Introduction
Diabetes mellitus (DM) is the most common metabolic
disease (1). There is increasing
evidence that diabetes is closely associated with male reproductive
dysfunction. Compared with non-diabetic individuals, male diabetic
patients showed an increased incidence of hypogonadism and
infertility (2). Oxidative stress
damage is regarded as the most influential harm-causing factor
affecting testicular function (2–4). The
increase in reactive oxygen species (ROS) causes non-specific
changes in nucleic acid, protein and phospholipid levels, resulting
in DNA, RNA and protein damage and alterations in antioxidant
enzyme levels, which lead to cellular and tissue damage (1). Diabetes inhibits reproductive
activity in experimental animals; for instance, the testicular
function of diabetic rats is impaired, including reduced testicular
weight, sperm count and sperm motility (5). Antioxidant vitamin E is capable of
improving diabetes-induced free radical damage in testicular tissue
(1).
A previous study showed that the apoptosis of
testicular germ cells increases in diabetic mice, leading to the
disruption of spermatogenesis (6).
Apoptosis occurs in the testes of diabetic animals, but the
mechanism of apoptosis has not yet been clarified (7). Apoptotic cell death is mediated by
the activation of apoptotic signaling pathways, including the Bcl-2
family proteins. Bcl-2 and Bcl-xL suppress apoptotic cell death
through anti-apoptotic function, whereas Bax and Bad promote
apoptotic death through pro-apoptotic function (7).
The overproduction of ROS promotes the process of
apoptosis by increasing caspase-3 activity and inhibiting Bcl-2
expression, which demonstrates that a crosstalk exists between
oxidative stress and apoptosis (2).
Previous studies have indicated that NADPH oxidase
is the main source of ROS, and that activated NADPH oxidase results
in increased ROS production (8–10).
ROS derived from NADPH oxidase play a potent role in initiating and
accelerating the development of diabetic complications (11). NADPH oxidase is composed of the
regulatory subunits, p67phox (phox, phagocyte oxidase),
p47phox, p22phox and p40phox, and
the catalytic subunit, gp91phox. The active oxidase
generates superoxide by transferring the electron from NADPH to
oxygen (12).
Apocynin has been previously shown to effectively
inhibit the increased NADPH oxidase activity in diabetic aortas and
to restore changes in nitric oxide synthase (NOS) expression,
thereby blocking the vicious cycle which results in
diabetes-associated endothelial dysfunction (13). A recent study also showed that
elevated activity of NADPH oxidase led to significant testicular
damage, and that the antioxidant, strontium fructose
1,6-diphosphate (FDP-Sr), restored the testicular function
(14).
The aim of this study was to examine whether
testicular dysfunction and apoptosis under diabetic conditions can
be ameliorated by the NADPH oxidase inhibitor, apocynin, by
suppressing oxidative stress.
Materials and methods
Experimental animals
Tongji Hospital, Tongji Medical College, Huazhong
University of Science (Wuhan, China) and the Technology Animal Care
and Use Committee approved all procedures undertaken in the current
study. Seven-week-old male Sprague-Dawley (SD) rats were obtained
from Tongji Medical College, Huazhong University of Science and
Technology. Diabetes in rats was induced by a single
intraperitoneal injection of streptozocin (STZ) at a dose of 60
mg/kg in citrate buffer (50 mM sodium citrate, pH 4.5), blood
glucose levels in serum samples obtained from the tail vein of all
rats were detected prior to diabetes induction and 72 h after
intraperitoneal injection of STZ with a blood glucose meter
(Johnson and Johnson, New Brunswick, NJ, USA). The rats with blood
glucose concentrations >11.1 mmol/l were accepted as being
diabetic.
Eight weeks after the induction of diabetes,
diabetic rats were randomly divided into 2 groups: the untreated
group and the apocynin-treatment group (16 mg/kg). The treatment
period was 4 weeks. Age-matched male SD rats were used as the
control group.
At the end of the treatment period, the blood
collected via cardiac puncture was allowed to clot and the serum
was obtained by centrifugation at 1,500 × g for 15 min. Serum
samples were stored at −80°C until analysis. The testes were
harvested after rats were sacrificed with an intraperitoneal
overdose of pentobarbital.
Measurement of serum testosterone
level
The serum testosterone level was detected using a
rat testosterone ELISA kit (R&D Systems, Inc., Minneapolis, MN,
USA) according to the manufacturer’s instructions.
Measurement of testicular mRNA
expression
Total RNA was isolated from testicular tissue using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. cDNA was synthesized from 500 ng of
RNA using the PrimeScriptTM RT reagent kit (Takara,
Dalian, China) according to the manufacturer’s instructions.
Real-time PCR was carried out with a Stratagene real-time PCR
system (Agilent Technologies, Inc., Santa Clara, CA, USA). The
real-time PCR reactions were performed using SYBR Premix Ex Taq
(Takara). The final volume of 25 μl contained 2.0 μl cDNA, 12.5 μl
SYBR Premix Ex Taq, 0.5 μl (10 mM) of forward and reverse primers,
0.5 μl ROX Reference Dye and 9.0 μl ddH2O. The cycling
conditions were as follows: predenaturation at 95°C for 30 sec,
followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec.
Immediately following amplification, melt curve protocols were
performed to ensure that primer dimers and other non-specific
products had been minimized or eliminated. As an endogenous
reference and for normalization purposes, the level of mRNA
expression for β-actin was measured. Relative quantification of the
expression levels of each transcript for each group was calculated
using the 2−ΔΔCt method. Using the non-diabetic control
as the calibrator, the data of other groups were presented as the
fold change in gene expression normalized to β-actin and relative
to the non-diabetic control. The sequences of the PCR primers used
are shown in Table I.
 | Table IPrimer sequences for real-time
quantitative polymerase chain reaction. |
Table I
Primer sequences for real-time
quantitative polymerase chain reaction.
| Gene | Primers |
|---|
|
p47phox | F:
5′-GAGACATACCTGACGGCCAAAGA-3′ |
|
p47phox | R:
5′-AGTCAGCGATGGCCCGATAG-3′ |
|
p67phox | F:
5′-GAAAGCATGAAGGATGCCTGG-3′ |
|
p67phox | R:
5′-ATAGCACCAAGATCACATCTCC-3′ |
| Bax | F:
5′-GTTACAGGGTTTCATCCAGG-3′ |
| Bax | R:
5′-CGTGTCCACGTCAGCAAT-3′ |
| Bcl-2 | F:
5′-CGGGAGAACAGGGTATGA-3′ |
| Bcl-2 | R:
5′-CAGGCTGGAAGGAGAAGAT-3′ |
| β-actin | F:
5′-AAGAGCTATGAGCTGCCTGA-3′ |
| β-actin | R:
5′-TACGGATGTCAACGTCACAC-3′ |
Measurement of testicular protein
expression
Testicular tissues were homogenized in lysis buffer
on ice. Protein was loaded on SDS-PAGE gel. The resolved proteins
were transferred onto 0.2-μm nitrocellulose membranes, and blots
were blocked in 5% non-fat dried milk for 1 h at 37°C to saturate
non-specific protein binding. The membranes were incubated
overnight at a 1:500 dilution of polyclonal primary antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The membranes were
then incubated with appropriate alkaline phosphatase-linked
secondary antibodies (Proteintech Group, Inc., Chicago, IL, USA)
for 1 h at 37°C and visualized using the BCIP/NBT Chromogen system
(Covance Inc., Princeton, NJ, USA). β-actin was used as the
internal control to standardize each sample with equal protein
quantity. Protein expression was quantified by computer-assisted
densitometry with the use of the Gel Pro version 4.0 software
(Media Cybernetics, Georgia, MD, USA).
Thiobarbituric acid reactive substances
(TBARS) assay
TBARS measures a family of lipid peroxidation
products and is a major indicator of oxidative stress (8). Frozen testicular tissue was
pulverized and resuspended in phosphate-buffered saline. TBARS
levels were analyzed using the TBARS assay kit (Cayman Chemical
Company, Ann Arbor, MI, USA) according to the manufacturer’s
instructions. In this assay, a malondialdehyde (MDA) standard curve
was constructed, and TBARS levels were expressed in terms of MDA
equivalents and normalized to the wet weight of testicular
tissue.
Detection of apoptosis
The apoptotic cells were detected by terminal
deoxynucleotidyl transferase-mediated dUTP nick end-labeling
(TUNEL) assay with the ApopTag peroxidase in situ apoptosis
detection kit (Chemicon International, Inc., Temecula, CA, USA), as
described in a previous study (15). Testicular tissue was fixed in 10%
formalin, embedded in paraffin and sectioned at 5 μm. The slides
were de-paraffinized and rehydrated, and treated with proteinase K
(20 mg/l) for 15 min, then treated with 3% hydrogen peroxide for 5
min to inhibit endogenous peroxidase and incubated with the TUNEL
reaction mixture containing terminal deoxynucleotidyl transferase
(TdT) and digoxigenin-11-dUTP at 37°C for 1 h. The TdT reaction was
performed in a humidified chamber at 37°C for 1 h, and then
3,3-diaminobenzidine (DAB) chromogen was used. Hematoxylin was used
as counterstaining.
Statistical analysis
Data were expressed as the means ± SD and analyzed
by one-way analysis of variance (ANOVA). Post hoc analysis was
carried out by Student Newman-Keuls or Dunnett’s tests as
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
General parameters
The final mean blood glucose concentration of
STZ-diabetic rats significantly increased compared with that of
initial mean blood glucose concentration, and apocynin treatment
for 4 weeks did not significantly affect the blood glucose
concentration in diabetic rats. The final mean bodyweight of the
diabetic rats significantly decreased compared with that of initial
mean bodyweight. Apocynin treatment did not significantly change
the body weight of diabetic rats (Table II).
 | Table IIWeight and blood glucose in control
and streptozocin (STZ) diabetic rats. |
Table II
Weight and blood glucose in control
and streptozocin (STZ) diabetic rats.
| Control (n=10) | Diabetes (n=10) | Diabetes + apocynin
(n=10) |
|---|
| Weight (g) |
| Initial | 272.7±6.9 | 273.4±6.8 | 274.3±6.5 |
| Final | 403.0±10.0a | 246.4±7.0a | 245.5±6.9a |
| Blood glucose
(mM) |
| Initial | 5.5±0.50 | 5.6±0.45 | 5.6±0.46 |
| Final | 5.7±0.39 | 30.5±1.20a | 30.2±1.20a |
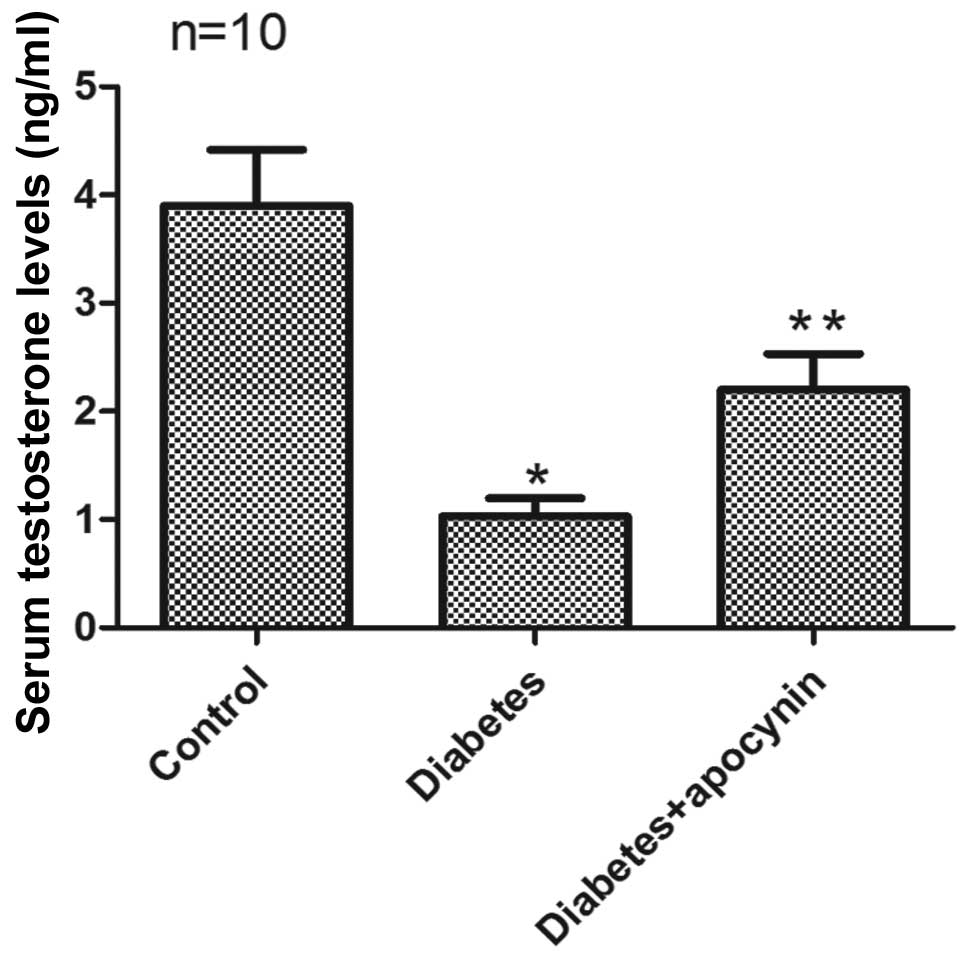
Total testosterone level
Serum testosterone levels in rats from the diabetic
group were significantly reduced compared with the control group
(P<0.05) (Fig. 1). Apocynin
treatment significantly increased the testosterone level in
diabetic rats (P<0.05) (Fig.
1).
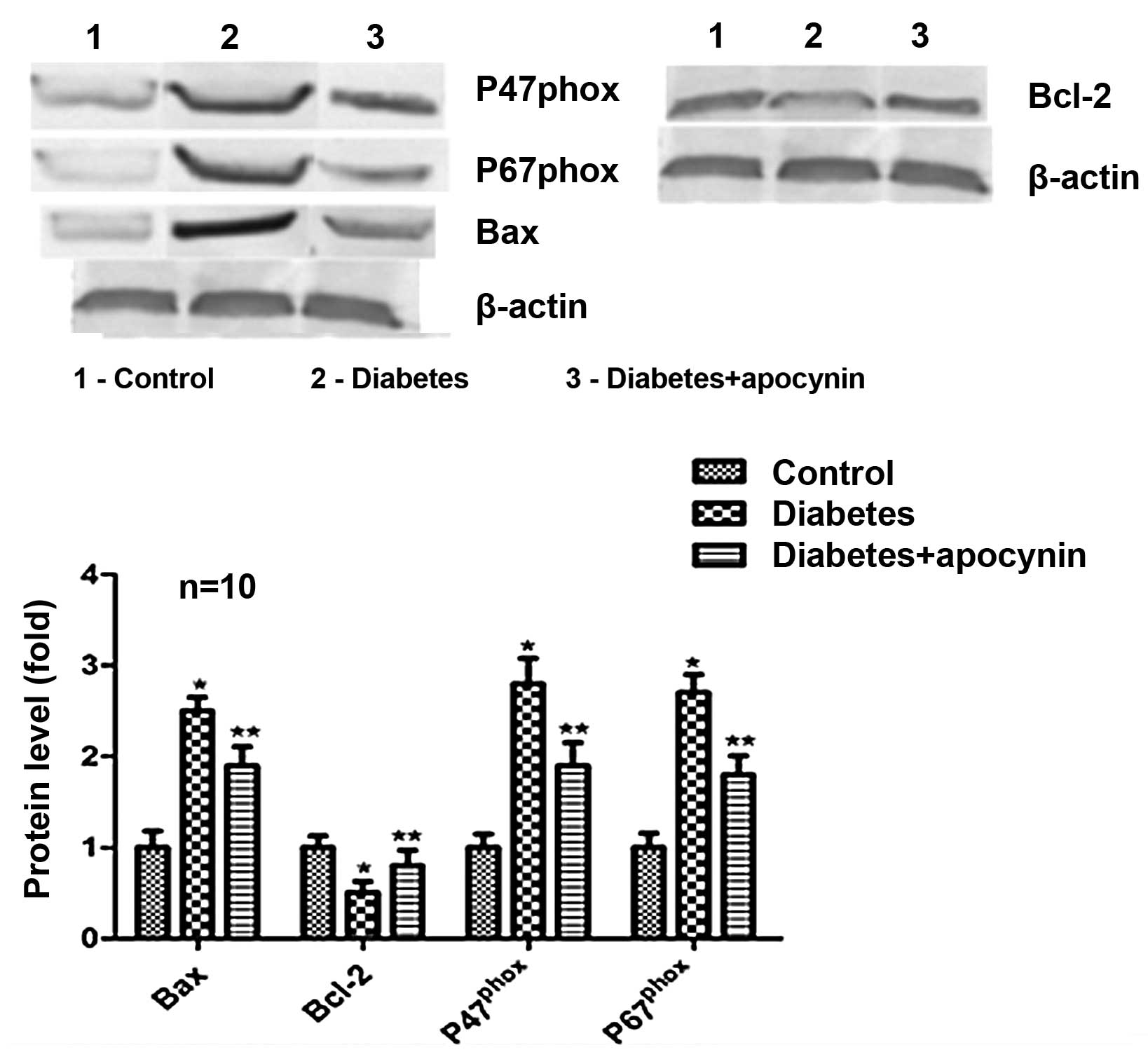
Protein expression
Western blot analysis revealed that Bcl-2 protein
expression was significantly decreased in rat testes of the
diabetic group (P<0.05) (Fig.
2), while the expression of Bax, p47phox and
p67phox significantly increased compared with the
control group (P<0.05) (Fig.
2). Treatment of diabetic rats with apocynin significantly
suppressed all down- and/or upregulation.
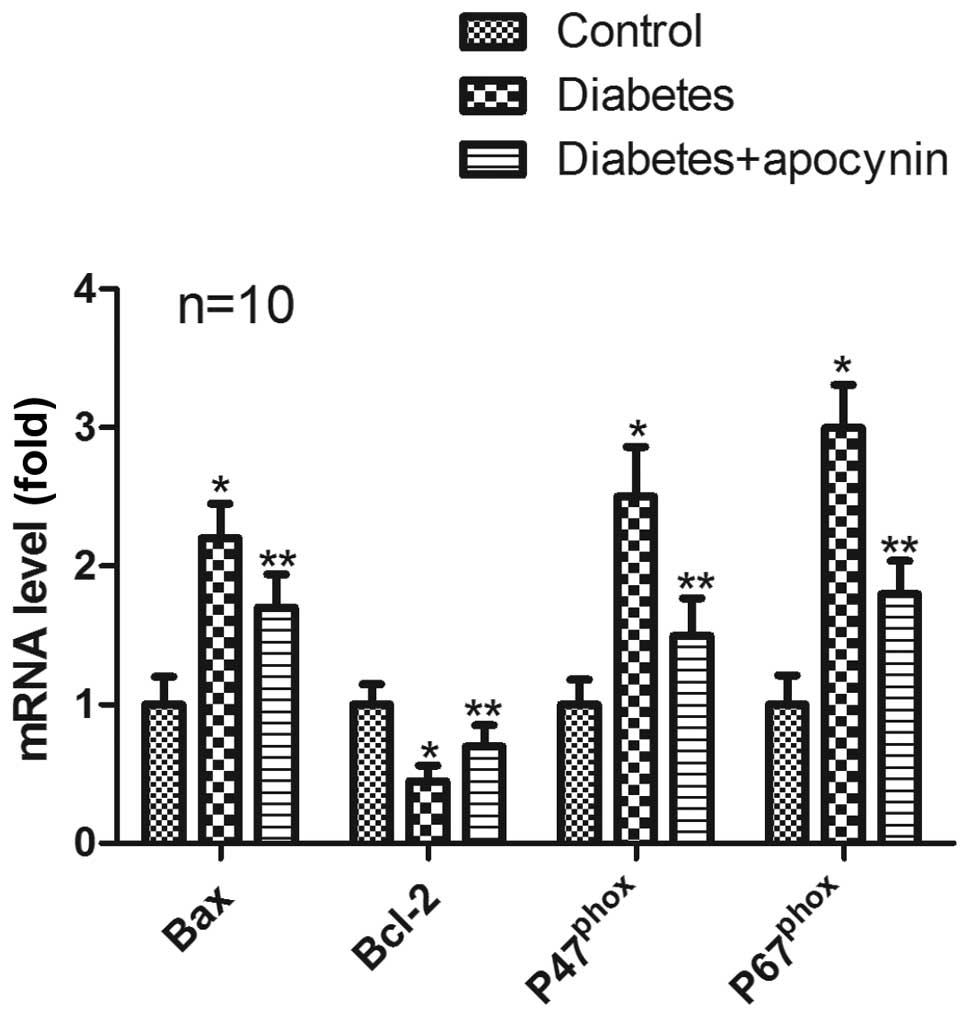
mRNA expression
In the testes of diabetic rats, mRNA expression of
Bcl-2 was significantly decreased (P<0.05) (Fig. 3), whereas mRNA expression of Bax,
p47phox and p67phox significantly increased
compared with control group (P<0.05) (Fig. 3). Treatment of diabetic rats with
apocynin significantly suppressed all down- and/or upregulation
(P<0.05) (Fig. 3).
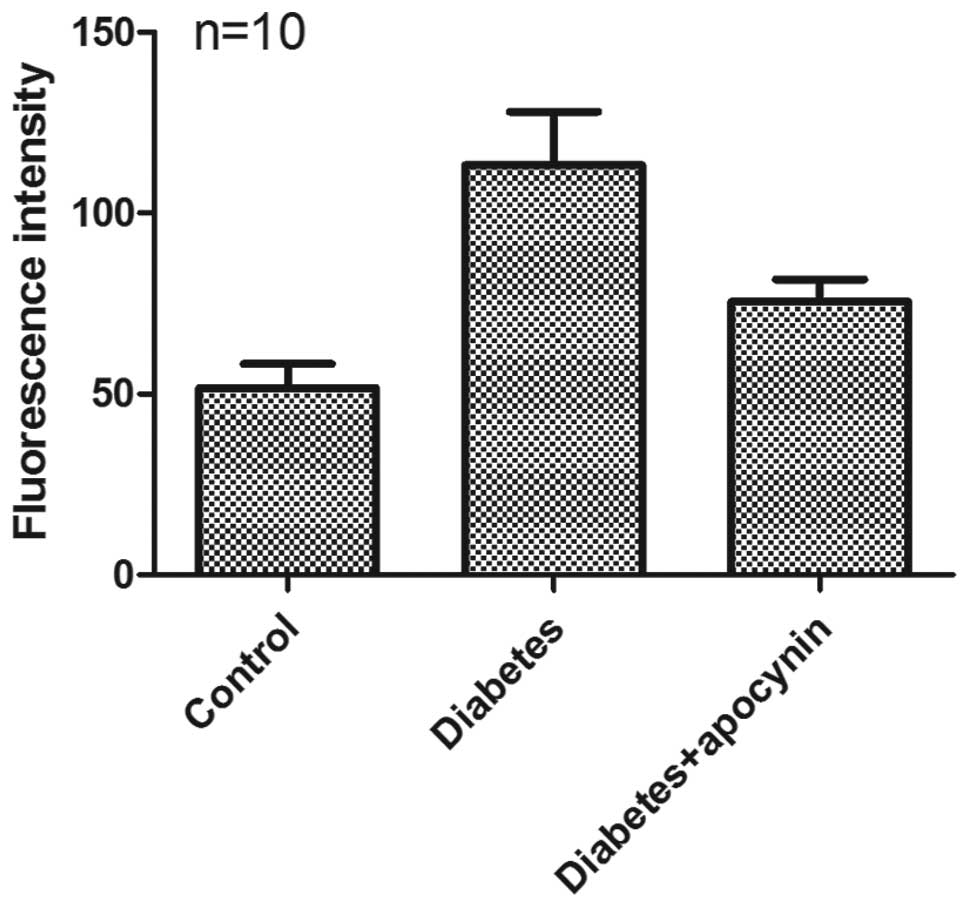
Production of ROS
A significant increase in the TBARS level was
observed in the testes of the diabetic rats when compared to those
of control rats (0.359±0.019 vs. 0.251±0.02 nmol MDA/mg wet tissue,
respectively) (P<0.05) (Fig.
4). Treatment with apocynin effectively reduced the TBARS level
in the testes of diabetic rats (0.302±0.018 nmol MDA/mg wet tissue)
(P<0.05) (Fig. 4).
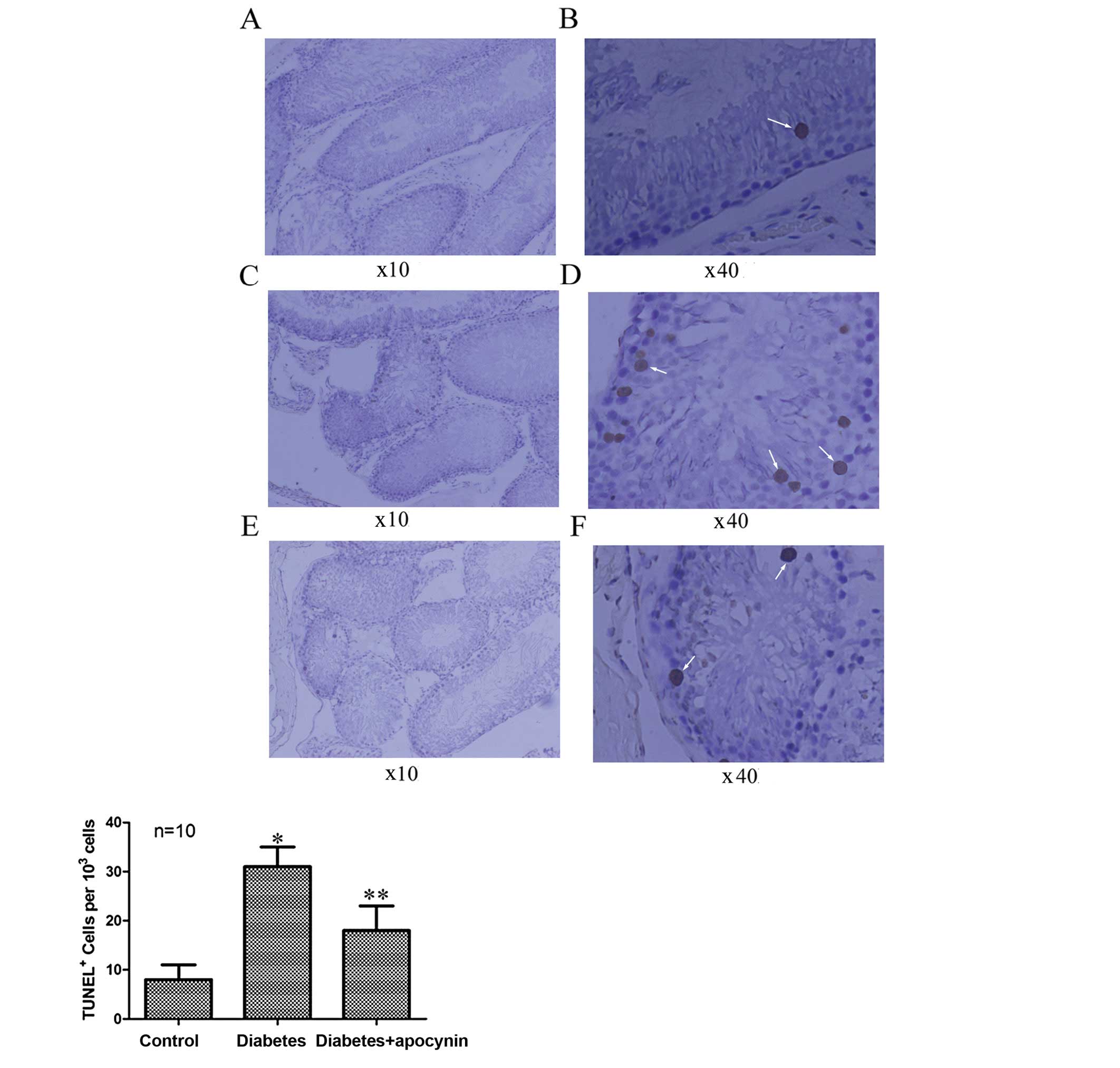
Apocynin decreases apoptosis
The testicular apoptotic cells significantly
increased in rats of the diabetic group compared with the control
group (P<0.05) (Fig. 5), as
evaluated by TUNEL staining. Treatment of diabetic rats with
apocynin significantly decreased apoptotic cell numbers (P<0.05)
(Fig. 5).
Discussion
The present study demonstrated that serum
testosterone levels and testicular apoptotic cells of diabetic rats
significantly declined, and testicular apoptotic cells of diabetic
rats significantly increased, which was associated with enhanced
oxidative stress and apoptosis in testes. The NADPH oxidase
inhibitor apocynin significantly ameliorated testicular dysfunction
by its antioxidant activity.
Apocynin was used at a dose of 16 mg/kg per day in
this study, with reference to a previous study on diabetic
neuropathy reporting that a daily dose of 100 mg/kg was no more
effective than 15 mg/kg in preventing neuropathy and reducing nerve
blood flow in diabetic rats (12).
Diabetes has been reported to cause testicular
dysfunction. Diabetes results in certain changes in seminiferous
tubules, including increased tubular wall thickness, severe
germ-cell depletion and Sertoli cell vacuolization, as well as
reduced testicular volumes, semen volume and numbers of Leydig
cells and their spontaneous secretion of testosterone (16). Oxidative stress plays a direct role
in the pathogenesis of various diabetic complications, which is one
of the major causes of testicular dysfunction (1,17).
Hyperglycemia is capable of increasing free radical formation and
reducing endogenous antioxidant capacity, leading to enhanced
oxidative stress in testicular tissue (1). Previous studies have suggested that
diabetic testicular lesions involve significant oxidative stress
(2). Overproduction of ROS results
in mitochondrial damage and lipid peroxidation in germ and Leydig
cells, which lead to dysfunction of testicular spermatogenesis and
steroidogenesis (2). Our data
showed that ROS production significantly increased and the
testosterone level significantly decreased in diabetic testicular
tissue, which confirmed the findings of previous studies (1,2).
A recent study suggested that NADPH oxidase be
considered as an important source of ROS in both physiological and
pathophysiological conditions (11). In another recent study, it was
demonstrated that NADPH oxidase p22, p47 and p67 subunits were
significantly increased in diabetic testes relative to normal
testes (14). In agreement with
these results, the results from our study demonstrated that NADPH
oxidase p47 and p67 subunits were significantly increased in
diabetic testes.
Other studies have reported a significant increase
in the apoptotic cell death in the testes of diabetic rats
(2,5,18).
There is increasing evidence showing that excessive ROS production
triggers an apoptosis cascade through the phosphorylation of JNK
and activation of Bax (18), and
by activating caspase, regulating the expression of Bcl-2 family
proteins (19). Moreover, the
decreased production of testosterone may also promote germ cell
apoptosis (20). In the present
study, increased ROS and decreased production of testosterone may
constitute the major reasons for testicular apoptosis, which
confirms the findings of previous studies (2,18–20).
Previous studies have shown that antioxidant vitamin
E and C relieve testicular injury by suppressing oxidative stress
(1). Antioxidant FDP-Sr and
endothelin type A receptor antagonist attenuated cell death by
suppressing oxidative stress (2,14,19).
NADPH oxidase is the main source of ROS, and elevated activity of
NADPH oxidase results in increased ROS production (8–10).
In our study, the NADPH oxidase inhibitor, apocynin, significantly
reduced ROS production and cell death by suppressing oxidative
stress, which was consistent with the results from a previous study
(14).
In conclusion, our results clearly demonstrate that
the increased rate of testicular cell death by apoptosis in
STZ-induced diabetic rats is driven by excessive ROS production,
and that apoptosis in testicular tissues can be ameliorated by the
NADPH oxidase inhibitor, apocynin, by suppressing the excessive
production of ROS.
References
|
1
|
Aybek H, Aybek Z, Rota S, Sen N and
Akbulut M: The effects of diabetes mellitus, age, and vitamin E on
testicular oxidative stress. Fertil Steril. 90:755–760. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang XY, Zhang Q, Dai DZ, Ying HJ, Wang QJ
and Dai Y: Effects of strontium fructose 1,6-diphosphate on
expression of apoptosis-related genes and oxidative stress in
testes of diabetic rats. Int J Urol. 15:251–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shrilatha B and Muralidhara: Early
oxidative stress in testis and epididymal sperm in
streptozotocin-induced diabetic mice: its progression and genotoxic
consequences. Reprod Toxicol. 23:578–587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boujbiha MA, Hamden K, Guermazi F,
Bouslama A, Omezzine A, Kammoun A and El Feki A: Testicular
toxicity in mercuric chloride treated rats: association with
oxidative stress. Reprod Toxicol. 28:81–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kosova B, Cetintaş VB, Yavaşoğlu A, Altay
B and Aktuğ H: From a molecular biological viewpoint, does
endothelin type A receptor antagonist therapy reduce
diabetes-induced testicular damage in rats? Urology. 77:250.e7–13.
2011. View Article : Google Scholar
|
|
6
|
Sainio-Pöllänen S, Henriksén K, Parvinen
M, Simell O and Pöllänen P: Stage-specific degeneration of germ
cells in the seminiferous tubules of nonobese diabetic mice. Int J
Androl. 20:243–253. 1997.PubMed/NCBI
|
|
7
|
Koh PO: Streptozotocin-induced diabetes
increases the interaction of Bad/Bcl-xL and decreases the binding
of pBad/14-3-3 in rat testis. Life Sci. 81:1079–1084. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin L, Lagoda G, Leite R, Webb RC and
Burnett AL: NADPH oxidase activation: a mechanism of
hypertension-associated erectile dysfunction. J Sex Med. 5:544–551.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin L and Burnett AL: NADPH oxidase:
recent evidence for its role in erectile dysfunction. Asian J
Androl. 10:6–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai H, Griendling KK and Harrison DG: The
vascular NAD(P)H oxidases as therapeutic targets in cardiovascular
diseases. Trends Pharmacol Sci. 24:471–478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taye A, Saad AH, Kumar AH and Morawietz H:
Effect of apocynin on NADPH oxidase-mediated oxidative
stress-LOX-1-eNOS pathway in human endothelial cells exposed to
high glucose. Eur J Pharmacol. 627:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cotter MA and Cameron NE: Effect of the
NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion
and function in diabetic rats. Life Sci. 73:1813–1824. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olukman M, Orhan CE, Celenk FG and Ulker
S: Apocynin restores endothelial dysfunction in streptozotocin
diabetic rats through regulation of nitric oxide synthase and NADPH
oxidase expressions. J Diabetes Complications. 24:415–423. 2010.
View Article : Google Scholar
|
|
14
|
Xu M, Dai DZ, Zhang Q, Cheng YS and Dai Y:
Upregulated NADPH oxidase contributes to diabetic testicular
complication and is relieved by strontium fructose 1,6-diphosphate.
Exp Clin Endocrinol Diabetes. 118:459–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Tan Y, Dai J, et al: Exacerbation
of diabetes-induced testicular apoptosis by zinc deficiency is most
likely associated with oxidative stress, p38 MAPK activation, and
p53 activation in mice. Toxicol Lett. 200:100–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Unlüçerçi Y, Bekpinar S and Koçak H:
Testis glutathione peroxidase and phospholipid hydroperoxide
glutathione peroxidase activities in aminoguanidine-treated
diabetic rats. Arch Biochem Biophys. 379:217–220. 2000.PubMed/NCBI
|
|
17
|
Shrilatha B and Muralidhara: Occurrence of
oxidative impairments, response of antioxidant defences and
associated biochemical perturbations in male reproductive milieu in
the Streptozotocin-diabetic rat. Int J Androl. 30:508–518. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koh PO: Streptozotocin-induced diabetes
increases apoptosis through JNK phosphorylation and Bax activation
in rat testes. J Vet Med Sci. 69:969–971. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaushal N and Bansal MP: Dietary selenium
variation-induced oxidative stress modulates CDC2/cyclin B1
expression and apoptosis of germ cells in mice testis. J Nutr
Biochem. 18:553–564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boekelheide K, Fleming SL, Johnson KJ,
Patel SR and Schoenfeld HA: Role of Sertoli cells in
injury-associated testicular germ cell apoptosis. Proc Soc Exp Biol
Med. 225:105–115. 2000. View Article : Google Scholar : PubMed/NCBI
|