Introduction
Pulmonary embolism (PE) consists of acute pulmonary
thromboembolism (APE) and chronic thromboembolic pulmonary
hypertension (CTEPH). PE together with deep venous thrombosis (DVT)
is termed venous thromboembolism (VTE). PE has become an
international health care problem due to its high morbidity,
misdiagnosis rate and mortality (1)
More than a century ago, Vichrow proposed a theory
known as Virchow’s triad, stating that thrombosis results from a
combination of hemodynamic changes (stasis, turbulence),
hypercoagulability and vessel wall injury (2). This theory forms the basis of the
pathogenesis of artery thrombosis and VTE. The American Collage of
Chest Physicians (ACCP) proposed a risk stratification for VTE and
recommended different methods of prevention for patients at
different risk levels (3). As a
matter of fact, some patients at the same risk level and in a
similar external environment, develop VTE after surgery, but the
majority do not. It is still not clear as to why the incidence of
VTE increases with age and why patients with a malignant disease
are prone to VTE. It is also still not clear as to why artery
thrombus is a ‘white clot’ and VTE a ‘red clot’. Although some
patients with DVT/PE received warfarin continuously, and the
D-dimer level was regulated within a good range, their pulmonary
artery pressure still increased gradually and they eventually
developed CTEPH. ACCP summarized the risk factors for VTE as:
trauma, surgery, increasing age, malignancy, pregnancy, heart
failure, immobility and estrogen-containing oral contraception
(3,4). Although guidelines for the
prevention, diagnosis and treatment of VTE have been published 8
times from 1995 to 2008 and have been updated continuously, the
clinical confusion associated with VTE remains. In 2008, Shackfored
reviewed the records of 37,615 patients with symptomatic VTE on
surgery services over the 10-year period since the initial
publication of the ACCP guidelines, of which 84% were either in
partial or complete compliance with the guidelines. The incidence
of VTE, however, increased gradually over the years of the study,
instead of decreasing (5). The
possible reason for theory and clinical practice being separated is
that the etiology and pathogenesis of VTE are not yet clear.
In 2006, Smeeth reported that VTE was associated
with infection, especially in the first 2 weeks following infection
(6). In 2010 it was also reported
that VTE was found in multiple organs in a patient, who died of
severe acute respiratory syndrome (SARS), indicating that a viral
infection caused systemic VTE (7).
In a previous study, we identified a virus-like structure in T
cells in a patient with CTEPH, which proved that T cells were
infiltrated by a virus (8). In
2011, it was also reported that CD3+ and CD8+
T cell-mediated immune deficiency or compromise occurred in
patients with CTEPH (9) and acute
PE (10). Our research focused on
the correlation between VTE and infection, inflammation, and
immunity.
There are numerous leukocytes aggregating at the
site of a thrombus in VTE. The adhesion among leukocytes,
endothelial cells and platelets occurs throughout the process of
VTE (11,12). Cell adhesion molecules (CAMs) are a
type of glycoprotein expressed on the cell surface mediating
cell-cell and cell-matrix interactions, which are the basis of cell
adhesion. CAMs participate in a series of physiological and
pathological processes, including signal transduction and
activation, morphogenic movements, cellular migrations, cell growth
and differentiation, as well as inflammation, thrombosis, wound
healing and metastasis. Hundreds of CAMs have been identified in
humans and are divided into 4 families: integrins, selectins and
the immunoglobulin and cadherin superfamily. In this research, a
whole human gene expression chip was applied to detect the
differences of CAM-related mRNA expression in patients with PE and
in a control group. The correlations among CAMs in activated
leukocytes, platelets and endothelial cells during the process of
PE were investigated.
Materials and methods
Patient information
The 20 patients enrolled in the PE group were those
who were admitted in hospital during the year 2007, and included 11
males and 9 females, with an average age of 70±14 years (44–89
years old). All patients were diagnosed with PE on the basis of at
least 2 of the following criteria: i) selective pulmonary
arteriography showing a filling defect or blockage; ii) pulmonary
ventilation perfusion scanning exhibiting single or multiple blood
flow perfusion defects with normal or abnormal ventilation and
mismatched ratio of ventilation/perfusion; iii) other clinical
characteristics, including a typical manifestation of PE, arterial
blood gas analysis, D-dimer test, ultrasound cardiogram (UCG) and
chest computerized tomography (CT) supported the diagnosis and
excluded other cardiac and pulmonary disorders. Another 20 patients
with ischemic heart disease admitted during the same period,
without PE, DVT and other congenital bleeding and thrombosis
diseases with comparative clinical presentation (11 males, 9
females; 44–91 years of age with a mean age of 72±14) were enrolled
in the control group. The study protocol was approved by the local
ethics committee and an informed consent was obtained from all the
patients in accordance with the declaration of Helsinki.
Total RNA isolation
A total of 5 ml of peripheral blood samples
anti-coagulated with EDTA were drawn from patients suspected of
having PE and from those without PE, immediately after being
admitted to the hospital. Mononuclear cells were obtained through
density gradient centrifugation with Ficoll solution and the
remaining red blood cells were destroyed by erythrocyte lysis
buffer (Qiagen, Hilden, Germany). Total mononuclear cell RNA was
extracted with TRIzol (Invitrogen, Carlsbad, USA) and purified with
Qiagen RNeasy column (Qiagen), according to the manufacturer’s
instructions. The isolated total RNA was tested and quantified
using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technology,
Cambrige, UK).
Gene expression clip
Agilent G4112A Whole Human Genome Oligo Microarrays
were purchased from Agilent (USA). A microarray is composed of
44,290 spots including 41,675 genes or transcripts, 314 negative
control spots, 1,924 positive control spots and 359 blank spots.
The functions of more than 70% of the genes in the microarray are
already known. All patients were subjected to clip analysis.
Target preparation and microarray
hybridization
The RNA samples of patients with confirmed diagnosis
of PE and controls were labeled using the indirect labeling method.
Briefly, 1 μg of total RNA was reverse transcribed. Second strand
cDNA was then produced and purified followed by in vitro
transcription (IVT) with T7 RNA Polymerase. During IVT, the
modified nucleotide, 5-(3-aminoallyl)-UTP (aaUTP) was incorporated
into the cDNA. Subsequently, the fluorescent Cy3 was chemically
coupled with the aaUTP which contains a reactive primary amino
group on the C5 position of uracil. The dye incorporation rate was
assessed with a Nanodrop ND-1000 spectrophotometer and was found to
be between 1.2–1.4 pmol/μl. Hybridization was carried out using the
Agilent Oligonucleotide Microarray in situ Hybridization
Plus kit (p/n 5,184–3,568), according to the manufacturer’s
instructions. Briefly, 750 ng of Cy3-labeled sample cDNA was
subjected to fragmentation (30 min at 60°C) and then hybridization
on 44K Human Whole-Genome 60-mer oligo-chips (G4112F, Agilent
Technologies) in a rotary oven (10 rpm, 60°C, 17 h). Slides were
disassembled and washed in solutions I and II, according to the
manufacturer’s instructions.
RT-PCR
Three differential genes in the microarray were
selected and their expressions were confirmed by RT-PCR. Among the
genes with differential expressions, 3 genes were randomly selected
and these genes and the house keeping gene (GAPDH) were subjected
to RT-PCR. The relative expression levels were indicated as the
expression of the target genes normalized to the expression of
GAPDH (2−ΔΔCt). The melting curve and the 2−
ΔΔCt-method were used to compare the differences in the
expressions between the control and the PE group. The results from
RT-PCR were consistent with from the microarray analysis.
Statistical analysis
The Agilent Feature Extraction software was used to
collect the original data from the microarray, followed by an
analysis with a robust multichip average (RMA). The gene intensity
data between the PE and control group were compared with a random
variance model-corrected t-test. Differentially expressed genes
were identified from whole genomes. A p-value <0.05 was
considered to indicate a statistically significant difference.
Results
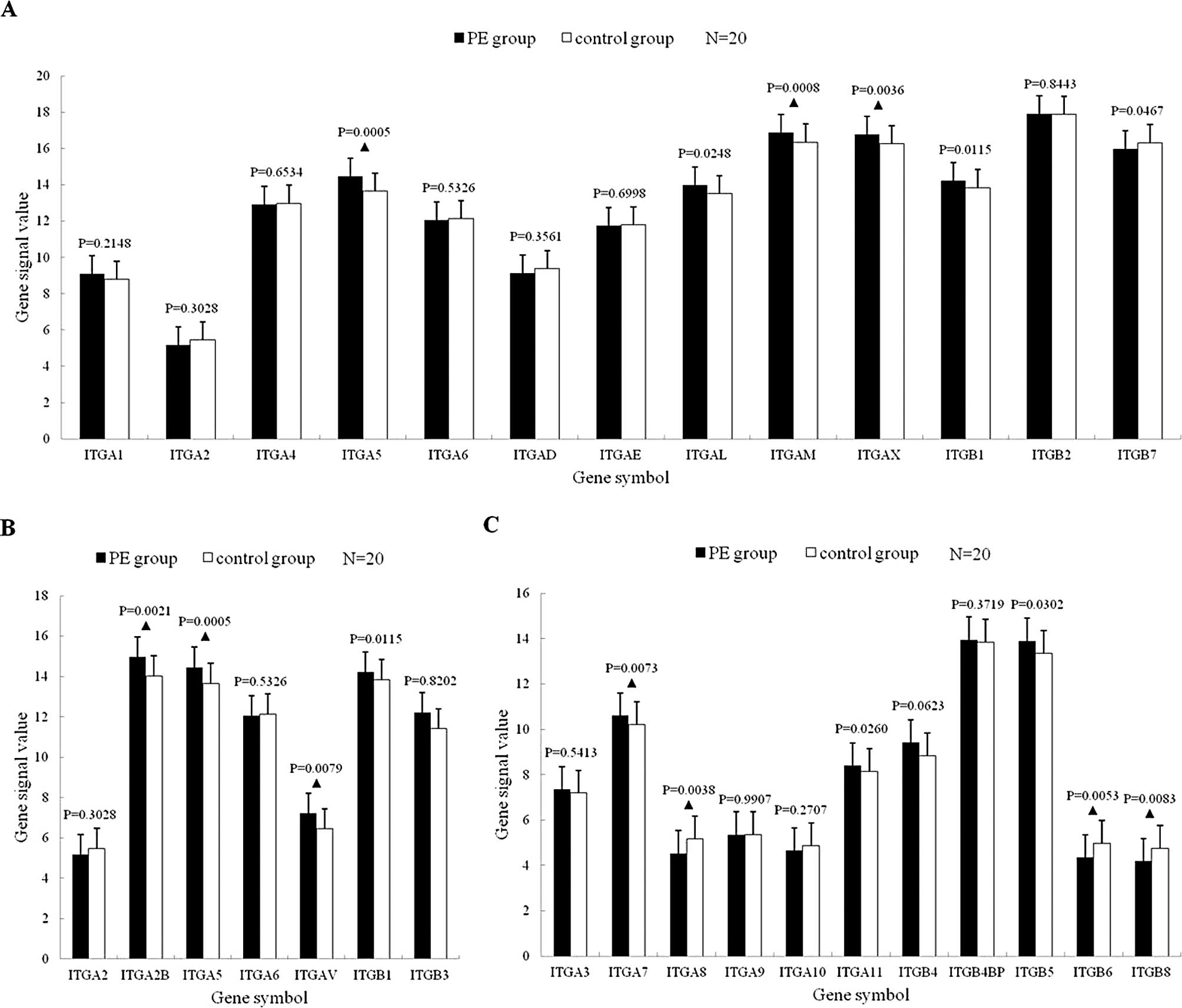
Integrin mRNA expression
Leukocyte-related integrin mRNA
expression
Among the 13 mRNAs, 7 were upregulated (of which 5
significantly) in the PE group, compared with the controls
(p<0.05); 6 were downregulated (of which 1 significantly) in the
PE group (p<0.05) (Fig.
1A).
Platelet-related integrin mRNA
expression
Of the 7 mRNAs, ITGA2, ITGA5, ITGA6 and ITGB1 were
also leukocyte-related integrin mRNAs. Five mRNAs were upregulated
(of which 4 significantly) (p<0.05); 2 mRNAs were downregulated,
but with no statistically significant difference (p>0.05)
(Fig. 1B).
Other integrin mRNA expression
Among the 11 mRNAs, 6 were upregulated (of which 3
significantly) in the PE group (p<0.05) and 5 were downregulated
(of which 3 significantly) (p<0.01) (Fig. 1C).
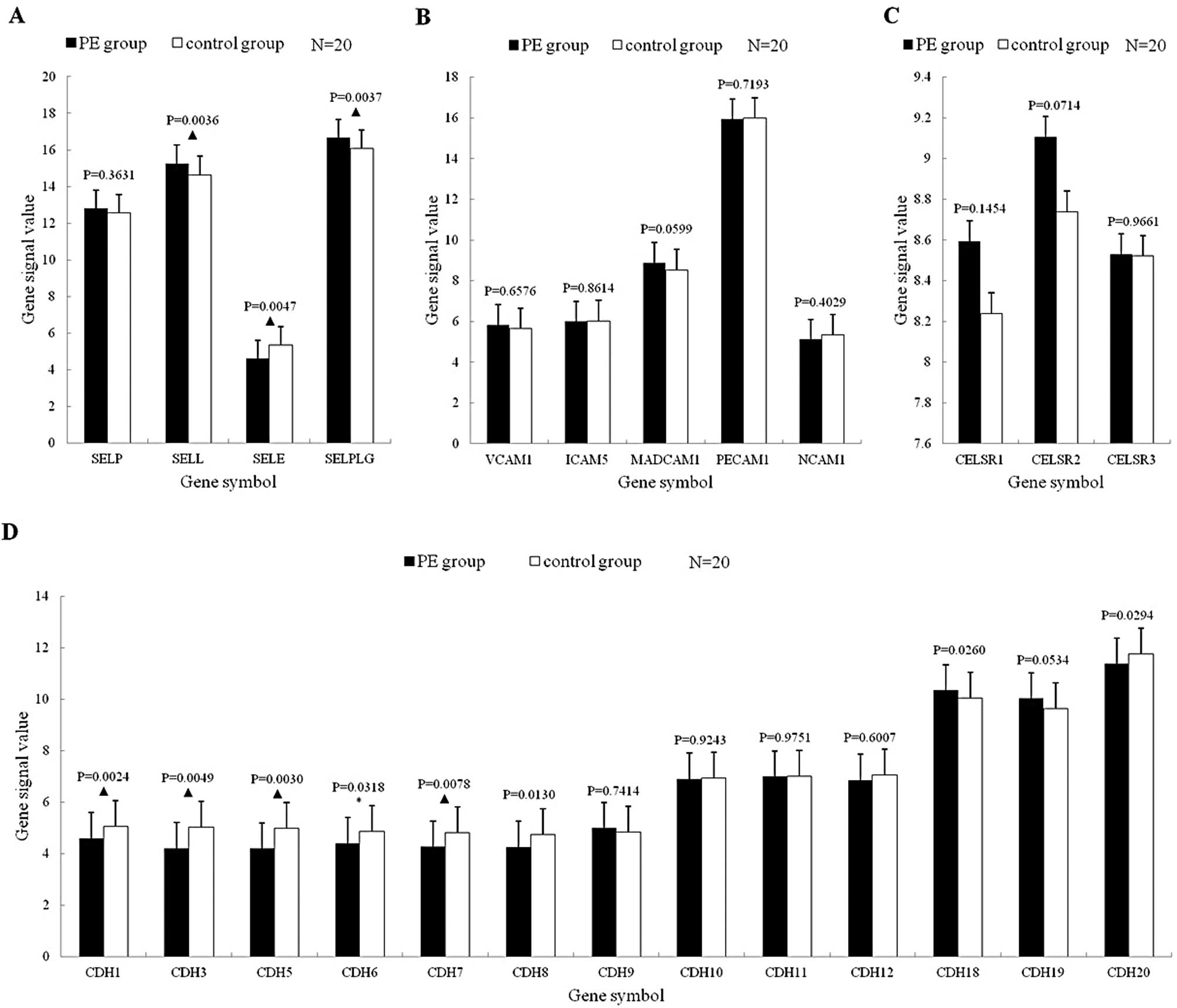
Selectin mRNA expression
Among the 4 mRNAs, SELL and SELPLG mRNA expressions
were significantly upregulated (p<0.01), while the SELE mRNA
expression was significantly downregulated (p<0.01) (Fig. 2A).
Immunoglobulin superfamily mRNA
expression
There were no statistically significant differences
in the 5 mRNAs between the PE and control group (p>0.05)
(Fig. 2B).
Cadherin superfamily mRNA expression
Classic cadherin mRNA expression
Among the 13 mRNAs, 10 were downregulated (of which
7 significantly) in the PE group (p<0.05) and 3 were upregulated
(of which 1 significantly) (p<0.05) (Fig. 2C).
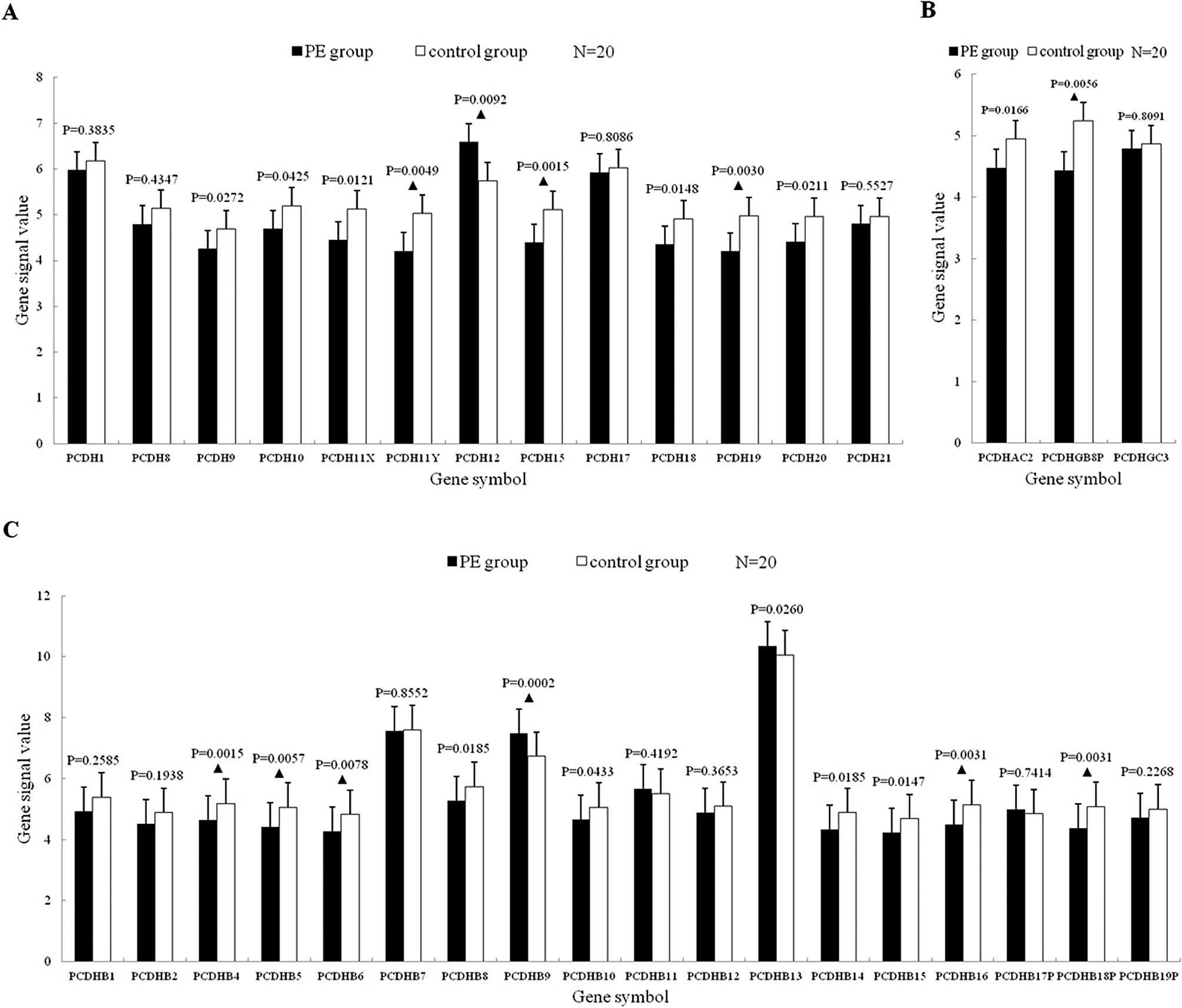
Protocadherin mRNA expression
The 34 mRNAs consisted of 13 non-clustered and 21
clustered protocadherins. A total of 19 mRNAs were significantly
downregulated (p<0.05), of which 9 more significantly
(p<0.01); 3 mRNAs were upregulated significantly (p<0.05), of
which 2 more significantly (p<0.01) (Fig. 3).
Flamingo cadherin mRNA expression
The 3 mRNAs were all upregulated, without a
statistically significant difference (p>0.05) (Fig. 2D).
Discussion
Expression profile of integrin mRNAs
Expression profile of
leukocyte-related integrin mRNAs
Among the 13 mRNAs, 5 (38.46%) were upregulated with
a statistically significant difference (p<0.05). In these 5
mRNAs, the ITGAL, ITGAM and ITGAX transcripts (subunit αL, αM and
αX) bind to subunit β2 to generate β2 integrins, and are expressed
on leukocytes specifically; the ITGA5 and ITGB1 transcripts are
subunits of α5 and β1. β1 binds to α1–α11 and αV to generate β1
integrins (13).
The 3 α subunits of β2 integrins were all
significantly upregulated with a statistically significant
difference, indicating that connections between β2 integrins and
their ligands were enhanced during the interaction between
leukocytes and vascular endothelial cells, a fact that suggests
that the adhesion of β2 integrins was enhanced. In the β1
integrins, only α5 and β1 were significantly upregulated with a
statistically significant difference. α5β1 was also expressed in
platelets, indicating that the upregulation of the subunits α5 and
β1 occurred in leukocytes and platelets at the same time.
Thus, we considered that the signal transduction of
β1 integrins was enhanced and leukocytes actively reinforced
adhesion to the vascular endothelial cells in PE patients. The αM
and αX mRNAs were significantly upregulated. αMβ2 was expressed in
monocytes, macrophages and neutrophils, while αXβ2 was expressed in
monocytes, NK cells, neutrophils and some lymphocytes. Caimi et
al reported that neutrophils were activated abnormally in VTE
(14,15). The genomics results were consistent
with the cytology results.
Expression profile of platelet-related
integrin mRNAs
There are 5 integrins expressed in platelets: αIIβ3
(GPIIb/IIIa), αVβ3, α2β1, α5β1 and α6β1. In our research, the 2
subunits of GPIIb/IIIa were both significantly upregulated. Most
GPIIb/IIIa copies are present on resting platelets, while another
small part is hidden in platelet α-granules. The GPIIb/IIIa copies
on α-granules become externalized on platelet secretion to increase
their surface expression by 25 to 50%, and expose their binding
sites for fibrinogens to become activated (16).
Expression profile of other integrin
mRNAs
In these 11 mRNAs, the ITGA11 and ITGA7 transcripts
(α11 and α7) bind to subunit β1 to generate α11β1 and α7β1; the
ITGB5 (β5) transcript binds to subunit αV to generate αVβ5. The
ligands of α11β1, α7β1 and αVβ5 are collagen, laminin and
fibronectin, respectively (17),
indicating that the signal transduction and the binding of
integrins to collagen, laminin and fibronectin are enhanced in PE
patients. In the 3 mRNA transcripts which were significantly
downregulated, the subunit α8 binds to β1 to generate α8β1; β6 and
β8 bind to αV to generate αVβ6 and αVβ8. Major ligands of α8β1,
αVβ6 and αVβ8 are all fibronectin.
In the PE group, most upregulated mRNAs of the
leukocyte-related integrin transcribed into β1 and β2 integrins;
60% of platelet-related integrin mRNAs were upregulated which
transcribed into β1 and β3 integrins, indicating that integrins
expressed in leukocytes and platelets play an important role in the
PE process, while the upregulation of β1 integrin mRNAs was related
to both leukocytes and platelets. Most transcripts of other
integrin mRNAs which were abnormally expressed bound to
fibronectin, indicating that fibronectin plays a role in the onset
of VTE.
Expression profile of selectin
mRNAs
P-selectin is stored in the a-granules of platelets
and the Weibel-Palade bodies of endothelial cells (18); E-selectin is expressed on the
surface of activated leukocytes specifically (19); L-selectin is expressed on the
surface of most leukocytes (20);
P-selectin glycoprotein ligand (PSGL-1) is expressed mainly on the
surface of leukocytes and platelets (21). PSGL-1 is a receptor of P-selectin
with high affinity that can bind both to L-selectin and E-selectin
to mediate interactions among leukocytes, platelets and endothelial
cells. In our research, L-selectin and PSGL-1 mRNAs were
significantly upregulated, indicating that leukocytes were
activated in PE patients; E-selectin mRNA was significantly
downregulated, indicating that the activation of endothelial cells
was obviously weakened, while no statistically significant
difference in P-selectin between the PE and the control group was
observed.
Expression profile of immunoglobulin
superfamily mRNAs
The expression trends of VCAM-1 mRNA and PECAM-1
mRNA in PE patients were similar to those in artery thrombosis
(22); no statistically
significant difference in the expression of MadCAM-1, ICAM-1,
NCAM-1 between the PE and the control group was observed,
indicating that the immunoglobulin superfamily did not play an
important role in PE.
Expression profile of cadherin
superfamily mRNAs
Expression profile of classic cadherin
mRNAs
Among the 13 mRNAs, 7 (53.84%) mRNAs were
downregulated statistically, of which 4 (30.77%) significantly.
Vascular endothelial cell cadherin (VE-cadherin) expressed on the
surface of endothelial cells is the major endothelial adhesion
molecule of the adherens junction, and negatively regulates the
transendothelial migration of leukocytes (23). During inflammation, leukocytes
weaken the negative regulatory effect of VE-cadherin by VE-cadherin
phosphorylation (24), as well as
the binding of VE-cadherin to endothial cells (25,26)
and cleave VE-cadherins (27) to
promote their transendothelial migration, enhance leukocyte
infiltration and cause inflammation. In this research, VE-cadherin
mRNA expression was significantly downregulated, indicating that
the adherens junction between vascular endothelial cells was
obviously weakened and that the permeability of the endothelium was
increased in PE patients.
Expression profile of protocadherin
mRNAs
A total of 19 (55.88%) mRNAs were significantly
downregulated among the 34 protocadherin mRNAs (p<0.05), of
which 9 (26.47) more significantly (p<0.01), indicating that
protocadherins were downregulated as a whole in VTE. The result was
similar to classic cadherins. Similar to VE-cadhrein, VE-cadherin 2
was also expressed on the adherens junction among endothelial
cells; however, in contrast to VE-cadherin, its mRNA expression was
upregulated in PE patients.
Expression profile of flamingo
cadherin mRNAs
Flamingo cadherins are a type of 7-pass
transmembrane protein (28). In
this research, the 3 mRNAs of this sub-family were all upregulated
in PE patients, but with no statistically significant
difference.
During the process of PE, leukocytes were
significantly activated with enhanced adhesion, the adhesion of
platelets was enhanced and the activation of vascular endothelial
cells and the adherens junction between endothelial cells were
weakened, with the endothelium becoming more permeable. During the
process of PE, leukocytes increased their ability to adhere and
were assisted by platelet adhesion. The vascular endothelial cell
adhesion, however, may be passive. The obvious weakness of the
adherens junction among endothelial cells provided the conditions
for the migration of inflammatory cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (No. 30570809) and the Key
Foundation of Science and Technology Commission of the Shanghai
Municipality (No. 05JC14038)
References
|
1
|
Spencer FA, Gore JM, Lessard D, Douketis
JD, Emery C and Goldberg RJ: Patient outcomes after deep vein
thrombosis and pulmonary embolism: the Worcester Venous
Thromboembolism Study. Arch Intern Med. 168:425–430. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Virchow RLK: Thrombosis and emboli.
Science History Publications; Massachusetts, MA: 1998
|
|
3
|
Geerts WH, Pineo GF, Heit JA, Bergqvist D,
Lassen MR, Colwell CW and Ray JG: Prevention of venous
thromboembolism: the Seventh ACCP Conference on Antithrombotic and
Thrombolytic Therapy. Chest. 126(Suppl 3): 338S–400S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geerts WH, Bergqvist D, Pineo GF, Heit JA,
Samama CM, Lassen MR and Colwell CW; American College of Chest
Physicians. Prevention of venous thromboembolism: American College
of Chest Physicians Evidence-Based Clinical Practice Guidelines
(8th Edition). Chest. 133(Suppl 6): 381S–453S. 2008. View Article : Google Scholar
|
|
5
|
Shackford SR, Rogers FB, Terrien CM,
Bouchard P, Ratliff J and Zubis R: A 10-year analysis of venous
thromboembolism on the surgical service: the effect of practice
guidelines for prophylaxis. Surgery. 144:3–11. 2008.PubMed/NCBI
|
|
6
|
Smeeth L, Cook C, Thomas S, Hall AJ,
Hubbard R and Vallance P: Risk of deep vein thrombosis and
pulmonary embolism after acute infection in a community setting.
Lancet. 367:1075–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiang-Hua Y, Le-Min W, Ai-Bin L, Zhu G,
Riquan L, Xu-You Z, Wei-Wei R and Ye-Nan W: Severe acute
respiratory syndrome and venous thromboembolism in multiple organs.
Am J Respir Crit Care Med. 182:436–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Gong Z, Liang A, Xie Y, Liu SL, Yu
Z, Wang L and Wang Y: Compromised T-cell immunity and virus-like
structure in a patient with pulmonary hypertension. Am J Respir
Crit Care Med. 182:434–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haoming S, Lemin W, Zhu G, Aibin L, Yuan
X, Wei L, Jinfa J, Wenjun X and Yuqin S: T cell-mediated immune
deficiency or compromise in patients with CTEPH. Am J Respir Crit
Care Med. 183:417–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Gong Z, Jiang J, Xu W, Duan Q, Liu
J and Qin C: Confusion of wide thrombolytic time window for acute
pulmonary embolism: mass spectrographic analysis for thrombus
proteins. Am J Respir Crit Care Med. 184:145–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prescott SM, Weyrich AS and Zimmerman GA:
Classification of venous thromboembolism (VTE). The clot is hot:
inflammation, myeloid leukocytes, and venous thromboembolism. J
Thromb Haemost. 3:2571–2573. 2005.PubMed/NCBI
|
|
12
|
Henke PK and Wakefield T: Thrombus
resolution and vein wall injury: dependence on chemokines and
leukocytes. Thromb Res. 123(Suppl 4): S72–S78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caimi G, Tozzi Ciancarelli MG, Ferrara F,
Montana M, Calandrino V, Canino B and Lo Presti R: Deep venous
thrombosis: behaviour of the polymorphonuclear leukocyte integrin
pattern at baseline and after in vitro activation. Clin Hemorheol
Microcirc. 33:11–17. 2005.PubMed/NCBI
|
|
15
|
Caimi G, Canino B, Ferrara F, Montana M
and Lo Presti R: Polymorphonuclear leukocyte integrins in deep
venous thrombosis. Clin Appl Thromb Hemost. 11:95–97. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quinn MJ, Byzova TV, Qin J, Topol EJ and
Plow EF: Integrin alphaIIbbeta3 and its antagonism. Arterioscler
Thromb Vasc Biol. 23:945–952. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Faralli JA, Schwinn MK, Gonzalez JM Jr,
Filla MS and Peters DM: Functional properties of fibronectin in the
trabecular meshwork. Exp Eye Res. 88:689–693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner DD: The Weibel-Palade body: the
storage granule for von Willebrand factor and P-selectin. Thromb
Haemost. 70:105–110. 1993.PubMed/NCBI
|
|
19
|
Bevilacqua MP, Stengelin S, Gimbrone MA Jr
and Seed B: Endothelial leukocyte adhesion molecule 1: An inducible
receptor for neutrophils related to complement regulatory proteins
and lectins. Science. 243:1160–1165. 1989. View Article : Google Scholar
|
|
20
|
Kansas GS: Selectins and their ligands:
Current concepts and controversies. Blood. 88:3259–3287.
1996.PubMed/NCBI
|
|
21
|
Wakefield TW, Myers DD and Henke PK:
Mechanisms of venous thrombosis and resolution. Arterioscler Thromb
Vasc Biol. 28:387–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong N and Chatterjee S: Platelet
endothelial cell adhesion molecule in cell signaling and
thrombosis. Mol Cell Biochem. 253:151–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muller WA: Mechanisms of transendothelial
migration of leukocytes. Circ Res. 105:223–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nottebaum AF, Cagna G, Winderlich M, Gamp
AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B,
Kamenyeva O, et al: VE-PTP maintains the endothelial barrier via
plakoglobin and becomes dissociated from VE-cadherin by leukocytes
and by VEGF. J Exp Med. 205:2929–2945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boggon TJ, Murray J, Chappuis-Flament S,
Wong E, Gumbiner BM and Shapiro L: C-cadherin ectodomain structure
and implications for cell adhesion mechanisms. Science.
296:1308–1313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hermant B, Bibert S, Concord E, Dublet B,
Weidenhaupt M, Vernet T and Gulino-Debrac D: Identification of
proteases involved in the proteolysis of vascular endothelium
cadherin during neutrophil transmigration. J Biol Chem.
278:14002–14012. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dejana E, Orsenigo F and Lampugnani MG:
The role of adherens junctions and VE-cadherin in the control of
vascular permeability. J Cell Sci. 121:2115–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shima Y, Kengaku M, Hirano T, Takeichi M
and Uemura T: Regulation of dendritic maintenance and growth by a
mammalian 7-pass transmembrane cadherin. Dev Cell. 7:205–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|