Introduction
The steroid receptor coactivator (SRC) family is
composed of SRC-1, SRC-2 and SRC-3. These cofactors interact with
nuclear receptors in a ligand-dependent manner and enhance
transcriptional activation by the receptor via histone
acetylation/methylation and recruitment of additional cofactors
(1–3). An indispensable member of the SCR
families, SRC-3 belongs to the p160 family of nuclear receptor
coactivators that significantly affect physiological function and
growth and development, involving cell proliferation, cell
migration, cell differentiation, somatic growth, sexual maturation,
female reproductive function, vaso-protection and breast cancer
(4–6).
Macrophages play an important role between the
natural non-specific immune and the specific immune responses.
Macrophages promote inflammatory reactions and activation of T
lymphocytic cells through secretion of cytokines or antigen
processing and presentation, thereby initiating specific immune
responses. In addition, the above-mentioned effects of macrophages
are induced by different stimuli, such as LPS and cytokines. The
ability of macrophages is restricted under normal physiological
conditions; however, detrimental effects are induced when the
responses to the stimuli of macrophages are altered.
The activation of the inflammatory cascade is
significantly involved in the development of resistance mechanism
disorders following severe trauma and infection (7). Over-activation of macrophages
following severe trauma may induce the synthesis of cytokines
(IL-1, TNF-α, IL-6 and TGF-β), and an increase in RNI and PGE2
(8,9). Subsequently, the level of
inflammatory mediators in the body are elevated (10). Finally, systemic inflammatory
response syndrome (SIRS) is caused by a local inflammatory response
induced by severe trauma and infection that is thought to be
mediated by the presence of circulating inflammatory mediators.
Therefore, SIRS plays a crucial role in the development of
resistance mechanism disorders following severe trauma and
infection (11,12). Over-activation of macrophages
increases the susceptibility of sepsis after severe trauma and
infection, which has been described as a ‘two-hit’ event (13). The first-hit of severe trauma
primarily induces the abnormal reactions in the body, such as the
release of inflammatory mediators, and the second hit (such as
sepsis) may lead to multiple organ failure and death.
To investigate the effect of SCR-3 in the
development of SIRS, we studied the regulation of the inflammatory
response by SCR-3 in peritoneal macrophages (PMs) based on the
primary cultures of PMs.
Materials and methods
Animals
SRC-3 knockout mice (SRC-3−/− mice) were
obtained as previously described (14), and wild-type (SRC-3+/+
mice) littermates served as control (both were of a C57/129 mouse
background). All experiments were performed using 3-month-old
female mice (20–25 g), that were acclimatized for 1 week prior to
the initiation of any procedures. All our experimental protocols
were approved by the Animal Care and Use Committee of our
institute.
Peritoneal macrophage preparation
Thioglycollate-elicited peritoneal exudate cells
were obtained from 3-month-old C57/129 female wild-type and
SRC-3−/− mice following intraperitoneal injection of 1
ml Brewer thioglycollate broth (4.05 g/100 ml; Sigma-Aldrich, USA)
and lavage of the peritoneal cavity with 5 ml of medium 3–4 days
later. The cells were washed twice and resuspended in RPMI-1640
containing 10% heat-inactivated fetal calf serum (FCS; Hyclone,
USA), penicillin (100 IU/ml) and streptomycin (100 g/ml; RPMI-FCS).
Macrophages were isolated from peritoneal exudate cells as
described (15). Peritoneal
exudate cells were seeded at densities of 5–6×105
cells/cm2 on Teflon-coated Petri dishes (100×15 mm), and
the macrophages were allowed to adhere for 2 h in a 5%
CO2 humidified atmosphere. The non-adherent cells were
removed by washing the dishes twice with 10 ml pre-warmed medium,
and the adherent cells were used as PMs.
Measurement of cytokines
Concentrations of TNF-α, IL-1β, IL-6 and IL-10 in
peritoneal fluid were determined using commercial ELISA kits
(Jingmei Biotech Co., Ltd., USA), according to the manufacturer's
instructions.
RT-PCR assay of cytokine mRNA
Total RNA was prepared from the PMs using Takara
reagent (Takara, Dalian, China) according to the manufacturer's
instructions and quantified spectrophotometrically. Primer
sequences were obtained from the literature or by Primer 5.0
software and were designed as shown in Table I. The PCR reactions for TNF-α,
IL-1β, IL-6 and IL-10 were carried out for 30, 28, 30 and 30
amplification cycles, respectively [94˚C/30 sec; 53˚C (TNF-α), 54°C
(IL-1β), 51˚C (IL-6), 52˚C (IL-10)/30 sec; 72˚C/60 sec], in an
RT-PCR system (Eppendorf, Germany). Ten microliters of each PCR
product was electrophoresed on a 1.5% agarose gel and visualized by
ethidium bromide staining.
 | Table IPrimer sequences used in RT-PCR assay
of cytokine mRNA. |
Table I
Primer sequences used in RT-PCR assay
of cytokine mRNA.
| Genes | Forward primer
sequence | Reverse primer
sequence |
|---|
| TNF-α |
5′-CGTGGAACTGGCAGAAGAGG-3′ |
5′-GGGCTACAGGCTTGTCACTC-3′ |
| IL-1β |
5′-GCATCCAGCTTCAAATCTCAC-3 |
5′-GTTCATCTCGGAGCCTGTAGT-3′ |
| IL-6 |
5′-CCTTCTTGGGACTGATGCTG-3′ |
5′-GGACTCTGGCTTTGTCTTTC-3′ |
| IL-10 |
5′-GCTGGACAACATACTGCTAAC-3′ |
5′-TAGACACCTTGGTCTTGGAG-3′ |
|
G3PDH |
5′-ACCACAGTCCATGCCATCAC-3′ |
5′-TCCACCACCCTGTTGCTGTA-3′ |
Western blot analysis of glucocorticoid
receptor (GR), NF-κB, AP-1 and IRF-1
PMs were collected, and 80 μl M-Per™ Mammalian
protein extraction reagent (Pierce, USA) was added; the mixture was
incubated in an ice bank for 10 min. Whole cell lysates were
obtained by subsequent centrifugation at 12,000 rpm for 20 min at
4˚C. Protein concentrations were determined using the Bradford
protein assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum
albumin (BSA; Hyclone, USA) as standard. Subsequently, 40 μg of
protein extracts was subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad) and
transferred to a Protran nitrocellulose membrane. The membrane was
incubated with rabbit antibody polyclonal antibody or rabbit
anti-Bcl-2 polyclonal antibody (Zhongshan Golden Bridge
Biotechnology Co., Zhongshan, China) at 4˚C overnight, after being
blocked with a 10% BSA solution. The membrane was washed with TBST
buffer (20 mmol/l Tris-HCl pH 7.4, 150 mmol/l NaCl and 0.1%
Tween-20), incubated with a secondary goat anti-rabbit horseradish
peroxidase (HRP)-conjugated antibody (Zhongshan Golden Bridge
Biotechnology Co.) for 2 h at room temperature, and finally
detected using the DAB kit (Sigma-Aldrich). β-actin (Sigma-Aldrich)
was used as an internal control for data analysis.
Statistical analysis
All the results are expressed as the means ± SEM.
The statistical significance of differences was analyzed using SPSS
software (SPSS for Windows 15.0; SPSS Inc., USA). P<0.05 was
indicative of statistical significance.
Results
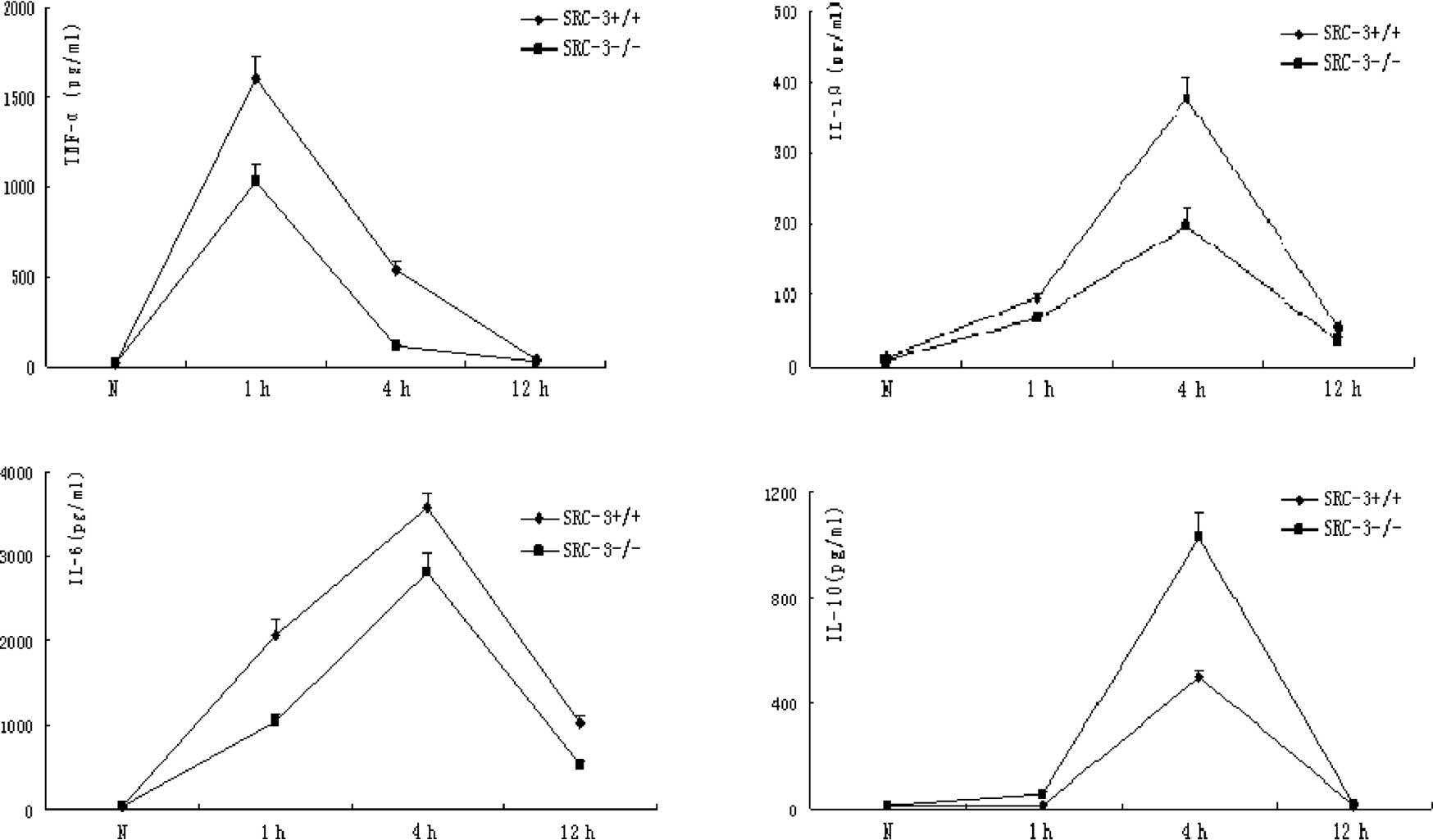
Results of ELISA assays
Normally, there is no expression difference in
TNF-α, IL-1β, IL-6 and IL-10 between SRC-3+/+ and
SRC-3−/− mice. The concentrations of TNF-α, IL-1β and
IL-6 in the serum of SRC-3+/+ and SRC-3−/−
mice were significantly increased at 1 and 4 h after injection of
LPS (i.p., 5 mg/kg). However, the concentrations of TNF-α, IL-1β
and IL-6 in the serum of SRC-3+/+ mice were
significantly higher compared to levels in the SRC-3−/−
mice. The IL-10 levels in the SRC-3+/+ and
SRC-3−/− mice at 1 h after injection of LPS were not
different at 0 h (normal phase), but they were significantly
increased at 4 h after injection. In addition, the concentration of
IL-10 in the serum of SRC-3−/− mice was significantly
higher compared to that in the SRC-3+/+ mice. The
concentration of IL-6 in the serum of SRC-3+/+ and
SRC-3−/− mice at 12 h after injection of LPS was
markedly higher than that at 0 h (normal phase), and the IL-6
levels in the SRC-3+/+ mice were relatively higher than
those in the SRC-3−/− mice (Fig. 1).
Results of the RT-PCR analysis of mRNA
expression
PMs were cultured in vitro, and stimulated
with LPS at the dose of 10 μg/ml. Cells were collected 4 h after
LPS treatment, and total RNA was isolated. RT-PCR analysis of TNF-α
mRNA was then performed (Fig. 2A,
right panels). Under normal conditions (0 h), there was no
significant difference in TNF-α mRNA expression in the PMs between
the SRC-3+/+ and SRC-3−/− mice. However, the
TNF-α mRNA expression of both groups was significantly increased
(Fig. 2A, left panels) 4 h after
LPS treatment, and the increase in TNF-α mRNA expression in the PMs
from SRC-3+/+ mice was markedly higher than that of the
SRC-3−/− mice (136.33 vs. 48.67%) (Fig. 2A, middle panel).
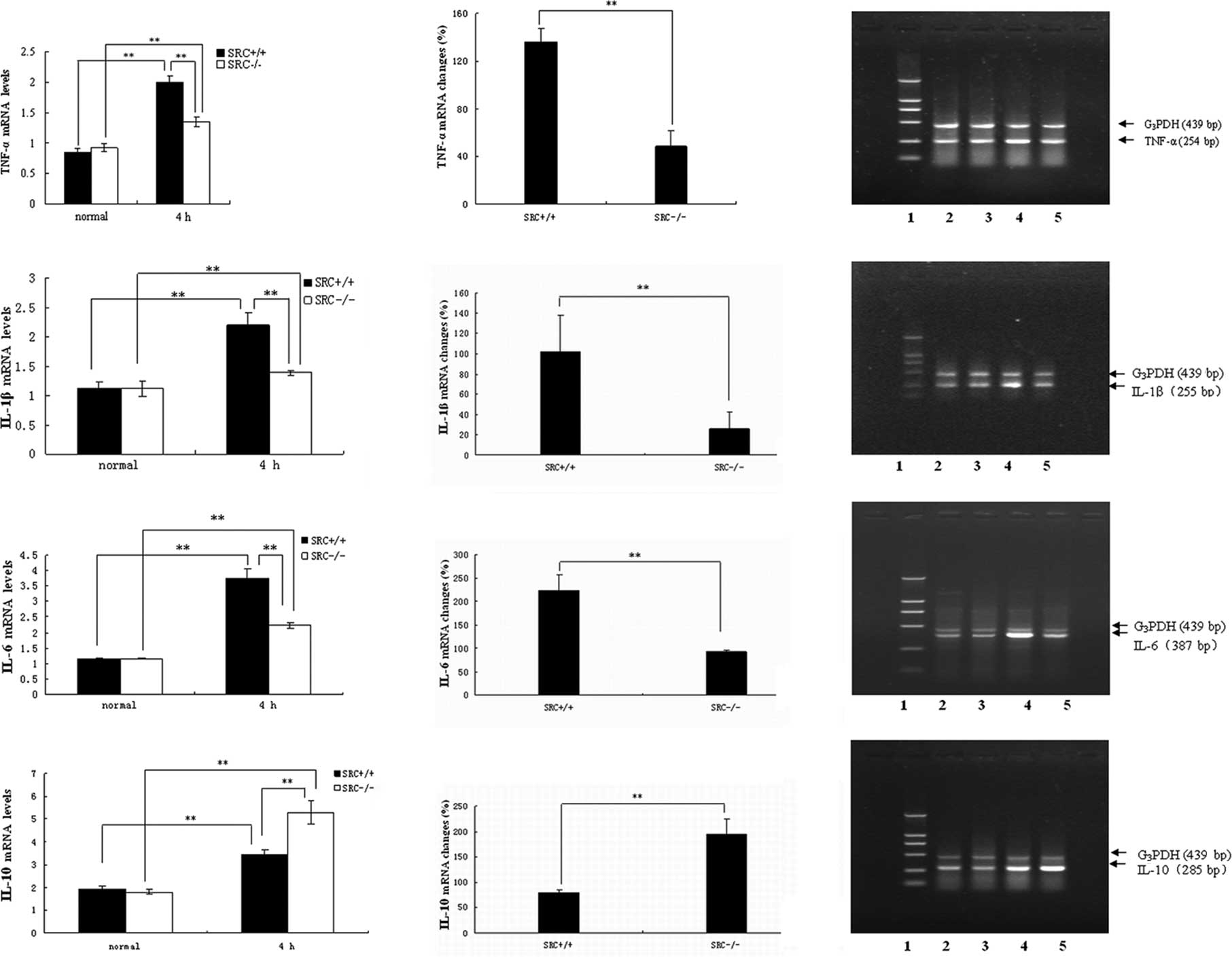 | Figure 2mRNA expression analysis by RT-PCR.
mRNA expression of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-10 in
serum from SRC-3+/+ and SRC-3−/− mice at 0 h
(normal) and 4 h after LPS treatment (10 μg/ml), respectively.
Analysis of the amplified PCR products by electrophoresis on 1.5%
agarose gels. Lane 1: DNA marker; the fragment lengths were 2,000,
1,000, 750, 500, 250 and 100 bp. Lanes 2 and 4: 0 and 4 h after
injection of SRC-3+/+ mice. Lanes 3 and 5: 0 and 4 h
after injection of SRC-3−/− mice. The fragment lengths
of G3PDH, TNF-α, IL-1β, IL-6 and IL-10 were 439, 254,
255, 387 and 285 bp, respectively. Data are expressed as the means
± SEM per time point (n=5). **p<0.01. |
Results of the RT-PCR analysis of IL-1β mRNA are
shown in Fig. 2B. Under normal
conditions, no statistical differences in the levels of IL-1β mRNA
in PMs were observed between the SRC-3+/+ and
SRC-3−/− mice. Following LPS stimulation in
vitro, the levels of IL-1β in the two mouse groups at 4 h were
markedly elevated (Fig. 2B, left
panels), and the increase in IL-1β mRNA in the SRC-3+/+
mice was significantly higher compared to that of the
SRC-3−/− group (101.78 vs. 25.69%; Fig. 2B, middle panel).
Similar results were observed in the RT-PCR analysis
of IL-6 mRNA (Fig. 2C). No
significant difference in IL-6 mRNA expression was observed between
the SRC-3+/+ and SRC-3−/− mice. Following LPS
treatment, the IL-6 mRNA expression in the SRC-3−/− mice
was also increased (Fig. 2C, left
panels). However, the increase in IL-6 mRNA in the
SRC-3+/+ mice was significantly higher compared to that
of the SRC-3−/− group (223.13 vs. 92.16%; Fig. 2C, middle panel).
As shown in Fig.
2D, under normal conditions there was no significant difference
in IL-10 mRNA expression in the PMs between the SRC-3+/+
and SRC-3−/− mice. The IL-10 mRNA expression of both
groups was markedly increased (Fig.
2D, left panels), however, the increase in IL-10 mRNA
expression of SRC-3+/+ mice was significantly lower
compared to that of the SRC-3−/− mice (79.63 vs.
193.86%) (Fig. 2D, middle
panel).
Results of the western blot analysis of
GR expression
PMs were cultured in vitro and stimulated
with LPS at the dose of 10 μg/ml. Cells were collected 4 h after
LPS treatment, and total protein was isolated. Subsequently,
SDS-PAGE and western blot analysis for GR were performed with 40 μg
total protein as isolated. Results of the GR expression analysis
are shown in Fig. 3A. Under normal
conditions, the expression levels of PM GR in the
SRC-3+/+ mice were not different than those of the
SRC-3−/− group. However, the expression levels of GR in
the PMs in the two groups at 4 h after LPS stimulation were
markedly reduced (Fig. 3A, top
panels), and the reduction in the SRC-3−/− group was
significantly lower compared to that of the SRC-3+/+
group (38.2 vs. 60.0%; Fig. 3A,
middle panel).
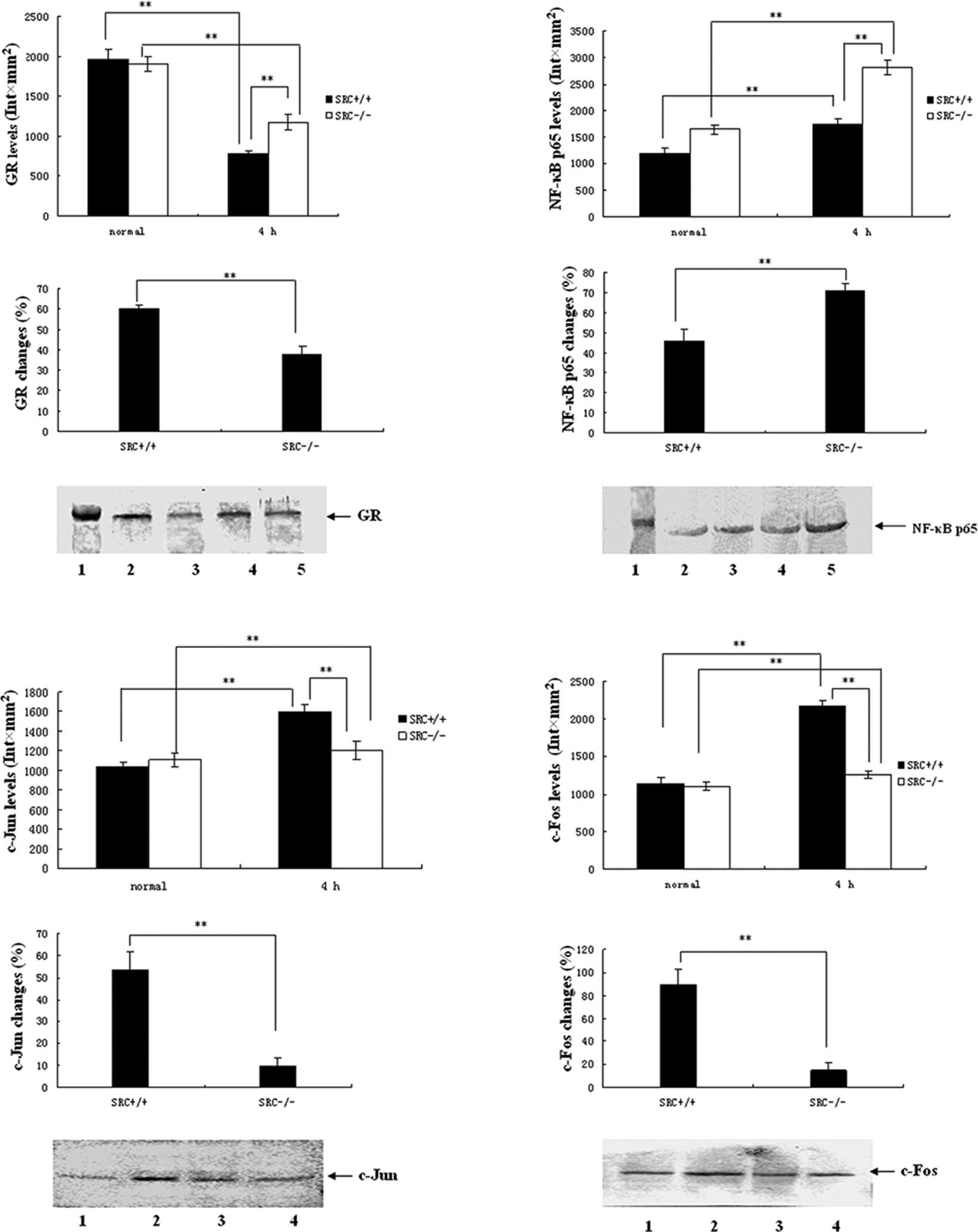 | Figure 3Protein expression analysis by
Western blotting. (A-D) Protein expression of GR, NF-κB p65 and
AP-1 (c-Jun and c-Fos) in PMs from SRC-3+/+ and
SRC-3−/− mice at 0 h (normal) and 4 h after injection of
LPS (i.p., 5 mg/kg), respectively. (A) Protein expression of GR in
PM. Lane 1, 98-kDa marker; lanes 2 and 3, 0 and 4 h after injection
of SRC-3+/+ mice; lanes 4 and 5, 0 and 4 h after
injection of SRC-3−/− mice. (B) Protein expression of
NF-κB p65 in PM. Lane 1, 66-kDa marker; lanes 2 and 3, 0 and 4 h
after injection of SRC-3+/+ mice; lanes 4 and 5, 0 and 4
h after injection of SRC-3−/− mice. (C) Protein
expression of c-Jun in PM. Lanes 1 and 2, 0 and 4 h after injection
of SRC-3+/+ mice; lanes 3 and 4, 0 and 4 h after
injection of SRC-3−/− mice. (D) Protein expression of
c-Fos in PM. Lanes 1 and 2, 0 and 4 h after injection of
SRC-3+/+ mice; lanes 3 and 4: 0 and 4 h after injection
of SRC-3−/− mice. Data are expressed as the means ± SEM
per time point (n=5). **p<0.01. |
Results of the western blot analysis of
NF-κB p65 expression
As shown in Fig.
3B, under normal conditions the expression levels of NF-κB p65
in PMs in the SRC-3+/+ group were markedly higher
compared to those of the SRC-3−/− group (Fig. 3A, top panels). The expression
levels of PM NF-κB p65 in the two groups at 4 h after LPS
stimulation were markedly elevated (Fig. 3A, top panels), but the increase in
the SRC-3−/− group was larger compared to that of the
SRC-3+/+ group (71.0 vs. 45.9%; Fig. 3B, middle panel).
Results of the western blot analysis of
AP-1 expression
Results of AP-1 expression analysis are presented in
Fig. 3C and D. Under normal
conditions, the expression levels of c-Jun/c-Fos in PMs in the
SRC-3+/+ group were not different than those of the
SRC-3−/− group. Following LPS stimulation, the
expression levels of c-Jun/c-Fos in PMs in the two groups were
markedly elevated at 4 h, but the increase in the
SRC-3−/− group was lower than that of the
SRC-3+/+ group (9.5 vs. 53.5%/14.8 vs. 89.6%).
Discussion
As immune effector cells, PMs are the major
components of cells in peritoneal fluid. PMs account for >90% of
abdominal lymphocytes, and play a critical role in the body's
immune and inflammatory reactions: anti-infection, antitumor and
participation in immune response and immuno-regulation. Abdominal
macrophages are normally dormant, but they are activated rapidly by
the stimulation of LPS, cytokines and pathogens. Subsequently,
cytokines, such as IL-1β, TNF-α, IL-6 and TGF-β, are produced and
lead to the activation of the inflammatory cascade and the
development of SIRS. In addition, a functional disorder and
suppression of macrophages are induced by the over-activation of
macrophages; suppression of the body's immune function is
accordingly induced. SCR-3 is involved in the activation of the
inflammatory cascade reaction, therefore, it is necessary to
investigate the changes after SCR-3 deficiency for clarification of
the mechanism of immune suppression following development of
SIRS.
The main mechanism for inflammation regulation by
macrophages is through the release of inflammatory cytokines.
Inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-10, are
mainly released by macrophages and are important inflammatory
mediators for the development of SRIS and immune function
disorders. PMs were cultured in vitro and stimulated with
LPS at a dose of 10 μg/ml. Cells were collected 4 h after LPS
treatment and total RNA was isolated. RT-PCR analysis revealed that
the increase in TNF-α, IL-1β and IL-6 mRNA in PMs treated with LPS
(10 μg/ml) from SRC-3−/− mice was significantly lower
than that in the SRC-3+/+ mice. These results revealed
that SCR-3 is involved in the activation of macrophages and the
synthesis of pro-inflammatory cytokines. Furthermore, SCR-3
deficiency suppressed the synthesis of pro-inflammatory cytokines
and reduced the activity of PMs following stimulation by LPS. The
synthesis and release of inflammatory cytokines from macrophages
induced by LPS are essential for the natural immune response. The
transcription of inflammatory cytokines may be suppressed by SCR-3
deficiency, showing that SCR-3 significantly affected the
maintenance of normal natural immune response as well.
IL-10, also known as a cytokine synthesis inhibitory
factor (CSIF), is mainly generated by TH2 cells and mononuclear
macrophages. IL-10 suppresses the generation of cytokines (such as
TNF-α, IL-1β, IL-6 and GM-CSF) from macrophages through the
negative feedback regulation; therefore, the anti-inflammatory
effect is stimulated, and expression of several costimulatory
molecules and MHC II molecules is down-regulated; antigen
presentation function of macrophages is accordingly suppressed
(16). Therefore, over-release of
TNF-α and severe disbalance of IL-10 may be a critical mechanism
for the development of SIRS and resistance mechanism disorders. Our
results showed that the expression of IL-10 mRNA in PMs from both
SRC-3+/+ and SRC-3−/− mice was significantly
elevated following treatment with LPS, and the elevated levels in
the SRC-3−/− mice were significantly higher compared to
those of the SRC-3+/+ mice. The mechanism by which the
expression of IL-10 mRNA was increased by SCR-3 deficiency may be
related to the signaling pathway for regulating the expression of
IL-10 and transcription factors.
Following treatment with LPS, TNF-α mRNA expression
upon SCR-3 deficiency was suppressed, and the IL-10 mRNA expression
was significantly higher. Our results showed that the balance
between TNF-α and IL-10 was broken to some extent, and revealed
that SRC-3 plays important roles not only in the regulation of
synthesis of inflammatory cytokines in PMs, but also in maintaining
the normal immune functions of PMs.
GR, NF-κB and AP-1 are known as important
inflammatory transcription factors. They participate in the
regulation of the body's immune response, and functional changes
are related to the development of SIRS. The activities of these
inflammatory transcription factors are related to the functional
status of PMs, therefore, it is important for the clarification of
the molecular mechanism involving the over-activation of PMs to
study the effects of SCR-3 deficiency on transcription factors in
PMs.
GC-GR reaction, an important stress and immune
reaction, regulates the development of SIRS and possesses
anti-inflammatory and immune suppressive effects; it also
suppresses T-lymphocyte activation. GR can be treated as a
ligand-dependent transcription factor, and GR can also regulate
gene expression (17), suppression
of T-cell activation and proliferation by GC, synthesis of
cytokines and transactivation of some transcription factors in the
process of T-cell activation (such as AP-1 and NF-κB) through
protein-protein interaction with other transcription factors
(18,19). The expression changes in the GR
protein directly reflect the inflammatory reaction activity and
immune status. Following LPS treatment, the decrease in the GR
protein expression in the PMs of the SRC-3−/− mice was
significantly lower than that in the SRC-3+/+ mice
(p<0.01).
The results of our study showed that SCR-3 is
associated with the inflammatory reaction activity of PMs, and
SCR-3 deficiency induces the responsiveness of PMs to stimulation
(such as LPS decrease), and even induces part of the PM immune
function suppression. The AP-1 and NF-κB inflammatory signal
transduction pathways are the main links to the activation of the
inflammatory cascade. GR interferes with the transcription
activation of inflammatory cytokines through protein-protein
interaction to suppress the synthesis and the release of
inflammatory cytokines (20). The
decline in the expression and function of GR in the PMs of
SRC-3+/+ mice relieved the suppression of GR on AP-1 and
NF-κB, and induced the over-activation of PMs. Furthermore, more
inflammatory cytokines are released and SIRS is induced by the
uncontrolled inflammatory reaction. However, the decrease in the GR
protein expression in SRC-3−/− mice was significantly
lower compared to that in the SRC-3+/+ mice. The results
revealed that SCR-3 deficiency protects the function of GR and
prevents the development of SIRS by restricting the extent of
glucocorticoid resistance. In addition, the suppression of the
immune function of PMs can be strengthened by SCR-3 deficiency.
These results demonstrate that SCR-3 may be related to the
co-activation of AP-1 and NF-κB, and SCR-3 deficiency affects the
expression and function changes of GR.
NF-κB specifically binds the κB sites in the
promoter or enhancer region of cytokines and adherence factors,
which is necessary for the regulation of the immune response,
inflammatory reaction, cell differentiation and development, cell
adhesion and cell apoptosis, and initiates and regulates the
transcription of these genes. Therefore, NF-κB plays important
roles in the body's immune response, inflammatory reaction and cell
differentiation and development (21). The p50/p65 heterodimer is the major
component of intracellular NF-κB (22). In our study, the levels of NF-κB
p65 protein expression in PMs of SRC-3+/+ mice were
significantly higher than those of the SRC-3−/− mice
under normal conditions. In view of the importance of NF-κB in the
process of the immune response, the results of our study showed
that the relative lack of NF-κB expression was induced by SCR-3
deficiency; accordingly, immunosuppression of normal PMs was
induced. SCR-3 is the co-activator of NF-κB, and overexpression of
SCR-3 significantly increases NF-κB transcription activity based on
TNF-α induction, in a dose-dependent manner, and is critically
involved in immune and inflammation reactions mediated by NF-κB
(23). NF-κB is an important
transcription factor, and the increase in its expression and
activity affects the function of GR through complex mechanisms, and
decreases the biological effect of GR or even induces GCR. The
presence of GCR in PMs from SRC-3+/+ mice promoted PMs
to release abundant inflammatory cytokines, and the inflammatory
cascade reaction was activated; accordingly, over-activation of PMs
was induced through positive feedback reaction.
The over-activation of PMs is an important cause for
resistance mechanism disorders in the body. The incidence and death
rate of infection is increased due to immunosuppression following
severe trauma, and the reason is the activation of cycloxygenase-2
(COX-2), which is induced by the activation of NF-κB; then the
production of PEG2 is increased. PEG2 is a powerful endogenous
immunodepressant which suppresses the mitosis of T-cells,
neutrophil chemotaxis and antibody synthesis of B-lymphocyte cells
(24). Chen et al (25) showed that LPS induces the
activation of NF-κB and AP-1 through the activation of the p38
MAPK, and induces the release of NO and PEG2. However, the increase
in NO and PEG2 induces the aggravation of inflammation and
immune-reaction suppression, respectively.
Therefore, SCR-3 deficiency prevents the
over-activation of PMs partly by suppressing the activity of NF-κB.
It appears that SCR-3 deficiency helps to prevent the occurrence of
the immune function suppression of the body. However, NF-κB plays
an important role in the normal immune response of PMs, the severe
regulation of NF-κB function induced by SCR-3 deficiency inevitable
induced a partial loss of immune response function of PMs.
Accordingly, resistance mechanism disorders will be induced or
aggravated. The bidirectional effects of SCR-3 maintain the dynamic
equilibrium between the inflammatory reaction and immune response
of PMs.
AP-1 consists of the Fos/Jun heterodimer or Jun/Jun
heterodimer, and the most common form of AP-1 is c-Jun/c-Fos, which
mediates cell proliferation, differentiation and expression of
inflammatory cytokines. The inflammatory protein regulated by AP-1
also includes TNF-α, IL-1, IL-6, IL-8, MIP-1α, MCP-1, ICAM-1,
VCAM-1 and iNOS. AP-1 is an important transcription factor of the
extracellular signal-regulated kinase (ERK) pathway (26). ERK pathway is an important pathway
related to cell proliferation and differentiation, which can be
activated by inflammatory cytokines, such as IL-6 and TNF-α.
Our study showed that the levels of c-Jun and c-Fos
protein expression in PMs from the SRC-3−/− mice were
not significant different from those of the SRC-3+/+
mice. However, c-Jun and c-Fos protein expression of PMs was
increased at 4 h following stimulation with LPS. The MAPK pathway
can be activated to different extents by LPS, and can induce an
increase in c-Jun and c-Fos expression; then, AP-1 is activated.
AP-1 regulates the transcription of abundant inflammatory
cytokines; on the one hand, the increase in its activity promotes
the release of inflammatory cytokines; on the other hand, the
down-regulation of GR expression and function is induced by
protein-protein interaction, and GCR and SIRS are induced.
Subsequently, the over-activation and resistance mechanism
disorders of PMs are induced. The results of our study showed that
the increase in c-Jun and c-Fos expression in PMs in
SRC-3−/− mice was significantly lower than that in
SRC-3+/+ mice (p<0.01). Therefore, SCR-3 deficiency
partly suppresses the expression and activation of AP-1. LPS
induces a large secretion of inflammatory cytokines, such as TNF-α
and IL-1β, and the synthesis and secretion of these inflammatory
cytokines are related to the expression and activity of AP-1.
Previous studies have demonstrated that SCR-3
deficiency suppresses the transcriptional activation of
inflammatory cytokines, such as TNF-α and IL-1β, in PMs and
decreases the synthesis and secretion of inflammatory cytokines
induced by LPS. The suppression of the expression and activity of
AP-1 is possible since the synthesis and secretion of inflammatory
cytokines (such as TNF-α and IL-1β) were relatively insufficient
after SCR-3 deficiency. The activation of AP-1 could be suppressed
to some extent by SCR-3 deficiency, and the over-activation of PMs
is reduced. However, immune suppression of PMs may be induced based
on the relative strengthening of GR's function. Accordingly, the
regulation of SCR-3 in macrophages is extremely complex, and SCR-3
may play a key role in the inflammation and immune responses in
macrophages.
Acknowledgements
This study was supported by the State Key Laboratory
Open Foundation of Trauma, Burns and Combined Injury (no.
SKLKF200910), and the China Postdoctoral Science Foundation (no.
20100471764).
References
|
1
|
JC WebsterRH OakleyCM JewellJA
CidlowskiProinflammatory cytokines regulate human glucocorticoid
receptor gene expression and lead to the accumulation of the
dominant negative beta isoform: a mechanism for the generation of
glucocorticoid resistanceProc Natl Acad Sci
USA9868656870200110.1073/pnas.121455098
|
|
2
|
A GoeckeJ GuerreroGlucocorticoid receptor
beta in acute and chronic inflammatory conditions: clinical
implicationsImmunobiology2118596200610.1016/j.imbio.2005.11.00216446173
|
|
3
|
L LiaoSQ KuangY YuanSM GonzalezBW
O'MalleyJ XuMolecular structure and biological function of the
cancer-amplified nuclear receptor coactivator SRC-3/AIB1J Steroid
Biochem Mol Biol83314200210.1016/S0960-0760(02)00254-612650696
|
|
4
|
O GojisB RudrarajuC AlifrangisJ KrellP
LibalovaC PalmieriThe role of steroid receptor coactivator-3
(SRC-3) in human malignant diseaseEur J Surg
Oncol36224229201010.1016/j.ejso.2009.08.00219716257
|
|
5
|
JP LydonBW O'MalleyMinireview: steroid
receptor coactivator-3: a multifarious coregulator in mammary gland
metastasisEndocrinology1521925201110.1210/en.2010-101221047941
|
|
6
|
I KuriharaH ShibataT SuzukiTranscriptional
regulation of steroid receptor coactivator-1 (SRC-1) in
glucocorticoid actionEndocr
Res2610331038200010.3109/0743580000904863511196413
|
|
7
|
H IwasakaT NoguchiTh1/Th2 balance in
systemic inflammatory response syndrome
(SIRS)Nippon-Rinsho62223722432004(In Japanese).
|
|
8
|
KP RumbaughJA ColmerJA GriswoldAN
HamoodThe effects of infection of thermal injury by Pseudomonas
aeruginosa PAO1 on the murine cytokine
responseCytokine16160168200110.1006/cyto.2001.096011792126
|
|
9
|
A PariharMS PariharS MilnerS BhatOxidative
stress and anti-oxidative mobilization in burn
injuryBurns34617200810.1016/j.burns.2007.04.00917905515
|
|
10
|
J LiYP SuHN LiuSF LouYS HuangJX JiangJP
WangDiazepam-ketamine inhibits 3 kinds of serum inflammatory
cytokines during the early stage in burned miceActa Academiae
Medicinae Militaris Tertiae261142004
|
|
11
|
MG SchwachaMacrophages and post-burn
immune
dysfunctionBurns29114200310.1016/S0305-4179(02)00187-012543039
|
|
12
|
PJ MackrellJM DalyJR MestreElevated
expression of cyclooxygenase-2 contributes to immune dysfunction in
a murine model of
traumaSurgery130826833200110.1067/msy.2001.11666911685192
|
|
13
|
EA DeitchMultiple organ failure.
Pathophysiology and potential future therapyAnn
Surg216117134199210.1097/00000658-199208000-000021503516
|
|
14
|
ZY DuYP SuBreeding, reproducing and
identifying SRC-3 knock-out miceActa Academiae Medicinae Militaris
Tertiae273733752005
|
|
15
|
DP SesterA TrieuK BrionLPS regulates a set
of genes in primary murine macrophages by antagonising CSF-1
actionImmunobiology21097107200510.1016/j.imbio.2005.05.00416164016
|
|
16
|
C GerardC BruynsA MarchantInterleukin 10
reduces the release of tumor necrosis factor and prevents lethality
in experimental endotoxemiaJ Exp
Med177547550199310.1084/jem.177.2.5478426124
|
|
17
|
N AuphanJA DiDonatoC
RosetteImmunosuppression by glucocorticoids: inhibition of NF-kappa
B activity through induction of I kappa B
synthesisScience270286290199510.1126/science.270.5234.2867569976
|
|
18
|
C RiccardiMG CifoneG
MiglioratiGlucocorticoid hormone-induced modulation of gene
expression and regulation of T-cell death: role of GITR and GILZ,
two dexamethasone-induced genesCell Death
Differ611821189199910.1038/sj.cdd.440060910637434
|
|
19
|
RI ScheinmanPC CogswellAK LofquistAS
Baldwin JrRole of transcriptional activation of I kappa B alpha in
mediation of immunosuppression by
glucocorticoidsScience270283286199510.1126/science.270.5234.2837569975
|
|
20
|
S OgawaJ LozachC BennerMolecular
determinants of crosstalk between nuclear receptors and toll-like
receptorsCell122707721200510.1016/j.cell.2005.06.02916143103
|
|
21
|
A WullaertRole of NF-kappaB activation in
intestinal immune homeostasisInt J Med
Microbiol3004956201010.1016/j.ijmm.2009.08.00719781989
|
|
22
|
L CaradonnaML MastronardiT
MagroneBiological and clinical significance of endotoxemia in the
course of hepatitis C virus infectionCurr Pharm
Des89951005200210.2174/138161202460698311945146
|
|
23
|
S WerbajhI NojekR LanzMA CostasRAC-3 is a
NF-kappaB coactivatorFEBS
Lett485195199200010.1016/S0014-5793(00)02223-711094166
|
|
24
|
CJ LoHG CryerM FuFR LoRegulation of
macrophage eicosanoid generation is dependent on nuclear factor
kappaBJ Trauma451923199810.1097/00005373-199807000-000049680006
|
|
25
|
C ChenYH ChenWW LinInvolvement of p38
mitogen-activated protein kinase in lipopolysaccharide-induced iNOS
and COX-2 expression in J774
macrophagesImmunology97124129199910.1046/j.1365-2567.1999.00747.x10447723
|
|
26
|
LH SigalBasic science for the clinician
43: the mitogen-activated protein kinase family in inflammatory
signalingJ Clin
Rheumatol139699200710.1097/01.rhu.0000260657.59520.4817414541
|