Introduction
Atherosclerosis is a chronic inflammatory disease of
the arterial wall that is characterized by the formation of
atherosclerotic lesions (1–3). In
the early stages of atherosclerosis, monocyte adhesion and
migration to the arterial wall is mediated by adhesion molecules
that are expressed on vascular cells, including intercellular
adhesion molecule 1 (ICAM-1) and E-selectin (2,4–6).
Previous studies demonstrated the importance of
aortic smooth muscle cells in the development of atherosclerosis
(1,7–10).
Specifically, adhesion molecules expressed in smooth muscle cells
in the intima of atherosclerotic lesions appear to facilitate the
accumulation of transmigrated leukocytes within the atherosclerotic
vascular wall (11–13). Adhesion molecule expression is
induced by the proinflammatory cytokine TNF-α, which is crucial for
the pathogenesis and progression of atherosclerosis (10,14).
The stem of Akebia quinata (A. quinata;
Lardizabalaceae) has been used as a crude drug for treating
urinary disorders and inflammatory disease in traditional Korean,
Chinese and Japanese Kampo medicine (15). A. quinata contains saponins
(triterpene and triterpene glycosides), chemical compounds
associated with a number of biological activities (16,17).
However, a limited number of studies have addressed the biological
and pharmacological effects of A. quinata. In particular,
the antiatherogenic effects of A. quinata in aortic smooth
muscle cells remain unclear.
In the present study, the effect of A.
quinata ethanol extract (AQEE) on TNF-α-induced adhesion
molecule expression in human aortic smooth muscle cells was
analyzed. In addition, the mechanisms underlying the
antiatherogenic effects of AQEE were investigated.
Materials and methods
Reagents
Antibodies against ICAM-1 and E-selectin were
purchased from R&D Systems (Minneapolis, MN, USA). Antibodies
against COX-2, p65, lamin A, β-actin, phospho-p38 and p38 were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell culture
Human aortic smooth muscle cells (HASMCs) were
purchased from ScienCell Research Laboratories (Carlsbad, CA, USA).
Cells were cultured as monolayers in smooth muscle cell medium
(ScienCell Research Laboratories) containing essential and
non-essential amino acids, vitamins, organic and inorganic
compounds, hormones, growth factors, trace minerals and 2% fetal
bovine serum (FBS) at 37°C in a humidified atmosphere with 5%
CO2. For subculturing, cells were detached using 0.125%
trypsin containing 0.01 M EDTA. Cells from passages 2–6 were used
for the study. THP-1 cells (ATCC, Manassas, VA, USA), a human
myelomonocytic cell line widely used to study monocyte/macrophage
biology in culture systems (18),
were used in the cell adhesion assay with HASMCs. THP-1 cells were
cultured in RPMI-1640 medium, supplemented with 2 mM L-glutamine,
100 μg/ml streptomycin, 100 IU/ml penicillin and 10% FBS.
Preparation and characterization of A.
quinata extract
A. quinata was purchased from Omniherb Co.,
Ltd. (Yeongcheon, Korea) and was authenticated based on its
microscopic and macroscopic characteristics by the Classification
and Identification Committee of the Korea Institute of Oriental
Medicine (KIOM, Daejeon, Korea). A voucher specimen has been
deposited at the herbarium of the Department of Herbal Resources
Research at KIOM. Dried A. quinata (200 g) was extracted
twice with 70% ethanol (with 2-h reflux). The extract was then
concentrated under reduced pressure at 40°C with a rotary
evaporator. The decoction was filtered, lyophilized and stored at
4°C until use. The lyophilized powder was dissolved in 10% dimethyl
sulfoxide and then filtered through a 0.22-μm syringe filter to
make the stock solution. The yield of the dried extract from the
starting crude materials was 12.01%.
Cell viability
Cells were seeded in 96-well flat-bottom plates
(2×104 cells/well) and incubated in the presence of
various concentrations of AQEE (0, 10, 50 and 250 μg/ml) for 8 h.
Cell counting Kit-8 (CCK-8) reagent (Dojindo, Kumamoto, Japan) was
added to each well and incubated for 1 h. Absorbance was measured
at 450 nm using a Benchmark Plus microplate reader (Bio-Rad
Laboratories, Hercules, CA, USA). The experiment was performed in
triplicate and cell viability was calculated relative to that of
the control.
THP-1 adhesion assay
Adhesion of THP-1 cells to HASMCs was measured as
described previously (19).
Briefly, HASMCs were grown in 96-well plates and pretreated with
AQEE (0, 10, 50 and 250 μg/ml) for 2 h at 37°C. Cells were washed
with medium and then incubated with fresh growth medium containing
TNF-α (10 ng/ml). Following 8 h, the medium was removed from the
wells and calcein AM-labeled THP-1 cells (2×105
cells/ml) in 0.2 ml medium were added to each well. Following 1-h
incubation at 37°C in 5% CO2, the microwells were washed
twice with 0.2 ml warm medium and the number of adherent cells was
detected by microscopy. Each experiment was performed in
triplicate.
Cell surface enzyme-linked immunosorbent
assay (ELISA)
The surface expression of adhesion molecules in
HASMCs was quantified by ELISA. Cells were seeded in 96-well
flat-bottom plates (2×104 cells/well), grown to
confluence and pretreated with AQEE (0, 10, 50 and 250 μg/ml) for 2
h at 37°C. The cells were then washed with medium and incubated for
8 h with fresh growth medium containing TNF-α (10 ng/ml). Following
incubation, the cells were washed with phosphate-buffered saline
(PBS, pH 7.4) and fixed with 0.1% glutaraldehyde for 30 min at 4°C.
Bovine serum albumin (BSA; 1.0% in PBS) was added to the cells to
reduce non-specific binding. The cells were then incubated
overnight at 4°C with primary monoclonal antibodies against ICAM-1
or E-selectin (0.25 g/ml, diluted in blocking buffer). The
following day, cells were washed with PBS and incubated with a
horseradish peroxidase-conjugated goat anti-mouse IgG secondary
antibody (1 μg/ml, diluted in PBS). The cells were then washed with
PBS and exposed to the peroxidase substrate (p-nitrophenyl
phosphate, 1 mg/ml in 0.1 M glycin buffer, pH 10.4, containing 1 mM
MgCl2 and 1 mM ZnCl2). Absorbance was
measured at 405 nm using an EnVision 2103 Multilabel Plate Reader
(Perkin-Elmer, Wellesley, MA, USA). Absorbance values of the
isotype-matched control antibody were used as the blank and
subtracted from the experimental values.
Western blot analysis
To determine the expression of COX-2, ICAM-1,
E-selectin, phospho-p38 and p38, HASMCs were pretreated with AQEE
(0, 10, 50 or 250 μg/ml) for 2 h. Cells were washed with medium and
incubated with fresh growth medium containing TNF-α (10 ng/ml) for
30 min or 8 h. Following treatment, cells were washed twice in PBS
and lysed in ice-cold lysis buffer [50 mM Tris-HCl, pH 7.4; 150 mM
NaCl; 1 mM EDTA; 0.5% (v/v) NP-40; and 0.1% (w/v) SDS] containing
protease inhibitor cocktail (Roche Diagnostics Corp., Indianapolis,
IN, USA) for 1 h. Lysates were collected following centrifugation
at 1,500 × g for 10 min at 4°C. To evaluate the nuclear
translocation of NF-κB p65 subunit, HASMCs were pretreated with
AQEE (0, 10, 50 or 250 μg/ml) as previously described and then
stimulated with TNF-α for 4 h. Cytosolic and nuclear extracts were
prepared using the Nuclear Extract kit (Active Motif, Carlsbad, CA,
USA) according to the manufacturer's instructions. Protein
concentration was determined using the Bio-Rad protein assay
(Bio-Rad Laboratories) with BSA as the standard. Protein lysates
(20 μg) were separated by 10% SDS-PAGE, electrophoretically
transferred to Immobilon polyvinylidene difluoride membranes
(Amersham, Arlington Heights, IL, USA) and probed with the
appropriate antibodies. Blots were developed using an enhanced
chemoluminescence kit (Amersham). In all immunoblotting
experiments, the blots were reprobed with an antibody against
β-actin or lamin A, which were used as protein loading
controls.
Statistical analysis
Results were presented as the mean ± SEM. Group
differences were determined by one-way analysis of variance,
followed by modified t-test with the Bonferroni correction for
comparisons between individual groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
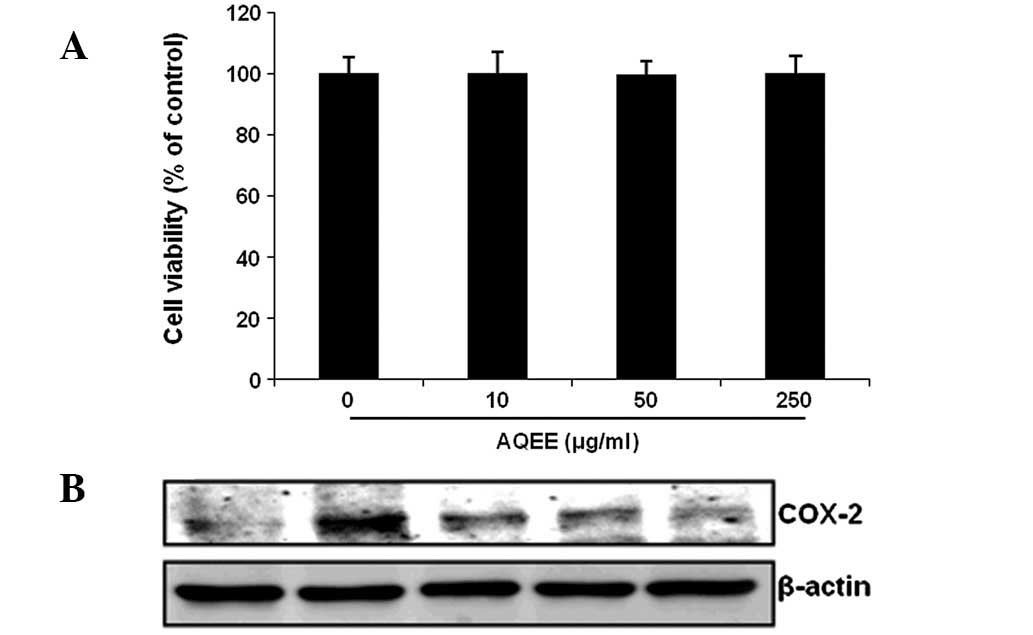
Effect of AQEE on cell viability and
cyclooxygenase 2 (COX-2) expression
The cytotoxic effects of AQEE on HASMCs were
assessed using the CCK-8 cell viability assay. Results demonstrated
that AQEE did not affect cell viability and was not cytotoxic to
HASMCs at the concentrations used (Fig. 1A). The effect of AQEE on COX-2
protein expression was determined by western blot analysis. COX-2
was identified to be upregulated in TNF-α-stimulated cells. AQEE
reduced TNF-α-induced COX-2 expression in a dose-dependent manner
(Fig. 1B).
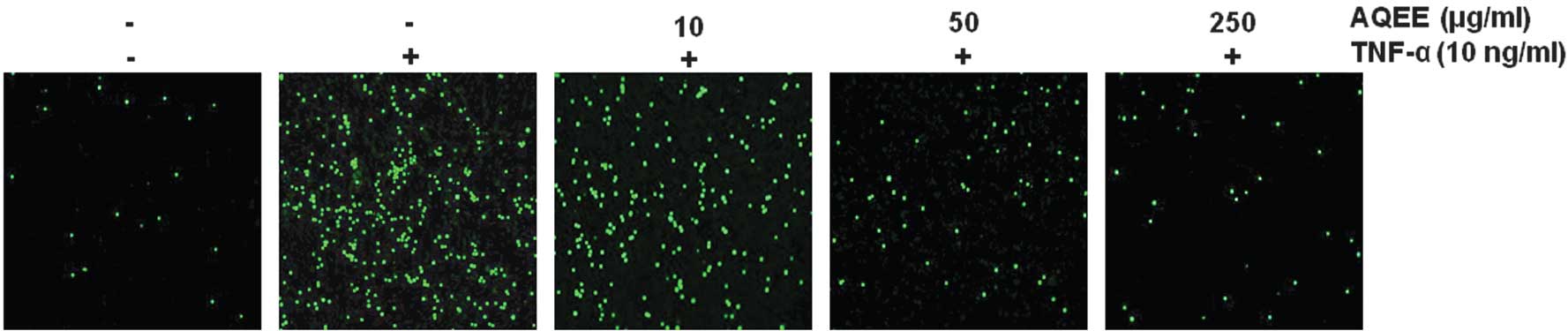
Effect of AQEE on TNF-α-stimulated
monocyte adhesion to HASMCs
A THP-1 adhesion assay was performed to evaluate the
effect of AQEE on monocyte adherence to TNF-α-activated HASMCs.
HASMCs were pretreated with AQEE (0, 10, 50 or 250 μg/ml) for 2 h
prior to TNF-α stimulation (10 ng/ml). TNF-α was identified to
significantly increase adhesion of THP-1 monocytic cells to HASMCs
(P<0.05). AQEE suppressed monocyte adhesion in a dose-dependent
manner (Fig. 2).
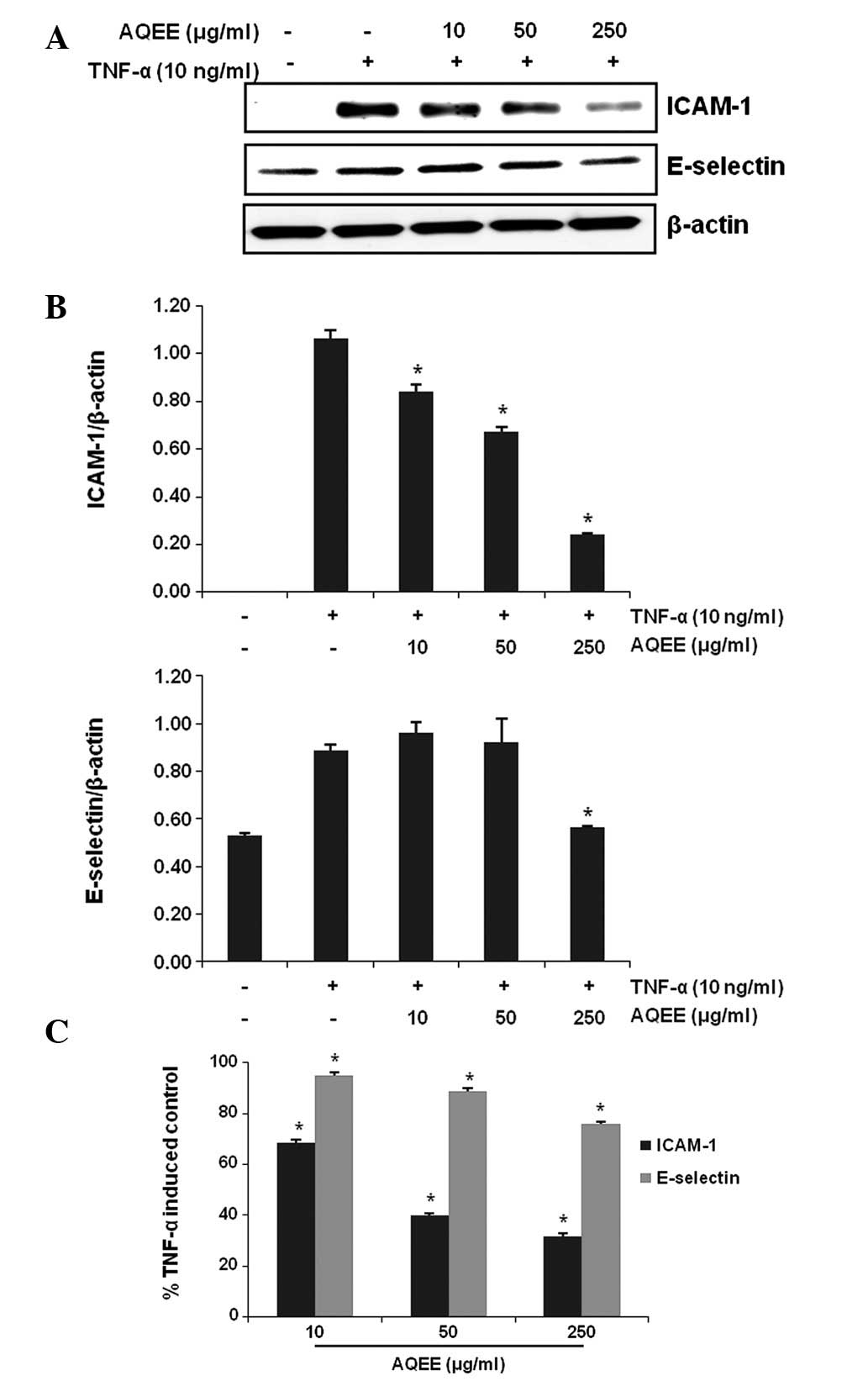
Effect of AQEE on TNF-α-induced
expression of adhesion molecules
The effect of AQEE on the TNF-α-induced expression
of adhesion molecules in HASMCs was assessed by western blot
analysis and cell surface ELISA. Results of the western blot
analysis revealed that TNF-α induced ICAM-1 and E-selectin
(Fig. 3A and B). AQEE pretreatment
inhibited the TNF-α-induced upregulation of the adhesion molecules
in a dose-dependent manner. Consistent with these observations,
ELISA results demonstrated that AQEE reduced adhesion molecule
expression on the cell surface of TNF-α-stimulated HASMCs (Fig. 3C).
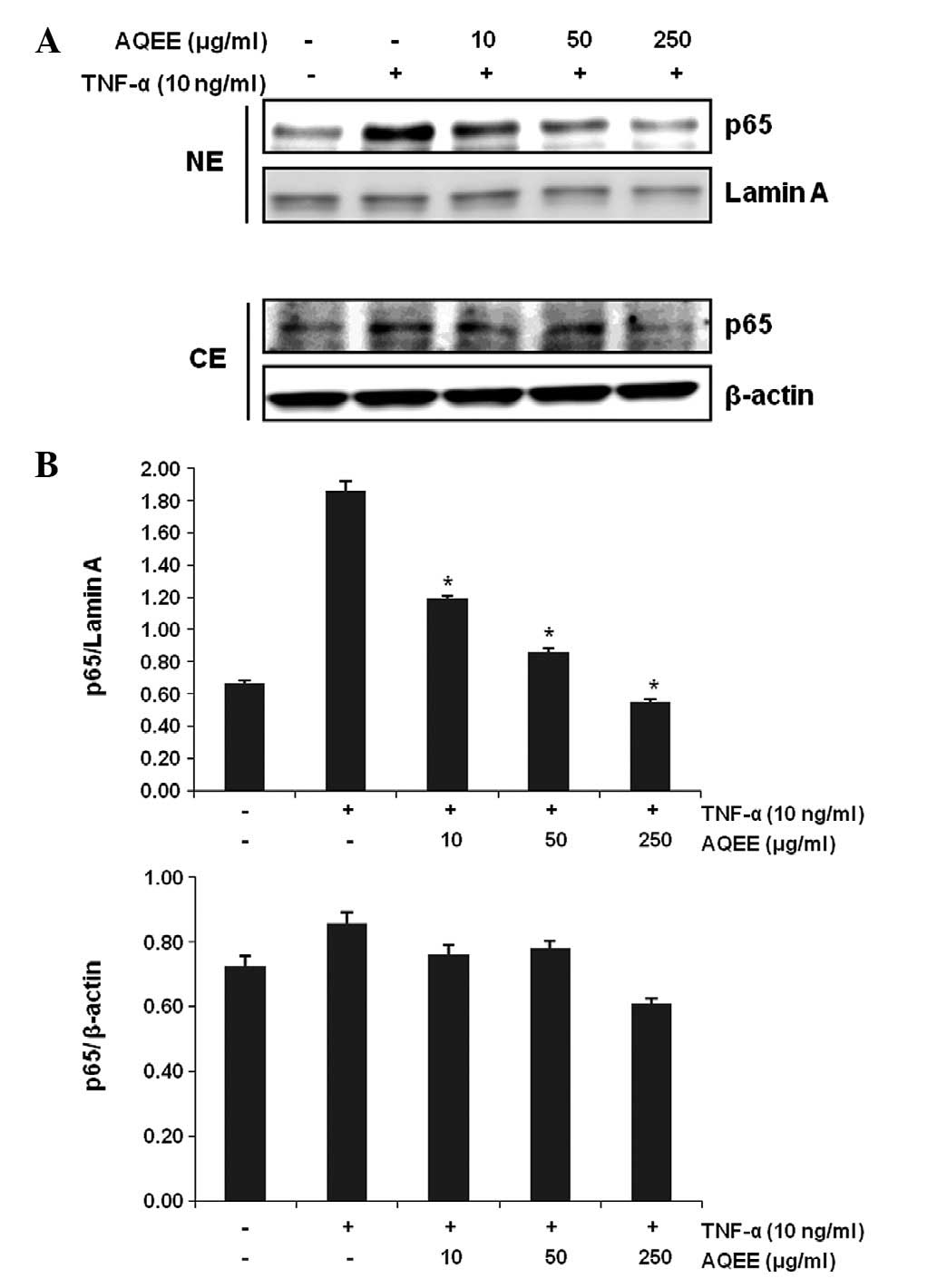
Effect of AQEE on TNF-α-induced NF-κB
translocation
NF-κB activation is crucial for the induction of
adhesion molecules by TNF-α (19,20).
Therefore, the effect of AQEE on TNF-α-induced nuclear
translocation of the activated NF-κB p65 subunit was investigated.
Cells were pretreated with various concentrations of AQEE for 2 h
and then stimulated with TNF-α for 4 h. Nuclear and cytoplasmic
extracts were then analyzed by western blot analysis. Fig. 4 demonstrates that AQEE decreased
p65 NF-κB nuclear translocation in a dose-dependent manner,
indicating that AQEE inhibits TNF-α-induced NF-κB activation.
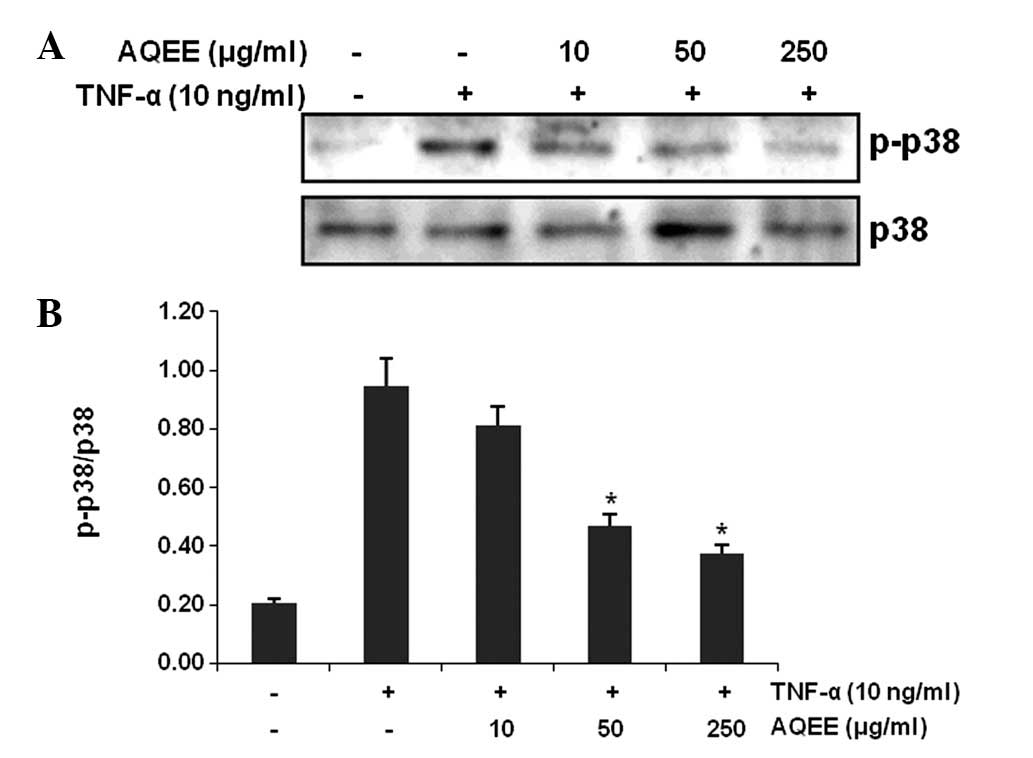
Effect of AQEE on p38 mitogen-activated
protein kinases (MAPKs) in TNF-α-stimulated HASMCs
MAPK signaling pathways are also involved in the
regulation of adhesion molecule expression (21,22).
We identified that 30-min treatment with TNF-α increased
phosphorylation (activation) of p38 MAPK. However, pretreatment
with AQEE for 2 h inhibited TNF-α-induced p38 MAPK phosphorylation
(Fig. 5).
Discussion
Interactions between aortic smooth muscle cells and
monocytes through proinflammatory mediators contribute to
inflammation in the vascular wall and the progression of
atherosclerosis (10). For
example, inflammatory cells secrete TNF-α, which upregulates the
adhesion molecule expression and promotes the formation of
atherosclerotic lesions (2).
In the present study, AQEE suppressed the
TNF-α-induced expression of adhesion molecules (ICAM-1 and
E-selectin) and COX-2 and inhibited monocyte adhesion to HASMCs.
These results indicate that AQEE is able to reduce TNF-α-induced
inflammatory response in aortic smooth muscle cells.
In the majority of unstimulated cells, NF-κB is
present as an inactive, IκB-bound complex in the cytoplasm.
Following cytokine stimulation, activated NF-κB translocates into
the nucleus and initiates transcription of genes involved in
inflammation, including COX-2 (23–25).
In addition, TNF-α activation of the transcription factor NF-κB is
required for the upregulation of muscle cell adhesion molecules
(20). In the present study, AQEE
was identified to attenuate TNF-α-induced NF-κB activation.
Previous studies (19) have shown
that MAPK signaling pathways are also involved in the regulation of
NF-κB activation in response to TNF-α. This study has demonstrated
that AQEE suppressed TNF-α-stimulated p38 phosphorylation, however,
the extract had little effect on extracellular signal-regulated
kinases (ERK)1/2 or c-Jun N-terminal kinase phosphorylation (data
not shown). These observations indicate that AQEE prevents the
upregulation of cell adhesion molecules by interfering with gene
transcription.
In summary, the present study has demonstrated that
AQEE inhibits vascular inflammation in TNF-α-stimulated HASMCs,
preventing the upregulation of adhesion molecules and COX-2 by
blocking NF-κB and p38 MAPK signaling pathways. These observations
may provide a foundation for the development of AQEE as an
anti-inflammatory agent to prevent vascular inflammatory
disorders.
Acknowledgements
The present study was supported by the Discovery of
Herbal Medicine for the Prevention of Prehypertension Project
(K12202) and the Construction of the Basis for Practical
Application of Herbal Resources (K11020) funded by the Ministry of
Education, Science and Technology of Korea to the Korea Institute
of Oriental Medicine.
Abbreviations:
|
AQEE
|
Akebia quinata ethanol
extract
|
|
CE
|
cytoplasmic extracts
|
|
COX-2
|
cyclooxygenase 2
|
|
HASMC
|
human aortic smooth muscle cell
|
|
ICAM-1
|
intercellular adhesion molecule 1
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NE
|
nuclear extracts
|
|
NF-κB
|
nuclear factor κB
|
|
TNF-α
|
tumor necrosis factor α
|
References
|
1
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar
|
|
3
|
Bevilacqua MP, Nelson RM, Mannori G and
Cecconi O: Endothelial-leukocyte adhesion molecules in human
disease. Annu Rev Med. 45:361–378. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bobryshev YV, Lord RS, Rainer SP and Munro
VF: VCAM-1 expression and network of VCAM-1 positive vascular
dendritic cells in advanced atherosclerotic lesions of carotid
arteries and aortas. Acta Histochem. 98:185–194. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien KD, McDonald TO, Chait A, Allen MD
and Alpers CE: Neovascular expression of E-selectin, intercellular
adhesion molecule-1 and vascular cell adhesion molecule-1 in human
atherosclerosis and their relation to intimal leukocyte content.
Circulation. 93:672–682. 1996. View Article : Google Scholar
|
|
6
|
O'Brien KD, Allen MD, McDonald TO, Chait
A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K and
Benjamin CD: Vascular cell adhesion molecule-1 is expressed in
human coronary atherosclerotic plaques. Implications for the mode
of progression of advanced coronary atherosclerosis. J Clin Invest.
92:945–951. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47(Suppl 8): C7–C12. 2006. View Article : Google Scholar
|
|
9
|
Braun M, Pietsch P, Schrör K, Baumann G
and Felix SB: Cellular adhesion molecules on vascular smooth muscle
cells. Cardiovasc Res. 41:395–401. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang Y, Lincoff AM, Plow EF and Topol EJ:
Cell adhesion molecules in coronary artery disease. J Am Coll
Cardiol. 24:1591–1601. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davies MJ, Gordon JL, Gearing AJ, Pigott
R, Woolf N, Katz D and Kyriakopoulos A: The expression of the
adhesion molecules ICAM-1, VCAM-1, PECAM and E-selectin in human
atherosclerosis. J Pathol. 171:223–229. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Libby P and Li H: Vascular cell adhesion
molecule-1 and smooth muscle cell activation during atherogenesis.
J Clin Invest. 92:538–539. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Printseva O, Peclo MM and Gown AM:
Various cell types in human atherosclerotic lesions express ICAM-1.
Further immunocytochemical and immunochemical studies employing
monoclonal antibody 10F3. Am J Pathol. 140:889–896. 1992.
|
|
14
|
Huo Y and Ley K: Adhesion molecules and
atherogenesis. Acta Physiol Scand. 173:35–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitaoka F, Kakiuchi N, Long C, Itoga M,
Mitsue A, Mouri C and Mikage M: Molecular characterization of
akebia plants and the derived traditional herbal medicine. Biol
Pharm Bull. 32:665–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mimaki Y, Doi S, Kuroda M and Yokosuka A:
Triterpene glycosides from the stems of Akebia quinata. Chem
Pharm Bull. 55:1319–1324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi J, Jung HJ, Lee KT and Park HJ:
Antinociceptive and anti-inflammatory effects of the saponin and
sapogenins obtained from the stem of Akebia quinata. J Med
Food. 8:78–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuchiya S, Yamabe M, Yamaguchi Y,
Kobayashi Y, Konno T and Tada K: Establishment and characterization
of a human acute monocytic leukemia cell line (THP-1). Int J
Cancer. 26:171–176. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Chou C, Sun Y and Huang W: Tumor
necrosis factor alpha-induced activation of downstream NF-kappaB
site of the promoter mediates epithelial ICAM-1 expression and
monocyte adhesion. Involvement of PKCalpha, tyrosine kinase and
IKK2, but not MAPKs, pathway. Cell Signal. 13:543–553. 2001.
View Article : Google Scholar
|
|
20
|
Collins T, Read MA, Neish AS, Whitley MZ,
Thanos D and Maniatis T: Transcriptional regulation of endothelial
cell adhesion molecules: NF-kappa B and cytokine-inducible
enhancers. FASEB J. 9:899–909. 1995.PubMed/NCBI
|
|
21
|
Ju JW, Kim SJ, Jun CD and Chun JS: p38
kinase and c-Jun N-terminal kinase oppositely regulates tumor
necrosis factor alpha-induced vascular cell adhesion molecule-1
expression and cell adhesion in chondrosarcoma cells. IUBMB Life.
54:293–299. 2002. View Article : Google Scholar
|
|
22
|
Ho AW, Wong CK and Lam CW: Tumor necrosis
factor-alpha up-regulates the expression of CCL2 and adhesion
molecules of human proximal tubular epithelial cells through MAPK
signaling pathways. Immunobiology. 213:533–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brasier AR: The nuclear
factor-kappaB-interleukin-6 signalling pathway mediating vascular
inflammation. Cardiovasc Res. 86:211–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waddick KG and Uckun FM: Innovative
treatment programs against cancer: II. Nuclear factor-kappaB
(NF-kappaB) as a molecular target. Biochem Pharmacol. 57:9–17.
1999. View Article : Google Scholar : PubMed/NCBI
|