progression were counteracted by the β-catenin inhibitor XAV939.
This was concurrent with decreased expression of β-catenin, PCNA,
c-Myc and Cyclin D1 proteins, and a rescue effect of TRIM52
upregulation on Wnt/β-catenin inhibition. These observations are in
agreement with those of previous reports, in that TRIM52 ablation
increases the proportion of cells in the G0/G1-phase (30,31,60),
which reveals that TRIM52 may regulate lung cancer cell
proliferation through activation of the Wnt/β-catenin signaling
pathway.
In summary, it was demonstrated that TRIM52 may act
as an oncogene in lung cancer progression. The downregulation of
TRIM52 significantly suppressed lung cancer cell proliferation via
blocking cell cycle progression, and TRIM52 upregulation promoted
proliferation and invasion, which may have occurred through
activation of Wnt/β-catenin signaling. Therefore, targeting TRIM52
is a potential therapeutic strategy for the treatment of lung
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by National Thirteenth
Five-Year Science and Technology Major Special Project for New Drug
Innovation and Development: The construction of a demonstration
technology platform for the clinical evaluation of new drugs for
malignant tumor and other diseases (grant no. 2017ZX09304001), and
Shanghai municipal health and Family Planning Commission: The
inhibition effects of Jinfukang Oral Solution on lung cancer
through TRIM52-Wnt/β-catenin pathway (grant no. 20174Y0053).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XM and HL conceived and designed the study. XM, LZ
and WX performed the experiments. XM and HL wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments conducted in this study were
approved by the Ethics Committee of Shanghai University of
Traditional Chinese Medicine and written informed consent was
obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin H, Bray F, Forman D,
Mathers C and Parkin D: GLOBOCAN, 2008 V1. 2, cancer incidence and
mortality Worldwide. IARC Cancer Base. 10:2010.
|
|
2
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. Ca Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blandin Knight S, Crosbie PA, Balata H,
Chudziak J, Hussell T and Dive C: Progress and prospects of early
detection in lung cancer. Open Biol. 7(pii): 1700702017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Center for Chronic Disease
Prevention Health Promotion (US) Office on Smoking Health, . The
health consequences of Smoking-50 years of progress: A report of
the surgeon general. Atlanta (GA): Centers for Disease Control and
Prevention (US); 2014
|
|
7
|
Stockwell HG, Goldman AL, Lyman GH, Noss
CI, Armstrong AW, Pinkham PA, Candelora EC and Brusa MR:
Enviromental tobacco smoke and lung cancer risk in nonsmoking
women. J Natl Cancer Inst. 84:1417–1422. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boffetta P, Agudo A, Ahrens W, Benhamou E,
Benhamou S, Darby SC, Ferro G, Fortes C, Gonzalez CA, Jöckel KH, et
al: Multicenter case-control study of exposure to environmental
tobacco smoke and lung cancer in europe. J Natl Cancer Inst.
90:1440–1450. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiyohara C, Wakai K, Mikami H, Sido K,
Ando M and Ohno Y: Risk modification by CYP1A1 and GSTM1
polymorphisms in the association of environmental tobacco smoke and
lung cancer: A case-control study in Japanese nonsmoking women. Int
J Cancer. 107:139–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nana-Sinkam SP and Powell CA: Molecular
biology of lung cancer: Diagnosis and management of lung cancer,
3rd edition: American College of Chest Physicians evidence-based
clinical practice guidelines. Chest 143 (5 Suppl). e30S–e39S. 2013.
View Article : Google Scholar
|
|
11
|
Nguyen DX, Chiang AC, Zhang HF, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF Signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen G, Shukeir N, Potti A, Sircar K,
Aprikian A, Goltzman D and Rabbani SA: Up-regulation of Wnt-1 and
beta-catenin production in patients with advanced metastatic
prostate carcinoma: Potential pathogenetic and prognostic
implications. Cancer. 101:1345–1356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang WM, Lo Muzio L, Rubini C and Yan G:
Effect of WNT-1 on beta-catenin expression and its relation to
Ki-67 and tumor differentiation in oral squamous cell carcinoma.
Oncol Rep. 13:1095–1099. 2005.PubMed/NCBI
|
|
14
|
Xu X, Sun PL, Li JZ, Jheon S, Lee CT and
Chung JH: Aberrant Wnt1/β-catenin expression is an independent poor
prognostic marker of non-small cell lung cancer after surgery. J
Thorac Oncol. 6:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tetsu O and Mccormick F: Beta-catenin
regulates expression of Cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hatakeyama S: TRIM family proteins: Roles
in autophagy, immunity, and carcinogenesis. Trends Biochem Sci.
42:297–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reymond A, Meroni G, Fantozzi A, Merla G,
Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, et
al: The tripartite motif family identifies cell compartments. EMBO
J. 20:2140–2151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mallery DL, McEwan WA, Bidgood SR, Towers
GJ, Johnson CM and James LC: Antibodies mediate intracellular
immunity through tripartite motif-containing 21 (TRIM21). Proc Natl
Acad Sci USA. 107:19985–19990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang T, Tang HM, Lu S, Yan DW, Yang YX
and Peng ZH: Up-regulation of tripartite motif-containing 29
promotes cancer cell proliferation and predicts poor survival in
colorectal cancer. Med Oncol. 30:7152013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren H, Xu Y, Wang Q, Jiang J, Wudumuli,
Hui L, Zhang Q, Zhang X, Wang E, Sun L and Qiu X: E3 ubiquitin
ligase tripartite motif-containing 71 promotes the proliferation of
non-small cell lung cancer through the inhibitor of
kappaB-α/nuclear factor kappaB pathway. Oncotarget. 9:10880–10890.
2017.PubMed/NCBI
|
|
22
|
Yamada Y, Takayama KI, Fujimura T,
Ashikari D, Obinata D, Takahashi S, Ikeda K, Kakutani S, Urano T,
Fukuhara H, et al: A novel prognostic factor TRIM44 promotes cell
proliferation and migration, and inhibits apoptosis in testicular
germ cell tumor. Cancer Sci. 108:32–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Xu C, Zhang X, Huang L, Zheng C,
Chen H, Wang Y, Ju H and Yao Q: TRIM11 upregulation contributes to
proliferation, invasion, and EMT of hepatocellular carcinoma cells.
Oncol Res. 25:691–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dükel M, Streitfeld WS, Tang TCC, Backman
LR, Ai L, May WS and Brown KD: The breast cancer tumor suppressor
TRIM29 is expressed via ATM-dependent signaling in response to
hypoxia. J Biol Chem. 291:21541–21552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Groner AC, Cato L, de Tribolet-Hardy J,
Bernasocchi T, Janouskova H, Melchers D, Houtman R, Cato ACB,
Tschopp P, Gu L, et al: TRIM24 is an oncogenic transcriptional
activator in prostate cancer. Cancer Cell. 29:846–858. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Ma C, Zhou T, Liu Y, Sun L and Yu Z:
TRIM65 negatively regulates p53 through ubiquitination. Biochem
Biophys Res Commun. 473:278–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XL, Shi WP, Shi HC, Lu SC, Wang K,
Sun C, He JS, Jin WG, Lv XX, Zou H, et al: Knockdown of TRIM65
inhibits lung cancer cell proliferation, migration and invasion: A
therapeutic target in human lung cancer. Oncotarget. 7:81527–81540.
2016.PubMed/NCBI
|
|
28
|
Li H, Zhang Y, Zhang Y, Bai X, Peng Y and
He P: TRIM31 is downregulated in non-small cell lung cancer and
serves as a potential tumor suppressor. Tumour Biol. 35:5747–5752.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malfavon-Borja R, Sawyer SL, Wu LI,
Emerman M and Malik HS: An evolutionary screen highlights canonical
and noncanonical candidate antiviral genes within the primate TRIM
gene family. Genome Biol Evol. 5:2141–2154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Wu SS, Chen XH, Tang ZH, Yu YS
and Zang GQ: Tripartite motif containing 52 (TRIM52) promotes cell
proliferation in hepatitis B virus-associated hepatocellular
carcinoma. Med Sci Monit. 23:5202–5210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Tao R, Wu SS, Xu CC, Wang JL,
Chen J, Yu YS, Tang ZH, Chen XH and Zang GQ: TRIM52 up-regulation
in hepatocellular carcinoma cells promotes proliferation, migration
and invasion through the ubiquitination of PPM1A. J Exp Clin Cancer
Res. 37:1162018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang W, Liu L, Li C, Luo N, Chen R, Li L,
Yu F and Cheng Z: TRIM52 plays an oncogenic role in ovarian cancer
associated with NF-κB pathway. Cell Death Dis. 9:9082018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong J, Kang B, Kim A, Hwang S, Ahn J, Lee
S, Kim J, Park JH and Cheon DS: Development of a highly sensitive
real-time one step RT-PCR combined complementary locked primer
technology and conjugated minor groove binder probe. Virol J.
8:3302011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nguyen VN, Mirejovský P, Mirejovský T,
Melínová L and Mandys V: Expression of Cyclin D1, Ki-67 and PCNA in
non-small cell lung cancer: Prognostic significance and comparison
with p53 and bcl-2. Acta Histochem. 102:323–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Woods AL, Hall PA, Shepherd NA, Hanby AM,
Waseem NH, Lane DP and Levison DA: The assessment of proliferating
cell nuclear antigen (PCNA)immunostaining in primary
gastrointestinal lymphomas and its relationship to histological
grade, S + G2 + M phase fraction (flow cytometric analysis) and
prognosis. Histopathology. 41:165–171. 2002.PubMed/NCBI
|
|
38
|
Yu CC, Hall PA, Fletcher CD, Camplejohn
RS, Waseem NH, Lane DP and Levison DA: Haemangiopericytomas: The
prognostic value of immunohistochemical staining with a monoclonal
antibody to proliferating cell nuclear antigen (PCNA).
Histopathology. 19:29–33. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tian XH, Hou WJ, Fang Y, Fan J, Tong H,
Bai SL, Chen Q, Xu H and Li Y: XAV939, a tankyrase 1 inhibitior,
promotes cell apoptosis in neuroblastoma cell lines by inhibiting
Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 32:1002013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu P, Wang Y, Liu X, Wang H, Zhang X, Wang
K, Wang Q and Hu R: Malignant gliomas induce and exploit astrocytic
mesenchymal-like transition by activating canonical Wnt/β-catenin
signaling. Med Oncol. 33:662016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim JH, Park JW and Chun YS: Human arrest
defective 1 acetylates and activates beta-catenin, promoting lung
cancer cell proliferation. Cancer Res. 66:10677–10682. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rui X, Hu J, Zhang T, Chao J and Wang HY:
TRIM29 overexpression is associated with poor prognosis and
promotes tumor progression by activating Wnt/β-catenin pathway in
cervical cancer. Oncotarget. 7:28579–28591. 2016.PubMed/NCBI
|
|
43
|
Kim PY, Tan O, Liu B, Trahair T, Liu T,
Haber M, Norris MD, Marshall GM and Cheung BB: High TDP43
expression is required for TRIM16-induced inhibition of cancer cell
growth and correlated with good prognosis of neuroblastoma and
breast cancer patients. Cancer Lett. 374:315–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee OH, Lee J, Lee KH, Woo YM, Kang JH,
Yoon HG, Bae SK, Songyang Z, Oh SH and Choi Y: Role of the focal
adhesion protein TRIM15 in colon cancer development. Biochim
Biophys Acta. 1853:409–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watanabe M and Hatakeyama S: TRIM proteins
and diseases. J Biochem. 161:135–144. 2017.PubMed/NCBI
|
|
46
|
Zhan W, Han T, Zhang C, Xie C, Gan M, Deng
K, Fu M and Wang JB: TRIM59 promotes the proliferation and
migration of non-small cell lung cancer cells by upregulating cell
cycle related proteins. PLoS One. 10:e01425962015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hart T, Chandrashekhar M, Aregger M,
Steinhart Z, Brown KR, Macleod G, Mis M, Zimmermann M,
Fradet-Turcotte A, Sun S, et al: High-resolution CRISPR screens
reveal fitness genes and genotype-specific cancer liabilities.
Cell. 163:1515–1526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang T, Birsoy K, Hughes NW, Krupczak KM,
Post Y, Wei JJ, Lander ES and Sabatini DM: Identification and
characterization of essential genes in the human genome. Science.
350:1096–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Peifer M and Polakis P: Wnt signaling in
oncogenesis and embryogenesis-a look outside the nucleus. Science.
287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
You Z, Saims D, Chen S, Zhang Z, Guttridge
DC, Guan K, Macdougald OA, Brown AM, Evan G, Kitajewski J and Wang
CY: Wnt signaling promotes oncogenic transformation by inhibiting
c-Myc-induced apoptosis. J Cell Biol. 157:429–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brown AM: Wnt signaling in breast cancer:
Have we come full circle? Breast Cancer Res. 3:351–355. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang X, Tan J, Li J, Kivimäe S, Yang X,
Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, et al: DACT3 is an
epigenetic regulator of Wnt/beta-catenin signaling in colorectal
cancer and is a therapeutic target of histone modifications. Cancer
Cell. 13:529–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Miller DM, Thomas SD, Islam A, Muench D
and Sedoris K: c-Myc and cancer metabolism. Clin Cancer Res.
18:5546–5553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hall M and Peters G: Genetic alterations
of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human
cancer. Adv Cancer Res. 68:67–108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yu Q, Geng Y and Sicinski P: Specific
protection against breast cancers by Cyclin D1 ablation. Nature.
411:1017–1021. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lukas J, Bartkova J, Rohde M, Strauss M
and Bartek J: Cyclin D1 is dispensable for G1 control in
retinoblastoma gene-deficient cells independently of cdk4 activity.
Mol Cell Biol. 15:2600–2611. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Griffey SM, Kraegel SA and Madewell BR:
Proliferation indices in spontaneous canine lung cancer:
Proliferating cell nuclear antigen (PCNA), Ki-67 (MIB1) and mitotic
counts. J Comp Pathol. 120:321–332. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Celis JE and Celis A: Cell cycle-dependent
variations in the distribution of the nuclear protein cyclin
proliferating cell nuclear antigen in cultured cells: Subdivision
of S phase. Proc Natl Acad Sci USA. 82:3262–3266. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bravo R, Frank R, Blundell PA and
Macdonaldbravo H: Cyclin/PCNA is the auxiliary protein of DNA
polymerase-delta. Nature. 326:515–517. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Benke S, Agerer B, Haas L, Stöger M,
Lercher A, Gabler L, Kiss I, Scinicariello S, Berger W, Bergthaler
A, et al: Human tripartite motif protein 52 is required for cell
context-dependent proliferation. Oncotarget. 9:13565–13581. 2018.
View Article : Google Scholar : PubMed/NCBI
|