Introduction
Lung cancer is the most common cancer, and the fifth
most common cause of death worldwide, primarily because of high
invasion, metastasis, and drug resistance (1,2).
Approximately 85% of all lung cancer cases are classified as
non-small cell lung cancer (NSCLC), including adenocarcinoma (Ade)
and squamous cell carcinoma (SCC) subtypes (3). Although multidisciplinary therapies are
widely used to treat NSCLC, its overall prognosis remains very
poor. In addition, currently there is no standard method to predict
the survival of patients with NSCLC (4). Hence, there is an urgent need for novel
and effective prognostic biomarkers for NSCLC.
Insulin-like growth factors (IGFs) are peptide
ligands that regulate cellular proliferation, differentiation,
apoptosis, and carcinogenesis (5,6). IGF
binding proteins (IGFBPs) are circulating proteins that modulate
IGF signaling by sequestering the circulating IGFs, thereby
regulating the mitogenic activity of the IGF receptors (7). The conventional IGFBP family has six
members (IGFBP1-6), which bind IGFs with high affinity (8). However, the concept of IGFBPs has
recently been redefined to include proteins that increase the
half-life of IGFs. Now, at least 10 members of the IGFBP
superfamily have been identified, including proteins that bind IGFs
with low affinity (9). Recently,
conventional IGFBPs have attracted increased attention due to their
roles in NSCLC. Previous studies have demonstrated abnormal
expression of IGFBPs in NSCLC, and assessed the diagnostic roles of
circulating IGFBP concentrations in the disease (10–17).
However, the prognostic roles of individual IGFBPs in NSCLC,
particularly at the mRNA level, remain unknown.
The development of microarray and RNA-sequencing
technology has revolutionized RNA and DNA research, providing a
wealth of data for bioinformatic analysis. In the present study,
data mining analysis was performed from patients with NSCLC using
various tools, with the purpose of exploring the differential
expression, potential functions, and distinct prognostic values of
IGFBP family members in NSCLC.
Materials and methods
Gene expression profiling
analysis
Gene Expression Profiling Interactive Analysis
(GEPIA; http://gepia.cancer-pku.cn) is a
newly developed interactive web server for the analysis of RNA
sequencing data derived from 9,736 tumors and 8,587 healthy samples
from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression
datasets. GEPIA provides customizable functions including
differential expression analysis, profile plotting, correlation
analysis, patient survival analysis, detection of similar genes,
and dimensionality reduction analysis (18). The expression of IGFBPS between tumor
and normal tissues was analyzed using Student's t-test, and
expression of IGFBPs in different tumor stages of NSCLC was
analyzed using F-test. P<0.01 and fold change (FC)>2 were
considered significant. In addition, IGFBP protein levels were
analyzed using the Human Protein Atlas database (HPA) (https://www.proteinatlas.org/) to confirm whether the
expression at the mRNA and protein levels matched (19).
Prognostic analysis
The prognostic value of the mRNA expression of IGFBP
family members was evaluated using an online tool, Kaplan-Meier
Plotter (www.kmplot.com) and GEPIA (http://gepia.cancer-pku.cn). To analyze the overall
survival (OS) of patients with NSCLC, patient samples were divided
into two groups (low and high expression) based on median mRNA
levels with a hazard ratio (HR) with 95% confidence intervals (CI)
and log-rank P-values (20). Log-rank
P-values <0.05 were considered statistically significant.
Univariate cox analysis was conducted with adjustments to smoking
status, clinical stages, chemotherapy, and sex of NSCLC.
Analysis of gene alteration
frequency
Known alterations in IGFBP genes in patients with
NSCLC were obtained from the cBioPortal for Cancer Genomics
(http://www.cbioportal.org) (21). Genomic profiles, including mutations,
putative copy-number alterations, and mRNA expression levels, were
selected by querying individual IGFBP family members.
Functional enrichment and
bioinformatics analysis
GeneMANIA (http://www.genemania.org), a prediction server that
acts as a biological network integrator for gene prioritization and
function prediction (22), was used
for correlation analysis of IGFBP family members at the gene level.
Enrichment analysis for gene ontology (GO) terms and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways (23,24) was
performed in the Database for Annotation, Visualization and
Integrated Discovery (DAVID; version 6.7, http://david.ncifcrf.gov/tools.jsp).
Results
IGFBP mRNA levels in patients with
NSCLC
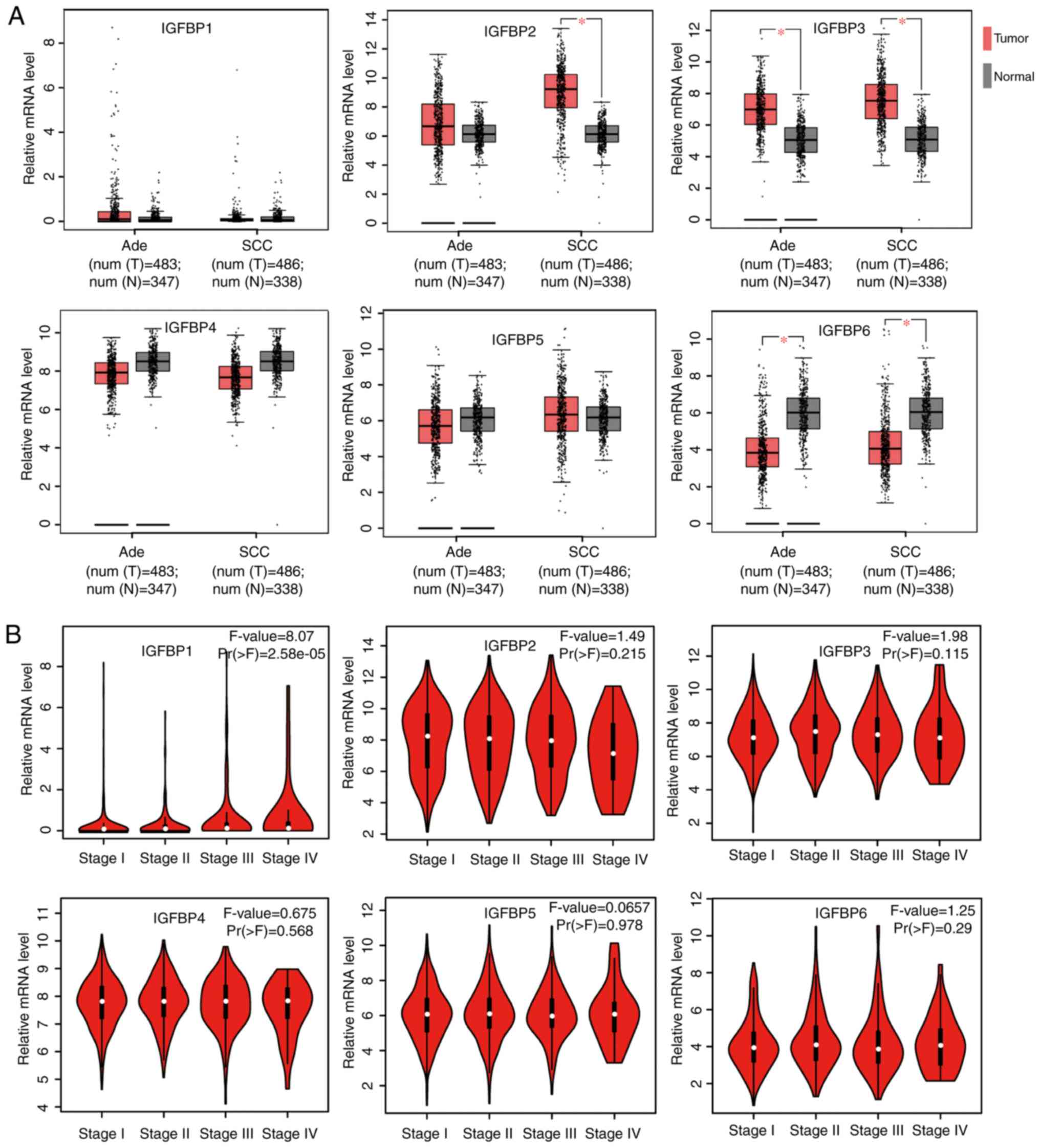
The relative mRNA expression of IGFBP genes in Ade
and SCC were examined, and compared to healthy tissue using GEPIA
analysis. Compared to healthy lung tissues, IGFBP2 mRNA expression
was significantly higher in SCC tissues, IGFBP3 mRNA expression was
significantly higher in both Ade and SCC tissues, and IGFBP6 mRNA
expression was significantly lower in both NSCLC subtypes.
Differences in expression between lung cancer and healthy tissues
were not observed for other IGFBPs (Fig.
1A). IGFBP expression was also investigated in different stages
of NSCLC. Only IGFBP1 expression changed significantly across
various tumor stages, whereas, the rest of the expression levels of
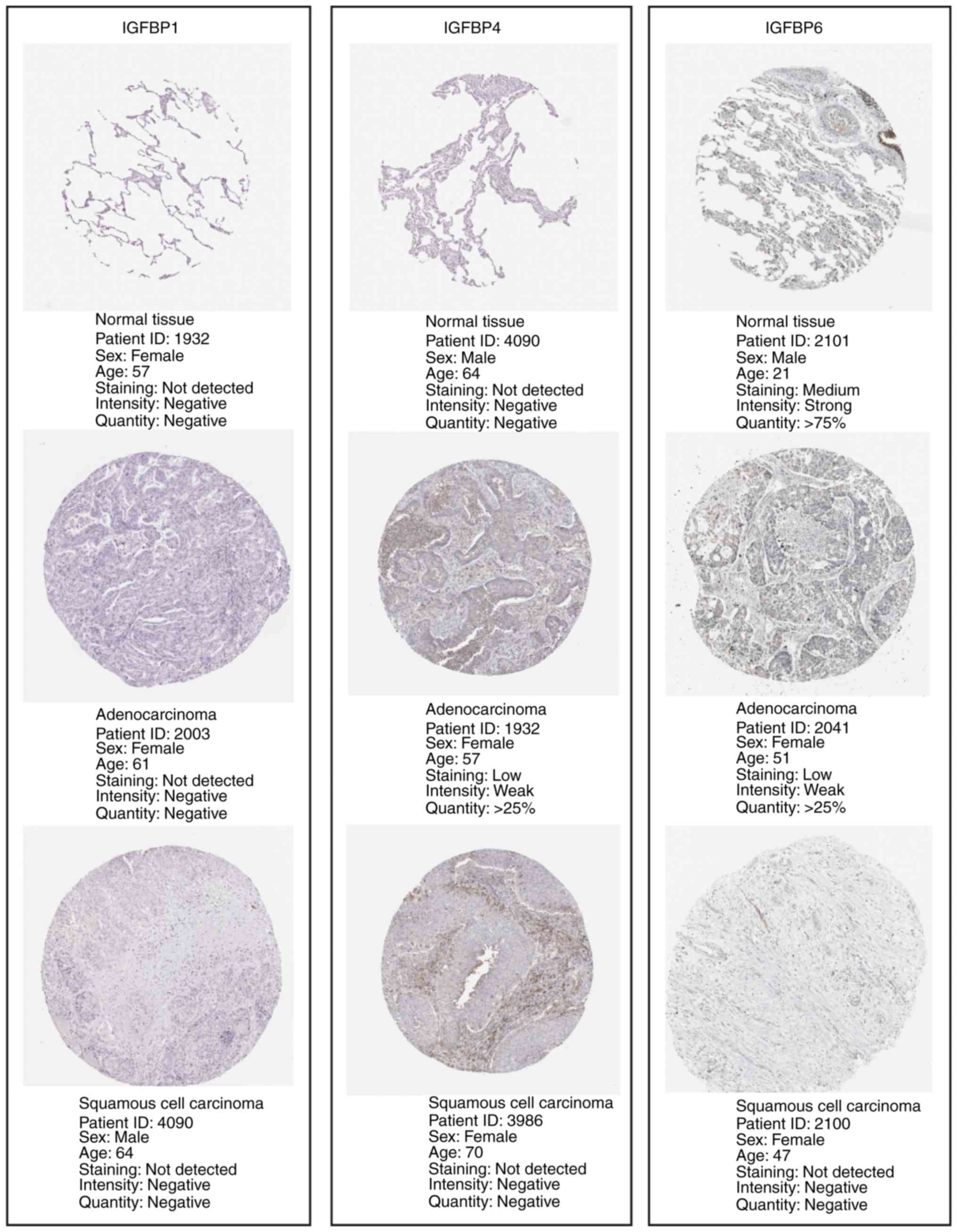
IGFBPs in various tumor stages were not differential (Fig. 1B). Additionally, the mRNA expression
levels of IGFBP1, IGFBP4, and IGFBP6 matched their reported protein
expression levels. However, representative images of the IGFBP2,
IGFBP3, and IGFBP5 protein levels were not available in the HPA
database (Fig. 2).
Prognostic value of IGFBP mRNA levels
in NSCLC
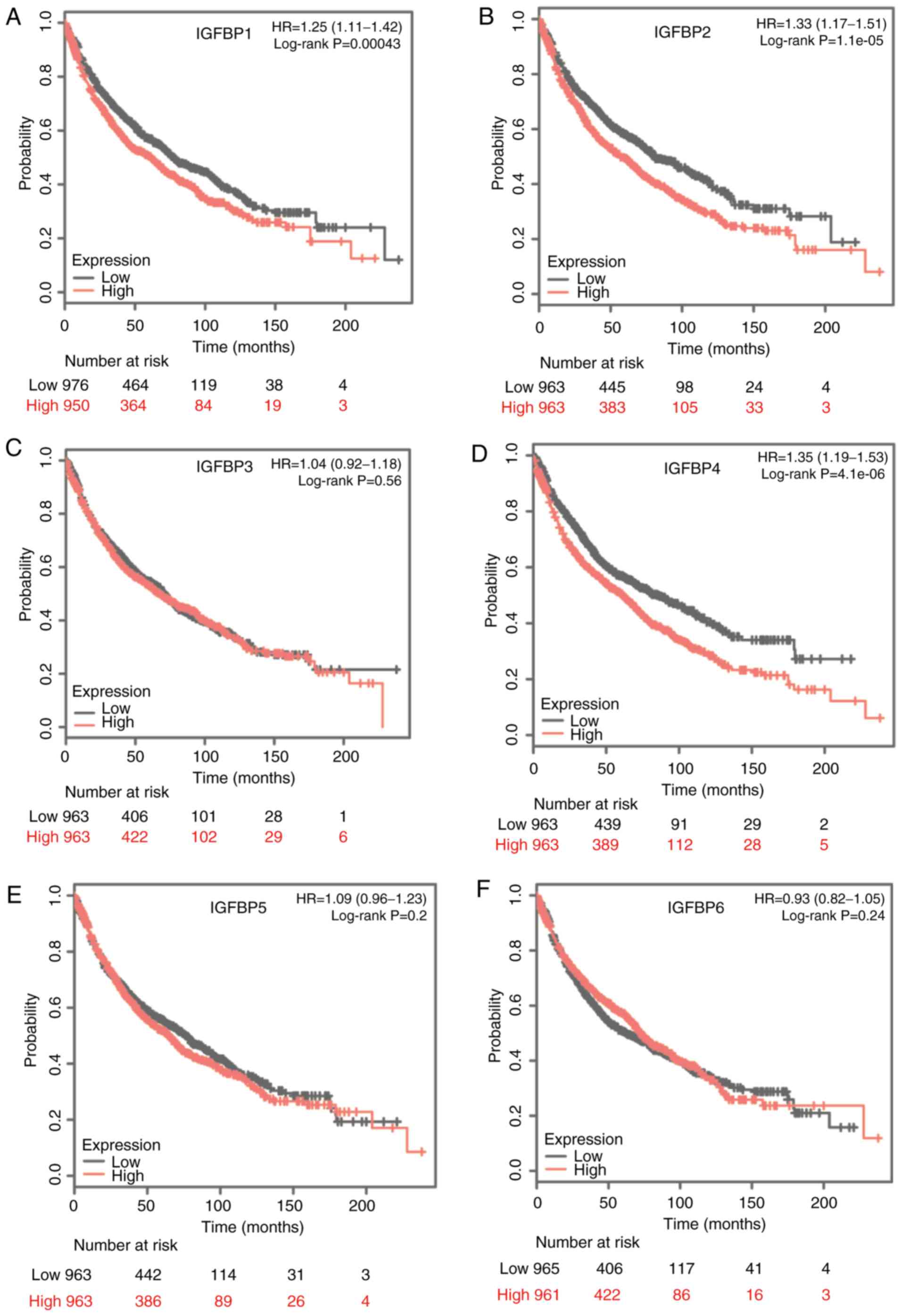
Next, the prognostic significance of IGFBP levels
were assessed, both in the total NSCLC cohort and in the Ade and
SCC subtypes, using Kaplan-Meier analysis. For the complete cohort,
increase in IGFBP1, IGFBP2, and IGFBP4 mRNA was strongly associated
with unfavorable OS, while IGFBP3, IGFBP5, and IGFBP6 mRNA levels
were not significantly correlated with the OS (Fig. 3). Increased IGFBP1, IGFBP2, and IGFBP4
mRNA levels were correlated with unfavorable OS in patients with
Ade, while IGFBP3, IGFBP5 and IGFBP6 mRNA levels were not
associated with the OS (Fig. 4).
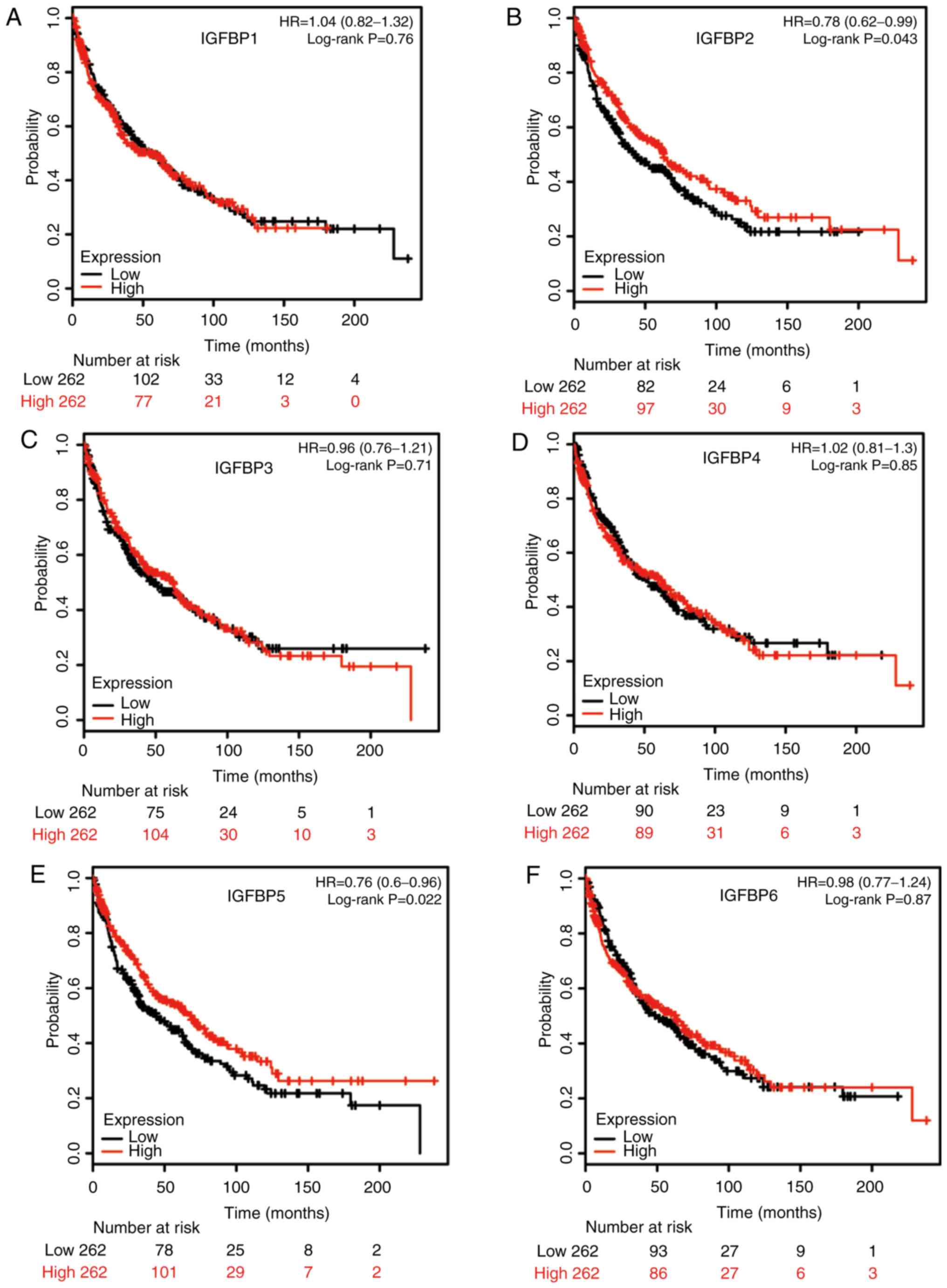
Additionally, increased IGFBP2 and IGFBP5 mRNA levels were
correlated with favorable OS in SCC patients, while the mRNA levels
of other IGFBPs were not significantly correlated with the OS
(Fig. 5). Notably, these results
indicated that IGFBP2 plays different prognostic roles in Ade and
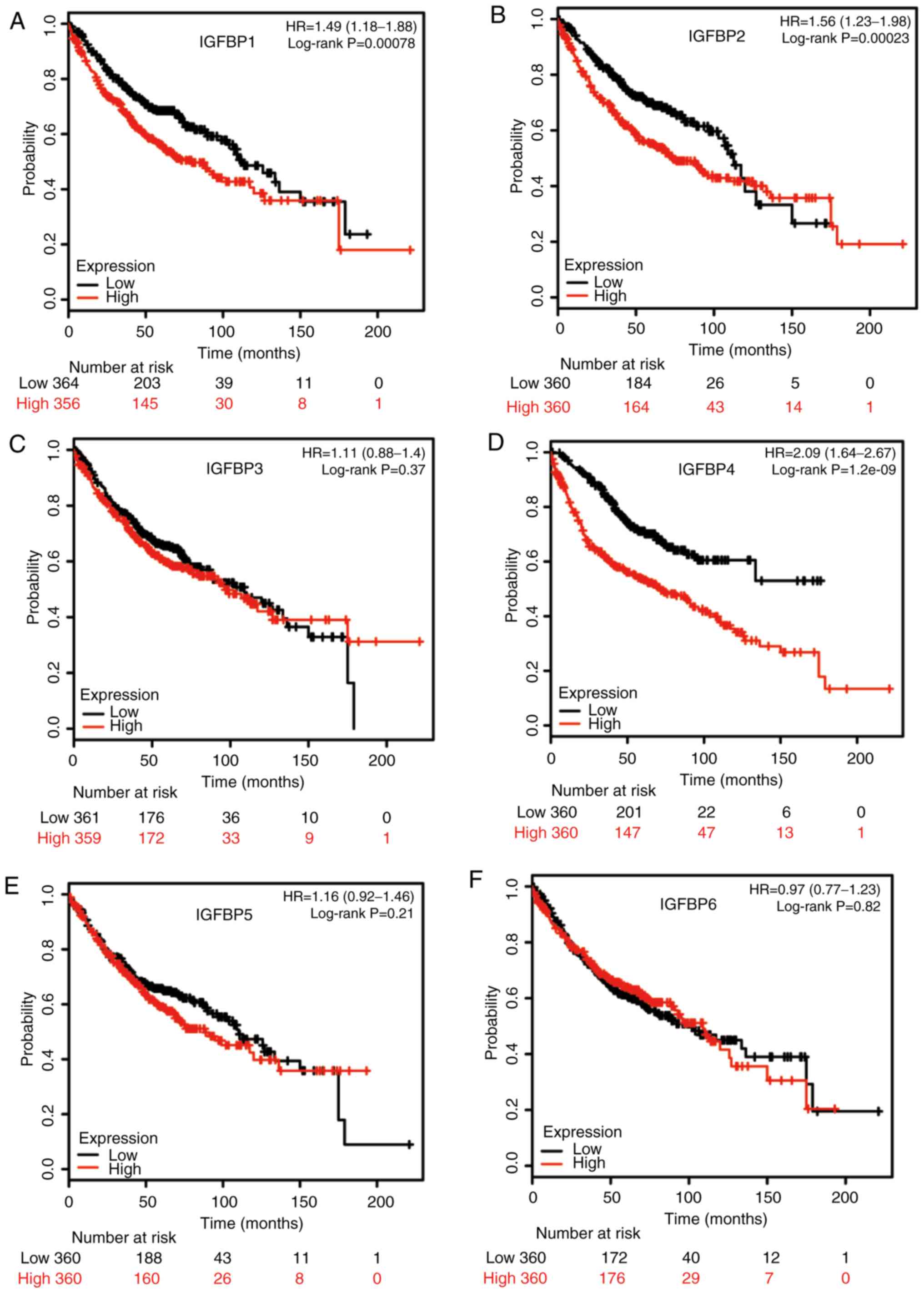
SCC. The prognostic values of IGFBP family members were validated
using the NSCLC data available in GEPIA. As revealed in Fig. 6, increased IGFBP1 and IGFBP3 mRNA was
correlated with unfavorable OS in NSCLC patients, while other
IGFBPs were not significantly correlated with the OS.
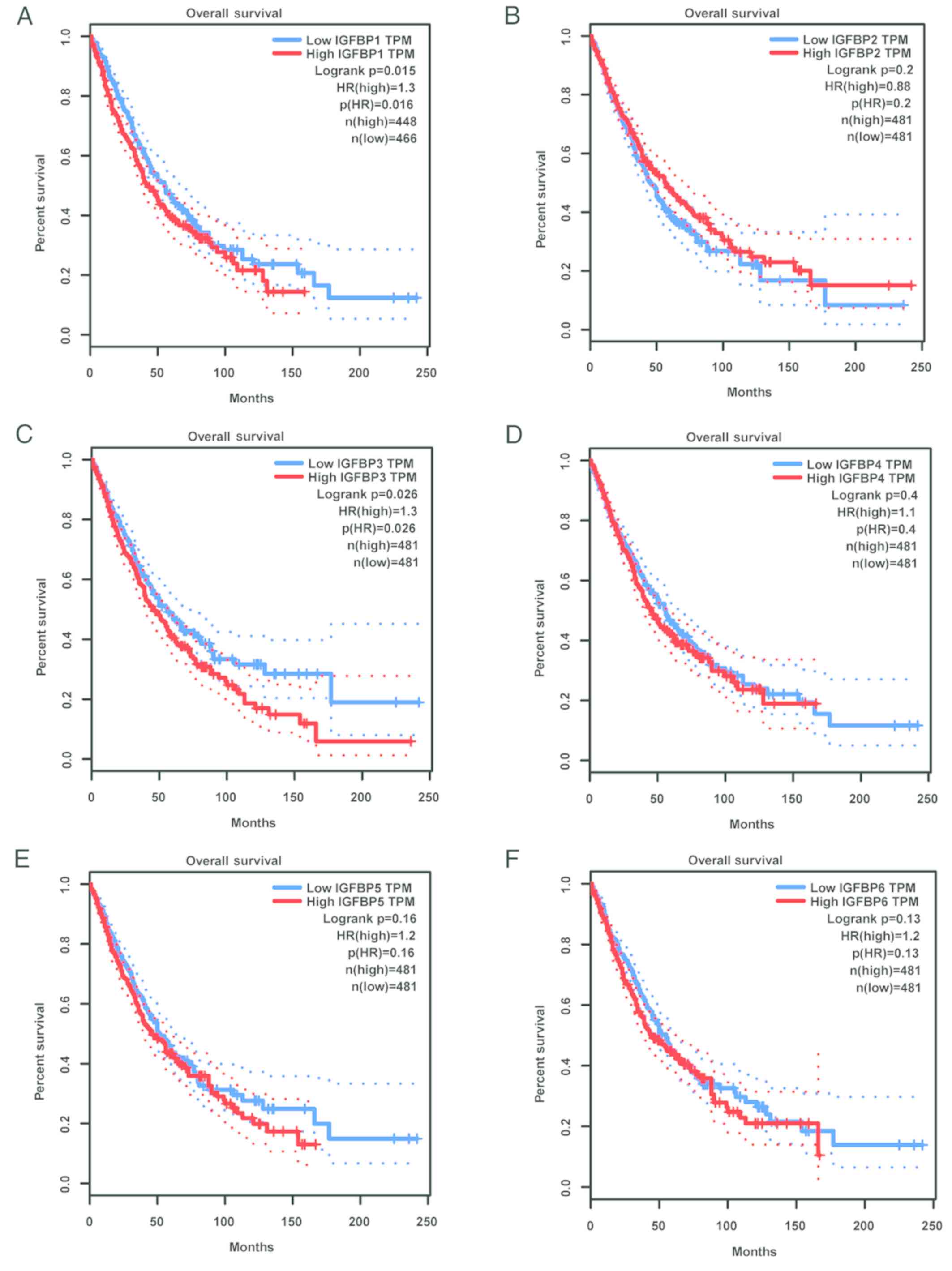 | Figure 6.Validation of IGFBP prognostic values
by GEPIA. OS curves for (A) IGFBP1, (B) IGFBP2, (C) IGFBP3, (D)
IGFBP4, (E) IGFBP5, and (F) IGFBP6 in all cases of NSCLC. Survival
curves marked as complete lines, and 95% confidence interval of
survival curves marked as dotted lines. Red represents high
expression and blue represents low expression. IGFBP, insulin-like
growth factor-binding protein; GEPIA, Gene Expression Profiling
Interactive Analysis; OS, overall survival; NSCLC, non-small cell
lung cancer. |
Prognostic values of IGFBP levels in
NSCLC subsets with different clinicopathological features
To assess for correlations between IGFBP expression
and other clinicopathological features, the smoking status
(Table I), clinical stages (Table II), chemotherapy treatments (Table III), and sex (Table IV) of patients with NSCLC were
examined. High IGFBP2, IGFBP3, and IGFBP4 mRNA levels were
associated with unfavorable OS in patients who had never smoked,
while high IGFBP1 and IGFBP4 mRNA levels were associated with
unfavorable OS in patients with a history of smoking (Table I). These results indicated that the
prognostic role of IGFBP4 in NSCLC is independent of the
smoking status.
 | Table I.Correlation between IGFBP mRNA level
and OS in NSCLC patients with smoking status. |
Table I.
Correlation between IGFBP mRNA level
and OS in NSCLC patients with smoking status.
| IGFBP
family | Smoking status | Cases | HR | 95% CI | P-value |
|---|
| IGFBP1 | Never smoked | 205 | 1.62 | 0.91–2.88 | 0.097 |
|
| smoked | 820 | 1.38 | 1.12–1.7 | 0.0025 |
| IGFBP2 | Never smoked | 205 | 2.75 | 1.5–5.03 | 0.00066 |
|
| smoked | 820 | 1.09 | 0.89–1.34 | 0.41 |
| IGFBP3 | Never smoked | 205 | 1.76 | 0.99–3.12 | 0.049 |
|
| smoked | 820 | 0.98 | 0.8–1.21 | 0.87 |
| IGFBP4 | Never smoked | 205 | 2.7 | 1.47–4.95 | 0.00083 |
|
| smoked | 820 | 1.46 | 1.18–1.8 | 0.00043 |
| IGFBP5 | Never smoked | 205 | 1.64 | 0.92–2.9 | 0.087 |
|
| smoked | 820 | 0.97 | 0.79–1.19 | 0.76 |
| IGFBP6 | Never smoked | 205 | 1.48 | 0.84–2.6 | 0.18 |
|
| smoked | 820 | 1.01 | 0.82–1.24 | 0.91 |
 | Table II.Correlation between IGFBP mRNA level
and OS in NSCLC patients with clinical stages. |
Table II.
Correlation between IGFBP mRNA level
and OS in NSCLC patients with clinical stages.
| IGFBP
family | Clinical
stages | Cases | HR | 95% CI | P-value |
|---|
| IGFBP1 | I | 577 | 1.65 | 1.26–2.17 | 0.00027 |
|
| II | 144 | 0.98 | 0.68–1.41 | 0.91 |
|
| III | 70 | 1.03 | 0.6–1.77 | 0.92 |
| IGFBP2 | I | 577 | 1.94 | 1.47–2.57 | 2.3e-06 |
|
| II | 144 | 1.45 | 1-2.09 | 0.047 |
|
| III | 70 | 1.12 | 0.65–1.94 | 0.68 |
| IGFBP3 | I | 577 | 1.06 | 0.81–1.39 | 0.68 |
|
| II | 144 | 1 | 0.69–1.44 | 1 |
|
| III | 70 | 1.2 | 0.69–2.08 | 0.53 |
| IGFBP4 | I | 577 | 1.87 | 1.42–2.47 | 6.9e-06 |
|
| II | 144 | 2.13 | 1.47–3.09 | 4.7e-05 |
|
| III | 70 | 0.97 | 0.56–1.69 | 0.92 |
| IGFBP5 | I | 577 | 1.23 | 0.94–1.62 | 0.13 |
|
| II | 144 | 0.94 | 0.65–1.35 | 0.72 |
|
| III | 70 | 0.97 | 0.56–1.66 | 0.9 |
| IGFBP6 | I | 577 | 1.01 | 0.77–1.32 | 0.96 |
|
| II | 144 | 1.01 | 0.7–1.46 | 0.95 |
|
| III | 70 | 0.81 | 0.47–1.4 | 0.45 |
 | Table III.Correlation between IGFBP mRNA level
and OS in NSCLC patients with chemotherapy status. |
Table III.
Correlation between IGFBP mRNA level
and OS in NSCLC patients with chemotherapy status.
| IGFBP
family | Chemotherapy | Cases | HR | 95% CI | P-value |
|---|
| IGFBP1 | No | 310 | 1.38 | 0.99–1.93 | 0.06 |
|
| Yes | 176 | 0.81 | 0.53–1.22 | 0.31 |
| IGFBP2 | No | 310 | 1.03 | 0.74–1.43 | 0.88 |
|
| Yes | 176 | 1.24 | 0.82–1.86 | 0.3 |
| IGFBP3 | No | 310 | 1.39 | 0.99–1.94 | 0.055 |
|
| Yes | 176 | 1.28 | 0.85–1.93 | 0.23 |
| IGFBP4 | No | 310 | 1.18 | 0.85–1.65 | 0.33 |
|
| Yes | 176 | 1.16 | 0.77–1.75 | 0.48 |
| IGFBP5 | No | 310 | 1.42 | 1.01–1.98 | 0.04 |
|
| Yes | 176 | 0.86 | 0.57–1.3 | 0.48 |
| IGFBP6 | No | 310 | 1.05 | 0.75–1.46 | 0.78 |
|
| Yes | 176 | 1.24 | 0.82–1.85 | 0.3 |
 | Table IV.Correlation between IGFBP mRNA level
and OS in NSCLC patients with different sex. |
Table IV.
Correlation between IGFBP mRNA level
and OS in NSCLC patients with different sex.
| IGFBP
family | Sex | Cases | HR | 95% CI | P-value |
|---|
| IGFBP1 | Female | 715 | 1.37 | 1.08–1.73 | 0.0085 |
|
| Male | 1,100 | 1.16 | 0.99–1.36 | 0.066 |
| IGFBP2 | Female | 715 | 1.1 | 0.88–1.39 | 0.4019 |
|
| Male | 1,100 | 1.3 | 1.11–1.52 | 0.0012 |
| IGFBP3 | Female | 715 | 1.04 | 0.82–1.31 | 0.77 |
|
| Male | 1,100 | 1.05 | 0.89–1.22 | 0.58 |
| IGFBP4 | Female | 715 | 1.32 | 1.05–1.67 | 0.019 |
|
| Male | 1,100 | 1.31 | 1.12–1.54 | 0.00067 |
| IGFBP5 | Female | 715 | 1.04 | 0.83–1.31 | 0.72 |
|
| Male | 1,100 | 1.02 | 0.87–1.2 | 0.79 |
| IGFBP6 | Female | 715 | 1.01 | 0.8–1.28 | 0.91 |
|
| Male | 1,100 | 0.87 | 0.75–1.02 | 0.095 |
High IGFBP1, IGFBP2, and IGFBP4 mRNA levels were
significantly correlated with unfavorable OS in patients with stage
I NSCLC (Table II), and high IGFBP2
and IGFBP4 mRNA levels were associated with unfavorable OS in stage
II NSCLC. These results indicated that IGFBP1, IGFBP2, and
IGFBP4 have prognostic roles in early-stage NSCLC. Increased
IGFBP5 mRNA was significantly associated with unfavorable OS in
patients who did not receive chemotherapy (Table III). Moreover, increased levels of
IGFBP1 mRNA were significantly associated with unfavorable OS in
female patients, and increased IGFBP2 mRNA levels were
significantly associated with unfavorable OS in male patients.
Increased IGFBP4 mRNA levels were significantly associated with
unfavorable OS in both female and male patients (Table IV).
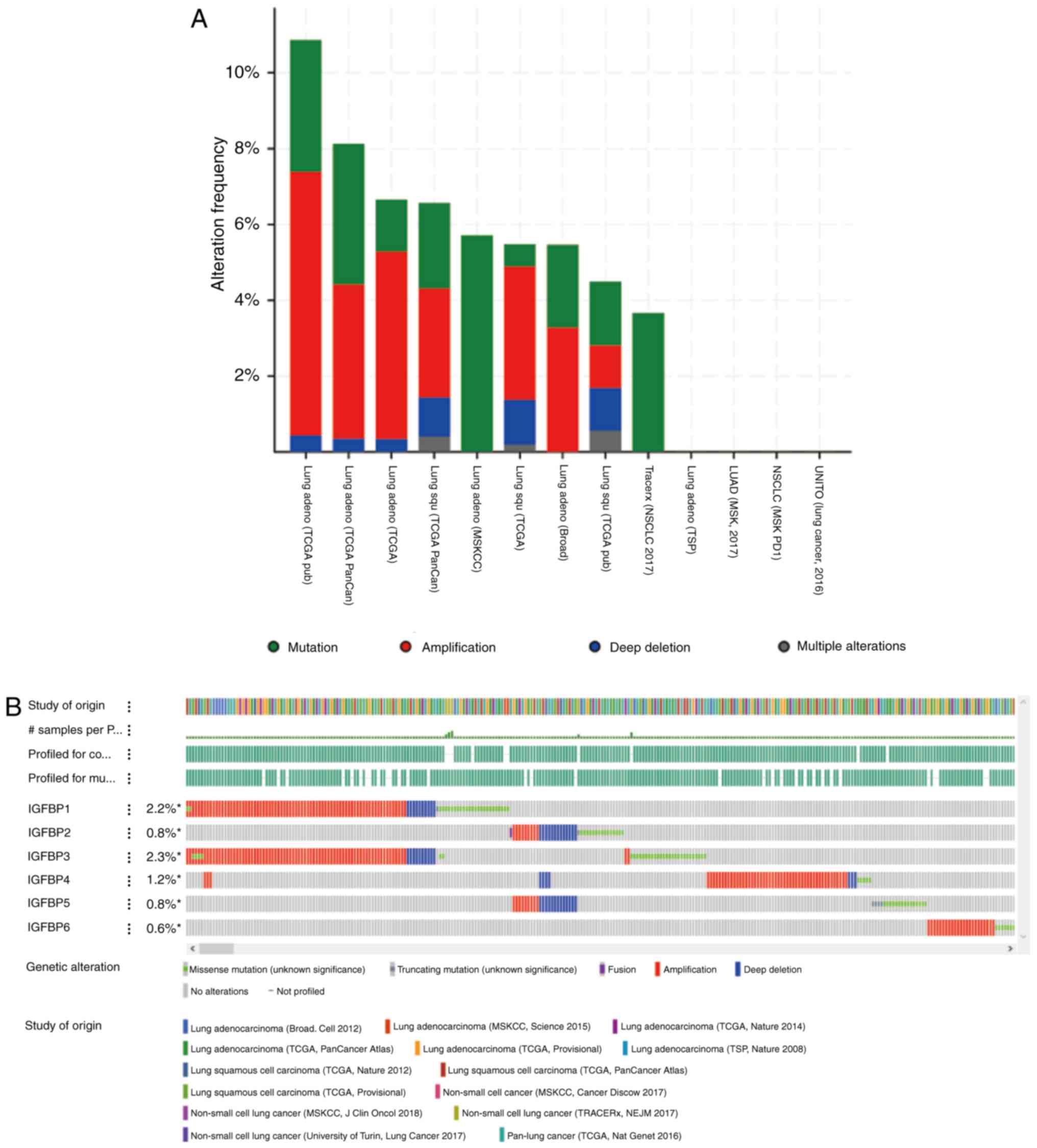
IGFBP alterations in NSCLC
The genetic alterations present in IGFBPs were
analyzed in NSCLC using cBioPortal. Thirteen NSCLC datasets were
analyzed. Among the datasets analyzed, the frequency of gene
alterations, including mutations, fusions, amplifications, deep
deletions, and multiple alterations ranged from 4.49% (8/178) to
10.87% (25/230), with mutations, amplifications, and deep deletions
being the most commonly observed alterations (Fig. 7A). The percentages of genetic
alterations in specific IGFBPs in NSCLC ranged from 0.6–2.3%
(IGFBP1, 2.2; IGFBP2, 0.8%; IGFBP3, 2.3%; IGFBP4, 1.2; IGFBP5,
0.8%; IGFBP6, 0.6%; Fig. 7B), and
were predominantly amplifications, deep deletions, and mutations;
these were consistent with the results in Fig. 7A. The prognostic roles of IGFBPs in
patients with NSCLC with or without alterations was analyzed, and
no significant correlation between the presence of alterations and
OS and disease-free survival (DFS) was observed (P=0.115 and
P=0.700, respectively; Fig. 7C and
D).
Next, GeneMANIA was used to construct a network of
IGFBPs and their functionally related genes. The database
identified 20 genes that were closely associated with IGFBPs.
Additionally, all IGFBPs had a protein binding domain, and IGFBP3
and IGFBP4 were co-expressed, and colocalized within the cell
(Fig. 7E).
Enrichment analysis of IGFBPs in
NSCLC
IGFBP functions were analyzed in DAVID, and 14 GO
terms were enriched (Table V). IGFBPs
were enriched in the following biological processes (BP): Type B
pancreatic cell proliferation, positive regulation of insulin-like
growth factor receptor signaling pathway, regulation of glucose
metabolic process, regulation of insulin-like growth factor
receptor signaling pathway, regulation of cell growth, and negative
regulation of smooth muscle cell migration. Molecular functions
(MF) associated with IGFBPs were fibronectin binding, insulin-like
growth factor II binding, and insulin-like growth factor I binding;
cellular components (CC) associated with IGFBPs were the
insulin-like growth factor ternary complex and the extracellular
space. No KEGG pathways were enriched for IGFBPs.
 | Table V.The GO function enrichment analysis
of IGFBPs in NSCLC. |
Table V.
The GO function enrichment analysis
of IGFBPs in NSCLC.
| Category | Term | Description | Count | P-value |
|---|
|
GOTERM_BP_DIRECT | GO:0044342 | Type B pancreatic
cell proliferation | 3 | 1.98E-06 |
|
GOTERM_BP_DIRECT | GO:0043568 | Positive regulation
of insulin-like growth factor receptor signaling pathway | 3 | 4.74E-06 |
|
GOTERM_BP_DIRECT | GO:0010906 | Regulation of
glucose metabolic process | 3 | 1.58E-05 |
|
GOTERM_BP_DIRECT | GO:0043567 | Regulation of
insulin-like growth factor receptor signaling pathway | 6 | 2.37E-17 |
|
GOTERM_BP_DIRECT | GO:0001558 | Regulation of cell
growth | 6 | 4.16E-14 |
|
GOTERM_BP_DIRECT | GO:0014912 | Negative regulation
of smooth muscle cell migration | 2 | 0.002837913 |
|
GOTERM_BP_DIRECT | GO:0014912 | Negative regulation
of smooth muscle cell migration | 2 | 0.002837913 |
|
GOTERM_MF_DIRECT | GO:0001968 | Fibronectin
binding | 2 | 0.002150352 |
|
GOTERM_MF_DIRECT | GO:0031995 | Insulin-like growth
factor II binding | 6 | 3.40E-18 |
|
GOTERM_MF_DIRECT | GO:0031994 | Insulin-like growth
factor I binding | 6 | 3.40E-18 |
|
GOTERM_CC_DIRECT | GO:0042567 | Insulin-like growth
factor ternary complex | 2 | 0.001436265 |
|
GOTERM_CC_DIRECT | GO:0005615 | Extracellular
space | 6 | 6.76E-07 |
Discussion
IGFBPs modulate cellular functions by
both IGF-dependent and -independent mechanisms
IGF proteins regulate cellular proliferation,
differentiation, apoptosis, and carcinogenesis, and IGFBPs modulate
their signaling through IGF sequestration. The IGF-independent
functions of IGFBPs depend on their interactions with many
signaling pathways, which include both stimulatory and inhibitory
cell-surface receptors such as the epidermal growth factor and
transforming growth factor (TGF)-β receptors. In addition, IGFBPs
regulate enzymes involved in sphingolipid metabolism. In this
manner, IGFBPs can affect the balance between growth-inhibitory
lipids, such as ceramides, and growth-stimulatory lipids, such as
sphingosine-1-phosphate (25). In the
present study, a bioinformatics approach was used to examine the
effects of these genes on NSCLC.
IGFBP1 mainly functions in the intracellular and
pericellular compartments to regulate cell growth and survival
(25). It interacts with several
proteins in addition to IGF ligands and plays an important role in
the development and progression of several cancer types (25–28). An
animal study revealed that IGFBP1 may function as a cell survival
factor by suppressing TGFβ1 activation (29). Sharma et al reported that
elevated IGFBP1 levels were associated with unfavorable OS in
prostate cancer (30). Recently,
however, Cao et al observed that low levels of IGFBP1
increased the risk of cancer (31).
However, in the present study, differential IGFBP1 expression was
not observed between tumor and healthy tissues, but differential
expression was observed in different tumor stages. High IGFBP1 mRNA
was correlated with unfavorable OS in the total NSCLC cohort, who
were followed for a 20-year period. High levels of IGFBP1 mRNA were
also correlated with unfavorable OS in Ade but not in SCC.
IGFBP2, a critical mediator of crosstalk between
several signaling pathways, is overexpressed in various cancer
types, including breast, ovarian, gastric, and colorectal cancer,
glioma, prostate cancer, leukemia, melanoma, rhabdomyosarcoma, as
well as lung cancer (32). High
IGFBP2 expression was revealed to be associated with poor prognosis
in lung cancer (11,33). IGFBP2 has tumorigenic functions, and
may act by regulating the phosphatase and tensin homolog
(PTEN)-phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway
(33). However, conflicting results
have also been reported. An in vitro study revealed that
IGFBP2 suppressed the growth of various types of lung cancer tumors
(34,35). In this study, IGFBP2 expression was
significantly upregulated in SCC tissues compared with normal
tissues. Consistent with previous research, the present study
revealed that high IGFBP2 mRNA expression was significantly
associated with unfavorable OS in the total NSCLC cohort and
patients with Ade specifically. However, high IGFBP2 mRNA levels
were significantly correlated with favorable OS in patients with
SCC. Thus, there is conflicting evidence as to whether IGFBP2 is
oncogenic or tumor suppressive and its exact mechanism of action
will require further investigation.
IGFBP3 was revealed to inhibit the mitogenic and
antiapoptotic functions of IGF1 (36). To date, many epidemiological studies
have demonstrated that low IGFBP3 expression increases the
incidence of cancer. In addition, IGFBP3 overexpression was
revealed to inhibit NSCLC cell growth and tumorigenicity in
vivo and in vitro (37–39).
IGFBP3 inhibited angiogenesis in lung tumors by blocking the
autocrine and paracrine loops of angiogenic factors (40); targeting of IGFBP3 by miRNA-125b was
associated with tumor invasion and poor patient outcomes in NSCLC
(41). Consistently, high plasma
levels of IGFBP3 were revealed to be correlated with good prognosis
in patients with advanced NSCLC (42). These results indicated that
circulating IGFBP3 levels may be a good prognostic marker in
patients with NSCLC. In the present study, it was revealed that
IGFBP3 mRNA expression was significantly higher in tumor tissues
than in normal tissues, and it was significantly associated with
unfavorable OS in patients with NSCLC. This could be attributed to
differing regulation at the mRNA and protein level, thus further
research would be helpful to explore the exact role of IGFBP3 in
NSCLC.
Studies on IGFBP4 in NSCLC are limited. However, in
epithelial ovarian tumors, IGFBP4 mRNA was highly expressed, but
was not associated with OS in patients with cancer (43). It was also observed that high IGFBP4
mRNA expression was significantly associated with unfavorable OS
for all patients with NSCLC and patients with Ade but not SCC.
However, differential IGFBP4 expression was not observed in tumor
and healthy tissues.
As with IGFBP4, studies on IGFBP5 in NSCLC are
limited. In breast cancer, IGFBP5 overexpression inhibited tumor
growth (44). However, the opposite
occured in other cancer types; IGFBP5 increased IGF-dependent and
-independent survival and proliferation in neuroblastoma and
pancreatic cancer (45,46). In the present study, differential
IGFBP5 expression was not observed between tumor and healthy
tissues, but high IGFBP5 levels were significantly correlated with
favorable OS in patients with SCC. The differential effects of
IGFBP5 may be attributed to the different microenvironments of
specific tumors.
IGFBP6 appears to have an inhibitory effect on lung
cancer. Consistent with a previous study, IGFBP6 expression was
lower in cancerous lungs than in normal lungs (47). A study by Sueoka et al
indicated that IGFBP6 is a potent inducer of programmed cell death
in NSCLC cells (48). Koyama et
al indicated that IGFBP6 mechanistically acted as an effector
of the tumor suppressor semaphorin 3B in lung cancer (49), and IGFBP6 was regulated by the
important tumor suppressor tumor protein p53 (50), and the molecular functions of IGFBPs
in other tumors were partially related to p53 (51–53).
However, in the present study, high IGFBP6 mRNA was not
significantly associated with OS in patients with NSCLC, Ade, or
SCC, presumably due to the TP53 status.
GEPIA was used to validate the prognostic value of
IGFBP mRNA expression in NSCLC. However, the results were not
completely consistent with the data from Kaplan-Meier analysis.
This may be due to the smaller sample size in GEPIA. Thus, well
designed studies with larger sample sizes should be performed in
the future.
The correlation between IGFBP mRNA levels and other
clinicopathological features was also evaluated. It was revealed
that IGFBP1, IGFBP2, IGFBP3, and IGFBP4 were significantly
associated with the smoking status of patients with NSCLC.
Nicotine, which promotes NSCLC growth and metastasis, is
responsible for 80% of all lung cancer cases (54). Further studies will be required to
investigate whether nicotine is directly related to aberrant IGFBP
expression in NSCLC patients. Moreover, it was also revealed that
high IGFBP1 and IGFBP4 mRNA levels were significantly correlated
with unfavorable OS in patients with stage I NSCLC. High IGFBP2 and
IGFBP4 mRNA expression levels were also associated with unfavorable
OS in stage II patients. Additionally, IGFBP5 was
significantly associated with unfavorable OS in patients who did
not receive chemotherapy.
As potential tumor suppressors and/or oncogenes,
IGFBP mutations may be associated with carcinogenesis and cancer
progression. Relatively consistent low levels of alterations were
revealed in each IGFBP in NSCLC, but these alterations had no
effect on OS or DFS, suggesting that these changes may not directly
impact NSCLC prognosis. To further investigate the potential
molecular mechanisms of IGFBPs in NSCLC, network analysis for each
IGFBP was performed. The genes were mainly enriched in
growth-related signaling pathways, highlighting their potential as
targets for anti-NSCLC therapeutics.
In summary, the results indicated that high IGFBP1,
IGFBP2, and IGFBP4 mRNA levels are associated with unfavorable OS
in all patients with NSCLC, and especially those with Ade.
Additionally, high IGFBP2 and IGFBP5 mRNA expression was
significantly correlated with favorable OS in patients with SCC.
Different IGFBPs were correlated with the smoking status, clinical
stage, and chemotherapeutic regimen. These results highlight the
heterogeneity and complexity of NSCLC signaling, and suggest that
IGFBP-based tools for accurate prognosis prediction and targeted
treatment strategies would be beneficial for patients with NSCLC.
Further research is required to explore IGFBP gene expression at
protein levels in different stages of lung cancer including lung
adenocarcinoma and lung squamous cell carcinoma, and to pursue the
exact molecular mechanisms of IGFBPs in NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Funds of China (nos. 81760351 and
81460015).
Availability of data and materials
The GEPIA database (http://gepia.cancer-pku.cn) was used to perform gene
expression profiling analysis and prognostic analysis. The
Kaplan-Meier Plotter (www.kmplot.com) was used to perform prognostic
analysis. The cBioPortal for Cancer Genomics (http://www.cbioportal.org) was used to perform
analysis of gene alteration frequency. GeneMANIA (http://www.genemania.org) was used for correlation
analysis. The DAVID database (http://david.ncifcrf.gov/) was used to perform
functional annotation and pathway enrichment analysis.
Authors' contributions
JW wrote the manuscript, carried out the research
methodology and acquired the data. DL and ZGH performed the data
analysis. JXX provided the technical support. ZGZ conceived and
designed the study. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu W, Ha M and Yin N: Combination of
platelet count and lymphocyte to monocyte ratio is a prognostic
factor in patients undergoing surgery for non-small cell lung
cancer. Oncotarget. 8:73198–73207. 2017.PubMed/NCBI
|
|
5
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: Biological actions.
Endocr Rev. 16:3–34. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Roith D: Seminars in medicine of the
Beth Israel deaconess medical center. Insulin-like growth factors.
N Engl J Med. 336:633–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
LeRoith D: Insulin-like growth factor
receptors and binding proteins. Baillieres Clin Endocrinol Metab.
10:49–73. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clemmons DR: Insulin-like growth factor
binding proteins and their role in controlling IGF actions.
Cytokine Growth Factor Rev. 8:45–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Q, Zhou Y, Ying K and Ruan W: IGFBP, a
novel target of lung cancer? Clin Chim Acta. 466:172–177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Y, Zhou Z, Hofstetter WL, Zhou Y, Hu W,
Guo C, Wang L, Guo W, Pataer A, Correa AM, et al: Aberrant
expression of proteins involved in signal transduction and DNA
repair pathways in lung cancer and their association with clinical
parameters. PLoS One. 7:e310872012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo C, Lu H, Gao W, Wang L, Lu K, Wu S,
Pataer A, Huang M, El-Zein R, Lin T, et al: Insulin-like growth
factor binding protein-2 level is increased in blood of lung cancer
patients and associated with poor survival. PLoS One. 8:e749732013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DY, Kim SJ and Lee YC: Serum
insulin-like growth factor (IGF)-I and IGF-binding proteins in lung
cancer patients. J Korean Med Sci. 14:401–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Wang Z, Liang Z, Liu J, Shi W, Bai
P, Lin X, Magaye R and Zhao J: Expression and clinical significance
of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues
from patients with non-small cell lung cancer. Onco Targets Ther.
6:1437–1444. 2013.PubMed/NCBI
|
|
14
|
Zhang Y, Ying X, Han S, Wang J, Zhou X,
Bai E, Zhang J and Zhu Q: Autoantibodies against insulin-like
growth factor-binding protein-2 as a serological biomarker in the
diagnosis of lung cancer. Int J Oncol. 42:93–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang YS, Kong G, Sun S, Liu D, El-Naggar
AK, Khuri FR, Hong WK and Lee HY: Clinical significance of
insulin-like growth factor-binding protein-3 expression in stage I
non-small cell lung cancer. Clin Cancer Res. 8:3796–3802.
2002.PubMed/NCBI
|
|
16
|
Kubasiak JC, Seder CW, Pithadia R, Basu S,
Fhied C, Phillips WW, Daly S, Shersher DD, Yoder MA, Chmielewski G,
et al: Value of circulating insulin-like growth factor-associated
proteins for the detection of stage I non-small cell lung cancer. J
Thorac Cardiovasc Surg. 149:727–734.e1-3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao H, Wang G, Meng L, Shen H, Feng Z, Liu
Q and Du J: Association between circulating levels of IGF-1 and
IGFBP-3 and lung cancer risk: A meta-analysis. PLoS One.
7:e498842012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovariancancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Montojo J, Zuberi K, Rodriguez H, Bader GD
and Morris Q: GeneMANIA: Fast gene network construction and
function prediction for Cytoscape. F1000Res. 3:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baxter RC: IGF binding proteins in cancer:
Mechanistic and clinical insights. Nat Rev Cancer. 14:329–341.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ammoun S, Schmid MC, Zhou L, Ristic N,
Ercolano E, Hilton DA, Perks CM and Hanemann CO: Insulin-like
growth factor-binding protein-1 (IGFBP-1) regulates human
schwannoma proliferation, adhesion and survival. Oncogene.
31:1710–1722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Figueroa JA, Sharma J, Jackson JG,
McDermott MJ, Hilsenbeck SG and Yee D: Recombinant insulin-like
growth factor binding protein-1 inhibits IGF-I, serum, and
estrogen-dependent growth of MCF-7 human breast cancer cells. J
Cell Physiol. 157:229–236. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamada H, Iijima K, Tomita O, Taguchi T,
Miharu M, Kobayashi K, Okita H, Saito M, Shimizu T and Kiyokawa N:
Effects of insulin-like growth factor-1 on B-cell precursor acute
lymphoblastic leukemia. Int J Hematol. 97:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leu JI, Crissey MA and Taub R: Massive
hepatic apoptosis associated with TGF-beta1 activation after Fas
ligand treatment of IGF binding protein-1-deficient mice. J Clin
Invest. 111:129–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma J, Gray KP, Evan C, Nakabayashi M,
Fichorova R, Rider J, Mucci L, Kantoff PW and Sweeney CJ: Elevated
insulin-like growth factor binding protein-1 (IGFBP-1) in men with
metastatic prostate cancer starting androgen deprivation therapy
(ADT) is associated with shorter time to castration resistance and
overall survival. Prostate. 74:225–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao Y, Nimptsch K, Shui IM, Platz EA, Wu
K, Pollak MN, Kenfield SA, Stampfer MJ and Giovannucci EL:
Prediagnostic plasma IGFBP-1, IGF-1 and risk of prostate cancer.
Int J Cancer. 136:2418–2426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao X, Sun S, Zhou X, Guo W and Zhang L:
IGF-binding protein 2 is a candidate target of therapeutic
potential in cancer. Tumour Biol. 37:1451–1459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maciejczyk A, Szelachowska J, Czapiga B,
Matkowski R, Hałoń A, Györffy B and Surowiak P: Elevated BUBR1
expression is associated with poor survival in early breast cancer
patients: 15-year follow-up analysis. J Histochem Cytochem.
61:330–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reeve JG, Morgan J, Schwander J and
Bleehen NM: Role for membrane and secreted insulin-like growth
factor-binding protein-2 in the regulation of insulin-like growth
factor action in lung tumors. Cancer Res. 53:4680–4685.
1993.PubMed/NCBI
|
|
35
|
Yazawa T, Sato H, Shimoyamada H, Okudela
K, Woo T, Tajiri M, Ogura T, Ogawa N, Suzuki T, Mitsui H, et al:
Neuroendocrine cancer-specific up-regulating mechanism of
insulin-like growth factor binding protein-2 in small cell lung
cancer. Am J Pathol. 175:976–987. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fürstenberger G and Senn HJ: Insulin-like
growth factors and cancer. Lancet Oncol. 3:298–302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee HY, Chun KH, Liu B, Wiehle SA,
Cristiano RJ, Hong WK, Cohen P and Kurie JM: Insulin-like growth
factor binding protein-3 inhibits the growth of non-small cell lung
cancer. Cancer Res. 62:3530–3537. 2002.PubMed/NCBI
|
|
38
|
Alami N, Page V, Yu Q, Jerome L, Paterson
J, Shiry L and Leyland-Jones B: Recombinant human insulin-like
growth factor-binding protein 3 inhibits tumor growth and targets
the Akt pathway in lung and colon cancer models. Growth Horm IGF
Res. 18:487–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hochscheid R, Jaques G and Wegmann B:
Transfection of human insulin-like growth factor-binding protein 3
gene inhibits cell growth and tumorigenicity: A cell culture model
for lung cancer. J Endocrinol. 166:553–563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim JH, Choi DS, Lee OH, Oh SH, Lippman SM
and Lee HY: Antiangiogenic antitumor activities of IGFBP-3 are
mediated by IGF-independent suppression of Erk1/2 activation and
Egr-1-mediated transcriptional events. Blood. 118:2622–2631. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang HH, Wang YC, Wu DW, Hung CS, Chen CY
and Lee H: Targeting insulin-like growth factor-binding protein-3
by microRNA-125b promotes tumor invasion and poor outcomes in
non-small-cell lung cancer. Tumour Biol.
39:10104283176943162017.PubMed/NCBI
|
|
42
|
Han JY, Choi BG, Choi JY, Lee SY and Ju
SY: The prognostic significance of pretreatment plasma levels of
insulin-like growth factor (IGF)-1, IGF-2, and IGF binding
protein-3 in patients with advanced non-small cell lung cancer.
Lung Cancer. 54:227–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Walker G, MacLeod K, Williams AR, Cameron
DA, Smyth JF and Langdon SP: Insulin-like growth factor binding
proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine
responsiveness in patients with ovarian cancer. Clin Cancer Res.
13:1438–1444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Butt AJ, Dickson KA, McDougall F and
Baxter RC: Insulin-like growth factor-binding protein-5 inhibits
the growth of human breast cancer cells in vitro and in vivo. J
Biol Chem. 278:29676–29685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tanno B, Cesi V, Vitali R, Sesti F,
Giuffrida ML, Mancini C, Calabretta B and Raschellà G: Silencing of
endogenous IGFBP-5 by micro RNA interference affects proliferation,
apoptosis and differentiation of neuroblastoma cells. Cell Death
Differ. 12:213–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Johnson SK and Haun RS: Insulin-like
growth factor binding protein-5 influences pancreatic cancer cell
growth. World J Gastroenterol. 15:3355–3366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yao R, Wang Y, Lubet RA and You M:
Differentially expressed genes associated with mouse lung tumor
progression. Oncogene. 21:5814–5821. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sueoka N, Lee HY, Wiehle S, Cristiano RJ,
Fang B, Ji L, Roth JA, Hong WK, Cohen P and Kurie JM: Insulin-like
growth factor binding protein-6 activates programmed cell death in
non-small cell lung cancer cells. Oncogene. 19:4432–4436. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koyama N, Zhang J, Huqun, Miyazawa H,
Tanaka T, Su X and Hagiwara K: Identification of IGFBP-6 as an
effector of the tumor suppressor activity of SEMA3B. Oncogene.
27:6581–6589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kannan K, Amariglio N, Rechavi G,
Jakob-Hirsch J, Kela I, Kaminski N, Getz G, Domany E and Givol D:
DNA microarrays identification of primary and secondary target
genes regulated by p53. Oncogene. 20:2225–2234. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bartram I, Erben U, Ortiz-Tanchez J,
Blunert K, Schlee C, Neumann M, Heesch S and Baldus CD: Inhibition
of IGF1-R overcomes IGFBP7-induced chemotherapy resistance in
T-ALL. BMC Cancer. 15:6632015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gutschner T, Hämmerle M, Pazaitis N, Bley
N, Fiskin E, Uckelmann H, Heim A, Groβ M, Hofmann N, Geffers R, et
al: Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1)
is an important protumorigenic factor in hepatocellular carcinoma.
Hepatology. 59:1900–1911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kawasaki T, Nosho K, Ohnishi M, Suemoto Y,
Kirkner GJ, Fuchs CS and Ogino S: IGFBP3 promoter methylation in
colorectal cancer: Relationship with microsatellite instability,
CpG island methylator phenotype, and p53. Neoplasia. 9:1091–1098.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bialous SA and Sarna L: Lung cancer and
tobacco: What is new? Nurs Clin North Am. 52:53–63. 2017.
View Article : Google Scholar : PubMed/NCBI
|