Introduction
Lung cancer, in which non-small cell lung cancer
(NSCLC) accounts for approximately 85% of cases, is the most lethal
type of cancer worldwide (1–3).
Studies have suggested that NSCLC is an inflammation-associated
carcinoma (3,4); however, although the production of
pro-inflammatory cytokines or mediators in the NSCLC
microenvironment formed by tumor cells, endothelial cells and
infiltrating inflammatory cells has been reported (5–7), the
roles of these molecules in NSCLC proliferation and growth remain
largely obscure.
Interleukin 17 (IL-17), also known as
IL-17A, is a newly identified pro-inflammatory cytokine
(8,9). A number of studies have demonstrated
that IL-17 production can promote cell proliferation and can
thus contribute to NSCLC growth and development (10–13).
However, the precise mechanisms underlying IL-17-induced NSCLC cell
proliferation are extremely complex (14,15),
and are not yet fully understood.
As is known, the expression of proliferation-related
genes is associated with the activation of certain molecules in
diverse signaling pathways, including various transcription factors
(14–17). High-mobility group A1
(HMGA1) is a transcription factor which plays a crucial role
in regulation of gene expression and biological process (18,19).
Emerging evidence has indicated the significant upregulation of
HMGA1 expression in several malignant types of cancer, such
as bladder cancer, thyroid cancer and NSCLC (20–22),
and the overexpression of HMGA1 has also been shown to
positively correlate with the cell proliferation and malignant
status of NSCLC (23,24). Reportedly, cyclin D1 is
widely overexpressed in certain types of human cancer, and several
growth factors, such as epidermal growth factor (EGF)
markedly enhance cancer cell proliferation by increasing cyclin
D1 expression (25,26). Moreover, transcription factors,
such as nuclear factor (NF)-κB can directly bind to the
cyclin D1 gene promoter and result in cyclin D1 gene
transcription and cell proliferation (26); the activation of the p15/cyclin
D1 pathway also greatly promotes NSCLC carcinogenesis (27).
The present study demonstrated that the production
of IL-17 and the expression levels of IL-17R, HMGA1
and cyclin D1 were significantly elevated in samples of
patients with NSCLC. Moreover, positive correlations were also
found between the expression of IL-17R, HMGA1 and cyclin
D1. Therefore, we wished to determine whether stimulation with
IL-17 would enhance NSCLC cell proliferation and increase
the expression of HMGA1 and cyclin D1 by binding to
IL-17R, as well as whether HMGA1 triggers cyclin
D1 gene transcription. In addition, we aimed to elucidate the
mechanisms involved in IL-17-induced NSCLC cell
proliferation. For this purpose, we performed a series of
experiments in order to shed light into these matters.
Materials and methods
Human specimens
Plasma samples from patients with NSCLC (n=40) and
healthy volunteers (n=40) were collected from the First Affiliated
Hospital of Nanjing Medical University (Nanjing, China). Patients
were eligible if they were diagnosed with NSCLC by biopsy and had
not been treated with chemotherapy or radiotherapy prior to sample
collection. Patients were excluded if they suffered from any other
disease (trauma, infections, allergies, autoimmune diseases or
other inflammatory diseases and cancers). Informed consent was
obtained from all patients participating in this research prior to
the experiment. This study was approved by the Ethics Committee of
Nanjing Medical University and conformed to the guidelines outlined
by the Declaration of Helsinki. Specifically, venous blood samples
were collected into K3EDTA tubes (Greiner Bio-One; Frickenhausen,
Germany) and were then fractionated by centrifugation (10 min,
3,000 × g). The plasma was aliquoted and stored at −80°C prior to
analysis. NSCLC tissue arrays (n=60, paired) were provided by the
National Engineering Center for BioChips (Shanghai, China).
Cell lines, reagents and antibodies
The human NSCLC cell lines, A549 (Cat. no. CCL-185),
H1299 (Cat. no. CRL-5803) and H1975 (Cat. no. CRL-5908) were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). The human adenocarcinoma cell lines, PC9 and SPC-A1, were
purchased from the European Collection of Authenticated Cell
Cultures (ECACC, Cat. no. 90071810) and the Institute of
Biochemistry and Cell Biology of the Chinese Academy of Science
(Cat. no. TCHu53), respectively. The human bronchial epithelial
cell line (16HBE) was supplied by Dr Gruenert (California Pacific
Medical Center, San Francisco, CA, USA). Recombinant human IL-17
(IL-17A) and anti-rabbit (Cat. no. HAF008) or anti-mouse (Cat.
no. HAF007) HRP-conjugated secondary antibodies were provided by
R&D Systems (Minneapolis, MN, USA). The anti-IL-17 (Cat.
no. ab9565), anti-IL-17R (Cat. no. ab180904),
anti-HMGA1 (Cat. no. ab129153) and anti-cyclin D1
(Cat. no. ab134175) antibodies were supplied by Abcam (Cambridge,
UK). The DAB substrate kit, BCA assay kit and Lipofectamine 2000
reagent were from Thermo Fisher Scientific (Waltham, MA, USA). The
cell counting kit-8 (CCK-8) was supplied by Dojindo Laboratories
(Kumamoto, Japan). Crystal violet was from Sigma-Aldrich (St.
Louis, MO, USA). The reverse transcription reagent kit, 2X Taq
Master Mix and qPCR SYBR-Green master mix were purchased from
Vazyme Biotech (Nanjing, China). The X-tremeGENE HP DNA
transfection reagent was from Roche Applied Science (Mannheim,
Germany). The dual-luciferase reporter assay system kit was
obtained from Promega (Madison, WI, USA). The chromatin
immunoprecipitation (ChIP) kit was provided by Cell Signaling
Technology (Danvers, MA, USA).
Detection by ELISA
The plasma IL-17 concentration was measured
using an anti-IL-17A coated ELISA kit (BMS2017; Thermo
Fisher Scientific). Briefly, standard, control and plasma samples
were added to the IL-17A antibody coated wells. Meantime, a
biotinylated IL-17A antibody was added, incubating for 2 h
at room temperature. The streptavidin-HRP was then added to all
washed wells and incubated for 1 h, followed by washing and TMB
substrate incubation for approximately 10 min. Finally, the enzyme
reaction was terminated by stop solution (provided with the kit)
and the absorbance at 450 nm (OD 450) of each well was measured on
a microplate reader (ELX 800, Biotek, Winooski, VT, USA) (28).
PCR and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from the NSCLC tissues and cells was
isolated using TRIzol reagent (Thermo Fisher Scientific) and the
cDNA was generated using the reverse transcription reagent kit
(Vazyme Biotech). The PCR assay was performed with a 50 µl
volume reaction containing 25 µl 2X Taq master mix, 1
µl forward primer (10 µM), 1 µl reverse primer
(10 µM) and 1 µl cDNA on an ABI 2720 thermal cycler
with the condition of 94°C/5 min, 35 cycles of 94°C/30 sec, 55°C/30
sec and 72°C/30 sec, 72°C/7 min. The amplification products were
then analyzed by agarose gel electrophoresis. The qPCR experiment
was performed with a 20 µl volume reaction containing 10
µl 2X qPCR SYBR-Green master mix, 0.4 µl forward
primer (10 µM), 0.4 µl reverse primer (10 µM),
1 µl cDNA and 0.4 µl 50X ROX reference dye 1 on an
ABI 7300 system in triplicate under the following conditions:
95°C/5 min, 40 cycles of 95°C/5 sec and 60°C/30 sec. The primers
used are shown in Table I. The
results were normalized to β-actin expression and analyzed using
the 2−ΔΔCq method (29,30).
 | Table ISpecific primers for qPCR and
plasmids construction. |
Table I
Specific primers for qPCR and
plasmids construction.
| Name | | Primers
(5′→3′) |
|---|
| HMGA1 | Forward |
GCTGGTAGGGAGTCAGAAGG |
| Reverse |
TTGGTTTCCTTCCTGGAGTT |
| Cyclin
D1 | Forward |
GCCACTTGCATGTTCG |
| Reverse |
GGGCTCCTCAGGTTCA |
| β-actin | Forward |
CAGCCATGTACGTTGCTATCCAGG |
| Reverse |
AGGTCCAGACGCAGGATGGCATG |
|
pIRES2-HMGA1 | Forward | CCGCTCGAGCACTCTTCCACCTGCTCCTTa |
| Reverse | CCGGAATTCATGGGTCACTGCTCCTCCTb |
| Cyclin
D1-FL | Forward | CGGGGTACCCTGGACGGCTCTTTACGCc |
| Reverse | CTAGCTAGCTCTGCTGCTCGCTGCTACTd |
| Truncate 1 | Forward | CGGGGTACCATGCTCTGAGGCTTGGCTATc |
| Reverse | CTAGCTAGCTCTGCTGCTCGCTGCTACTd |
| Truncate 2 | Forward | CGGGGTACCAAATTCTAAAGGTGAAGGGACGc |
| Reverse | CTAGCTAGCTCTGCTGCTCGCTGCTACTd |
| Truncate 3 | Forward | CGGGGTACCCTCAGGGATGGCTTTTGGc |
| Reverse | CTAGCTAGCTCTGCTGCTCGCTGCTACTd |
Immunohistochemical (IHC) staining
The slides from the NSCLC tissue array were
incubated with the antibodies against IL-17R (dilution 1:100),
HMGA1 (dilution 1:250) and cyclin D1 (dilution 1:250), followed by
incubation with secondary antibodies (dilution 1:500). The reaction
was visualized with a DAB HRP substrate kit. Finally, sections were
viewed under a light microscope (Eclipse 90i; Nikon, Tokyo, Japan).
The tissues subjected to IHC were scored according to the staining
intensity and stained area, and 5 randomly selected fields were
analyzed. The scoring of the staining intensity was as follows:
negative, 0; weak, 1; moderate, 2; and strong, 3. The scoring of
the stained area was as follows: 0%, 0; 1–25%, 1; 26–50%, 2;
51–75%, 3; and 76–100%, 4. These scores were multiplied to produce
the final score: 0–1, negative expression; 2–4, weak positive
expression; and 6–12, strong positive expression (31,32).
Plasmid construction
The HMGA1 expression plasmid was constructed
by inserting the complete open reading frame (ORF) of the human
HMGA1 gene (NM_145899.2) into the pIRES2-EGFP vector
(Clontech/Takara Bio, Shiga, Japan). The specific primer sequences
are shown in Table I. The
pGpU6/GFP/Neo vector carrying small hairpin RNA targeting
HMGA1 (shHMGA1) was provided by GenePharma (Shanghai,
China). The shRNA-targeted sequences of HMGA1 gene were as
follows: shHMGA1-1, 5′-CAACTCCAGGAAGGAAACCAA-3′;
shHMGA1-2, 5′-CCTTGGCCTCCAAGCAGGAAA-3′; shHMGA1-3,
5′-GAAGGAGGAAGAGGAGGG CAT-3′; and scrambled shRNA control (shCTR),
5′-GTTCTCCGAACGTGTCACGT-3′. In addition, the reporter plasmids
carrying cyclin D1 full-length (FL) and truncated promoter
plasmids were constructed by inserting the corresponding fragments
into the pGL3-basic vector (Promega). The detailed primers are
listed in Table I.
Cell culture, stimulation and
transfection
The cells were maintained in DMEM with 10% FBS in an
incubator containing 5% CO2 at 37°C. For IL-17
stimulation, the cells were cultured overnight and starved for 24
h; IL-17 was then added into the medium at various
concentrations (0, 0.5, 5, 50 and 500 ng/ml) for different periods
of time (according to the needs of distinct experiments). For
plasmid transient transfection, 3×105 cells were seeded
per well in a 6-well plate. A mixture of 3 µg plasmid and 6
µl transfection reagent was then added followed by
incubation for 48 h. The transfection efficiency was determined by
GFP expression at 24 h following transfection. For IL-17R
siRNA (siIL-17R) transfection, the cells were seeded in a
6-well plate and cultured overnight, and the mixture of 100 pmol
siIL-17R and 5 µl Lipofectamine 2000 was then added
followed by 24 h of incubation. The siIL-17R was supplied by
GenePharma and the sequences were as follows: forward,
5′-CCUGCAGCUGAACACCAAUTT-3′ and reverse,
5′-AUUGGUGUUCAGCUGCAGGTT-3′.
Western blot analysis
The cells were lysed using RIPA buffer (Beyotime,
Beijing China) and the total protein concentration was measured by
BCA assay. The protein samples were loaded (50 µg per well)
and electrophoresed on a 10% SDS-PAGE gel and transferred onto PVDF
membranes (Pall Corp., Port Washington, NY, USA). The blots were
then blocked with 5% non-fat milk for 1 h at room temperature, and
probed with the IL-17R, HMGA1 or cyclin D1
antibody overnight at 4°C, followed by incubation with the rabbit
IgG HRP-conjuncated (dilution 1:1,000) antibody for 30 min at room
temperature, and then exposed using regular X-ray film. The
dilution of anti-IL-17R, anti-HMGA1 and
anti-cyclin D1 antibodies was 1:500, 1:10,000 and 1:1,000
respectively. The control antibody was β-actin antibody (Cat. no.
AF0003; Beyotime Beijing, China) and the dilution was 1:1,000.
Autoradiograms were quantified by densitometry (Quantity One
software; Bio-Rad, Hercules, CA, USA).
Prediction of HMGA1 response
elements
The HMGA1 response elements on cyclin
D1 gene promoter were predicted by using the online software
JASPAR (http://jaspar.genereg.net/). Briefly,
the matrix model of HMGA1 binding element was found and
selected, and the promoter sequence of Cyclin D1 gene was
then input into the scan window, with the relative profile score
threshold set at about 80%. After scanning, the putative sites were
displayed.
Luciferase reporter assay
The afore-mentioned promoter reporters were
transfected into the A549 cells with a pRL-SV40 vector (Promega),
separately. A dual-luciferase reporter reagent was used to measure
the promoter activity according to the manufacturer's instructions
and as previously described (33).
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed using ChIP-grade
HMGA1 antibody and ChIP-grade protein G agarose beads in
accordance with the manufacturer's instructions provided with the
SimpleChIP Plus Enzymatic Chromatin IP kit and as previously
described (34). A non-specific
rabbit IgG was used as a negative control. PCR and RT-qPCR were
performed to analyze the cyclin D1 promoter in the ChIP
materials. The primers for the cyclin D1 promoter fragment
in the ChIP assay were as follows: forward,
5′-CCCCATAAATCATCCAGGC-3′; and reverse,
5′-CCCGAGCACCCACAATC-3′.
Cell proliferation assay
Cell proliferation was measured by CCK-8 and colony
formation assays. For CCK-8 assay, based on the manufacturer's
instructions, the optical density (OD) values at 450 nm were
documented using a microplate reader (Biotek). For colony formation
assay, the cells were seeded in a 6-well plate at 500 cells/per
well. Following culture for 10 days, the cells were fixed and
stained with 0.1% crystal violet. Visible colonies were
counted.
Statistical analysis
All data are presented as the means ± SE. Data
analysis was carried out using GraphPad Prism 6.01 (GraphPad
Software, San Diego, CA, USA). The significant difference between 2
groups was determined by a Student's t-test. Multi-group
comparisons were carried out by one-way ANOVA with Dunnett's post
hoc test. Using a Chi-square test, the association of the clinical
parameters of the patients with NSCLC with the expression of
proteins was analyzed. The correlation between the IHC scores was
determined by computing Pearson's correlation coefficient. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Elevated plasma IL-17 levels, and tissue
IL-17R, HMGA1 and cyclin D1 expression levels in patients with
NSCLC
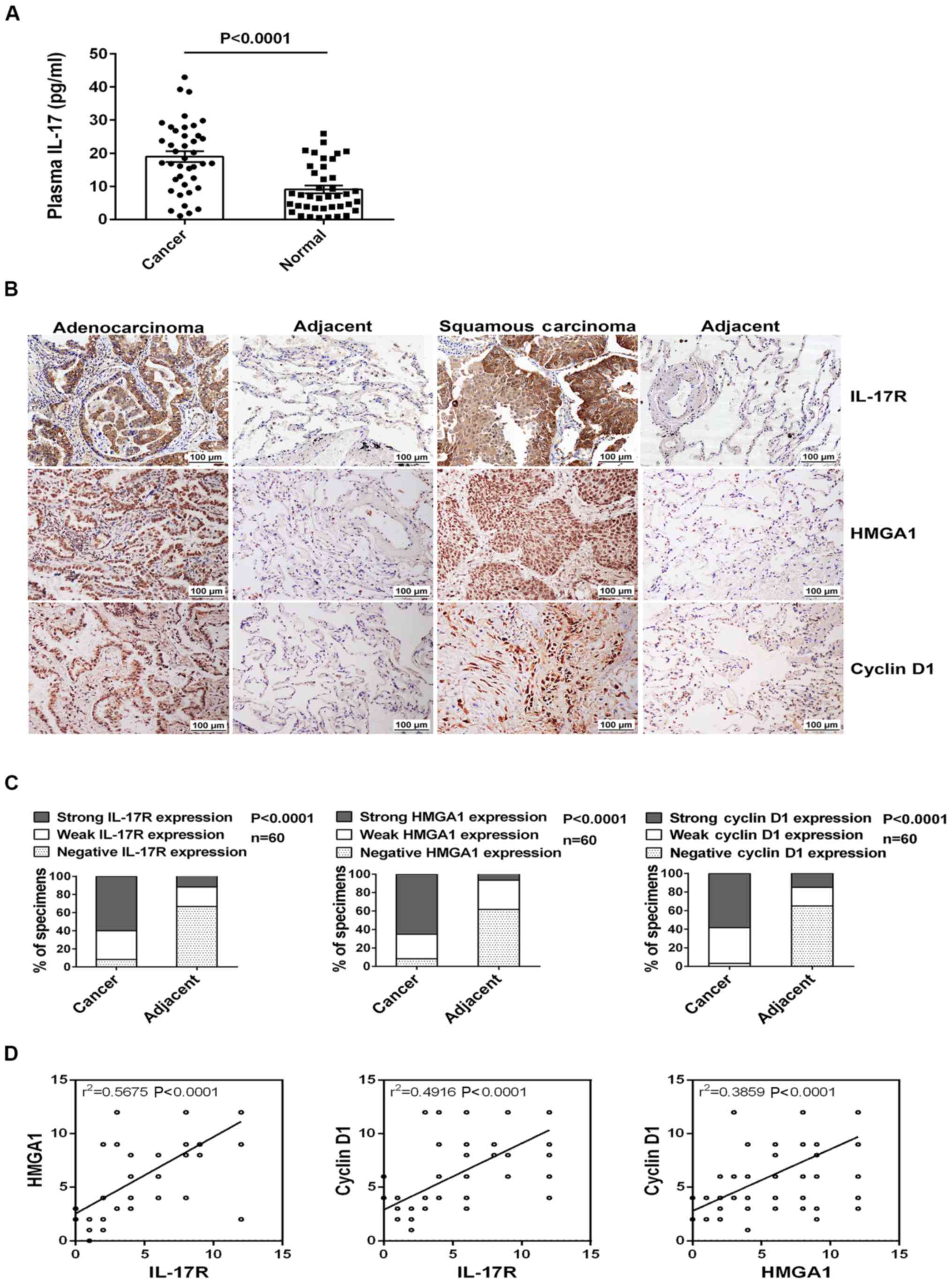
At the beginning of the present study, we examined
the concentration of IL-17 in plasma and the expression
levels of IL-17R, HMGA1 and cyclin D1 in the
tumor tissues from patients with NSCLC. Using ELISA, we found that
the plasma IL-17 levels were significantly increased in the 40
cases of NSCLC (the clinical characteristics of the patients are
summarized in Table II) compared
with the 40 healthy donors (Fig.
1A). Moreover, a marked upregulation in the expression of
IL-17R, HMGA1 and cyclin D1 in the NSCLC
tissues in comparison with the adjacent normal tissues was
demonstrated by IHC staining in the NSCLC tissue microarrays (n=60,
Fig. 1B and C). Furthermore, we
also discovered that the expression of IL-17R, HMGA1
and cyclin D1 was associated with the tumor size, lymph node
metastasis and TNM stage in these patients with NSCLC (Table III). Notably, positive
correlations were also found between the expression of the
above-mentioned 3 genes in the NSCLC tissues (Fig. 1D). These data thus suggest that the
production of IL-17, IL-17R, HMGA1 and cyclin D1 may
probably contribute to the development of NSCLC.
 | Table IIThe characteristics of the patients
with non-small cell lung cancer (NSCLC) detected by ELISA. |
Table II
The characteristics of the patients
with non-small cell lung cancer (NSCLC) detected by ELISA.
|
Characteristics | No. (%) |
|---|
| Total | 40 |
| Sex | |
| Male | 25 (62.5) |
| Female | 15 (37.5) |
| Age | |
| <60 | 7 (17.5) |
| ≥60 | 33 (82.5) |
| Tumor size | |
| <5 cm | 16 (40) |
| ≥5 cm | 24 (60) |
| Lymph node
metastasis | |
| Negative | 11 (27.5) |
| Positive | 29 (72.5) |
| TNM stage | |
| I | 3 (7.5) |
| II | 11 (27.5) |
| III | 16 (40) |
| IV | 10 (25) |
| Pathological
type | |
| Squamous
carcinoma | 15 (37.5) |
|
Adenocarcinoma | 17 (42.5) |
| Large cell
carcinoma | 2 (5) |
| Bronchioloalveolar
carcinoma | 6 (15) |
 | Table IIIAssociation between
IL-17R/HMGA1/cyclin D1 expression with the
clinicopathological characteristics of the patients with non-small
cell lung cancer (NSCLC). |
Table III
Association between
IL-17R/HMGA1/cyclin D1 expression with the
clinicopathological characteristics of the patients with non-small
cell lung cancer (NSCLC).
| Characteristic | Total | IL-17R
expression
| P-valuea | HMGA1
expression
| P-valuea | Cyclin D1
expression
| P-valuea |
|---|
| Weak and
negative | Strong | Weak and
negative | Strong | Weak and
negative | Strong |
|---|
| 60 | 24 | 36 | | 21 | 39 | | 25 | 35 | |
| Sex | | | | | | | | | | |
| Male | 45 | 15 | 30 | 0.0679 | 17 | 28 | 0.4346 | 17 | 28 | 0.2899 |
| Female | 15 | 9 | 6 | | 4 | 11 | | 8 | 7 | |
| Age (years) | | | | | | | | | | |
| <60 | 26 | 11 | 15 | 0.7497 | 10 | 16 | 0.623 | 11 | 15 | 0.9298 |
| ≥60 | 34 | 13 | 21 | | 11 | 23 | | 14 | 20 | |
| Tumor size | | | | | | | | | | |
| <5 cm | 35 | 22 | 13 | <0.0001b | 19 | 16 | 0.0002b | 4 | 31 | <0.0001b |
| ≥5 cm | 25 | 2 | 23 | | 2 | 23 | | 21 | 4 | |
| Lymph node
metastasis | | | | | | | | | | |
| Negative | 30 | 18 | 12 | 0.0016b | 16 | 14 | 0.0029b | 19 | 11 | 0.0007b |
| Positive | 30 | 6 | 24 | | 5 | 25 | | 6 | 24 | |
| TNM stage | | | | | | | | | | |
| I | 19 | 16 | 3 | <0.0001b | 11 | 8 | 0.0374b | 3 | 16 | 0.0002b |
| II | 27 | 7 | 20 | | 6 | 21 | | 10 | 17 | |
| III | 14 | 1 | 13 | | 4 | 10 | | 12 | 2 | |
| Pathological
type | | | | | | | | | | |
| Squamous
carcinoma | 20 | 6 | 14 | 0.3728 | 6 | 14 | 0.0861 | 7 | 13 | 0.2566 |
|
Adenocarcinoma | 20 | 9 | 11 | | 5 | 15 | | 11 | 9 | |
| Large cell
carcinoma | 10 | 3 | 7 | | 7 | 3 | | 5 | 5 | |
| Bronchioloalveolar
carcinoma | 10 | 6 | 4 | | 3 | 7 | | 2 | 8 | |
IL-17 induces A549 cell proliferation and
upregulates HMGA1 or cyclin D1 expression
Given that IL-17, IL-17R, HMGA1 and cyclin
D1 expression levels were all elevated in the patients with
NSCLC, and the expression of IL-17R positively correlated
with HMGA1 or cyclin D1 expression, we assumed that
IL-17, as an extracellular stimulus, may trigger NSCLC cell
proliferation and upregulate HMGA1 and cyclin D1 by
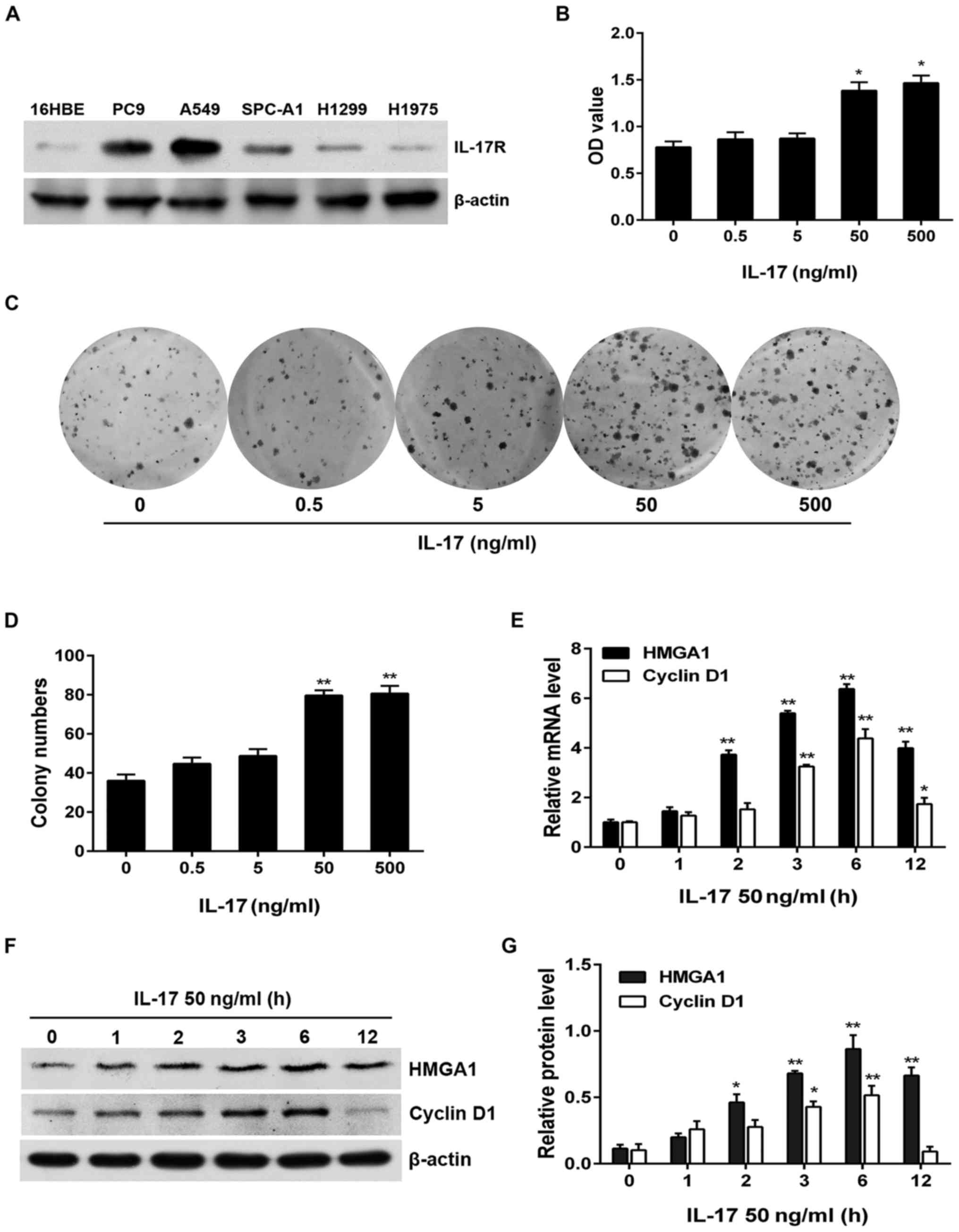
binding to IL-17R. Firstly, to determine the most
susceptible NSCLC cell line to IL-17 stimulation, we
assessed IL-17R expression in different NSCLC cell lines.
The results revealed that IL-17R was markedly overexpressed
in the PC9, A549 and SPC-A1 cells in comparison with the human
bronchial epithelial 16HBE cells, particularly in the A549 cells
(Fig. 2A). Subsequently, we
selected the A549 cells and treated the cells with human
recombinant IL-17 (i.e., IL-17A) and found that A549
cell proliferation (examined by CCK-8 assay and colony formation
assay) was prominently upregulated by IL-17 stimulation in a
dose-dependent manner, particularly when the IL-17
concentration reached 50 and 500 ng/ml (Fig. 2B–D). In addition, to ascertain
whether IL-17 increases HMGA1 and cyclin D1
expression as well as the optimal time-point for the
IL-17-induced production of HMGA1 and cyclin
D1, we stimulated the A549 cells with IL-17 (50 ng/ml)
for different periods of time and detected the transcription and
expression of the afore-mentioned two genes by RT-qPCR and western
blot analysis. The results revealed that the mRNA and protein
expression levels of HMGA1 and cyclin D1 were
significantly increased at 2 h (HMGA1) or 3 h (cyclin
D1) and both peaked at 6 h (Fig.
2E–G). These results indicate that IL-17 not only
induces A549 cell proliferation, but also increases the expression
of HMGA1 and cyclin D1.
IL-17R knockdown or IL-17 neutralization
suppresses IL-17- induced A549 cell proliferation, HMGA1 and cyclin
D1 upregulation
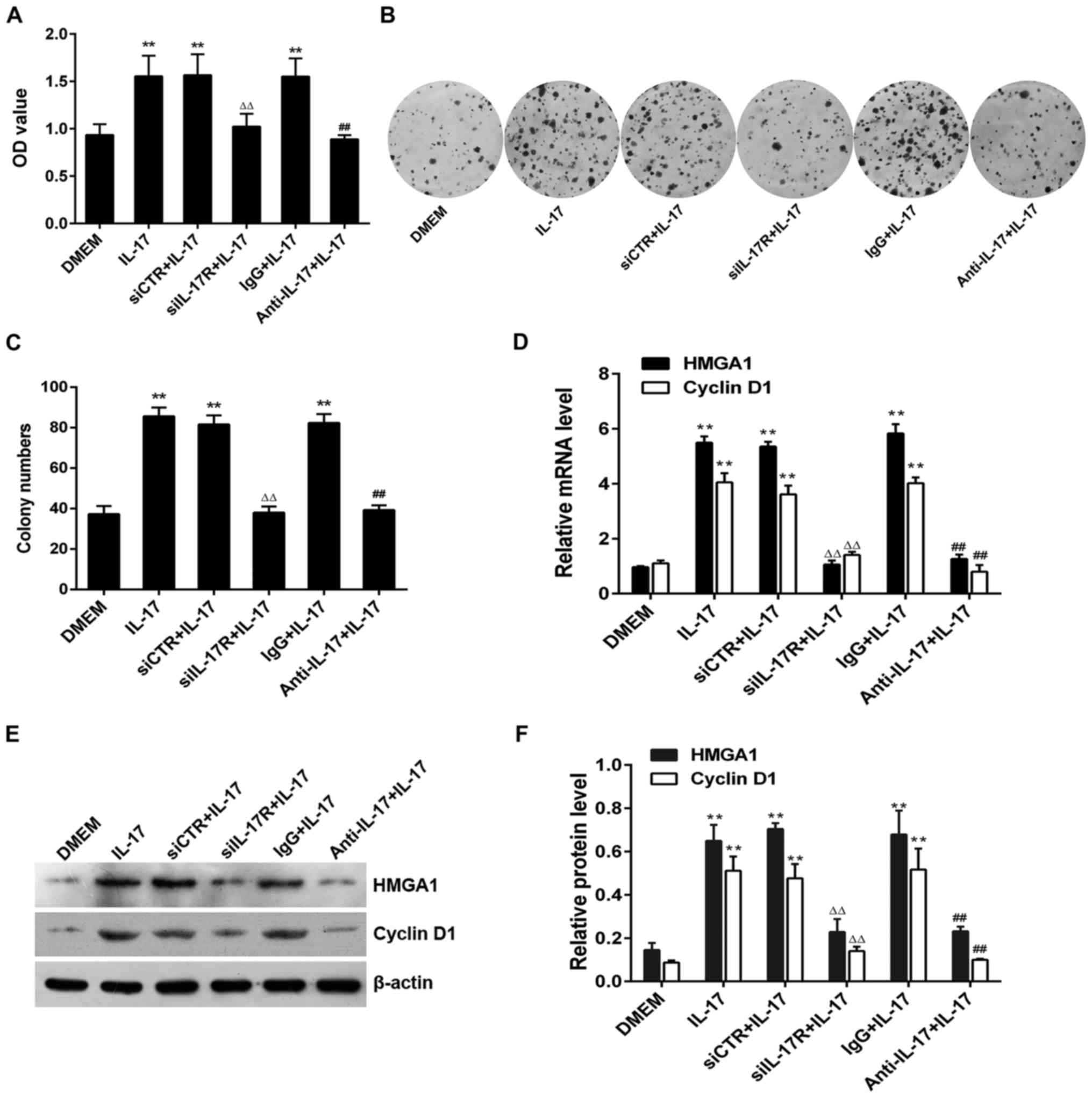
To further confirm that IL-17 stimulation
promotes A549 cell proliferation and increases the expression of
HMGA1 or cyclin D1 by binding to IL-17R, we
subjected the A549 cells to siIL-17R transfection or
IL-17 antibody incubation, prior to treatment with
IL-17 and then examined cell proliferation and the levels of
the two above-mentioned proteins. The results of CCK-8 assay and
colony formation assay revealed that the viability (OD value) and
the colony numbers of A549 cells were greatly multiplied following
treatment with IL-17; however, these effects were notably
reversed by the silencing of IL-17R with siIL-17R or
by the neutralization of IL-17 with anti-IL-17
antibody (Fig. 3A–C). Similarly,
transfection with siIL-17 or incubation with
anti-IL-17 also significantly decreased the
IL-17-induced mRNA and protein expression of HMGA1
and cyclin D1 in the A549 cells (Fig. 3D–F). These results further denote
that IL-17 effectively promotes the proliferation, and
increases the expression of HMGA1 and cyclin D1 in
A549 cells through its interaction with IL-17R.
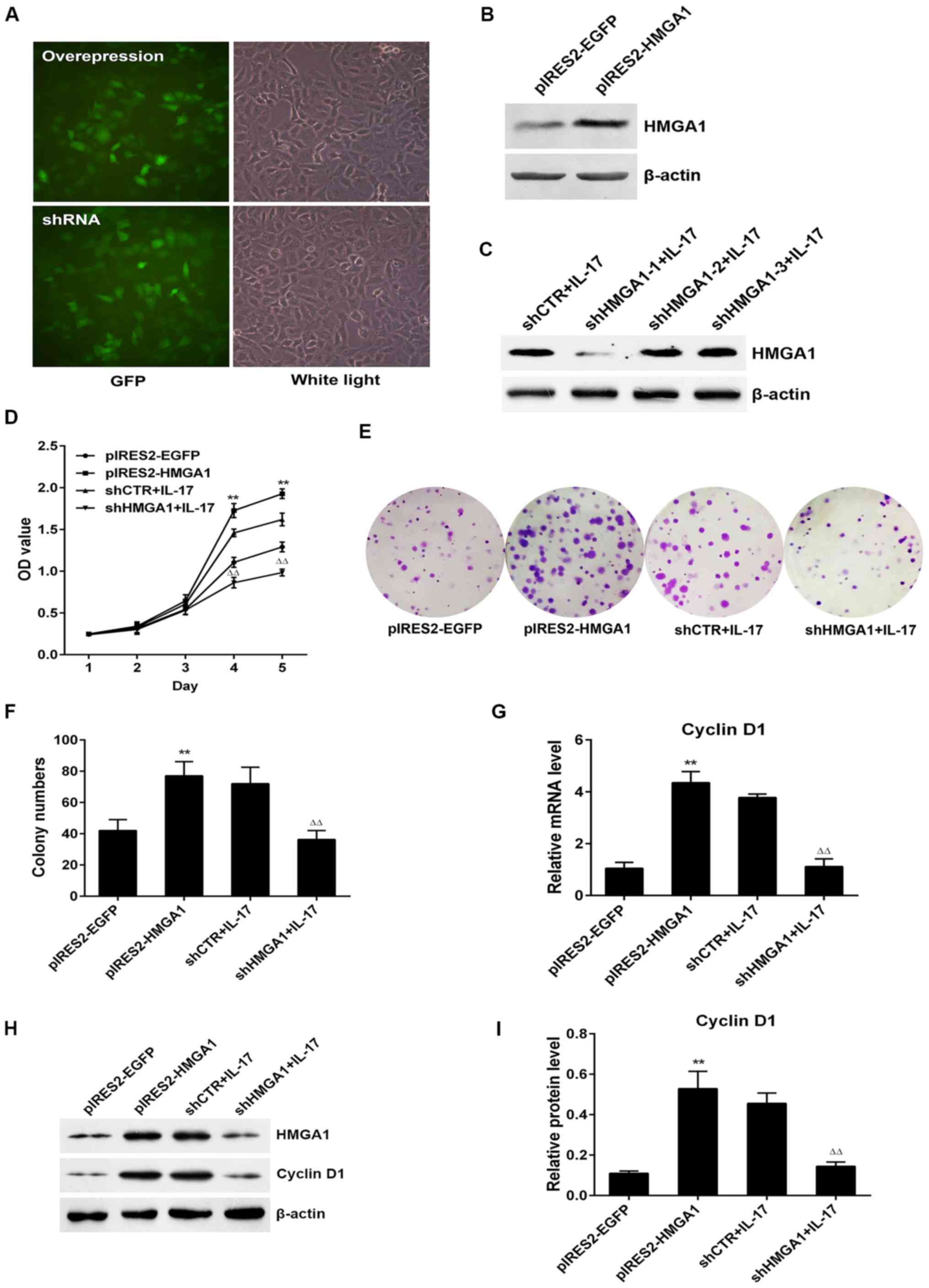
HMGA1 contributes to IL-17-induced A549
cell proliferation and cyclin D1 production
It has been reported that HMGA1 is a
transcription factor which can activate the transcription of
downstream target genes and finally increase their expression
(35,36). Since our experiments verified that
HMGA1 and cyclin D1 expression levels were increased
in the process of IL-17-induced A549 cell proliferation, and
the expression phase of HMGA1 was earlier than that of
cyclin D1, we hypothesized that HMGA1 itself may
enhance cyclin D1 transcription and expression, leading to
an increment in cell proliferation, and that the absence of
HMGA1 may impede the afore-mentioned phenomena mediated by
IL-17. To confirm our hypothesis, we first constructed the
HMGA1 overexpression plasmid (pIRES2-HMGA1) and the
plasmid carrying small hairpin RNA targeting HMGA1
(shHMGA1) and examined the transfection efficiency of these
plasmids (Fig. 4A–C).
Subsequently, CCK-8 and colony formation assays were performed. As
was expected, A549 cell proliferation was markedly elevated
following HMGA1 overexpression, whereas the knockdown of
HMGA1 markedly suppressed cell proliferation upon
IL-17 stimulation (Fig.
4D–F). In addition, the mRNA and protein levels of cyclin
D1 were eminently upregulated or downregulated when
HMGA1 was overexpressed or silenced with IL-17
treatment, respectively (Fig.
4G–I), indicating that IL-17-induced HMGA1
expression exerts a promoting effect on the proliferation of and
cyclin D1 expression in A549 cells mediated by IL-17.
IL-17 activates the cyclin D1 gene
promoter through HMGA1 binding to its response element on the
promoter
As mentioned earlier, IL-17 stimulation
increased cyclin D1 expression via the transcription factor,
HMGA1; hence, we wished to determine whether IL-17
activates the cyclin D1 gene promoter through HMGA1.
For this purpose, we carried out luciferase reporter assay, and
found that IL-17 markedly increased cyclin D1
promoter activity at 3 h (peaked at 6 h) after the A549 cells were
stimulated (Fig. 5A). In addition,
the overexpression of HMGA1 markedly activated the full
length of the cyclin D1 promoter (cyclin D1-FL,
−2,099 to +86 nt), whereas the activity of cyclin D1-FL markedly
decreased in accordance with HMGA1 knockdown (Fig. 5B). Subsequently, to locate the
region in which HMGA1 binds to on the cyclin D1
promoter, we first predicted three potential HMGA1 response
elements (−1,700 to −1,691 nt, −1,026 to −1,017 nt, and −139 to
−130 nt) using the online software JASPAR (http://jaspar.genereg.net/), and then constructed
three truncated promoter reporters based on the prediction
(Fig. 5C), which were truncate 1
(T1, −1,672 to +86 nt), truncate 2 (T2, −1,026 to +86 nt) and
truncate 3 (T3, −139 to +86 nt). By luciferase assay, we confirmed
that in the A549 cells treated with IL-17 or transfected
with the HMGA1 overexpression plasmid, the activity of all
cyclin D1 truncated promoters was markedly decreased
compared to that of the cyclin D1-FL. However, no
statistically significant differences were observed between the
luciferase activity in these three truncated reporters (Fig. 5D and E), suggesting that the most
effective HMGA1 binding element may be located in the region
of −2,099 to −1,673 nt on the cyclin D1 promoter (probably
−1,700 to −1,691 nt). Finally, to determine the exact binding of
HMGA1 to the above-mentioned element, a ChIP assay was
performed using antibody against HMGA1, and the region
−1,752 to −1,622 nt (containing −1,700 to −1,691 nt) was then
amplified by RT-qPCR. The results revealed that the binding of
HMGA1 to the indicated fragment of the cyclin D1
promoter was prominently increased both in the A549 cells subjected
to IL-17 stimulation or in those transfected with the
HMGA1 overexpression vector; however, this binding was
diminished when HMGA1 was silenced (Fig. 5F and G). These findings imply that
IL-17 enhances cyclin D1 promoter activity via
HMGA1, directly binding to its response element in the
region −1,752 to −1,622 nt, which eventually results in an
increased cyclin D1 expression and A549 cell
proliferation.
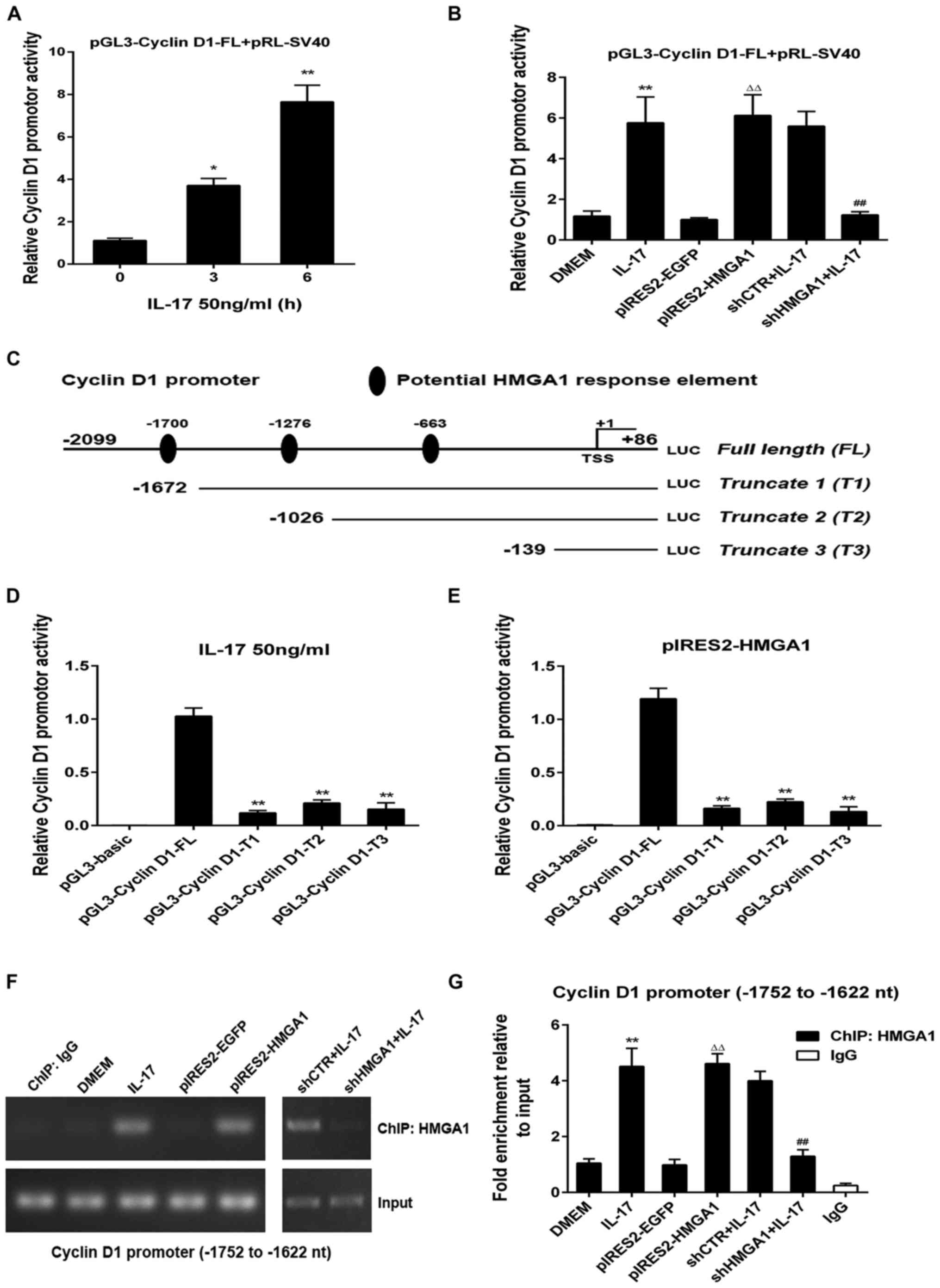 | Figure 5Identification of cyclin D1 promoter
activity in A549 cells upon IL-17 stimulation and the HMGA1 binding
element on the cyclin D1 promoter. (A) A549 cells were transfected
with the full-length promoter of cyclin D1 (cyclin D1-FL, −2,099 to
+86 nt), followed by stimulation with IL-17 at 50 ng/ml for 3 or 6
h. Luciferase reporter assay was performed to examine the activity
of the cyclin D1 promoter in A549 cells at different time-points.
*P<0.05 and **P<0.01 vs. 0 h. (B)
Cyclin D1-FL was co-transfected with pIRES2-HMGA1 or with shHMGA1
into A549 cells, followed by IL-17 stimulation. The activity of the
cyclin D1-FL promoter was assessed by reporter assay.
**P<0.01 vs. DMEM, ΔΔP<0.01 vs.
pIRES2-EGFP, ##P<0.01 vs. shCTR + IL-17 group. (C)
Schematic representation of the cyclin D1 promoter and the
predicted HMGA1 response elements on it. The reporter plasmids
carrying truncated promoter regions were constructed as indicated.
(D and E) Using luciferase assay, the activity of cyclin D1-FL and
three truncated promoters (cyclin D1-T1, cyclin D1-T2 and cyclin
D1-T3) was measured either in the presence of (D) IL-17 stimulation
or (E) HMGA1 overexpression. **P<0.01 vs. pGL3-cyclin
D1-FL. (F and G) A549 cells were transfected with pIRES2-HMGA1 or
with shHMGA1 followed by exposure to IL-17. Anti-HMGA1 was used to
perform ChIP assay. (F) PCR and (G) RT-qPCR were then applied to
quantify the binding of HMGA1 to the indicated region of the cyclin
D1 promoter. **P<0.01 vs. DMEM,
ΔΔP<0.01 vs. pIRES2-EGFP, ##P<0.01 vs.
shCTR + IL-17 group. All data are shown as the means ± SE from 3
independent experiments. HMGA1, high-mobility group A1. |
Disscussion
NSCLC is one of the most common types of malignancy
worldwide (1,2). Accumulating evidence suggests that
NSCLC is a typical inflammation-associated cancer (3,4,37,38),
and IL-17 as a pro-inflammatory cytokine, has been reported
to be closely associated with NSCLC cell proliferation and
development (7,39); however, the mechanisms underlying
IL-17-induced NSCLC cell proliferation have not yet been
fully elucidated.
It has been well documented that cell proliferation
is associated with extracellular stimuli and proliferative molecule
expression (7,40,41).
IL-17A (namely IL-17), as a stimulus, is mainly
secreted by activated T cells, mononuclear cells, dendritic cells
(DCs) and other cells, including tumor cells (5,6).
Recent studies have revealed that IL-17 overproduction
contributes to the inflammatory microenvironment for NSCLC cell
proliferation and growth (5,11).
Moreover, HMGA1 as a transcription factor and cyclin
D1 as a proliferative protein can also promote cancer cell
proliferation (40–43). Hence, in this study, in order to
better understand the pathogenesis of IL-17-induced NSCLC
cell proliferation, we first detected the level of IL-17 in
plasma, and the expression of IL-17R, HMGA1 as well as that
of cyclin D1 in the tumor tissues of patients with NSCLC.
Our results revealed the markedly elevated production of IL-17,
IL-17R, HMGA1 and cyclin D1, and positive correlations
between IL-17R, HMGA1 and cyclin D1 expression.
Besides, we also confirmed the positive correlation between
IL-17R, HMGA1 and cyclin D1 expression with tumor
size, lymph node metastasis and the TNM stage of patients with
NSCLC. These results indicate that the overexpression of these
molecules mentioned above, not only exists in patients with NSCLC,
but may also be related to NSCLC growth.
As is known, IL-17 plays a crucial role in
controlling inflammation (8) and
promoting tumor cell proliferation such as colitis-associated
cancer (44) and NSCLC (39). Moreover, IL-17, as an
extracellular stimulus, can activate several cell signaling
pathways, such as p38/c-Fos and JNK/c-Jun (45), and HMGA1 has been reported
to be a direct transcriptional target of c-Jun (46), indicating that IL-17 may
promote the transcription of the HMGA1 gene. However,
whether HMGA1 and cyclin D1 expression is upregulated
in NSCLC cells upon IL-17 stimulation remains elusive. Our
in vitro experiments demonstrated an enhanced proliferation
of, as well as an increased HMGA1 and cyclin D1
expression in A549 cells exposed to IL-17. Additionally,
IL-17R knockdown with siIL-17R or the neutralizing of
IL-17 with anti-IL-17 antibody significantly
decreased the proliferation and HMGA1 or cyclin D1
expression in the A549 cells. These data thus suggest that
IL-17 markedly induces A549 cell proliferation through
HMGA1 and cyclin D1 production.
Reportedly, HMGA1 protein acts within the
nucleus of mammalian cells as an architectural transcription factor
(40,42); however, the
cytoplasmic/mitochondrial localization of HMGA1 protein in
multiple cell types has also been found (35), suggesting that HMGA1 may
undergo nucleocytoplasmic translocation during some biological
processes. Moreover, HMGA1 can modulate gene expression by
altering the chromatin structure and orchestrating the assembly of
transcription factor complexes to augment target gene promoter
activity (42). Furthermore,
HMGA1 markedly facilitates the proliferation of several
types of cancer cells, such as pancreatic cancer (36), breast cancer (47), ovarian cancer (48), colon cancer (49) and thyroid cancer (21). Additionally, cyclin D1 also
regulates cyclin-dependent kinase (CDK)4 and CDK6 to
promote cell cycle transition from the G1 to the S phase (50–52).
However, even though it has already been mentioned in the
literature that during the process of pancreatic cancer cell
proliferation, HMGA1 regulates the transcription of the
cyclin D1 gene, which promotes cell cycle G1/S transition
through CDK4 and CDK6 (36), the effects of HMGA1 on
cyclin D1 gene transcription, expression and cell
proliferation in NSCLC and the specific mechanisms involved have
not yet been fully determined. In this study, the
IL-17-induced expression phase of HMGA1 was slightly
earlier than that of cyclin D1; we thus speculated that the
upregulation of HMGA1 induced by IL-17 may have
effect on cyclin D1 expression and the proliferation of A549
cells. By the overexpression or knockdown of HMGA1 in the
presence of IL-17 stimulation, we found that the
proliferation of and cyclin D1 expression in A549 cells were
positively associated with HMGA1 expression, and so was the
cyclin D1 promoter activity, suggesting that HMGA1
expression can trigger cyclin D1 gene transcription. Further
experiments affirmed that HMGA1 can directly bind to the
promoter of the cyclin D1 gene, and the site of HMGA1
binding to the cyclin D1 promoter within the region of
−1,752 to −1,622 nt was uncovered for the first time, at least to
the best of our knowledge. Collectively, these results suggest that
IL-17-induced A549 cell proliferation is linked with
HMGA1 boosting cyclin D1 gene transcription and
expression, indicating that the activation of the HMGA1/cyclin
D1 axis is indispensable in the mechanisms of NSCLC A549 cell
proliferation upon IL-17 stimulation.
In conclusion, the present study verified that the
expression level of IL-17, IL-17R, HMGA1 and cyclin
D1 was significantly increased in samples from patients with
NSCLC. Moreover, IL-17R, HMGA1 and cyclin D1 were
positively associated with the malignancy grade of NSCLC. Besides,
we revealed that IL-17 stimulation prominently upregulated
the expression of HMGA1 and cyclin D1 in the A549
cells, and induced cell proliferation in vitro. Furthermore,
elevated HMGA1 expression directly binds to its response
element on the cyclin D1 gene promoter, resulting in
cyclin D1 gene transcription and expression, and finally
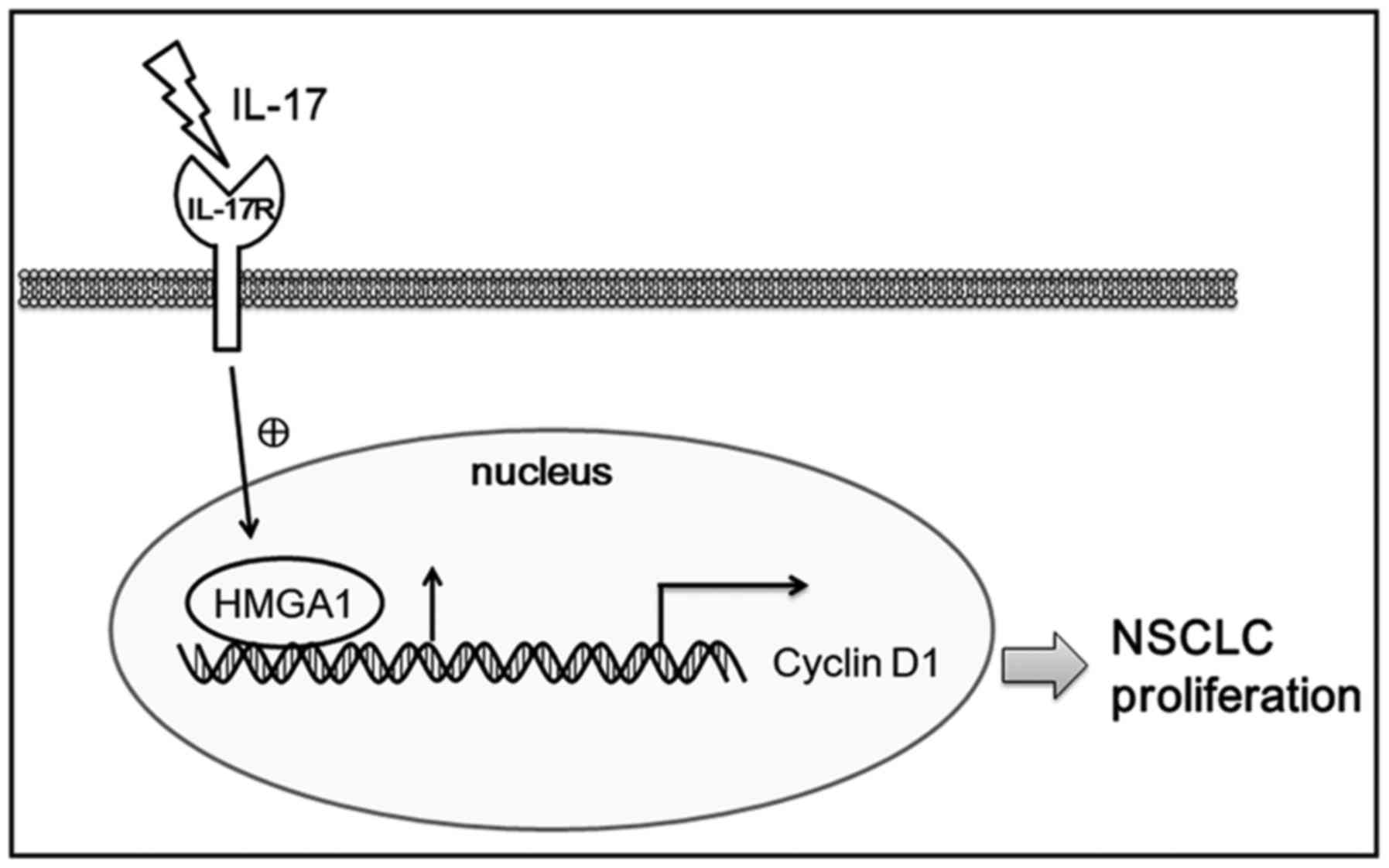
promoting A549 cell proliferation (Fig. 6). Overall, our data suggest that
the IL-17/HMGA1/cyclin D1 axis promotes NSCLC cell
proliferation, and may provide new insight into the pathogenesis of
NSCLC.
Acknowledgments
The authors would like to thank Miss Samjhana
Pandey for modifying the language.
Notes
[1]
Funding
This study was supported by grants from the
National Natural Science Foundations of China (nos. 81272532,
81472626 and 81672896).
[2] Availability
of data and materials
The analyzed datasets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
YS and WQ designed the study and CZ wrote the
manuscript. CZ and YL carried out experiments. WZ collected and
provided the samples of NSCLC patients. DZ, PM, LM and FY
participated in the experiments and analyzed the data. YS and YW
supervised the study. All authors have read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
This study was approved by the Ethics Committee of
Nanjing Medical University and conformed to the guidelines outlined
by the Declaration of Helsinki. Informed consent was obtained from
all patients participating in this research prior to the
experiment.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Oh IJ and Ahn SJ: Multidisciplinary team
approach for the management of patients with locally advanced
non-small cell lung cancer: Searching the evidence to guide the
decision. Radiat Oncol J. 35:16–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGranahan T and Nagpal S: A
Neuro-oncologist's perspective on management of brain metastases in
patients with EGFR mutant non-small cell lung cancer. Curr Treat
Options Oncol. 18:222017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu HX, Shi L, Zhang Y, Zhu YC, Bai CX,
Wang XD and Zhou JB: Myocyte enhancer factor 2D provides a
cross-talk between chronic inflammation and lung cancer. J Transl
Med. 15:652017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu F, Xu J, Huang Q, Han J, Duan L, Fan J,
Lv Z, Guo M, Hu G, Chen L, et al: The role of interleukin-17 in
lung cancer. Mediators Inflamm. 2016:84940792016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwiecien I, Stelmaszczyk-Emmel A,
Polubiec-Kownacka M, Dziedzic D and Domagala-Kulawik J: Elevated
regulatory T cells, surface and intracellular CTLA-4 expression and
interleukin-17 in the lung cancer microenvironment in humans.
Cancer Immunol Immunother. 66:161–170. 2017. View Article : Google Scholar :
|
|
6
|
Chang SH, Mirabolfathinejad SG, Katta H,
Cumpian AM, Gong L, Caetano MS, Moghaddam SJ and Dong C: T helper
17 cells play a critical pathogenic role in lung cancer. Proc Natl
Acad Sci USA. 111:5664–5669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei L, Wang H, Yang F, Ding Q and Zhao J:
Interleukin-17 potently increases non-small cell lung cancer
growth. Mol Med Rep. 13:1673–1680. 2016. View Article : Google Scholar
|
|
8
|
Kuwabara T, Ishikawa F, Kondo M and
Kakiuchi T: The role of IL-17 and related cytokines in inflammatory
autoimmune diseases. Mediators Inflamm. 2017:39080612017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Q, Du J, Fan J, Lv Z, Qian X, Zhang
X, Han J, Chen C, Wu F and Jin Y: The effect of proinflammatory
cytokines on IL-17RA expression in NSCLC. Med Oncol. 31:1442014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng S, Shao Z, Liu X, Guo L, Zhang X, Na
Q, Chen X, Ma Y, Zheng J, Song B, et al: Interleukin 17A
polymorphism elevates gene expression and is associated with
increased risk of nonsmall cell lung cancer. DNA Cell Biol.
34:63–68. 2015. View Article : Google Scholar
|
|
11
|
Cao Y, Zhao D, Li P, Wang L, Qiao B, Qin
X, Li L and Wang Y: MicroRNA-181a-5p impedes IL-17-induced nonsmall
cell lung cancer proliferation and migration through targeting
VCAM-1. Cell Physiol Biochem. 42:346–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Han Y, Fei G, Guo Z, Ren T and Liu
Z: IL-17 promoted metastasis of non-small-cell lung cancer cells.
Immunol Lett. 148:144–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirshberg S, Izhar U, Amir G, Demma J,
Vernea F, Beider K, Shlomai Z, Wald H, Zamir G, Shapira OM, et al:
Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression.
PLoS One. 6:e248562011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin
S, Cao S, Che D, Liu F and Yu Y: Interleukin-17 promotes
angiogenesis by stimulating VEGF production of cancer cells via the
STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep.
5:160532015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu L, Chen X, Zhao J, Martin B, Zepp JA,
Ko JS, Gu C, Cai G, Ouyang W, Sen G, et al: A novel IL-17 signaling
pathway controlling keratinocyte proliferation and tumorigenesis
via the TRAF4-ERK5 axis. J Exp Med. 212:1571–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu W, Zhang Y, Liu X, Zhou J, Li Y, Zhou
Y, Shan K, Xia M, Che N, Feng X, et al: Sublytic C5b-9 complexes
induce proliferative changes of glomerular mesangial cells in rat
Thy-1 nephritis through TRAF6-mediated PI3K-dependent Akt1
activation. J Pathol. 226:619–632. 2012. View Article : Google Scholar
|
|
17
|
Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S
and Yu Y: Interleukin-17-induced EMT promotes lung cancer cell
migration and invasion via NF-κB/ZEB1 signal pathway. Am J Cancer
Res. 5:1169–1179. 2015.
|
|
18
|
Sumter TF, Xian L, Huso T, Koo M, Chang
YT, Almasri TN, Chia L, Inglis C, Reid D and Resar LM: The high
mobility group A1 (HMGA1) transcriptome in cancer and development.
Curr Mol Med. 16:353–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esposito F, De Martino M, D'Angelo D,
Mussnich P, Raverot G, Jaffrain-Rea ML, Fraggetta F, Trouillas J
and Fusco A: HMGA1-pseudogene expression is induced in human
pituitary tumors. Cell Cycle. 14:1471–1475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin Y, Chen H, Hu Z, Mao Y, Xu X, Zhu Y,
Xu X, Wu J, Li S, Mao Q, et al: miR-26a inhibits proliferation and
motility in bladder cancer by targeting HMGA1. FEBS Lett.
587:2467–2473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong J, Liu C, Zhang QH, Chen L, Shen YY,
Chen YJ, Zeng X, Zu XY and Cao RX: TGF-β1 induces HMGA1 expression:
The role of HMGA1 in thyroid cancer proliferation and invasion. Int
J Oncol. 50:1567–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sekimoto N, Suzuki A, Suzuki Y and Sugano
S: Expression of miR-26a exhibits a negative correlation with HMGA1
and regulates cancer progression by targeting HMGA1 in lung
adenocarcinoma cells. Mol Med Rep. 15:534–542. 2017. View Article : Google Scholar :
|
|
23
|
Sarhadi VK, Wikman H, Salmenkivi K, Kuosma
E, Sioris T, Salo J, Karjalainen A, Knuutila S and Anttila S:
Increased expression of high mobility group A proteins in lung
cancer. J Pathol. 209:206–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Wang Q, Chen F and Liu J:
Elevated expression of HMGA1 correlates with the malignant status
and prognosis of non-small cell lung cancer. Tumour Biol.
36:1213–1219. 2015. View Article : Google Scholar
|
|
25
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016. View Article : Google Scholar
|
|
27
|
Tian XP, Jin XH, Li M, Huang WJ, Xie D and
Zhang JX: The depletion of PinX1 involved in the tumorigenesis of
non-small cell lung cancer promotes cell proliferation via
p15/cyclin D1 pathway. Mol Cancer. 16:742017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shan K, Pang R, Zhao C, Liu X, Gao W,
Zhang J, Zhao D, Wang Y and Qiu W: IL-17-triggered downregulation
of miR-497 results in high HIF-1α expression and consequent IL-1β
and IL-6 production by astrocytes in EAE mice. Cell Mol Immunol.
14:1–15. 2017.
|
|
29
|
Qiu W, Zhou J, Zhu G, Zhao D, He F, Zhang
J, Lu Y, Yu T, Liu L and Wang Y: Sublytic C5b-9 triggers glomerular
mesangial cell apoptosis via XAF1 gene activation mediated by
p300-dependent IRF-1 acetylation. Cell Death Dis. 5:e11762014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
31
|
Al-Azhri J, Zhang Y, Bshara W, Zirpoli G,
McCann SE, Khoury T, Morrison CD, Edge SB, Ambrosone CB and Yao S:
Tumor expression of Vitamin D receptor and breast cancer
histopathological characteristics and prognosis. Clin Cancer Res.
23:97–103. 2017. View Article : Google Scholar :
|
|
32
|
Surowiak P, Materna V, Györffy B,
Matkowski R, Wojnar A, Maciejczyk A, Paluchowski P, Dziegiel P,
Pudełko M, Kornafel J, et al: Multivariate analysis of oestrogen
receptor alpha, pS2, metallothionein and CD24 expression in
invasive breast cancers. Br J Cancer. 95:339–346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Li Y, Shan K, Wang L, Qiu W, Lu
Y, Zhao D, Zhu G, He F and Wang Y: Sublytic C5b-9 induces IL-6 and
TGF-β1 production by glomerular mesangial cells in rat Thy-1
nephritis through p300-mediated C/EBPβ acetylation. FASEB J.
28:1511–1525. 2014. View Article : Google Scholar
|
|
34
|
He F, Zhou M, Yu T, Zhao D, Zhang J, Qiu
W, Lu Y, Liu Y, Wang L and Wang Y: Sublytic C5b-9 triggers
glomerular mesangial cell apoptosis in rat Thy-1 nephritis via
Gadd45 activation mediated by Egr-1 and p300-dependent ATF3
acetylation. J Mol Cell Biol. 8:477–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dement GA, Treff NR, Magnuson NS,
Franceschi V and Reeves R: Dynamic mitochondrial localization of
nuclear transcription factor HMGA1. Exp Cell Res. 307:388–401.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kolb S, Fritsch R, Saur D, Reichert M,
Schmid RM and Schneider G: HMGA1 controls transcription of insulin
receptor to regulate cyclin D1 translation in pancreatic cancer
cells. Cancer Res. 67:4679–4686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vendramini-Costa DB and Carvalho JE:
Molecular link mechanisms between inflammation and cancer. Curr
Pharm Des. 18:3831–3852. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JJ, Kim HJ, Yang CS, Kyeong HH, Choi
JM, Hwang DE, Yuk JM, Park K, Kim YJ, Lee SG, et al: A
high-affinity protein binder that blocks the IL-6/STAT3 signaling
pathway effectively suppresses non-small cell lung cancer. Mol
Ther. 22:1254–1265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan B, Che D, Cao J, Shen J, Jin S, Zhou
Y, Liu F, Gu K, Man Y, Shang L, et al: Interleukin-17 levels
correlate with poor prognosis and vascular endothelial growth
factor concentration in the serum of patients with non-small cell
lung cancer. Biomarkers. 20:232–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Quintavalle C, Burmeister K, Piscuoglio S,
Quagliata L, Karamitopoulou E, Sepe R, Fusco A, Terracciano LM,
Andersen JB, Pallante P, et al: High mobility group A1 enhances
tumorigenicity of human cholangiocarcinoma and confers resistance
to therapy. Mol Carcinog. 56:2146–2157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahlin C, Lundgren C, Embretsén-Varro E,
Jirström K, Blomqvist C and Fjällskog M: High expression of cyclin
D1 is associated to high proliferation rate and increased risk of
mortality in women with ER-positive but not in ER-negative breast
cancers. Breast Cancer Res Treat. 164:667–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huso TH and Resar LM: The high mobility
group A1 molecular switch: Turning on cancer - can we turn it off?
Expert Opin Ther Targets. 18:541–553. 2014. View Article : Google Scholar
|
|
43
|
Li Z, Qu L, Luo W, Tian Y, Zhai H, Xu K
and Zhong H: Mig-6 is down-regulated in HCC and inhibits the
proliferation of HCC cells via the P-ERK/Cyclin D1 pathway. Exp Mol
Pathol. 102:492–499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hyun YS, Han DS, Lee AR, Eun CS, Youn J
and Kim HY: Role of IL-17A in the development of colitis-associated
cancer. Carcinogenesis. 33:931–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fabre T, Kared H, Friedman SL and Shoukry
NH: IL-17A enhances the expression of profibrotic genes through
upregulation of the TGF-β receptor on hepatic stellate cells in a
JNK-dependent manner. J Immunol. 193:3925–3933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hommura F, Katabami M, Leaner VD,
Donninger H, Sumter TF, Resar LM and Birrer MJ: HMG-I/Y is a
c-Jun/activator protein-1 target gene and is necessary for
c-Jun-induced anchorage-independent growth in Rat1a cells. Mol
Cancer Res. 2:305–314. 2004.PubMed/NCBI
|
|
47
|
Zhou WB, Zhong CN, Luo XP, Zhang YY, Zhang
GY, Zhou DX and Liu LP: miR-625 suppresses cell proliferation and
migration by targeting HMGA1 in breast cancer. Biochem Biophys Res
Commun. 470:838–844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Y, Wang Y, Zhang Y, Fu J and Zhang G:
Knockdown of HMGA1 expression by short/small hairpin RNA inhibits
growth of ovarian carcinoma cells. Biotechnol Appl Biochem. 59:1–5.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang Q, Wang X, Tang C, Chen X and He J:
H19 promotes the migration and invasion of colon cancer by sponging
miR-138 to upregulate the expression of HMGA1. Int J Oncol.
50:1801–1809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Żuryń A, Litwiniec A, Safiejko-Mroczka B,
Klimaszewska-Wiśniewska A, Gagat M, Krajewski A, Gackowska L and
Grzanka D: The effect of sulforaphane on the cell cycle, apoptosis
and expression of cyclin D1 and p21 in the A549 non-small cell lung
cancer cell line. Int J Oncol. 48:2521–2533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chikara S, Lindsey K, Dhillon H, Mamidi S,
Kittilson J, Christofidou-Solomidou M and Reindl KM: Enterolactone
induces G1-phase cell cycle arrest in nonsmall cell lung cancer
cells by downregulating cyclins and cyclin-dependent kinases. Nutr
Cancer. 69:652–662. 2017. View Article : Google Scholar : PubMed/NCBI
|