Introduction
Bone morphogenetic proteins (BMPs) are members of
the transforming growth factor-β (TGF-β) superfamily that play
important roles in the majority of morphogenetic processes during
development. BMPs are able to induce the formation of bone at
non-bony sites in adult animals by influencing the differentiation
of mesenchymal progenitor cells along the cartilage lineage pathway
(1,2). BMPs act not only on osteoblasts and
chondrocytes, but also on numerous other cell types, including
neuronal cells (3).
BMP-2 signaling involves two types of transmembrane
serine/threonine kinases; type I (BRI) and type II (BRII) receptors
(4–8). These two types of receptors are
required to form a functional complex to initiate further signaling
events. BRIs phosphorylate Smad1, Smad5 and Smad8 (R-Smads), which
then assemble into heteromeric complexes with Smad4 (Co-Smad), and
translocate into the nucleus to regulate the transcription of
target genes (9,10). In addition, BMP receptors initiate
other signaling pathways, distinct from the Smad pathway, resulting
in the activation of p38 MAPK and JNK (11–13).
It has been revealed that BMP-2 is able to inhibit
the proliferation of smooth muscle cells, primary glomerular
mesangial cells and adrenocortical tumor cells (14–16).
In breast cancer cell lines or tumor tissues, BMP-2 expression is
often decreased (17,18). Ghosh-Choudhury et al studied
the function of BMP2 and reported that BMP-2 led to G1 arrest by
inducing the expression of p21 to inhibit the proliferation of
breast cancer cells (19,20). However, a number of different lines
of evidence have shown that BMP-2 inhibits apoptosis (21,22)
rather than inhibiting the proliferation of breast cancer cells
(23). The effect of BMP-2 on
breast cancer is complicated and unclear, and therefore requires
further study.
In this study, BMP-2 significantly inhibited the
proliferation of the breast cancer cell lines MDA-MB-231 and MCF-7,
by promoting cell cycle G1 arrest and apoptosis, and also inhibited
the formation of cancer xenografts in nude mice in vivo.
Materials and methods
Cell culture and animals
Human breast cancer cell lines MDA-MB-231 and MCF-7
were obtained from the American Type Culture Collection (ATCC,
Manassas, VA, USA). MDA-MB-231 cells were maintained in Leiboviz’s
L15 medium and MCF-7 cells were maintained in DMEM medium, both
supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories, Logan, UT, USA) in humidified air at 37°C.
Anti-β-actin, anti-phospho-Smad1/5/8 and anti-cleaved-caspase-3
were obtained from Cell Signaling Technology (Beverly, MA, USA) and
anti-Smad1/5/8, HRP-goat anti-rabbit conjugate and HRP-goat
anti-mouse conjugate were from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). BALB/c nude mice (4–5 weeks) were obtained
from the Experimental Animal Research Centre of Zhongshan
University and were maintained in a specific pathogen-free
laboratory. All procedures involving animals were performed in
accordance with the institutional animal welfare guidelines of the
Experimental Animal Research Center of Sun Yat-Sen University.
Quantitative polymerase chain reaction
(qPCR)
Total cellular RNA was extracted from cell cultures
using a TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions, and resuspended
in 50 μl of water treated with 0.1% DEPC (Sigma-Aldrich, Seelze,
Germany). RNA concentration was determined using a BioPhotometer
(Eppendorf Scientific, Hamburg, Germany). Reverse transcription of
total RNA (2 μg) primed with an oligo(dT) oligonucleotide was
performed using a SuperScript™ III First-Strand Synthesis SuperMix
reverse transcription kit (Invitrogen) according to the
manufacturer’s instructions. qRT-PCR was performed using an IQ
SYBR-Green mix in an iCycler PCR machine (Bio-Rad, Hercules, CA,
USA). β-actin was used as the housekeeping control gene. The mean
value of 3 wells was calculated, and each experiment was repeated 3
times. The primers (Invitrogen, Shanghai, China) designed for the
respective genes are shown in Table
I.
 | Table IPrimers used for real-time PCR. |
Table I
Primers used for real-time PCR.
| Genes | Forward | Reverse |
|---|
| β-actin |
ATTGCCGACAGGATGCAGA |
GAGTACTTGCGCTCAGGAGGA |
| BMPR1b |
AATGCCACCATTGTCCA |
CTAGGCAACCAGAAGTGACCACAG |
| BMPR1a |
AGTGTCTCCAGTCAAGCTCTGGGTA |
CCATCTCTGCTGCTGCGCTCATTTA |
| BMPR2 |
TGCAGATGGACGCATGGAA |
CGGCAAGAGCTTACCCAGTCA |
| Smad4 |
CAGCACTACCACCTGGACTGGA |
CTGGAATGCAAGCTCATTGTGAA |
| p21 |
TGAGCCGCGACTGTGATG |
GTCTCGGTGACAAAGTCGAAGTT |
Western blot analysis
To determine the effect of BMP-2 on the activation
of Smad1/5/8, MDA-MB-231 and MCF-7 cells were starved in serum-free
medium overnight to knock down the endogenous level of
phosphorylated kinases. Cells were pretreated with 20 μg/l BMP-2
for 15 min, and then solubilized by incubation for 15 min at 4°C in
cell lysis solution (Upstate Biotechnology, Inc., Waltham, MA, USA)
containing a protease inhibitor cocktail (Roche). The protein
concentration of the soluble extracts was determined using a BCA
protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
Separation of 50 μg of total protein was performed on 12%
SDS-polyacrylamide gels and was transferred to a polyvinylidene
difluoride membrane before immunoblotting with primary antibodies.
Equal loading of protein samples was verified with antibodies
(dilution, 1:3000) against Smad1/5/8, pi-Smad1/5/8 and β-actin. The
specific proteins were detected using an enhanced chemiluminescence
detection system (Thermo Fisher Scientific).
MTT assay
MDA-MB-231 and MCF-7 cells were used to detect the
antitumor effects of BMP-2. Cells were cultured in 96-well plates
(~5,000 cells per well) for 24 h. Cells were then starved with
their respective medium plus 1% dialyzed fetal calf serum (A15-107;
PAA Laboratories, Linz, Austria) for 24 h. The experiment included
a control group and BMP-2 groups (2.5, 5, 10, 20 or 30 μg/l). After
48 h of BMP-2 induction, cell growth was measured using an MTT
assay. Absorbance was recorded at 570 nm, with a reference at 630
nm serving as the blank. The effect of BMP-2 on cell viability was
assessed as the percentage of cell viability compared with the
control cells, which were arbitrarily assigned 100% viability. The
mean value of 5 wells was calculated, and each experiment was
repeated 3 times.
Flow cytometry
Human breast cancer cell lines MDA-MB-231 and MCF-7
in the logarithmic phase of growth were incubated with 20 μg/l
BMP-2 for 24 h. For cell cycle analysis, samples (1×106
cells) were fixed and permeabilized by the addition of 1 ml of
ice-cold 70% ethanol for 15 min on ice. Following washing, the
cells were resuspended in 125 μl of 1.12% (w/v) sodium citrate
containing 0.2 mg/ml RNase (Roche) and incubated for 15 min at
37°C. Propidium iodide (PI; 50 mg/ml; Sigma-Aldrich, St. Louis, MO,
USA) was added to the cells for 30 min at room temperature in the
dark. The cells were then stored at 4°C until they were assayed by
flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). Cell cycle analysis was performed using ModFit LT software
(Verity Software House, Inc., Topsham, ME, USA).
Quantification of apoptotic cells
Apoptosis induced by BMP-2 in MDA-MB-231 and MCF-7
cells was determined by flow cytometry using the Annexin
V-conjugated Alexa Fluor 488 (Alexa488) Apoptosis Detection kit
(Millipore), according to the manufacturer’s instructions.
Following overnight serum starvation, cells were treated with BMP-2
(20 μg/l) for 48 h. The cells were then collected, washed in PBS
and incubated with Alexa488 and PI for cell staining in the dark at
room temperature for 10 min. The stained cells were analyzed by
FACS using a FACSCalibur instrument (BD Biosciences) equipped with
CellQuest 3.3 software.
Nude mice xenograft model of breast
cancer cell lines
BALB/c 4- to-6-week-old female mice, weighing 18–22
g, were randomly divided into 4 groups. Two groups were
intravenously injected with 1×107 MDA-MB-231 cells, and
the other two groups were intravenously injected with
1×107 MCF-7 cells. The mice (n=6 per group) were treated
with either PBS (vehicle) or 20 μg/l BMP-2 via the tail vein daily
for 14 days. After the animals were sacrificed, the tumors were
weighed.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for Social Sciences 13.0 (SPSS). Data were
presented as the mean ± SEM. The Student’s t-test (two-tailed) was
used to compare the two groups for independent samples assuming
equal variances among all experimental data sets. P<0.05 was
considered to indicate a statistically significant difference.
Results
BMP-2 signaling pathway assay in
MDA-MB-231 and MCF-7 cells
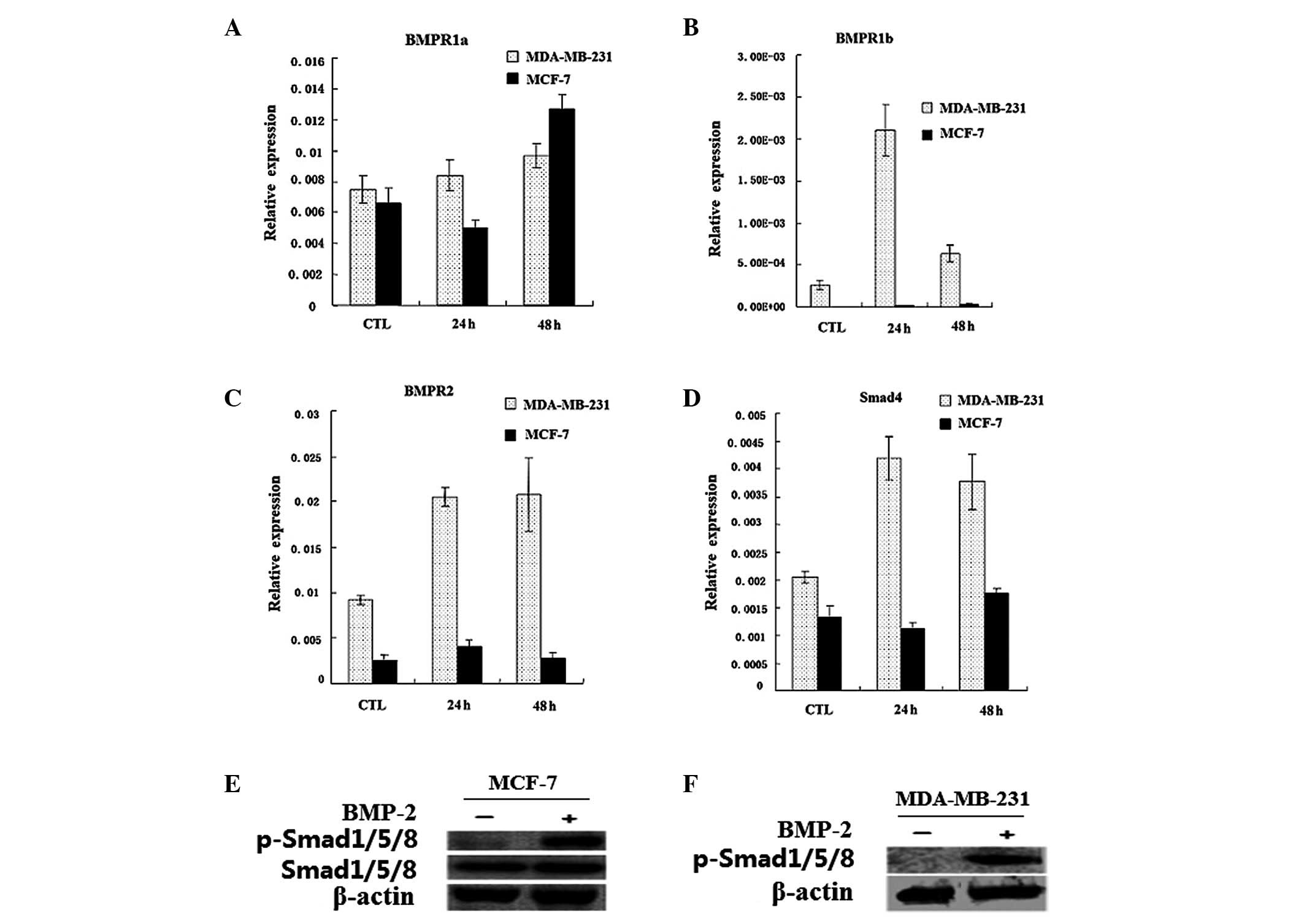
The mRNA expression levels of BMPR1a, BMPR1b, BMPR2
and Smad4 in MDA-MB-231 and MCF-7 breast cancer cell lines are
shown in Fig. 1A-D. The expression
of the genes increased to varying degrees in the MDA-MB-231 cells
when induced by BMP-2 for 24 or 48 h. However, the level of the
genes was lower in the MCF-7 cells; only BMPR1a was distinctly
upregulated by BMP-2 following 48 h.
Results of the western blot analysis showed that 20
μg/l BMP-2 is able to activate the BMP signaling pathway, which led
to the rapid phosphorylation of Smad1/5/8 (Fig. 1E-F). This finding indicated that
the BMP signaling pathway remained intact in the MDA-MB-231 and
MCF-7 cells.
Inhibitory effect of BMP-2 on the
proliferation of breast cancer cells
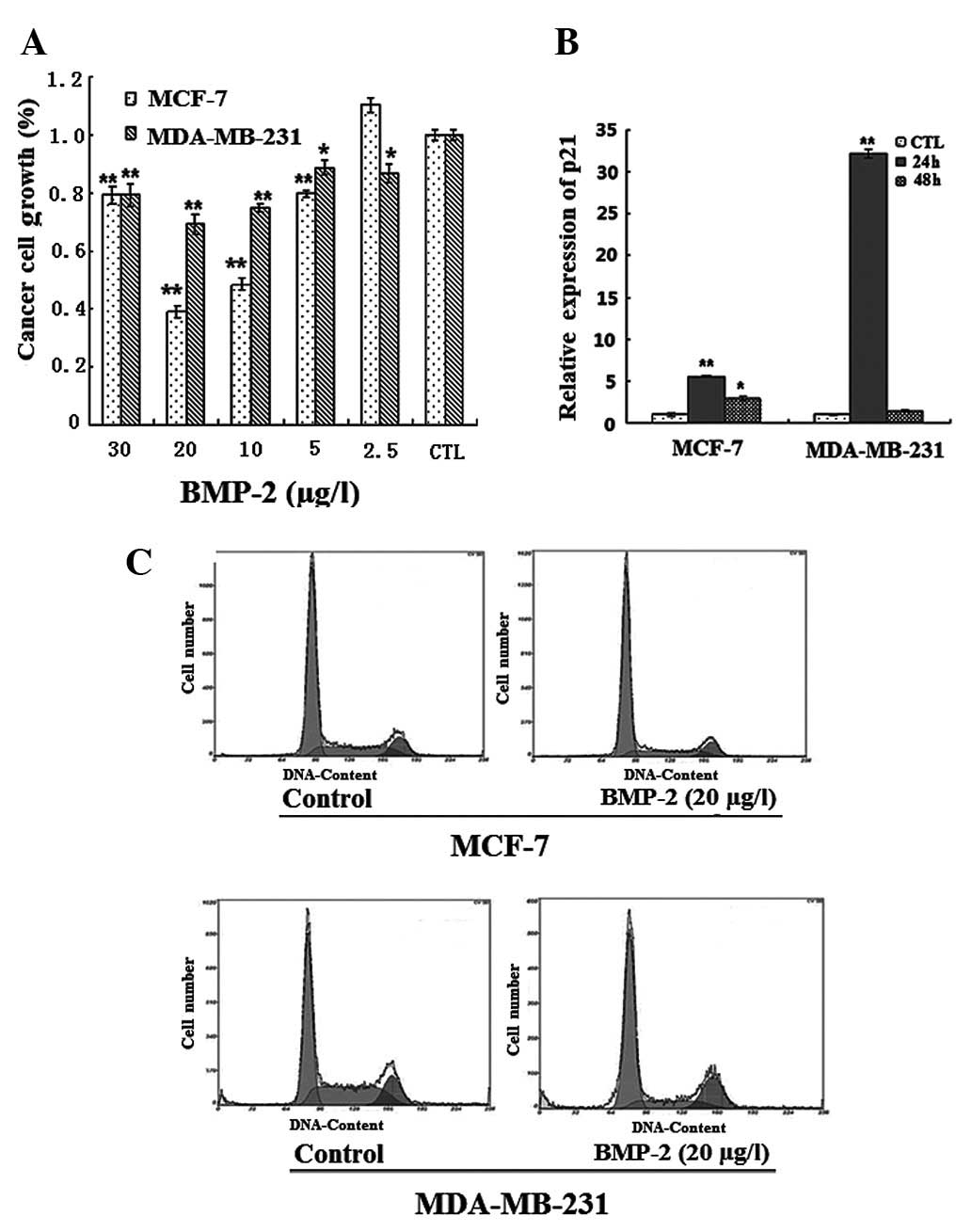
Cells were induced with 2.5, 5, 10, 20 or 30 μg/l
BMP-2 for two days. Results of the MTT assay indicated that the
strongest inhibition of BMP-2 on the proliferation of MDA-MB-231
and MCF-7 cells was 30 and 60%, respectively, following the
addition of 20 μg/l BMP-2 (Fig.
2A). This finding may be due to the more rapid growth rate of
MDA-MB-231 cells compared to MCF-7 cells.
Effect of BMP-2 on the breast cancer cell
cycle
The flow cytometric analysis demonstrated that cells
treated with 20 μg/l BMP-2 for two days remained in the G1 phase
from 46.7 to 62% in the MDA-MB-231 cells and 63.7 to 70.8% in the
MCF-7 cells, respectively. This finding indicated that BMP-2
effectively inhibited cell proliferation by initating G1 arrest
(Fig. 2C). BMP-2 was also able to
induce a rapid expression of p21 (Fig.
2B), which was in accordance with results by Ghosh-Choudhury
et al which revealed that the increase in p21 led to G1
arrest (19,20).
Pro-apoptotic function of BMP-2 on breast
cancer cells
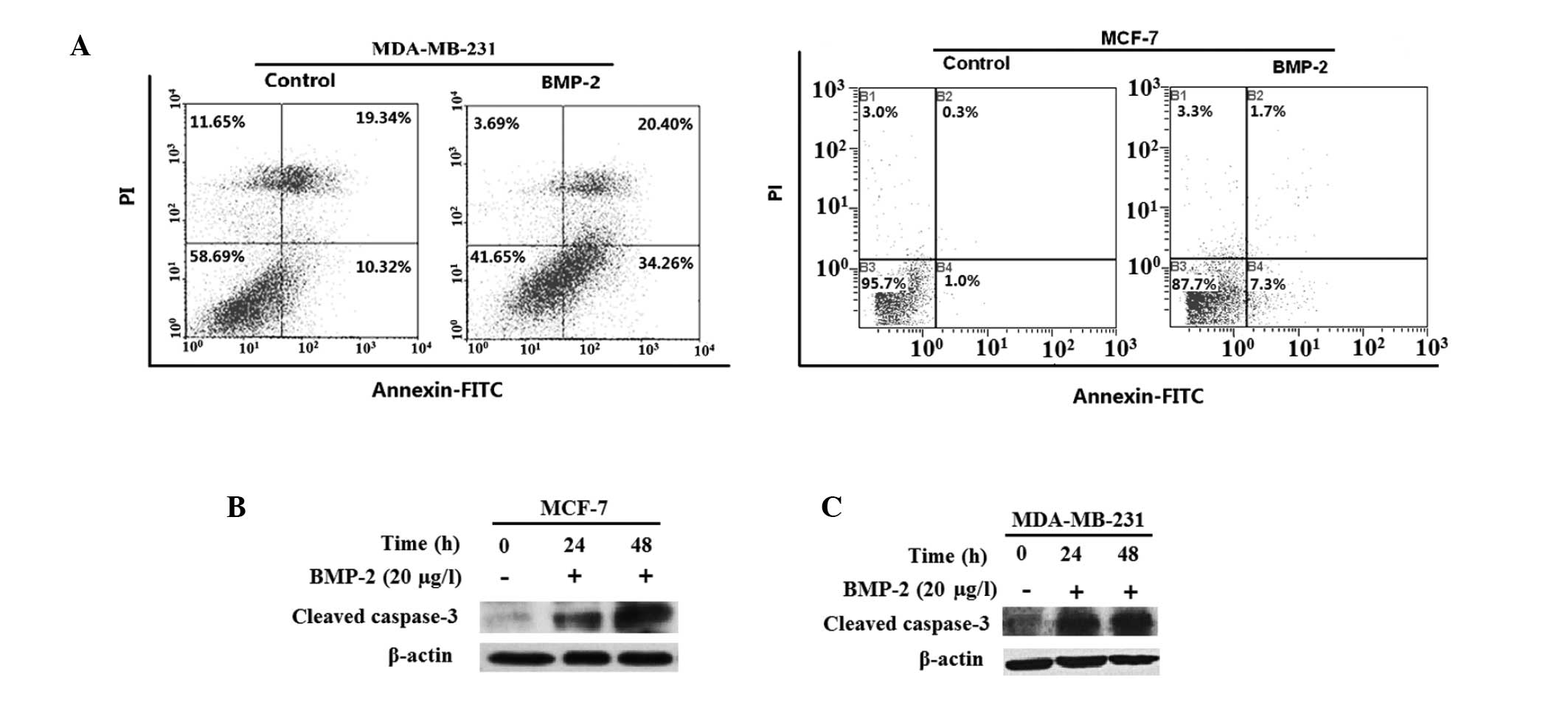
In MDA-MB-231 and MCF-7 cells, BMP-2 was able to
promote apoptosis following the addition of 20 μg/l BMP-2 for two
days. The early apoptotic cells increased from 10.3 to 34.3% in
MDA-MB-231 cells (Fig. 3A) and
from 1.0 to 7.3% in MCF-7 cells. In addition, there were ~20% of
necrotic cells in the MDA-MB-231 cells. We also observed that the
expression of cleaved caspase-3 in the two cell lines was
upregulated (Fig. 3B and C).
Inhibitory effect of BMP-2 on the
tumorigenesis of MDA-MB-231 and MCF-7 breast cancer cells in the
Balb/c (nu/nu) nude mice xenograft model
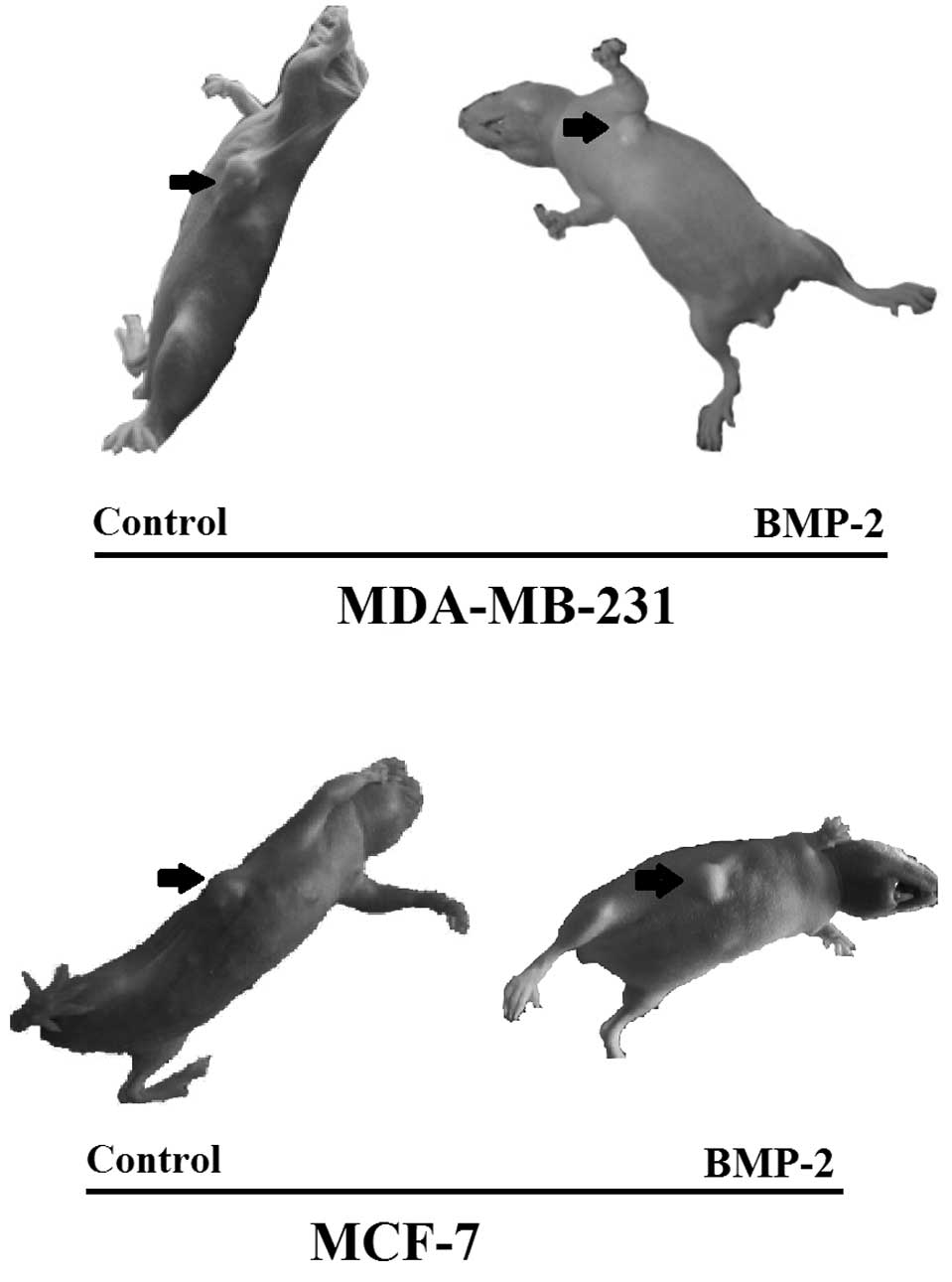
Balb/c (nu/nu) nude mice were administered with
breast cancer cells and xenograft tumors were allowed to form for
14 days. In the MDA-MB-231 group, subsequent tumor analysis
demonstrated that 5/6 mice in the control subgroup generated
tumors, while only 1/6 mice in the BMP-2 treatment subgroup
generated tumors (Table II). In
the remaining 5/6 mice, the cancer cells transplanted were necrotic
and pulp-like (Fig. 4). We also
found in the MCF-7 group that the mean tumor weights of the control
subgroup and BMP-2 treatment subgroup were 0.4690 and 0.1793 g,
respectively, demonstrating that BMP-2 was also an inhibitor of
tumor formation in MCF-7 cells.
 | Table IIBMP-2 inhibits tumor formation in the
MDA-MB-231 and MCF-7 breast cancer cells in nude mice. |
Table II
BMP-2 inhibits tumor formation in the
MDA-MB-231 and MCF-7 breast cancer cells in nude mice.
| Group | Tumor weight (g) | Mean (g) | P-value |
|---|
| MDA-MB-231 |
| Control | 0.3126 | 0.2755 | 0.539 | 0.2963 | 0.3252 | 0.2422 | 0.3318 | 0.004 |
| BMP-2 | - | - | - | - | - | 0.3602 | 0.0600 | |
| MCF-7 |
| Control | 0.8612 | 0.4201 | 0.3815 | 0.2831 | 0.6200 | 0.2480 | 0.4690 | 0.026 |
| BMP-2 | 0.0814 | 0.2292 | 0.3655 | 0.1021 | 0.2974 | - | 0.1793 | |
Thus, BMP-2 significantly inhibited the formation of
tumors in MDA-MB-231 and MCF-7 cells in nude mice (Fig. 4).
Discussion
As previously reported, BMPs described as either
growth stimulators or growth inhibitors appear to depend on dosage,
type of cells or tissue and tumor microenvironment (24,25).
In this study, BMP-2 was found to inhibit cancer cell growth in
vitro and in vivo by inducing G1 arrest and apoptosis in
MDA-MB-231 and MCF-7 human breast cancer cell lines. The inhibitory
effect on cell growth appears to be due to the G1 phase arrest
caused by BMP-2-induced p21 upregulation. p21 is able to bind to
cyclin E/CDK2 and inhibit CDK2 activity, thereby preventing cells
progressing from G1 to S phase (26). Furthermore, the observed increase
in cell apoptosis may be correlated with the upregulation of the
caspase signaling pathway by BMP-2.
In this study, the expression of BMPRs and Smad4 in
MDA-MB-231 cells was higher than that in MCF-7 cells. Furthermore,
there was evident auto-enhancement of BMP signaling following the
induction of BMP-2 in MDA-MB-231 cells. These results may explain
the reason for the inhibitory effect of BMP-2 on tumor formation in
MDA-MB-231 cells being stronger than that in MCF-7 cells in
vivo.
To the best of our knowledge, this is the first
study demonstrating that BMP-2 was able to inhibit MDA-MB-231 and
MCF-7 tumor formation in nude mice. Although certain lines of
evidence have demonstrated that BMP-2 promotes the metastasis of
breast cancer (23,27–28),
such an event was not observed in this study. It is worth
mentioning that in this study the formation of breast tumors was
terminated after two weeks, which may have been an insufficient
period of time to observe the metastasis of breast xenograft
tumors.
In conclusion, BMP-2 was an inhibitor of early tumor
development through the induction of G1 arrest and apoptosis.
Acknowledgements
This study was funded by the Ministry of Science and
Technology of China (2009ZX09103-632).
References
|
1
|
Wozney JM, Rosen V, Celeste AJ, et al:
Novel regulators of bone formation: molecular clones and
activities. Science. 242:1528–1534. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hogan BL: Bone morphogenetic proteins:
multifunctional regulators of vertebrate development. Genes Dev.
10:1580–1594. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reddi AH: Role of morphogenetic proteins
in skeletal tissue engineering and regeneration. Nat Biotechnol.
16:247–252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoodless PA, Haerry T, Abdollah S, et al:
MADR1, a MAD-related protein that functions in BMP2 signaling
pathways. Cell. 85:489–500. 1996. View Article : Google Scholar
|
|
5
|
Nohe A, Hassel S, Ehrlich M, et al: The
mode of bone morphogenetic protein (BMP) receptor oligomerization
determines different BMP-2 signaling pathways. J Biol Chem.
277:5330–5338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Ventura F, Doody J and Massague J:
Human type II receptor for bone morphogenic proteins (BMPs):
extension of the two-kinase receptor model to the BMPs. Mol Cell
Biol. 15:3479–3486. 1995.PubMed/NCBI
|
|
7
|
Nohno T, Ishikawa T, Saito T, et al:
Identification of a human type II receptor for bone morphogenetic
protein-4 that forms differential heteromeric complexes with bone
morphogenetic protein type I receptors. J Biol Chem.
270:22522–22526. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenzweig BL, Imamura T, Okadome T, et
al: Cloning and characterization of a human type II receptor for
bone morphogenetic proteins. Proc Natl Acad Sci USA. 92:7632–7636.
1995. View Article : Google Scholar
|
|
9
|
Massague J and Chen YG: Controlling
TGF-beta signaling. Genes Dev. 14:627–644. 2000.
|
|
10
|
Attisano L and Wrana JL: Smads as
transcriptional co-modulators. Curr Opin Cell Biol. 12:235–243.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimura N, Matsuo R, Shibuya H, Nakashima K
and Taga T: BMP2-induced apoptosis is mediated by activation of the
TAK1-p38 kinase pathway that is negatively regulated by Smad6. J
Biol Chem. 275:17647–17652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee KS, Hong SH and Bae SC: Both the Smad
and p38 MAPK pathways play a crucial role in Runx2 expression
following induction by transforming growth factor-beta and bone
morphogenetic protein. Oncogene. 21:7156–7163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guicheux J, Lemonnier J, Ghayor C, Suzuki
A, Palmer G and Caverzasio J: Activation of p38 mitogen-activated
protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their
implication in the stimulation of osteoblastic cell
differentiation. J Bone Miner Res. 18:2060–2068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnsen IK, Kappler R, Auernhammer CJ and
Beuschlein F: Bone morphogenetic proteins 2 and 5 are
down-regulated in adrenocortical carcinoma and modulate adrenal
cell proliferation and steroidogenesis. Cancer Res. 69:5784–5792.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh Choudhury G, Kim YS, Simon M, et al:
Bone morphogenetic protein 2 inhibits platelet-derived growth
factor-induced c-fos gene transcription and DNA synthesis in
mesangial cells. Involvement of mitogen-activated protein kinase. J
Biol Chem. 274:10897–10902. 1999.
|
|
16
|
Wong GA, Tang V, El-Sabeawy F and Weiss
RH: BMP-2 inhibits proliferation of human aortic smooth muscle
cells via p21Cip1/Waf1. Am J Physiol Endocrinol Metab.
284:E972–E979. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reinholz MM, Iturria SJ, Ingle JN and
Roche PC: Differential gene expression of TGF-beta family members
and osteopontin in breast tumor tissue: analysis by real-time
quantitative PCR. Breast Cancer Res Treat. 74:255–269. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alarmo EL and Kallioniemi A: Bone
morphogenetic proteins in breast cancer: dual role in
tumourigenesis? Endocr Relat Cancer. 17:R123–R139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghosh-Choudhury N, Ghosh-Choudhury G,
Celeste A, et al: Bone morphogenetic protein-2 induces cyclin
kinase inhibitor p21 and hypophosphorylation of retinoblastoma
protein in estradiol-treated MCF-7 human breast cancer cells.
Biochim Biophys Acta. 1497:186–196. 2000. View Article : Google Scholar
|
|
20
|
Ghosh-Choudhury N, Woodruff K, Qi W,
Celeste A, Abboud SL and Ghosh Choudhury G: Bone morphogenetic
protein-2 blocks MDA MB 231 human breast cancer cell proliferation
by inhibiting cyclin-dependent kinase-mediated retinoblastoma
protein phosphorylation. Biochem Biophys Res Commun. 272:705–711.
2000. View Article : Google Scholar
|
|
21
|
Raida M, Clement JH, Ameri K, Han C, Leek
RD and Harris AL: Expression of bone morphogenetic protein 2 in
breast cancer cells inhibits hypoxic cell death. Int J Oncol.
26:1465–1470. 2005.PubMed/NCBI
|
|
22
|
Steinert S, Kroll TC, Taubert I, et al:
Differential expression of cancer-related genes by single and
permanent exposure to bone morphogenetic protein 2. J Cancer Res
Clin Oncol. 134:1237–1245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pouliot F, Blais A and Labrie C:
Overexpression of a dominant negative type II bone morphogenetic
protein receptor inhibits the growth of human breast cancer cells.
Cancer Res. 63:277–281. 2003.PubMed/NCBI
|
|
24
|
Alarmo EL, Parssinen J, Ketolainen JM,
Savinainen K, Karhu R and Kallioniemi A: BMP7 influences
proliferation, migration, and invasion of breast cancer cells.
Cancer Lett. 275:35–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim M and Choe S: BMPs and their clinical
potentials. BMB Rep. 44:619–634. 2011. View Article : Google Scholar
|
|
26
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fong S, Itahana Y, Sumida T, et al: Id-1
as a molecular target in therapy for breast cancer cell invasion
and metastasis. Proc Natl Acad Sci USA. 100:13543–13548. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clement JH, Marr N, Meissner A, et al:
Bone morphogenetic protein 2 (BMP-2) induces sequential changes of
Id gene expression in the breast cancer cell line MCF-7. J Cancer
Res Clin Oncol. 126:271–279. 2000. View Article : Google Scholar : PubMed/NCBI
|