Introduction
Cancer immunotherapy has been suggested as a new
generation of antineoplastic strategies, especially after the great
success of T cell modulatory therapies (1,2).
Complement-mediated immunotherapy as well as T cell immunotherapy
can be a target of antineoplastic strategies (3). There is some evidence that complement is
an effector mechanism in tumor immunotherapy (3), therapeutic antitumor monoclonal
antibodies, including atumumab and rituximab (4,5), and
several anticancer drugs, including paclitaxel (6) and cisplatin (7), lead to the activation of
complement-dependent cytotoxicity.
Among complement regulatory proteins, the present
study focused on decay-accelerating factor (CD55 or DAF or
791Tgp72), an inhibitor of complement-mediated lysis (8,9). A murine
monoclonal antibody recognizing 791Tgp72 has been suggested as a
potential therapeutic agent by stimulating T-cell responses
(10). A
177Lu-DTPA-anti-CD55 antibody was developed targeting
the pleural cavity in pleural metastatic mouse models, suggesting
both diagnostic and therapeutic roles for malignant pleural
effusion (11). However, the efficacy
of the novel anti-CD55 chimeric monoclonal antibody and its
inhibition of CD55 on colorectal cancer were not carefully studied.
In fact, even the level of CD55 expression in colorectal cancer is
not well established (12).
Therefore, the levels of CD55 were assessed using tissues from
colon cancer patients and studied the role of the new chimeric CD55
antibody in the remission of colorectal cancer.
Colorectal cancer is the third most prevalent cancer
affecting both men and women worldwide (13). Colorectal cancer is divided into
groups of patients with microsatellite-unstable and
microsatellite-stable tumors (14).
The former tumors contain many gene mutations and may lead to new
immunogenic antigens that provoke immune responses. Indeed, tumors
that contain many mutations demonstrate better clinical benefit
from immune checkpoint blockade with pembrolizumab than do
microsatellite-stable tumors (15).
Notably, in contrast to other cancer types, colorectal cancer has
shown low efficacy to pembrolizumab (16). Therefore, it was hypothesized that
colorectal cancers with many mutations may have better outcomes in
response to CD55 blockade, which is related to the immune
system.
The discovery of 5-fluorouracil (5-FU) in 1957 was a
landmark advance for patients with colorectal cancer and has since
been used as one of the first-line treatments in advanced
colorectal cancer (17). However,
5-FU is mainly limited due to its resistance (18). Therefore, combination therapies, such
as FOLFOX (5-FU, leucovorin, and oxaliplatin), FOLFIRI (5-FU,
leucovorin, and irinotecan), FLOT (5-FU, oxaliplatin, and
docetaxel), and ECF (epirubicin, cisplatin, and 5-FU), have been
established as efficacious cytotoxic regimens (19). Notably, these regimens are further
combined with a monoclonal antibody, such as cetuximab and
bevacizumab, according to the molecular characteristics (19,20).
The aim of the present study was to validate a novel
anti-CD55 antibody as an effective therapy for managing colorectal
cancer. Herein, it is demonstrated that anti-CD55 is a promising
therapeutic agent as both a monotherapy and a combined therapy with
5-FU in colorectal cancer.
Materials and methods
Selection of CD55-specific scFvs and
preparation of anti-CD55 IgG
Construction of a naïve chicken phage-displayed scFv
library, biopanning to select CD55-specific scFvs, and preparation
of anti-CD55 IgG were performed by SG Medical, Inc., as previously
described (11). Flow cytometric
analysis was used to validate the anti-CD55 antibodies and
CD55-expressing colorectal cancer cell lines. CD55-positive cells
were stained with an Alexa Fluor 647 (A-20186; Molecular Probes;
Thermo Fisher Scientific, Inc.)-conjugated anti-CD55 antibody and
measured by a BD FACS Canto II. The anti-CD55 Ab except for the IHC
(AP14798A; Abgent, Inc.) and immunoblotting (ab54595; Abcam) is not
commercially available. The sequence of the antibody is also not
publicly available because patent application is in progress.
Cell culture
HT-29 (ATCC HTB-38), DLD-1 (ATCC CCL-221), SW-620,
HCT-15 (ATCC CCL-225), and LoVo (ATCC CCL-229) colorectal cancer
cells and THP-1 (ATCC TIB-202) monocytes were maintained in
RPMI-1640 (11875119; Gibco; Thermo Fisher Scientific, Inc.) with
10% FBS. Mutation data were obtained from the Sanger Institute
(21), according to which LoVo cells
contain somatic mutations in KRAS, MSH2, APC, and FBXW7 genes.
Cells were preincubated with 100 µM of 5-FU (F6627; Sigma-Aldrich;
Merck KGaA) for 1 h prior to incubation with 100 µg/ml anti-CD55
antibody unless otherwise indicated.
C5a release
DLD-1 or SW-620 cells were incubated in the presence
of human complement (S1764; Sigma-Aldrich; Merck KGaA). Cell
supernatants in triplicate of standards and samples containing 100
µg/ml chimeric anti-CD55 antibody or human IgG were assayed for C5a
using a commercial human C5a ELISA kit (HK349; Hycult Biotech). C5a
release was quantified using a microplate reader at 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR) of cytokines
The effect of anti-CD55 antibody on cytokine
production in THP-1 cells was measured by RT-qPCR. THP-1 cells were
pretreated with or without anti-CD55 (100 ng/ml) or control IgG
(100 ng/ml) for 1 h and then stimulated for 3 h with LPS (1 µg/ml,
tlrl-peklps; Invivogen; Thermo Fisher Scientific, Inc.). Total RNA
was isolated with TRIzol Reagent (15596026; Ambion; Thermo Fisher
Scientific, Inc.) according to the protocol of the manufacturer.
For RT-qPCR, cDNA was synthesized from 2 µg of total RNA using
oligo dT and SuperScript Reverse Transcriptase IV (18090050;
Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
protocol of the manufacturer. The cDNA was amplified with a set of
gene-specific primers and SYBR Green (4309155; Invitrogen; Thermo
Fisher Scientific, Inc.) and then subjected to RT-qPCR
quantification using the Light Cycler 480 II (Roche Diagnostics).
The thermocycling conditions were as follows: Pre-denaturation at
95°C for 5 min followed by denaturation at 95°C for 10 sec, 60°C
annealing for 10 sec and 72°C extension for 10 sec, for 45 cycles;
finally denaturation occurred at 95°C for 5 sec and 65°C for 1 min.
Gene expression was normalized to that of GAPDH. RT-qPCR primer
sequences are listed in Table I.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Target | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| Human TNF |
CAGGGAGCCTTTGGTTCTGG |
CCGTGTCTCAAGGAAGTCTGG |
| Human IL-6 |
TCTGCGCAGCTTTAAGGAGT |
CCCAGTGGACAGGTTTCTGA |
| Human IL-1β |
CCATCAGCCAGGACAGTCAG |
TCAGGCGGGCTTTAAGTGAG |
| Human GAPDH |
AATCCCATCACCATCTTCCA |
TGGACTCCACGACGTACTCA |
Statistical analysis
Statistical analysis for each of the experiments
(Figs. 2 and 3) was determined by Student's t-test. For
the comparison of multiple groups (Fig.
4C and D) the one-way analysis of variance (ANOVA) followed by
Tukey's post hoc test, a method that takes into account the
repeated measurements, was appropriate instead of the t-test
(23). P<0.05 was considered
significant. In addition, cancer outlier profile analysis (COPA) of
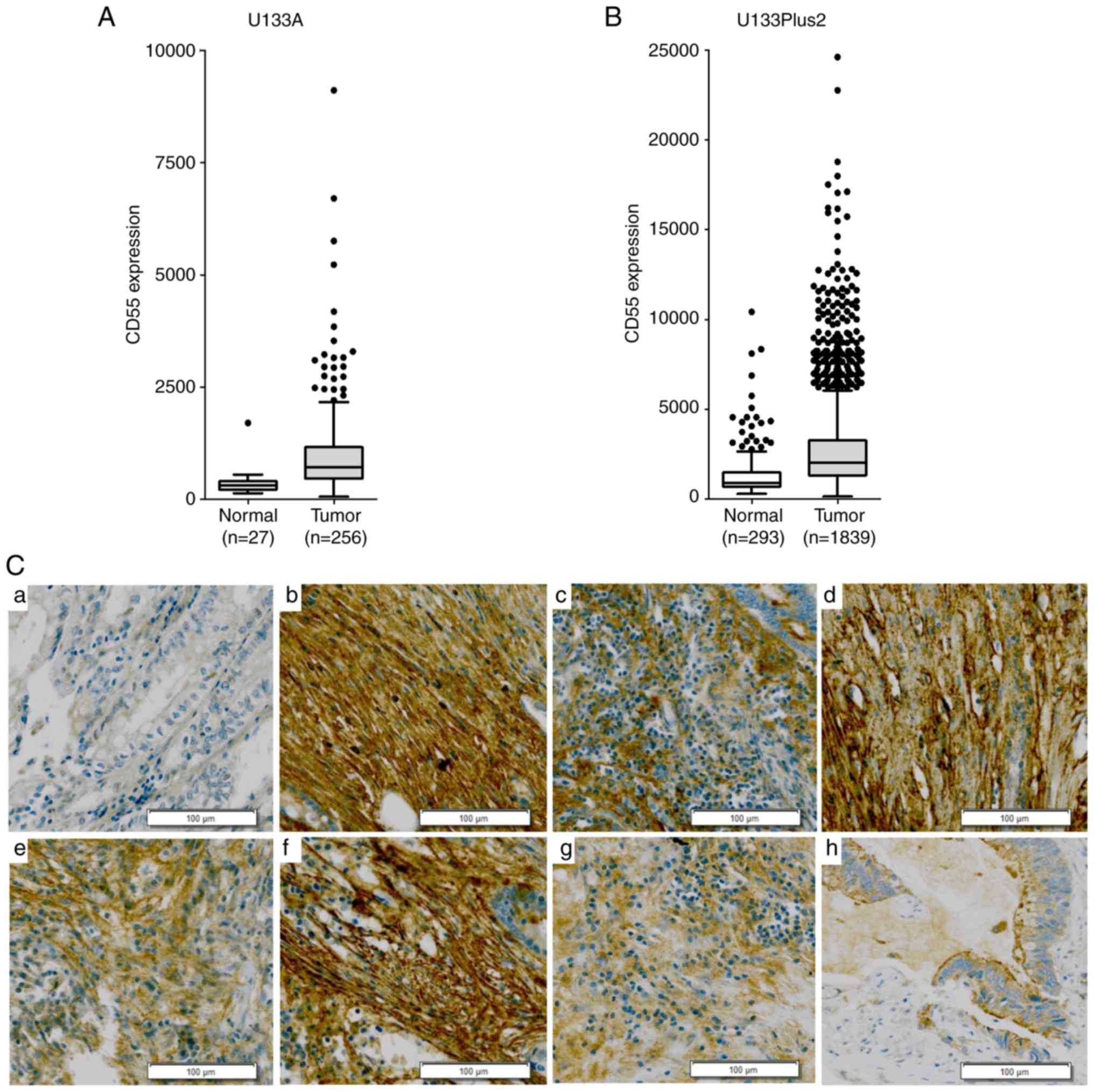
CD55 was applied to the GENT database (http://medical-genome.kribb.re.kr/GENT/) using the
Affymetrix U133A and U133Plus2 platforms (22) (Fig. 1A and
B). Supporting materials and methods are presented in Data
S1.
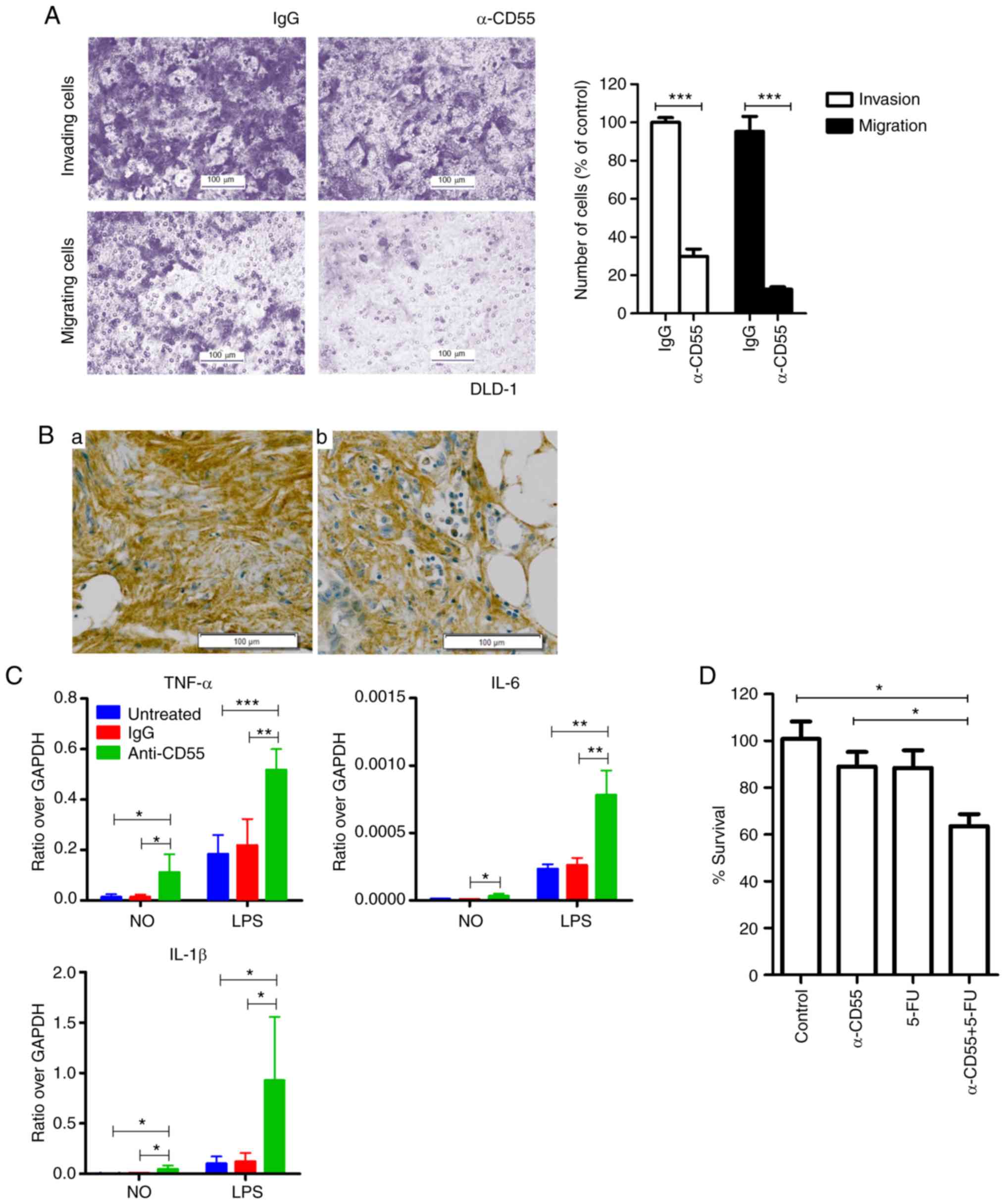 | Figure 4.The effect of the anti-CD55 antibody
on invasion and migration of colorectal cancer cells. (A) Invasion
and migration of DLD-1 cells treated with IgG and the anti-CD55
antibody. The results are presented as the mean ± SEM (n=8;
***P<0.001, Student's t test). (B) Immunohistochemical analysis
of CD55 in metastatic cancer tissues. (a) Metastatic liver tissue;
(b) metastatic omentum tissue. Scale bars, 100 µm. (C) RT-qPCR of
cytokines in THP-1 cells, including TNF-α, IL-6, and IL-1β. Cells
were treated with LPS in the presence of IgG or anti-CD55. The
results are presented as the mean ± SEM (n=4; *P<0.05,
**P<0.01, ***P<0.001, one-way ANOVA). (D) Cell viability
assays of LoVo cells treated with anti-CD55 in the presence or
absence of 5-FU. The results are presented as the mean ± SEM (n=3;
*P<0.05, one-way ANOVA). TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6; IL-1β, interleukin-1β; 5-FU, 5-fluorouracil. |
Results
Upregulation of CD55 during tumor
progression of colorectal cancer
CD55 mRNA was overexpressed in subsets of colorectal
cancer tissues rather than across all colorectal cancer cases
(Fig. 1A and B). To validate the CD55
mRNA overexpression in colorectal cancer tissues,
immunohistochemical analysis was performed in 62 samples. Of the 41
colorectal adenocarcinoma tissues, 21 (51.2%) were strongly
positive for CD55 (Table SI). Strong
CD55 expression was not observed in Stage I colorectal cancer
(Table SII). CD55 was strongly
positive in the later clinical stages of colorectal cancer. CD55
was expressed on cell membranes and in the cytoplasm, particularly
in differentiated colon adenocarcinoma (Fig. 1C). This suggests that the
overexpression of CD55 is required in the progressive stage of
colorectal cancer but not in the early stage of colorectal
cancer.
Validation of a novel chimeric
anti-CD55 monoclonal antibody
To validate CD55 as a target of colorectal cancer, a
novel chimeric human anti-CD55 monoclonal antibodies constructed
from phage-displayed antibody fragments was developed. In the
present study, the focus for novel anti-CD55 monoclonal antibodies
was the specific targeting of colorectal cancer cells expressing
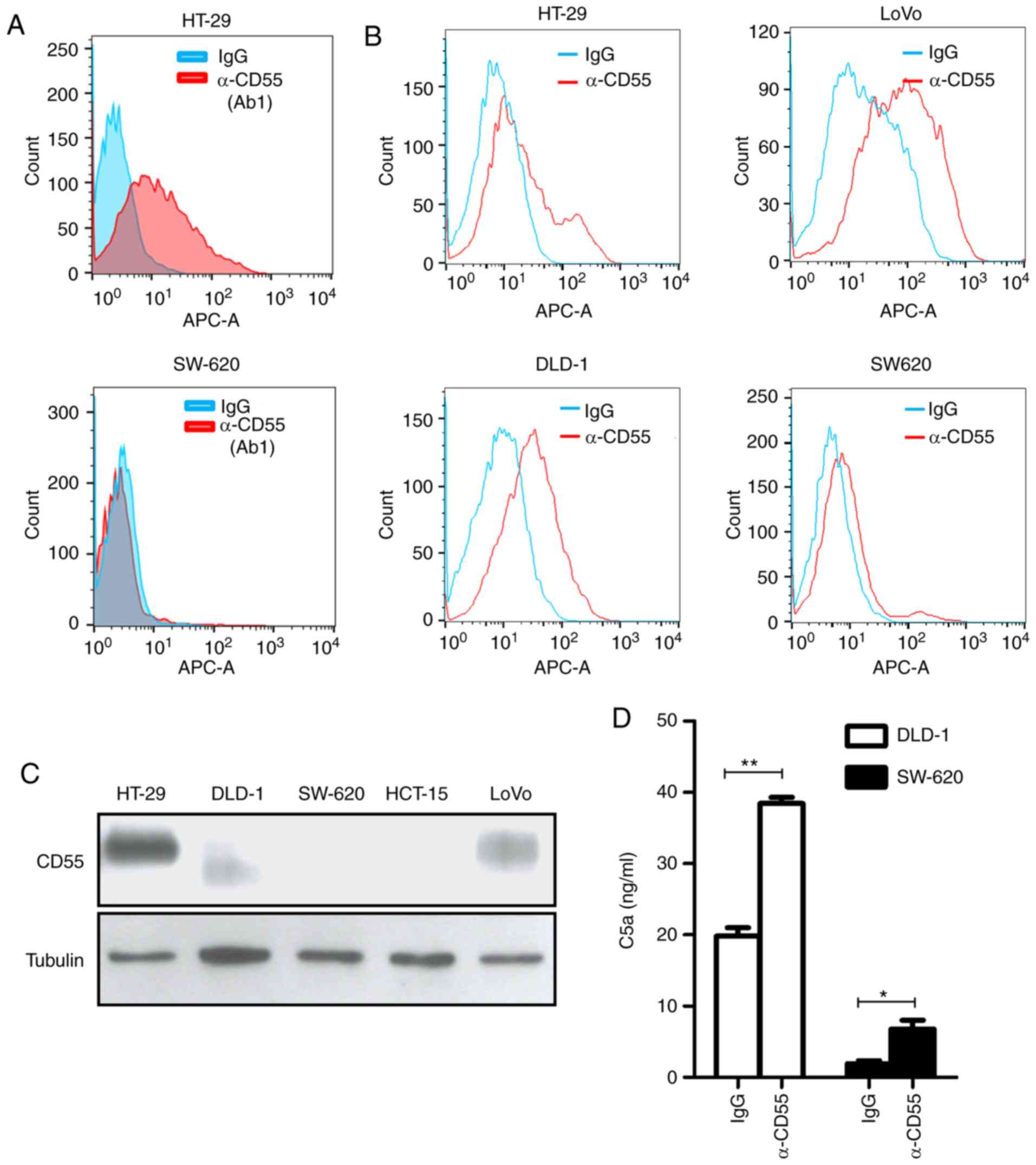
CD55. HT-29 and SW-620 colorectal cancer cells were used as
positive (outlier) and negative (nonoutlier) controls,
respectively. Notably, only the Ab1 anti-CD55 antibody specifically
bound to HT-29 cells by flow cytometry among the strong possible
candidates (Fig. 2A). This result
suggests that the Ab1 anti-CD55 antibody (herein referred to as
‘anti-CD55 antibody’) is a promising therapeutic candidate for
treating colorectal cancer.
For application as a colorectal cancer therapy, the
binding activity of anti-CD55 was analyzed in colorectal cancer
cells expressing various levels of CD55: CD55-positive HT-29, DLD-1
and LoVo cells and CD55-negative SW-620 cells (Fig. 2B). The expression of CD55 in
colorectal cancer cell lines was validated with a commercially
available anti-CD55 antibody by immunoblotting. Different sizes of
CD55 in DLD-1 cells might be caused by different glycosylation
(Fig. 2C).
Next, it was confirmed whether the anti-CD55
antibody activates complement-dependent responses. Complement
activity was measured by a product of the complement system, C5a,
through an enzyme-linked immunosorbent assay (ELISA). Indeed, a
clear increase in C5a release was demonstrated after anti-CD55
antibody treatment (Fig. 2D). These
findings demonstrate that the validated anti-CD55 antibody has the
potential to treat colorectal cancer cells by activation of the
complement system.
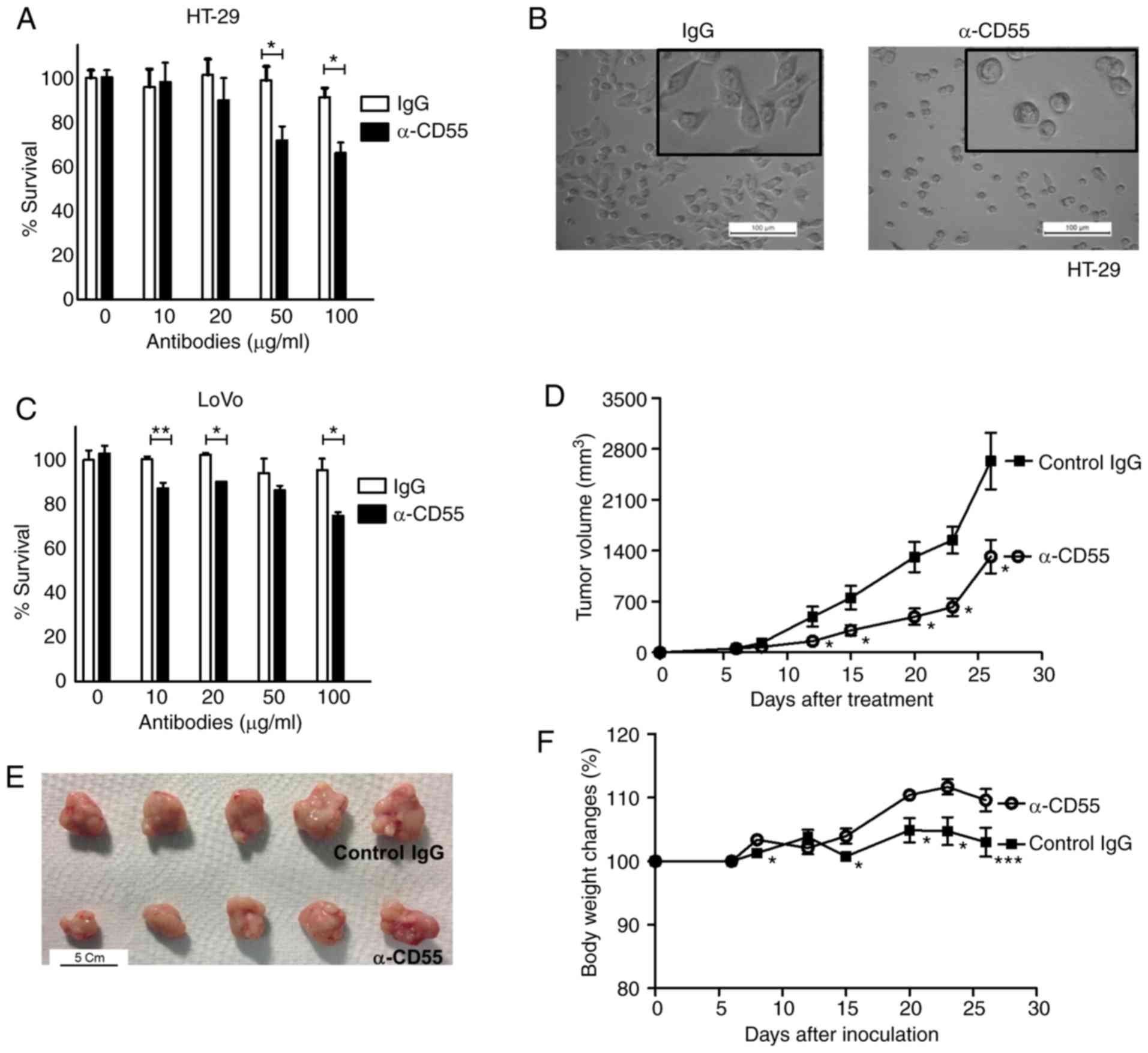
Therapeutic efficacy of the anti-CD55
antibody in colorectal cancer cells
The therapeutic effect of neutralizing antibody
against CD55 was determined both in vitro and in
vivo. The anti-CD55 antibody decreased the viability of HT-29
cells when the cells were treated with anti-CD55 antibody (Fig. 3A). Anti-CD55-treated HT-29 cells
rounded up and detached, whereas IgG-treated cells remained normal
in shape (flat and attached) (Fig.
3B). Consistent with the HT-29 cell results, anti-CD55 reduced
the viability of LoVo cells by 25.1% (Fig. 3C).
Since LoVo cells contain mutations which may
irritate the immune system, it was suspected that the anti-CD55
antibody may attenuate LoVo-bearing tumors. Importantly,
LoVo-induced tumorigenesis and, consequently, tumor volume were
significantly attenuated by treating LoVo-bearing xenograft mice
with anti-CD55 antibody (Fig. 3D and
E). Finally, to further examine the potential toxicity of
anti-CD55 antibody, the body weight of IgG- and anti-CD55
antibody-treated mice was analyzed. LoVo-bearing xenograft mice
tolerated treatment with the anti-CD55 antibody (Fig. 3F), suggesting that the toxicity of the
anti-CD55 antibody was insignificant. Surprisingly, an increase in
body weight was observed in anti-CD55 antibody-treated mice
(Fig. 3F). Since cachexia and weight
loss are problems among cancer patients, anti-CD55 would likely
have a beneficial effect on quality of life.
The anti-metastatic effect of the
anti-CD55 antibody in colorectal cancer cells
Even though the anti-CD55 antibody did not attenuate
the viability of DLD-1 cells (Fig.
S1), invasion and migration were significantly reduced in
anti-CD55 antibody-treated DLD-1 cells (Fig. 4A). Therefore, anti-CD55 was able to
inhibit metastasis at least in DLD-1 cells. Since an overexpression
of CD55 was observed in metastatic liver and omentum tissues
(Fig. 4Ba and Bb), CD55 induces
metastasis at least in certain contexts of colorectal cancer
tissues.
The possibility that anti-CD55 induces
proinflammatory cytokines to prevent metastasis was assessed. The
RT-qPCR analysis confirmed lipopolysaccharide-induced upregulation
of proinflammatory cytokine transcription in THP-1 cells by
anti-CD55 antibody treatment (Fig.
4C). These findings suggest that the anti-CD55 antibody induces
proinflammatory cytokine release and ultimately prevents
metastasis.
Enhanced antitumor effect of 5-FU in
combination with the anti-CD55 antibody
The present findings suggest that anti-CD55 is able
to enhance the antitumor efficacy of 5-FU. To examine the
combinatorial efficacy of anti-CD55 and 5-FU, LoVo cells were
treated with anti-CD55 antibody in the presence or absence of 5-FU.
The combined treatment of anti-CD55 antibody and 5-FU led to a
reduction in cell viability at all time points assessed (Fig. S2). At day 2, treatment of LoVo cells
with either anti-CD55 or 5-FU alone decreased cell viability. A
combinatorial treatment of these two agents diminished cell
viability, which was indicative of a synergistic effect (Fig. 4D). Therefore, the anti-CD55 antibody
demonstrated promising therapeutic effects in colorectal cancer and
would be more curative as a combined therapy.
Discussion
The expression of CD55 and its clinical relevance in
colorectal cancer have not yet been established. CD55 upregulation
has been demonstrated in some colorectal cancers (24–26);
however, several groups have reported that CD55 is not highly
expressed in colorectal tumor tissues (12).
Herein, it is demonstrated that CD55 is
overexpressed in subsets of colorectal cancer tissues and the novel
anti-CD55 antibody suppresses colorectal tumorigenesis. The
anti-CD55 antibody activated complement, stimulated the production
of proinflammatory cytokines and ultimately caused cancer cell
death. Considering that CD55 suppresses T cell immunity (27) and natural killer cells (28), the anti-CD55 antibody might activate
both, thus impeding tumor initiation and growth. Additionally,
natural killer cell and/or macrophage-mediated antibody-dependent
cell-mediated cytotoxicity might be expanded by the anti-CD55
antibody. Importantly, combinational therapy with the novel CD55
antibody with 5-FU enhanced the therapeutic effect against
colorectal cancer. Interestingly, a previous study showed that
neutralization of CD55 augments the therapeutic effect of Herceptin
in lung carcinoma cells (29).
Therefore combined therapy with the novel anti-CD55 antibody and
Herceptin may also be an important strategy in lung cancer even if
anti-CD55 antibody alone did not reduce the viability of H460 lung
cancer cells (11).
In the immunohistochemistry assay, only 2 of 10
metastatic tissues were strongly positive for CD55, even given the
limited sample size (Table SI). This
finding was not expected since 51.2% of colorectal adenocarcinoma
tissues were strongly positive for CD55. In contrast, it has been
suggested that CD55 facilitates metastasis via CD97 binding and
oncogenic tyrosine kinase pathways (28). Therefore, the possibility of anti-CD55
antibody affecting the metastasis of colorectal cancer cells was
examined. Notably, whereas HT-29 and LoVo cells were not properly
metastasized in the transwell assay, DLD-1 colorectal cancer cells
migrated and invaded appropriately in the same system and it was
revealed that the novel therapeutic anti-CD55 monoclonal antibody
inhibits metastasis of colorectal cancer cells.
It has also been reported that the complement
component C3, a downstream factor of CD55, facilitates
leptomeningeal metastasis (30),
suggesting that CD55 could also be used for therapy against this
disease. These data suggest that the novel anti-CD55 antibody can
be used to treat a broad range of tumors as both a monotherapy and
a combination therapy.
Weight loss is one of the critical factors which
determine quality of life for cancer patients since they suffer
from cachexia and malnutrition, which lead to wasted energy with
atrophy of fat and skeletal muscle (31). Therefore, considering the lack of
weight loss due to anti-CD55 treatment (Fig. 3F), this antibody could be considered
as a promising antineoplastic candidate able to minimize cancer
patients' discomfort and extend their survival. Additionally, low
kidney toxicity is crucial for cancer patients. The anti-CD55
monoclonal antibody was radiolabeled with 177Lu to
detect kidney deposition and the levels of
177Lu-anti-CD55 in the kidney were not high (2.79–6.19%
ID/g) (11). Efforts are being made
to shorten the half-life of anti-CD55 antibody by Ab Fc engineering
to minimize toxicity.
Interestingly, soluble CD55 is present in the stool
of colorectal cancer patients, possibly mediated by
metalloproteinase-7 (32). This
suggests that CD55 may be used as a marker for colorectal cancer
and that the anti-CD55 antibody could potentially be utilized for
colorectal cancer diagnosis and therapeutics.
It has been reported that CD55 is associated with a
number of diseases, including malaria (33), protein-losing enteropathy (34) and autoimmune diseases (35). Interestingly, targeting CD55 on
erythrocytes would not cause substantial toxicity considering the
existence of hematologically normal individuals lacking CD55
(33). Thus, the anti-CD55 antibody
may be a promising vaccine or therapy, especially for malaria.
Notably, eculizumab, a monoclonal antibody against C5 that inhibits
terminal complement activation, has been used to treat paroxysmal
nocturnal hemoglobinuria (36).
Therefore, the anti-CD55 antibody might be a therapy for paroxysmal
nocturnal hemoglobinuria because CD55 is another complement
inhibitor.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Korea Atomic Energy
Research Institute major project: Development of Radioisotope
Production and Application Technology (grant no. 525330-18).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SHD, JCL and LKK designed the study and wrote the
manuscript. SHD, EHC, JYL, SYL and SHJ planned and analyzed all
experiments. JCL, SHJ and LKK supervised the study.
Ethics approval and consent to
participate
Animal care and experimental protocols were approved
by the Institutional Animal Care and Use Committee at Korea Atomic
Energy Research Institue (KAERI) (KAERI–IACUC-2017-025).
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macor P and Tedesco F: Complement as
effector system in cancer immunotherapy. Immunol Lett. 111:6–13.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pawluczkowycz AW, Beurskens FJ, Beum PV,
Lindorfer MA, van de Winkel JG, Parren PW and Taylor RP: Binding of
submaximal C1q promotes complement-dependent cytotoxicity (CDC) of
B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab
(RTX): Considerably higher levels of CDC are induced by OFA than by
RTX. J Immunol. 183:749–758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer S, Leusen JH and Boross P:
Regulation of complement and modulation of its activity in
monoclonal antibody therapy of cancer. MAbs. 6:1133–1144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szebeni J, Alving CR, Savay S, Barenholz
Y, Priev A, Danino D and Talmon Y: Formation of
complement-activating particles in aqueous solutions of Taxol:
Possible role in hypersensitivity reactions. Int Immunopharmacol.
1:721–735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gilbert RD, Stanley LK, Fowler DJ, Angus
EM, Hardy SA and Goodship TH: Cisplatin-induced haemolytic uraemic
syndrome associated with a novel intronic mutation of CD46 treated
with eculizumab. Clin Kidney J. 6:421–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spendlove I, Ramage JM, Bradley R, Harris
C and Durrant LG: Complement decay accelerating factor (DAF)/CD55
in cancer. Cancer Immunol Immunother. 55:987–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Spendlove I, Morgan J and Durrant
LG: CD55 is over-expressed in the tumour environment. Br J Cancer.
84:80–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Durrant LG, Doran M, Austin EB and Robins
RA: Induction of cellular immune responses by a murine monoclonal
anti-idiotypic antibody recognizing the 791Tgp72 antigen expressed
on colorectal, gastric and ovarian human tumours. Int J Cancer.
61:62–66. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dho SH, Kim SY, Chung C, Cho EH, Lee SY,
Kim JY, Kim LK, Min SW, Lee J, Jung SH and Lim JC: Development of a
radionuclide-labeled monoclonal anti-CD55 antibody with theranostic
potential in pleural metastatic lung cancer. Sci Rep. 8:89602018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thorsteinsson L, O'Dowd GM, Harrington PM
and Johnson PM: The complement regulatory proteins CD46 and CD59,
but not CD55, are highly expressed by glandular epithelium of human
breast and colorectal tumour tissues. APMIS. 106:869–878. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marisa L, de Reyniès A, Duval A, Selves J,
Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D,
Ayadi M, et al: Gene expression classification of colon cancer into
molecular subtypes: Characterization, validation, and prognostic
value. PLoS Med. 10:e10014532013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Timmermann B, Kerick M, Roehr C, Fischer
A, Isau M, Boerno ST, Wunderlich A, Barmeyer C, Seemann P, Koenig
J, et al: Somatic mutation profiles of MSI and MSS colorectal
cancer identified by whole exome next generation sequencing and
bioinformatics analysis. PLoS One. 5:e156612010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gustavsson B, Carlsson G, Machover D,
Petrelli N, Roth A, Schmoll HJ, Tveit KM and Gibson F: A review of
the evolution of systemic chemotherapy in the management of
colorectal cancer. Clin Colorectal Cancer. 14:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Russo A, Maiolino S, Pagliara V, Ungaro F,
Tatangelo F, Leone A, Scalia G, Budillon A, Quaglia F and Russo G:
Enhancement of 5-FU sensitivity by the proapoptotic rpL3 gene in
p53 null colon cancer cells through combined polymer nanoparticles.
Oncotarget. 7:79670–79687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Modest DP, Neumann UP and Pratschke J:
FOLFOXIRI plus bevacizumab as conversion-therapy for liver
metastases in colorectal cancer: A necessity? Eur J Cancer.
73:71–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Launay M, Dahan L, Duval M, Rodallec A,
Milano G, Duluc M, Lacarelle B, Ciccolini J and Seitz JF: Beating
the odds: Efficacy and toxicity of dihydropyrimidine
dehydrogenase-driven adaptive dosing of 5-FU in patients with
digestive cancer. Br J Clin Pharmacol. 81:124–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bamford S, Dawson E, Forbes S, Clements J,
Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR
and Wooster R: The COSMIC (Catalogue of Somatic Mutations in
Cancer) database and website. Br J Cancer. 91:355–358. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin G, Kang TW, Yang S, Baek SJ, Jeong YS
and Kim SY: GENT: Gene expression database of normal and tumor
tissues. Cancer Inform. 10:149–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HY: Analysis of variance (ANOVA)
comparing means of more than two groups. Restor Dent Endod.
39:74–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawa M, Mizuno M, Kawada M, Uesu T,
Nasu J, Takeuchi K, Okada H, Endo Y, Fujita T and Tsuji T:
Polymorphic expression of decay-accelerating factor in human
colorectal cancer. J Gastroenterol Hepatol. 16:184–189. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koretz K, Brüderlein S, Henne C and Möller
P: Decay-accelerating factor (DAF, CD55) in normal colorectal
mucosa, adenomas and carcinomas. Br J Cancer. 66:810–814. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niehans GA, Cherwitz DL, Staley NA, Knapp
DJ and Dalmasso AP: Human carcinomas variably express the
complement inhibitory proteins CD46 (membrane cofactor protein),
CD55 (decay-accelerating factor), and CD59 (protectin). Am J
Pathol. 149:129–142. 1996.PubMed/NCBI
|
|
27
|
Liu J, Miwa T, Hilliard B, Chen Y, Lambris
JD, Wells AD and Song WC: The complement inhibitory protein DAF
(CD55) suppresses T cell immunity in vivo. J Exp Med. 201:567–577.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mikesch JH, Schier K, Roetger A, Simon R,
Buerger H and Brandt B: The expression and action of
decay-accelerating factor (CD55) in human malignancies and cancer
therapy. Cell Oncol. 28:223–232. 2006.PubMed/NCBI
|
|
29
|
Zhao WP, Zhu B, Duan YZ and Chen ZT:
Neutralization of complement regulatory proteins CD55 and CD59
augments therapeutic effect of herceptin against lung carcinoma
cells. Oncol Rep. 21:1405–1411. 2009.PubMed/NCBI
|
|
30
|
Boire A, Zou Y, Shieh J, Macalinao DG,
Pentsova E and Massagué J: Complement component 3 adapts the
cerebrospinal fluid for leptomeningeal metastasis. Cell.
168:1101.e13–1113.e13. 2017. View Article : Google Scholar
|
|
31
|
Kir S, White JP, Kleiner S, Kazak L, Cohen
P, Baracos VE and Spiegelman BM: Tumour-derived PTH-related protein
triggers adipose tissue browning and cancer cachexia. Nature.
513:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morgan J, Spendlove I and Durrant LG: The
role of CD55 in protecting the tumour environment from complement
attack. Tissue Antigens. 60:213–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Egan ES, Jiang RH, Moechtar MA, Barteneva
NS, Weekes MP, Nobre LV, Gygi SP, Paulo JA, Frantzreb C, Tani Y, et
al: Malaria. A forward genetic screen identifies erythrocyte CD55
as essential for Plasmodium falciparum invasion. Science.
348:711–714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ozen A, Comrie WA, Ardy RC, Domínguez
Conde C, Dalgic B, Beser ÖF, Morawski AR, Karakoc-Aydiner E, Tutar
E, Baris S, et al: CD55 deficiency, early-onset protein-losing
enteropathy, and thrombosis. N Engl J Med. 377:52–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Visser L, de Vos AF, Hamann J, Melief MJ,
van Meurs M, van Lier RA, Laman JD and Hintzen RQ: Expression of
the EGF-TM7 receptor CD97 and its ligand CD55 (DAF) in multiple
sclerosis. J Neuroimmunol. 132:156–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hillmen P, Young NS, Schubert J, Brodsky
RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, et al:
The complement inhibitor eculizumab in paroxysmal nocturnal
hemoglobinuria. N Engl J Med. 355:1233–1243. 2006. View Article : Google Scholar : PubMed/NCBI
|