Introduction
Mannan-binding lectin (MBL; also known as
mannose-binding lectin), a member of the family of collectins
(C-type lectins with a collagen-like domain), is considered a
classical pattern recognition molecule of innate immunity. MBL
recognizes and binds to conserved carbohydrate motifs, such as
mannose, mannan, N-acetyl-mannosamine, N-acetyl-D-glucosamine and
fucose, MBL and is present on the surface of a variety of
micro-organisms via its carbohydrate recognition domain (CRD) in a
calcium-dependent manner; its biological effect is thought to be
mediated by a collagen-like region (CLR) (1,2). MBL
constitutes a crucial part of innate immunity as it triggers
immediate cell lysis or phagocytosis by initiating the lectin
pathway of complement activation. MBL also promotes phagocytosis by
directly binding to cell surface receptors without the involvement
of the complement (3,4). The lectin pathway activation is
mediated by three MBL-associated serine proteases (MASPs), MASP1,
MASP-2 and MASP-3 (5). MBL is also
involved in the clearance of abnormal self-components, including
cell debris and apoptotic cells (6).
Mounting evidence suggests that MBL gene mutations,
a common genetic disorder, are associated with increased
susceptibility to a broad spectrum of infections, particularly when
immunity is already compromised by immunological immaturity,
comorbidity or chemotherapy (7,8). MBL
mutations may also contribute to the morbidity of autoimmune
disorders, such as rheumatoid arthritis (RA) and systemic lupus
erythematosus (SLE) (9,10). Moreover, variant MBL alleles are
associated with disease progression in concomitant diseases, such
as cystic fibrosis and chronic granulomatous disease (11,12).
The wild-type human MBL2 gene (MBL1 is a pseudogene) is referred to
as A, and three variant alleles in CLR are B (GGC54GAC), C
(GGA57GAA) and D (CGT52TGT), resulting in the amino acid changes
from Gly to Asp (34Asp, mature peptide without a signal sequence of
20 amino acids), Gly to Glu (37Glu) and Arg to Cys (32Cys),
respectively. The frequencies of these mutations vary among ethnic
groups, but are notably high overall. Allele B is undoubtedly the
most common basis for low MBL levels in Europeans, with frequency
ranging from 0.11 to 0.15. C is found most frequently in
sub-Saharan African populations, with frequency ranging from 0.23
to 0.29, but D is rare in all populations examined thus far
(0.06–0.08) and appears to be limited to Caucasian and northern
East African populations (13).
Our previous study showed that the frequencies of allele B are
0.138, 0.168 and 0.100 in Chinese Han, Uygur and Pai (Bai)
populations, respectively, but alleles C and D were not detected in
these populations (14,15).
It is widely accepted that possession of variant
alleles conferring low MBL concentrations is associated with a high
risk of recurrent infections. However, there is a large variation
of MBL levels in individuals with identical genotypes. For example,
it was found that the plasma levels range from 0 to 5 ng/ml (median
1.2) in individuals homozygous for allele A, but from 0 to 1.2
ng/ml (median 0.2) in individuals heterozygous for allele B
(16). Therefore, the mechanisms
involved in the correlation between MBL mutations and
susceptibility to infection have yet to be defined. Whether or not
the MBL deficiency results from the decreased plasma level or the
impaired functions should also be investigated.
The aim of the present study was to preliminarily
define the associations of human MBL variant alleles with
immunodeficiency, including the secretion levels and functions of
MBL. We expressed the wild-type as well as variant forms of MBL in
COS-7 and Chinese hamster ovary (CHO) cells by transient and stable
transfection, respectively. Antigenic MBL levels were analyzed by
indirect and sandwich enzyme-linked immuno- sorbent assay (ELISA),
the molecular weights of MBL proteins by SDS-PAGE and western
blotting, and functional activities by the mannan-binding,
MASP-binding and C4 deposition assays. The results indicated that
the mutations in the MBL gene may affect the oligomer formation of
MBL, which in turn leads to a defective ability to bind ligand and
to activate the complement lectin pathway, rather than synthesis or
secretion of the protein.
Materials and methods
Cloning and vector construction of MBL
genes
The intact cDNA encoding MBL polypeptide, including
the signal sequence, was amplified from the liver tissue of a
5-month-old Chinese foetus, cloned in a pGEM-T vector. The
recombinant plasmid obtained was designated pMBLw. pMBLw was used
as a template, and MBL variants GGC54GAC, CGT52TGT and GGA57GAA
were created using the Megaprimer-PCR or Takara MutanBEST kit. The
products of site-directed mutagenesis were inserted into the pGEM-T
vector, and three recombinant plasmids, pMBLm52, pMBLm54 and
pMBLm57, were obtained. The target sequences in four recombinant
plasmids were amplified by PCR and inserted into eukaryotic
expression vectors PcDNA4/HisMaxC. The resulting recombinant
expression vectors were designated pcMBLw, pcMBLm52, pcMBLm54 and
pcMBLm57, respectively.
Transient expression of wild-type and
variant forms of MBL in COS-7 cells
COS-7 cells (ATCC) were plated at a density of
5×104 cells/ml in 24-well plates (Nunc, Roskilde,
Denmark) in Iscove's modified Dulbecco's medium (IMDM; Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco)
for 24 h prior to transfection. The cells were then transfected
with 10 μl of Lipofectamine™ reagent (Invitrogen, Carlsbad, CA,
USA) complexed with 1 μg of DNA (pcMBLm52, pcMBLm54, pcMBLm57 or
pcMBLw). Seventy-two hours later, the culture supernatants were
collected and analyzed by sandwich ELISA.
Stable expression of wild-type and
variant forms of MBL in CHO cells
CHO cell transfectants were generated by
electroporation of CHO cells with the recombinant plasmids
previously mentioned. Zeocin (800 mg/l) was added into the cultures
for 30 days to select CHO cells and a further 200 mg/l were then
added for another 30 days to obtain stable Zeocin-resistant
transfectants. The expression of mRNAs was analyzed by RT-PCR. The
selected clones were subcloned in 96-well microtitre plates using a
limiting dilution method and subsequently incubated in serum-free
medium (JRH Bioscience, Victoria, Australia). The recombinant
proteins were purified from the culture supernatants by
Ni2+-NTA agarose chromatography (Invitrogen) and
identified by indirect ELISA.
SDS-PAGE and western blotting
SDS-PAGE was performed using 10% Tris acetate gels
and stained with Coomassie brilliant blue. Western blotting was
carried out on polyvinylidene difluoride (PVDF) membrane.
Non-specific binding was blocked by incubating the membrane with
TBST [20 mM Tris-HCl, 150 mM NaCl, 0.1% (v/v) Tween-20] containing
5% (w/v) skim milk at 4˚C overnight. The membrane was then
incubated with anti-MBL monoclonal antibody (mAb) HYB131-11 (Abcam,
Cambridge, UK) for 1 h at room temperature. After washing with
TBST, the membrane was further incubated with horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG for 30 min. The
peroxidase reaction was finally performed using TMB as a substrate
after washing the membrane.
Indirect ELISA
Flat-bottomed microtitre plates (Nunc) were coated
with recombinant wild-type and variant forms of MBL proteins were
purified by Ni2+-NTA agarose chromatography at 4˚C
overnight, and then incubated with anti-MBL mAb HYB131-11,
anti-MBL-CRD mAb (prepared in our laboratory) or anti-His mAb
(Invitrogen) at 37˚C for 1 h, followed by HRP-conjugated anti-mouse
IgG. The amount of binding proteins was determined by measuring the
absorbance at 450 nm (A450 nm) using TMB as a substrate for the
peroxidase reaction.
Sandwich ELISA
Flat-bottomed microtitre plates were coated with
anti-MBL polyclonal antibody (1:100) (prepared in our lab) at 4˚C
overnight, followed by incubation with the culture supernatants of
the transient expression system at 37˚C for 1 h, and HRP-conjugated
anti-His polyclonal antibody (R&D Systems, Minneapolis, MN,
USA) at 37˚C for 1 h. The amount of binding proteins was determined
by measuring A450 nm using TMB as a substrate. The MBL standard was
a kind offer from Professor Jens Chr. Jensenius (Aarhus University,
Aarhus, Denmark).
Binding of MBL to mannan
Mannan (10 mg/l; Sigma-Aldrich, St. Louis, MO, USA)
was placed onto microtitre wells. The wells were then incubated
with purified proteins in TBS containing 5 mM CaCl2 and
5% (w/v) bovine serum albumin (TBS-Ca-BSA) at 37˚C for 1 h,
anti-MBL mAb HYB131-11 at 37˚C for 1 h, and HRP-conjugated
anti-mouse IgG at 37˚C for 30 min. The amount of proteins binding
to mannan was determined by measuring A450 nm using TMB as a
substrate. In certain experiments, proteins were simultaneously
incubated with mannan in the presence of mannose,
N-acetylglucosamine or EDTA.
Binding of MBL to MASPs
A purified recombinant N terminal fragment of MASP1
or MASP2 (10 mg/l, prepared in our laboratory) was placed onto
microtitre wells. The wells were then incubated with four MBL
proteins and the MBL-CLR protein (prepared in our laboratory) in
TBS, anti-MBL mAb HYB131-11, and HRP-conjugated anti-mouse IgG at
37˚C for 1 h. The amount of proteins binding to MASP was determined
by measuring A450 nm using TMB as a substrate.
C4 deposition assay
The ability of variant MBL proteins as well as
wild-type MBL to activate the lectin pathway of the complement was
determined by the C4 deposition assay. Microtitre plates were
coated with mannan (50 mg/l) at 4˚C overnight. The indicated
concentrations of purified MBL proteins in TBST containing 5% (w/v)
skim milk were then added and incubated at 37˚C for 1 h. After
being washed, the plates were incubated with MBL-deficiency human
serum (genotype LYPB/LYPB, collected in our lab) at 37˚C for 1 h,
anti-human C4d mAb (Quidel, San Diego, CA, USA) at 37˚C for 1 h,
and HRP-conjugated anti-mouse IgG at 37˚C for 30 min. The amount of
C4d deposition was determined by measuring A450 nm values.
Statistical analysis
Statistical significance of data was determined by
comparing the results between wild-type and variant MBL proteins
using a one-way analysis of variance (ANOVA) with the Tukey post
hoc test. P<0.05 was considered to denote statistical
significance.
Results
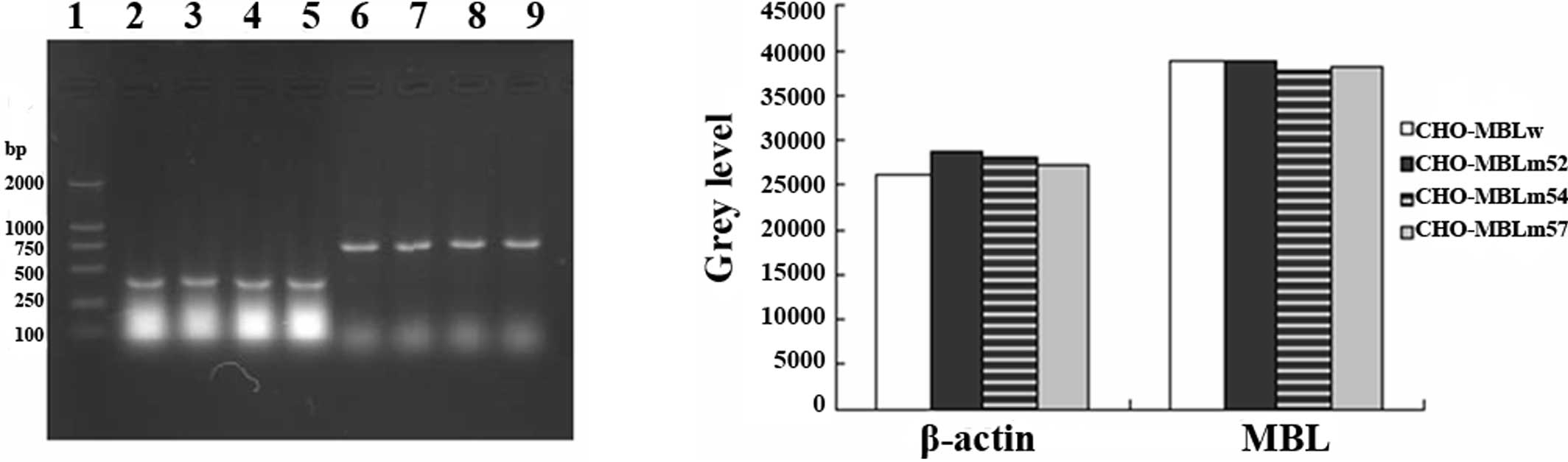
Stable expression of MBL genes in CHO
cells
To investigate the biological behavior of MBL
variants, we transfected pcMBLw, pcMBLm52, pcMBLm54 and pcMBLm57
into CHO cells by electroperforation. The same number of
transfected cells was collected and identified by RT-PCR. mRNAs of
variants and the wild-type MBL gene were effectively expressed in
CHO cells (Fig. 1A). No difference
was found among the MBL variants and the wild-type MBL gene
(Fig. 1B).
Transient expression of MBL genes in
COS-7 cells
The same mass number expression vectors of wild-type
and three variants of MBL were transiently transfected into the
same amount of COS-7 cells. Then, 72 h later, proteins secreted in
the culture supernatant were analyzed by a sandwich ELISA assay. No
difference of the concentrations among the variants and wild-type
of MBL was found (P>0.05) (Fig.
2).
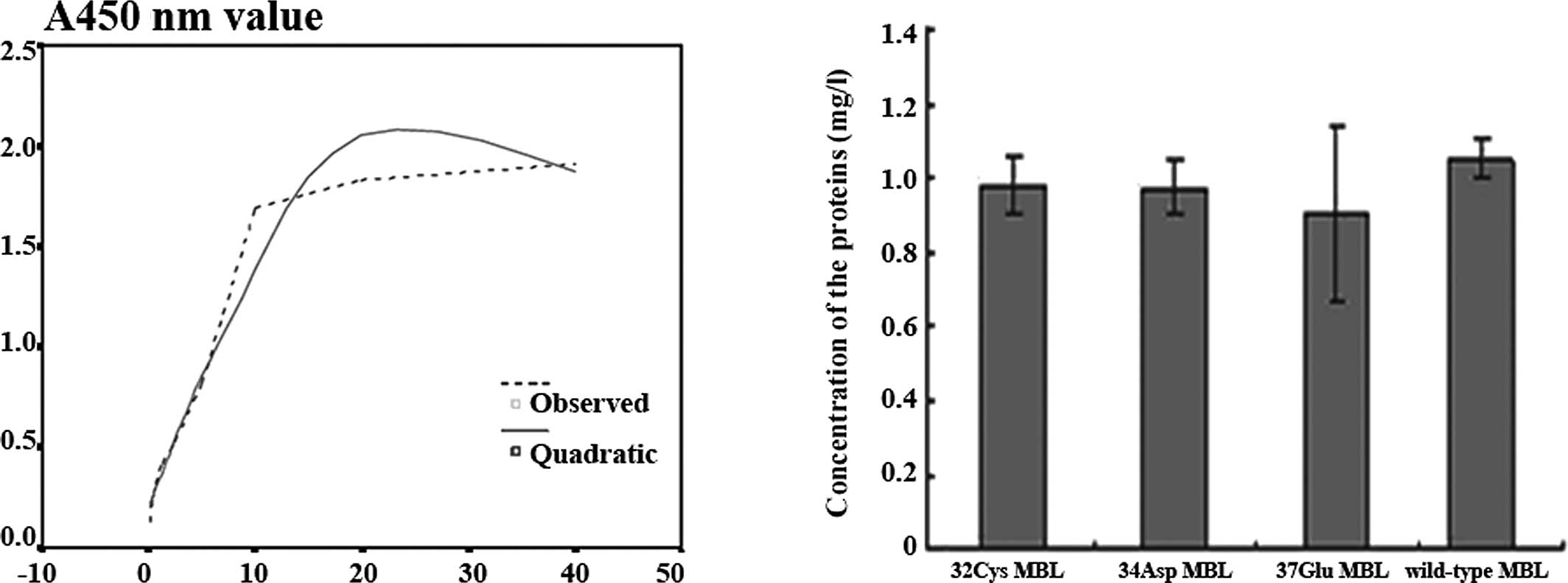 | Figure 2Protein levels in the culture
supernatants of the transient expression system. COS-7 cells were
plated at a density of 5×104 cells/ml in 24-well plates
for 24 h and then transfected with 1 μg of DNA (pcMBLm52, pcMBLm54,
pcMBLm57 or pcMBLw). Seventy-two hours later, culture supernatants
were collected and analyzed by sandwich ELISA. (A) The MBL standard
is diluted from 40 to 0.3125 mg/l. Using the non-linear regression
analysis, the protein concentration is independent X, A450 nm value
is dependent Y, R2=0.96563. (B) Y-values were obtained
from the equation Y=-0.002536X2+0.143289X+0.200338. The
mean values of 32Cys, 34Asp, 37Glu and wild-type MBL proteins were
0.980, 0.971, 0.900 and 1.047 mg/l, respectively. No differences
were observed in the concentrations between the mutated MBL
proteins and wild-type MBL (P>0.05). |
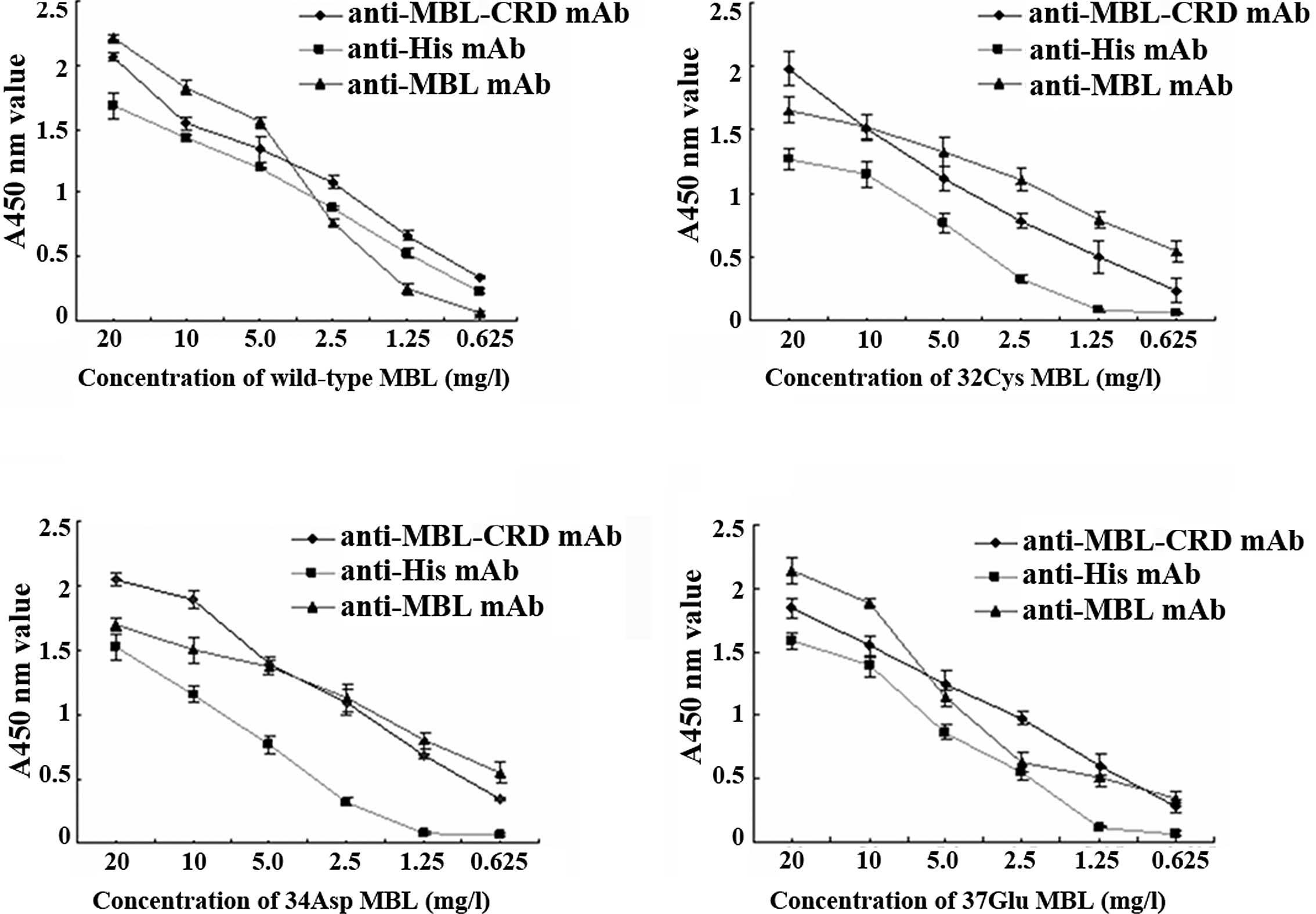
Identification of proteins by indirect
ELISA
Recombinant MBL proteins were purified and
identified by indirect ELISA. Each of the proteins may be
recognized by self-prepared (anti-MBL-CRD mAb) and commercialized
(HYB131-11) mAbs (Fig. 3),
indicating that they are MBL proteins.
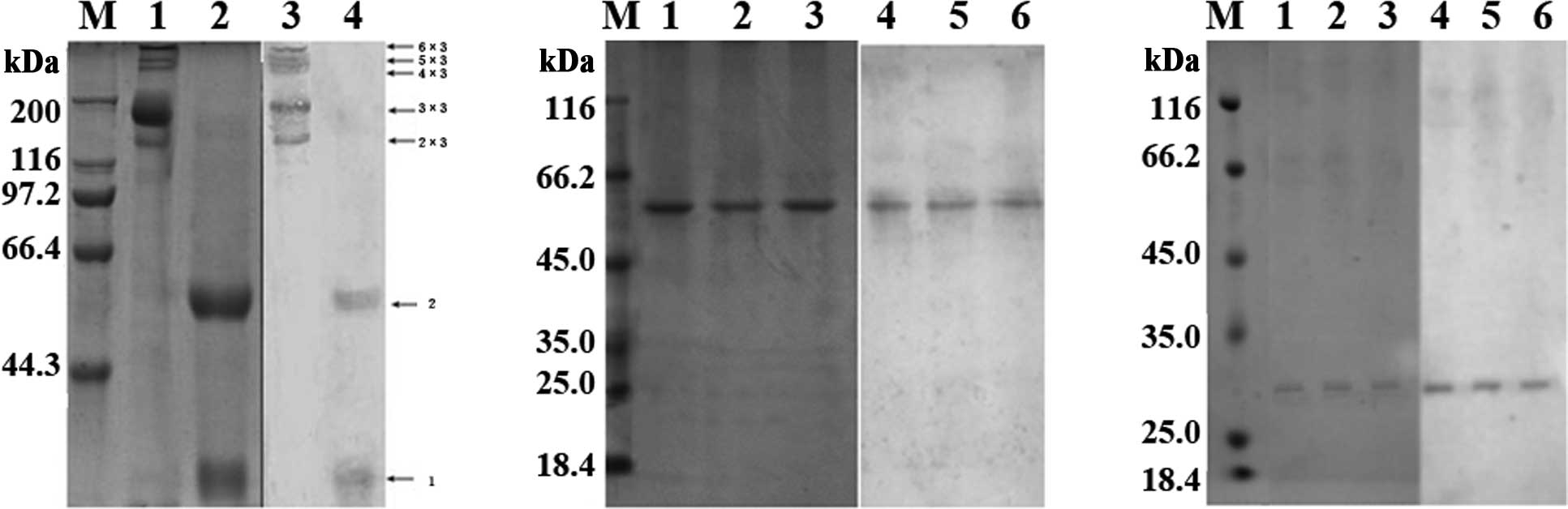
Detection of MBL expression by SDS-PAGE
and western blotting
The oligomeric composition of recombinant wild-type
and mutated MBL proteins was analyzed on non-reduced and reduced
SDS-PAGE followed by western blotting (Fig. 4). As shown in Fig. 4A, under non-reducing conditions,
the recombinant wild-type MBL forms oligomers containing two, three
and four chains (150, 225 and 300 kDa, respectively), whereas
mutated MBL proteins form covalent oligomers containing two
polypeptide chains (~60,000) (Fig.
4B), indicating a much lower oligomerization in the mutated MBL
proteins, 32Cys, 34Asp and 37Glu. Wild-type MBL was reduced to a
single chain (25 kDa) and an oligomer containing two polypeptide
chains (50 kDa) (Fig. 4A), whereas
the mutated proteins were reduced to a single chain with a
molecular mass of ~32 kDa (Fig.
4C).
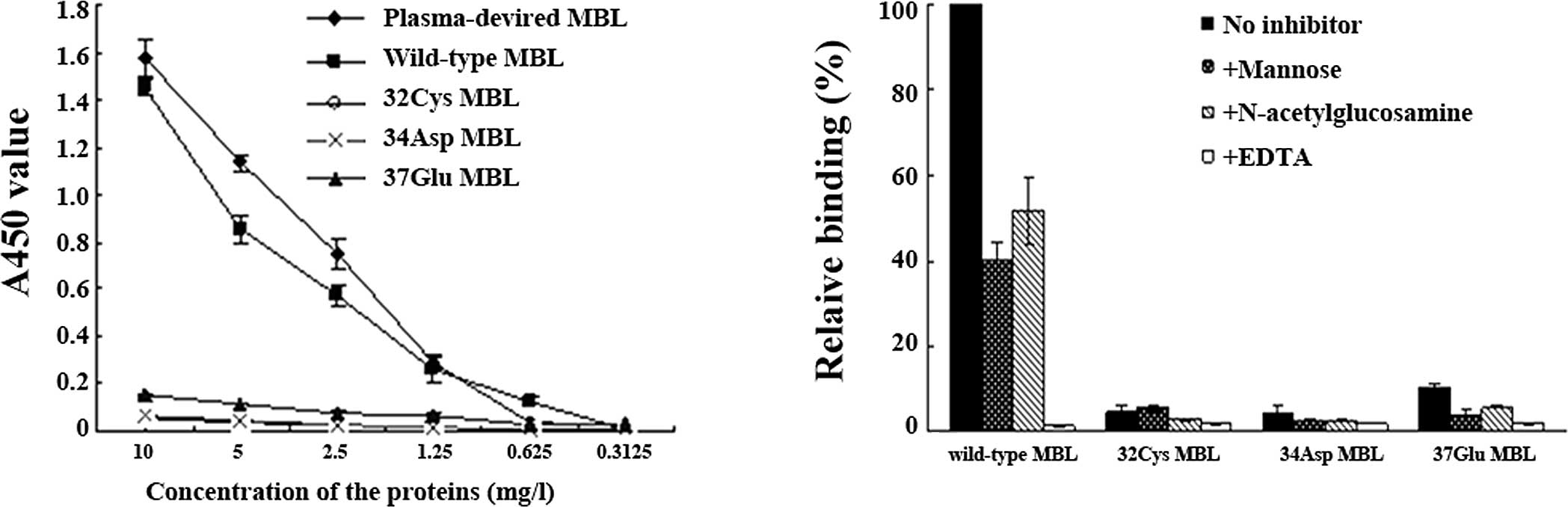
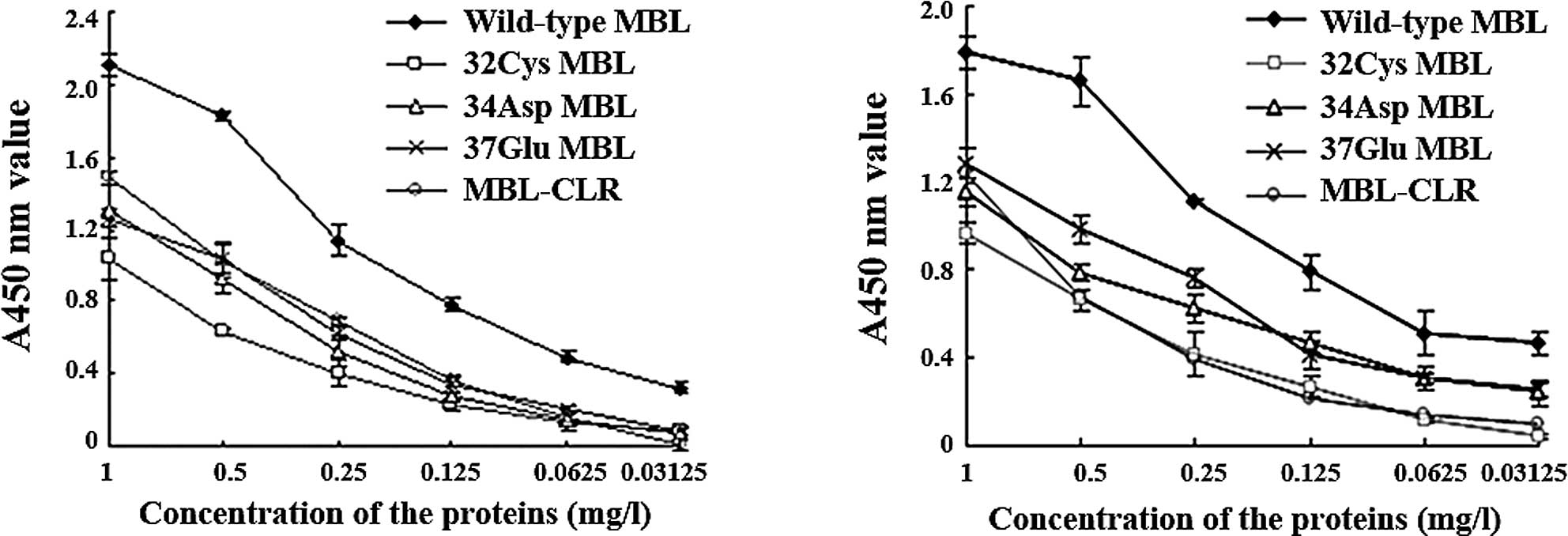
Binding of proteins to mannan
The mannan-binding assay showed that the mutated MBL
proteins lose the ability to bind to mannan (Fig. 5A), while mannose and
N-acetylglucosamine partly inhibited binding of the wild-type MBL
protein to mannan, and EDTA totally inhibited the binding (Fig. 5B).
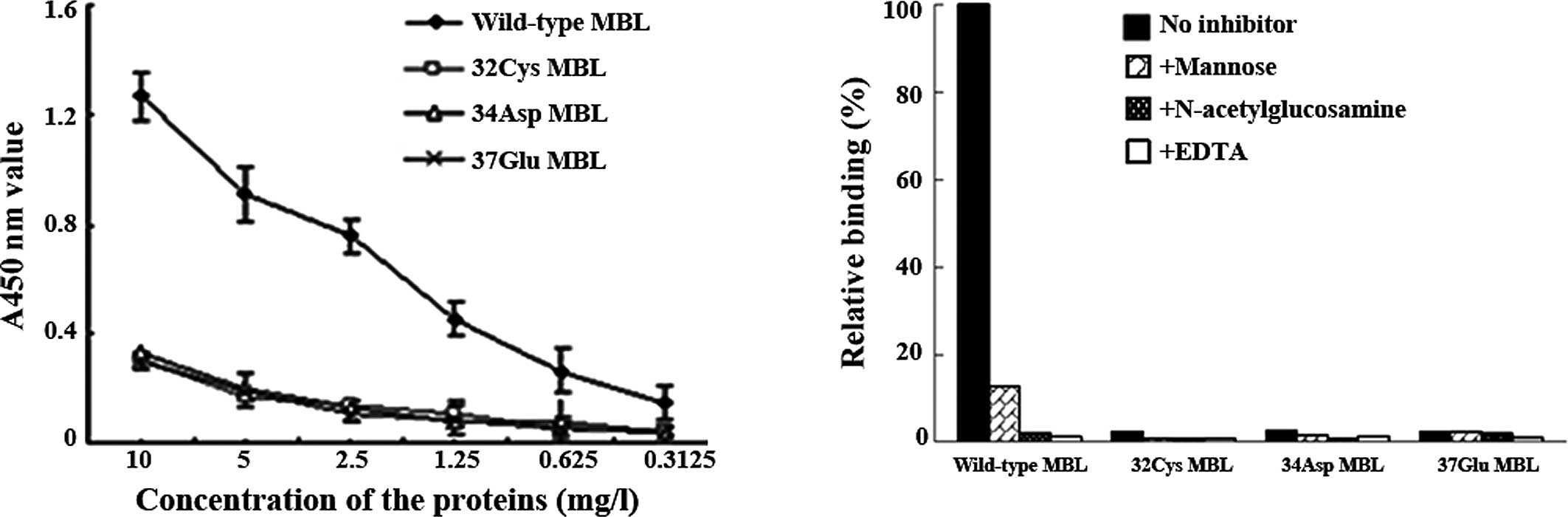
Binding of MBL to MASP as determined by
ELISA
To reveal the interaction between mutated MBL and
MASPs, we assessed the binding of N-terminal fragments of MASP1 and
MASP2 to four MBL proteins and the MBL-CLR protein by ELISA. Three
variant MBL proteins bound to MASP1 (Fig. 6A) and MASP2 (Fig. 6B) with a lower ability compared to
the wild-type MBL, without, however, completely losing this binding
ability.
Activities of MBL as determined by C4
cleavage assay
Not all the mutated proteins activated the
complement system (Fig. 7A).
Mannose and N-acetylglucosamine partly inhibited C4d deposition
resulting from the wild-type MBL, whereas EDTA completely inhibited
the activity (Fig. 7B).
Discussion
The aim of the present study was to investigate the
relationship among MBL gene mutations, antigenic MBL secretion
levels and MBL functional activities. The MBL encoding gene was
amplified by RT-PCR. The sequencing result indicated that the
sequence of cDNA is identical to that of mRNA for human MBL
(accession no. X15422), with the exception of the two nucleotides
ACC24GCC (Thr→Ala) and GGC185GGT (Gly→Gly). The nucleotide sequence
was deposited in GenBank with the accession number AF360991. Using
a MBL cDNA from the HepG2 human hepatocellular carcinoma cell line,
Larsen et al (17) reported
that mutations in the human MBL gene compromise the oligomerization
and activity of the protein. We obtained MBL cDNA from the liver of
a Chinese foetus, which is exists naturally. GGC54GAC, CGT52TGT and
GGA57GAA variants were created with pMBLw as the template, whose
sequences contain the expected point mutations without any other
changes. The recombinant eukaryotic expression vectors, pcMBLw,
pcMBLm52, pcMBLm54 and pcMBLm57, were constructed by inserting the
target sequences into eukaryotic expression vectors PcDNA4/HisMaxC,
and were transfected into COS-7 and CHO cells for transient and
stable expression studies, respectively.
MBL is a macroprotein with complicated functions,
and it is impossible to obtain an intact protein in the protokaryon
expression system. Ohtani et al (18) reported a high level and effective
production of human wild-type MBL in CHO cells. We transfected
recombinant plasmids into CHO cells and found that there is no
difference among the mRNAs of variants. Additionally, the wild-type
MBL gene is capable of being effectively expressed in CHO cells.
From these results, we inferred that the point mutations in exon 1
of the MBL gene do not interrupt the expression of the MBL
gene.
To ivestigate the effect of the mutations on the
secretion of MBL protein, Larsen et al (17) attempted to achieve a transient
expression in COS-7 cells; however, they failed due to low
expression levels. We successfully expressed variant and wild-type
MBL proteins in COS-7 cells, and gained insight into the
relationship between MBL gene mutations and MBL protein synthesis
or secretion, because protein levels are affected with few factors
in the transient transfection system. Results of the sandwich ELISA
assay showed that the gene mutations may not affect the MBL level
in human plasma.
The product of the human MBL gene is a 25–32 kDa
polypeptide chain containing 228 amino acids, consisting of four
domains: the cysteine-rich N-terminal region responsible for the
formation of intra- and inter-subunit disulfide bonds, an extended
CLR (repeating Gly-X-Y triplets), a short-helical neck region
initiating trimerization of the collagen-like sequence, and a
C-terminal CRD that constitutes a globular head. Three identical
polypeptide chains combine to form a subunit, with a tail
consisting mainly of a collagen-like triple helix and a
three-headed cluster of globular CRDs. Several homotrimeric
subunits may then oligomerize to form a series of oligomers via
disulfide bonding in the N-terminal region. The cysteine-rich
N-terminal region and CLR are essential for effective
oligomerization. MBL appears to exist in plasma as a mixture of 2–6
trimeric subunits. Only high-order oligomers (teramers or larger
oligomers) interact with carbohydrates with higher affinity and
efficiently activate the complement. The common character of the
three mutations is that all of them affect the Gly-X-Y repeats in
the CLR (17).
To further study the relationship between gene
mutations and the protein activities of human MBL, we purified
recombinant wild-type as well as mutated MBL proteins and found
that mutated MBL has less oligomerization compared to wild-type
MBL. Mutated MBL proteins lose the mannan binding and complement
activation ability. Binding of MBL to MASPs showed that the three
variant MBL proteins bind to MASP1 and MASP2 with a lower ability
compared to wild-type MBL, although they do not completely lose
this binding ability. This gives rise to the possibility that the
three point mutation sites are not involved in the binding of
MASPs. This assumption is consistent with the finding that the
mutation sites of MBL are not involved in the binding sites for
MASPs, as demonstrated by our group using synthetic peptides
(19). Therefore, the inability of
the variant MBL proteins to activate the complement results from
lower oligomerization, which impairs the interaction of MBL with
mannan and MASPs. As for CGT52TGT, a Cys introduced by the mutation
may form another disulfide bond that may disrupt the structure of
the MBL molecule, as well as its function. GGC54GAC and GGA57GAA
mutations introduce Asp or Glu with a large side chain and charge
instead of neutral Gly, and may affect formation of the CLR
α-helix, resulting in the formation of fewer oligomers.
Since Valdimarsson et al (20) reported that MBL replacement therapy
with a plasma-derived product is both safe and promising, attention
has focused on MBL in clinical research. Terms such as ‘MBL
deficiency’ and ‘MBL insufficiency’ have not been well-defined due
to high variability in different individuals. The plasma levels of
MBL are affected by various factors, such as age, race, gene
polymorphism and individual status. In individuals with the same
MBL genotype, a MBL concentration of five times or more exists.
Therefore, susceptibility to infections may not be affected simply
by the plasma MBL levels. Furthermore, it was reported that variant
alleles in humans give rise to relatively high levels of MBL in the
circulation (21). MBL deficiency
does not only involve lower MBL levels, but also structural
changes. In this study, we found that the variant forms of MBL
differ in the forms of oligomers. These variant forms all form a
dominant band with a molecular mass of approximately 60 kDa,
responding in the decrease of their functions. However, we did not
find that these point mutations affect the synthesis or secretion
of the MBL protein. Immunodeficiency caused by MBL mutations may be
the result of less oligomerization, rather than the decrease in
plasma MBL levels. Therefore, we noted that the immunological
methods based on the antigen-antibody binding may not distinguish
normal MBL from variant MBL. The most important tool for the
dissection of MBL deficiency involves two ELISA systems that detect
MBL levels; one is based on the double-antibody sandwich ELISA,
whereas the other is the mannan-binding ELISA. The former detects
all the forms of MBL, including monomers and oligomers, while the
latter only detects MBL oligomers, but may be interfered with by
classical pathway activation by anti-mannan antibodies (22). Special consideration with these
ELISA systems should be given to the detection of polymers as
opposed to various forms of MBL, in order that the actual levels of
functional MBL are reflected.
In conclusion, three forms of MBL gene mutations
decrease the functions of their products, including the binding to
mannan and MASPs and the ability to activate the complement, which
result from lowered protein oligomerization, although they do not
affect the synthesis or secretion of the MBL protein. The data
suggest that MBL deficiency associated with three polymorphic
variants may result from impaired oligomerization of the MBL
protein rather than the decrease of MBL levels in plasma.
Additionally, amino acids Arg32, Gly34 and Gly37 are required to
maintain the structure and function of MBL.
Acknowledgements
This study was supported by the Natural Scientific
Foundation of China (39970286), and the Natural Scientific
Foundation Team Research Project of Guangdong Province
(015003).
Abbreviations:
|
MBL
|
mannan-binding lectin
|
|
CRD
|
carbohydrate recognition domain
|
|
CLR
|
collagen-like region
|
|
MASP
|
MBL-associated serine protease
|
|
RA
|
rheumatoid arthritis
|
|
SLE
|
systemic lupus erythematosus
|
|
CHO
|
Chinese hamster ovary
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
PVDF
|
polyvinylidene difluoride
|
|
HRP
|
horseradish peroxidase
|
References
|
1
|
G GuptaA SuroliaCollectins: sentinels of
innate immunityBioessays29452464200710.1002/bies.2057317450595
|
|
2
|
MW TurnerThe role of mannose-binding
lectin in health and diseaseMol
Immunol40423429200310.1016/S0161-5890(03)00155-X14568388
|
|
3
|
RM DommettN KleinMW TurnerMannose-binding
lectin in innate immunity: past, present and futureTissue
Antigen68193209200610.1111/j.1399-0039.2006.00649.x16948640
|
|
4
|
DL JackNJ KleinMW TurnerMannose-binding
lectin: targeting the microbial world for complement attack and
opsonophagocytosisImmunol
Rev1808699200110.1034/j.1600-065X.2001.1800108.x11414367
|
|
5
|
K HajelaM KojimaG AmbrusThe biological
functions of MBL-associated serine proteases
(MASPs)Immunobiol205467475200212396008
|
|
6
|
SS BohlsonDA FraserAJ TennerComplement
proteins C1q and MBL are pattern recognition molecules that signal
immediate and long-term protective immune functionsMol
Immunol443343200710.1016/j.molimm.2006.06.02116908067
|
|
7
|
DP EisenRM MinchintonImpact of
mannose-binding lectin on susceptibility to infectious diseasesClin
Infect Dis3714961505200310.1086/37932414614673
|
|
8
|
DL WorthleyPG BardyCG
MullighanMannose-binding lectin: biology and clinical
implicationsIntern Med
J35548555200510.1111/j.1445-5994.2005.00908.x16105157
|
|
9
|
OA MonticieloT MucenicRM XavierJC BrenolJA
ChiesThe role of mannose-binding lectin in systemic lupus
erythematosusClin
Rheumatol27413419200810.1007/s10067-008-0838-818214570
|
|
10
|
CP MauryJ AittoniemiS TiitinenK LaihoK
KaarelaM HurmeVariant mannose-binding lectin 2 genotype is a risk
factor for reactive systemic amyloidosis in rheumatoid arthritisJ
Intern Med262466469200710.1111/j.1365-2796.2007.01838.x
|
|
11
|
M CarlssonAG SjöholmL ErikssonS ThielJC
JenseniusM SegelmarkL TruedssonDeficiency of the mannan-binding
lectin pathway of complement and poor outcome in cystic fibrosis:
bacterial colonization may be decisive for a relationshipClin Exp
Immunol139306313200510.1111/j.1365-2249.2004.02690.x15654829
|
|
12
|
CB FosterT LehrnbecherF MolHost defense
molecule polymorphisms influence the risk for immune-mediated
complications in chronic granulomatous diseaseJ Clin
Invest10221462155199810.1172/JCI5084
|
|
13
|
P GarredF LarsenJ SeyfarthR FujitaHO
MadsenMannose-binding lectin and its genetic variantsGenes
Immun78594200610.1038/sj.gene.636428316395391
|
|
14
|
XP YuCW LvZM GeJC LiL MaZL
ChenInvestigation of single nucleotide polymorphisms, haplotypes
and genotypes of mannan-binding lectin gene in Bai (Pai)
nationality in ChinaChinese J Immunol (in Chinese)227387422006
|
|
15
|
CQ ZhongXP YuFY WangT KurexijiangZL
ChenFrequencies of CGT52TGT, GGC54GAC and GGA57GAA point mutations
in MBL gene in Chinese Uyghur populationNan Fang Yi Ke Da Xue Xue
Bao (In Chinese)2617641767200617259116
|
|
16
|
SV PetersenS ThielJC JenseniusThe
mannan-binding lectin pathway of complement activation: biology and
disease associationMol
Immunol38133149200110.1016/S0161-5890(01)00038-411532276
|
|
17
|
F LarsenHO MadsenRB SimC KochP
GarredDisease-associated mutations in human mannose-binding lectin
compromise oligomerization and activity of the final proteinJ Biol
Chem2792130221311200410.1074/jbc.M400520200
|
|
18
|
K OhtaniY SuzukiS EdaHigh-level effective
production of human mannan-binding lectin MBL in Chinese hamster
ovary CHO cellsJ Immunol
Methods222135144199910.1016/S0022-1759(98)00190-210022380
|
|
19
|
DM ZuoXM CaiN ZhaoLY ZhangZL ChenLocation
of MBL-associated serine proteases binding motifs on human
mannan-binding lectin (MBL)Protein Peptide
Lett17131136201010.2174/09298661078990956620214636
|
|
20
|
H ValdimarssonM StefanssonT
VikingsdottirGJ ArasonC KochS ThielJC JenseniusReconstitution of
opsonizing activity by infusion of mannan-binding lectin (MBL) to
MBL-deficient humansScand J
Immunol48116123199810.1046/j.1365-3083.1998.00396.x9716101
|
|
21
|
P GarredF LarsenHO MadsenaC
KochMannose-binding lectin deficiency - revisitedMol
Immunol407384200310.1016/S0161-5890(03)00104-412914814
|
|
22
|
MA SeelenA RoosaJ WieslanderFunctional
analysis of the classical, alternative, and MBL pathways of the
complement system: standardization and validation of a simple
ELISAJ Immunol
Methods296187198200510.1016/j.jim.2004.11.01615680163
|