Introduction
In 2008, the number of new cases of pancreatic
cancer in developed countries was ranked ninth worldwide in males
and females, however, the estimated number of mortalities was
ranked fourth and fifth worldwide in females and males,
respectively (1). Pancreatic
cancer remains a challenging disease worldwide. In 2012, the
mortality of pancreatic cancer continues to increase. Pancreatic
cancer is the fourth leading cause of cancer mortality in the USA
(2). These statistics indicate
that current chemotherapeutic medicines are unsatisfactory and
highlight the requirement for identification of new treatments.
Traditional herbs are widely accepted as a valid method of
treatment of various forms of human cancer and a considerable
effort to develop alternative medicines is currently underway
(3). Tanshinone IIA (Tan-IIA;
C19H18O3) is one of the active
constituents of Danshen (4,5).
Tan-IIA is toxic to numerous human cancer cells, including Colo205
colon cancer (6), MDA-MB-231
breast cancer (7), A-549 non-small
cell lung cancer (8), H-146 small
cell lung cancer (9) and Hep-J5
hepatocellular carcinoma cells (10). Previously, it was reported that
Tan-IIA has cytotoxic effects in MIAPaCa-2 human pancreatic tumor
cell lines as the half-maximal inhibitory concentration
(IC50) was calculated as 1.9 μM (11). However, the mechanism has not been
established. In the present study, the efficacy and molecular
mechanisms of Tan-IIA in human pancreatic cancer BxPC-3 cells was
investigated.
Materials and methods
Chemicals and reagents
Tan-IIA was purchased from Sigma-Aldrich (no.
568-72-9; St. Louis, MO, USA). The BxPC-3 human pancreatic cancer
cell line (BCRC no. 60283) was obtained from the Food Industry
Research and Development Institute (Hsinchu, Taiwan).
3-(4,5-Dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT),
sodium deoxycholate, leupeptin, Triton X-100, Tris-HCl,
ribonuclease-A, sodium orthovanadate, sodium pyruvate, HEPES,
RPMI-1640 medium, trypsin-EDTA, mouse anti-β-actin and
penicillin-streptomycin were obtained from Sigma-Aldrich. Dimethyl
sulfoxide (DMSO), potassium phosphates and TE buffer were purchased
from Merck Co. (Darmstadt, Germany). Fetal bovine serum (FBS) and
glutamine were obtained from Gibco-BRL (Grand Island, NY, USA).
Buffer (10X TG-SDS), Tween-20 and glycine were obtained from
Amresco LLC (St. Louis, MO, USA). BioMax film was obtained from
Kodak (Rochester, NY, USA). Antibodies against Bax (#2774), Bcl-xL
(#2764), Bcl-2 (#2872), MCL-1 (#2764) and TCTP (#2764) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Other materials and reagents not specified were obtained from
Sigma-Aldrich or Merck Co.
Cell culture
BxPC-3 cells were maintained in RPMI-1640 medium
containing 10% FBS, 1% penicillin-streptomycin (10,000 U/ml
penicillin and 10 mg/ml streptomycin) at 37°C in a humidified
atmosphere containing 5% CO2.
Cytotoxicity assay
Cells were plated in 96-well plates at a density of
1×104 cells/well for 16 h. Following this, the cells
were treated with various concentrations of Tan-IIA for 24 and 48
h. Following this, cells were incubated with 1 mg/ml MTT in fresh
complete RPMI-1640 medium for 2 h. The surviving cells converted
MTT to formazan by forming a blue-purple color when dissolved in
DMSO. The intensity of formazan was measured at 590 nm using a
microplate reader. The relative percentage of cell viability was
calculated by dividing the absorbance of treated cells by that of
the control in each experiment.
Cell cycle analysis
Cell cycle progression following treatment with
Tan-IIA was measured by flow cytometry. The cells were plated at a
density of 1×106 cells/6-cm dish in complete medium for
16 h. Following treatment, the cells were collected and fixed with
ice-cold 70% ethanol overnight at -20°C. Cells were centrifuged and
the cell pellets were treated with 4 μg/ml PI solutions containing
100 μg/ml RNase at 37°C for 30 min. Subsequently, samples were
analyzed in a Cytomics™ FC500 Flow Cytometer (Beckman Coulter,
Miami, FL, USA). A minimum of 10,000 cells were analyzed for DNA
content and the percentage of cell cycle phases was quantified.
Western blot analysis
Following drug treatment, cells were lysed in
ice-cold whole cell extract buffer containing protease inhibitors.
The lysate was agitated for 30 min at 4°C and centrifuged at 10,000
rpm for 10 min. Protein concentration was measured using a BCA
protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of
protein was subjected to electrophoresis using 12% sodium dodecyl
sulfate-polyacrylamide gels. To verify equal protein loading and
transfer, proteins were then transferred to polyvinylidene
difluoride membranes and the membranes were blocked overnight at
4°C using blocking buffer [5% non-fat dried milk in solution
containing 50 mM Tris/HCl (pH 8.0), 2 mM CaCl2, 80 mM
sodium chloride, 0.05% Tween-20 and 0.02% sodium azide]. Membranes
were then incubated for 2 h at 25°C with specific primary
antibodies followed by anti-rabbit or anti-mouse immunoglobulin G
horseradish peroxidase-conjugated secondary antibodies. The
membranes were washed three times for 10 min with washing solution.
Finally, the protein bands were visualized on the X-ray film using
an enhanced chemiluminescence detection system (Perkin-Elmer,
Waltham, MA, USA).
Statistical analysis
Values are presented as the mean ± SD. The Student's
t-test was used to analyze statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
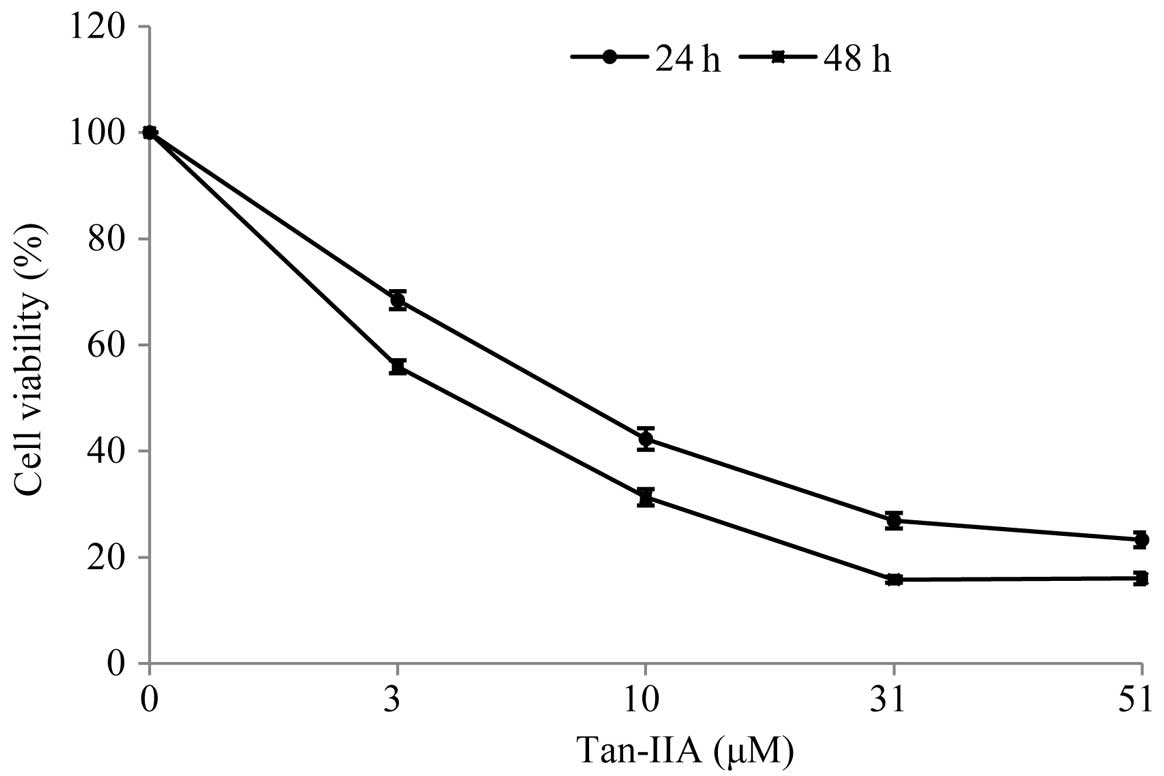
Cytotoxicity of Tan-IIA in BxPC-3
cells
When cultured with various concentrations of Tan-IIA
(0, 3, 10, 31 and 51 μM) for 24 and 48 h, the viable cell
percentages relative to the control were 68.41±1.69, 42.3±2.02,
26.91±1.47 and 23.31±1.40% for 24 h and 55.90±1.20, 31.32±1.54,
15.83±0.56 and 16.04±1.09% for 48 h, respectively. During Tan-IIA
treatment for 24 and 48 h, the half-maximum inhibitory
concentration (IC50) was 8.5 and 4.0 μM, respectively.
The results revealed that Tan-IIA inhibits the proliferation of
human pancreatic cancer BxPC-3 cells in a time- and dose-dependent
manner (Fig. 1).
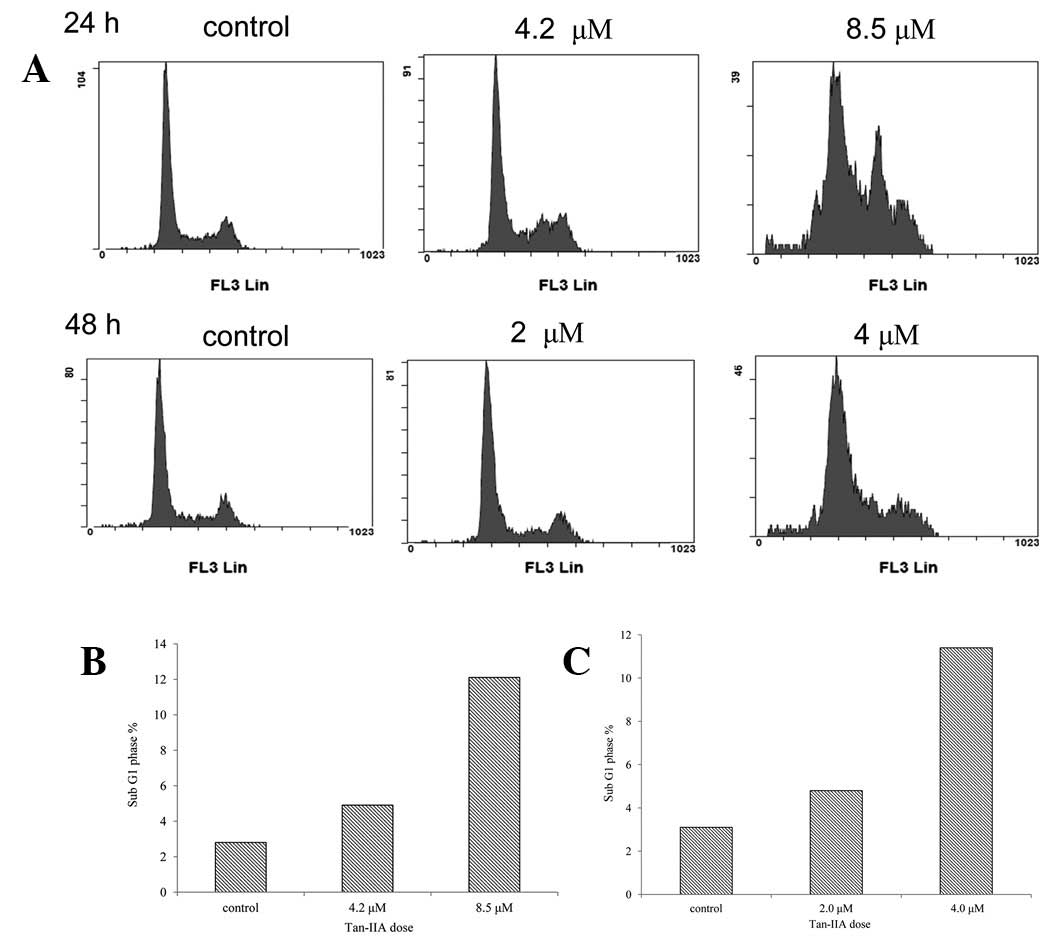
Tan-IIA induced apoptosis in BxPC-3
cells
BxPC-3 cells were plated in 6-cm dishes at a density
of 1×106 cells and treated with Tan-IIA (0, 4.2 and 8.5
μM) for 24 h. Cell cycle analysis was performed by FACS (Fig. 2A). Results indicate that the
percentages of sub-G1 cells were 2.8, 4.9 and 12.1,
respectively (Fig. 2B). The BxPC-3
cells were plated in 6-cm dishes at a density of 1×106
cells and then were treated with Tan-IIA (0, 2 and 4 μM) for 48 h.
Percentages of sub-G1 cells were 3.1, 4.8 and 11.4%,
respectively (Fig. 2C). These
results demonstrate that Tan-IIA induces apoptosis in a time- and
dose-dependent manner.
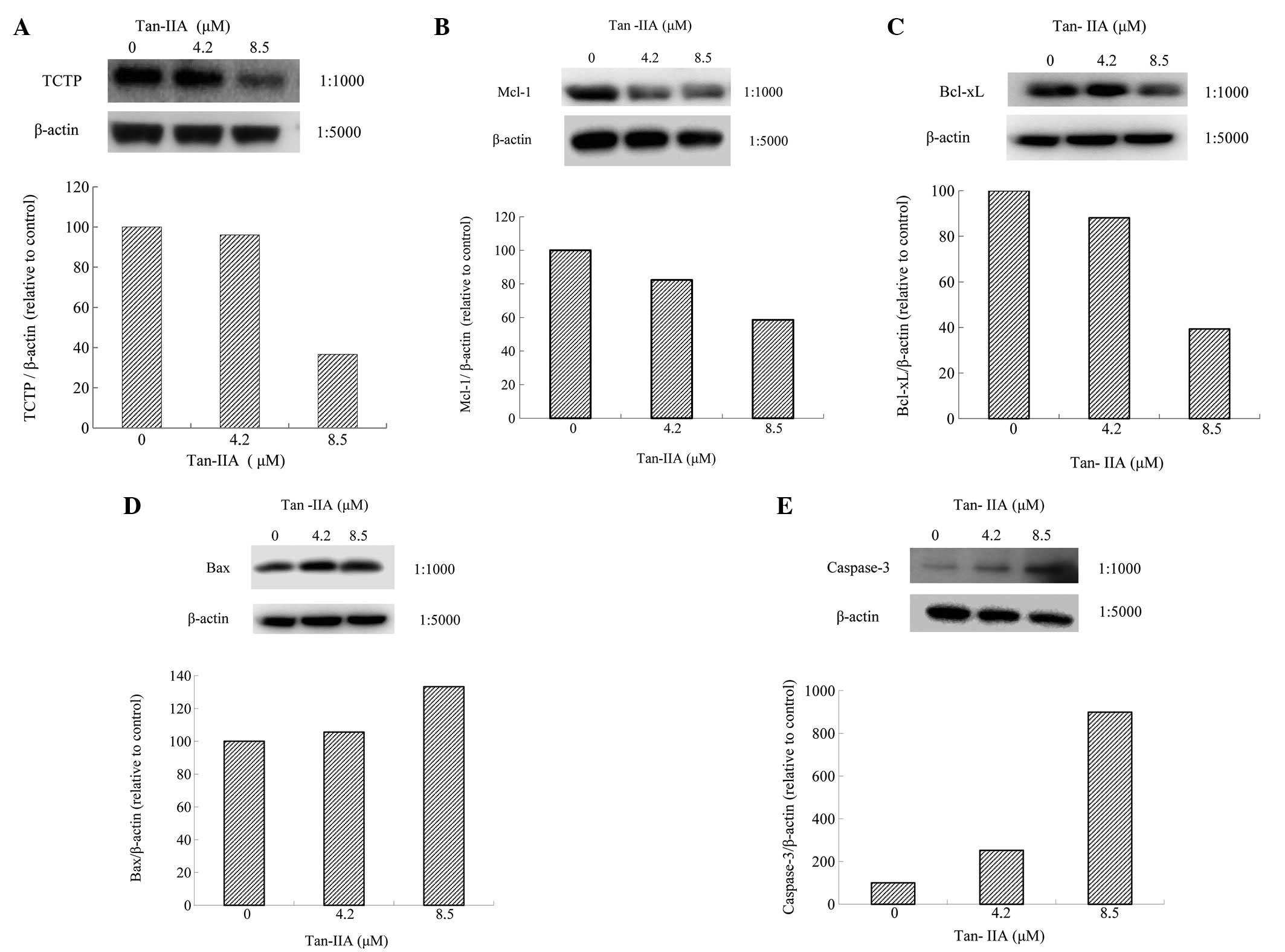
Effect of Tan-IIA on protein expression
of TCTP, MCL-1, Bcl-xl, Bax and Caspase-3 in BxPC-3 cells
BxPC-3 cells were treated with various
concentrations (0, 4.2 and 8.5 μM) of Tan-IIA for 24 h and the
protein expression levels were evaluated by western blot analysis.
The results revealed that Tan-IIA decreased expression of TCTP
(Fig. 3A), MCL-1 (Fig. 3B) and Bcl-xl (Fig. 3C) and increased Bax (Fig. 3D) and Caspase-3 expression
(Fig. 3E).
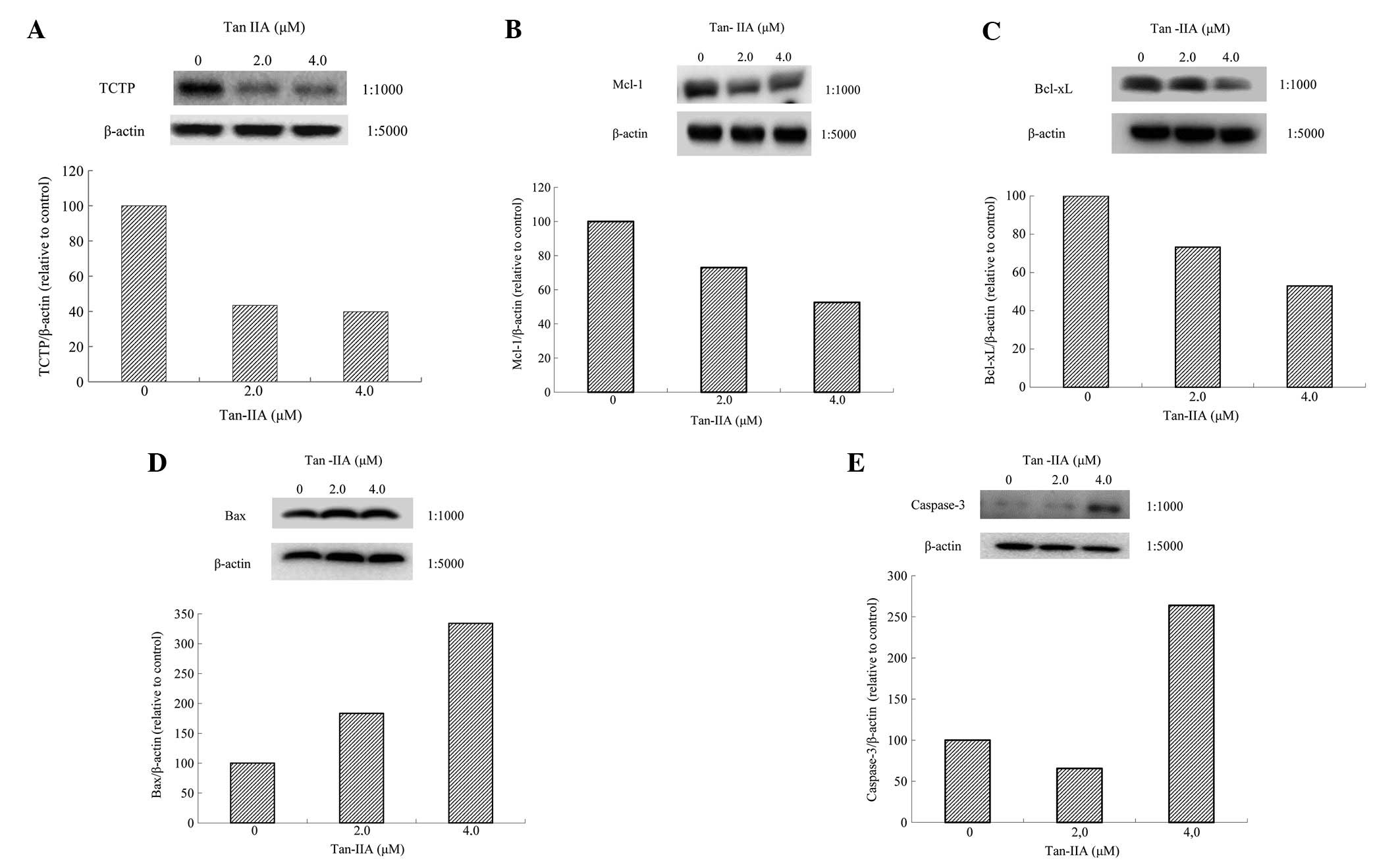
BxPC-3 cells were treated with various
concentrations (0, 2.0 and 4.0 μM) of Tan-IIA for 48 h and protein
expression was evaluated by western blot analysis. Tan-IIA
decreased expression of TCTP (Fig.
4A), MCL-1 (Fig. 4B) and
Bcl-xl (Fig. 4C) and increased Bax
(Fig. 4D) and Caspase-3 expression
(Fig. 4E).
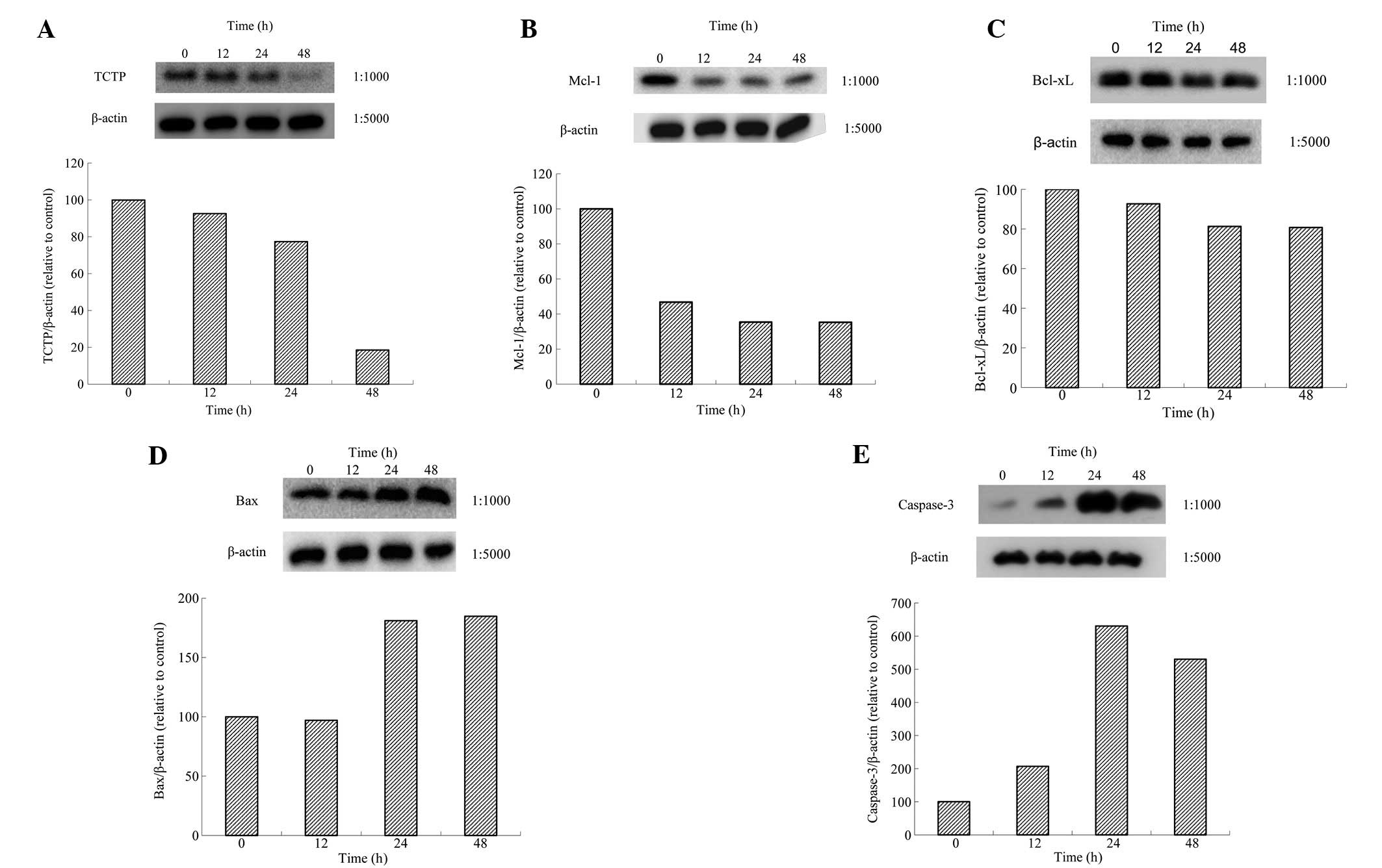
BxPC-3 cells were treated with Tan-IIA (8.5 μM) for
various durations (0, 24, 48 and 72 h) and protein expression
levels were evaluated by western blot analysis. Tan-IIA decreased
expression of TCTP (Fig. 5A),
MCL-1 (Fig. 5B) and Bcl-xl
(Fig. 5C) and increased Bax
(Fig. 5D) and Caspase-3 expression
(Fig. 5E). Results demonstrate
that Tan-IIA treatment of BxPC-3 cells inhibited TCTP, Bcl-xl and
MCL-1 expression.
TCTP is a 18-23-kDa hydrophilic protein, identified
over 30 years ago in Ehrlich acites tumor cells (12-14).
Overexpression of TCTP inhibits apoptosis and previous studies
using antisense and siRNA knockdown identified increased apoptosis
following knockdown of TCTP (15-17).
It is well known that TCTP binds MCL-1 (16,18,19)
and Bcl-xL (20) to inhibit
apoptosis. In addition, the anti-apoptotic mechanism of TCTP has
also been associated with antagonization of Bax (21). Tan-IIA also downregulates
expression of the mitochondrial protective Bcl-2 family memeber
MCL-1, inducing apoptosis in prostate cancer cells (22). These observations indicate that
Tan-IIA inhibits protein expression of TCTP, MCL-1 and Bcl-xl to
destroy mitochondrial function and increase Bax and Caspase-3
expression, inducing apoptosis in human pancreatic cancer BxPC-3
cells in vitro. The current study is the first to
demonstrate inhibition of BxPC-3 cells by Tan-II through
downregulation of TCTP, Bcl-xl and MCL-1 expression. The
chemotherapeutic potential of Tan-IIA in human pancreatic cancer
requires additional studies in the future.
Acknowledgements
The present study was supported by a grant from the
Research Section of the Changhua Christian Hospital (Changhua,
Taiwan; no. 101-CCH-IRP-11).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3
|
Verhoef MJ, Balneaves LG, Boon HS and
Vroegindewey A: Reasons for and characteristics associated with
complementary and alternative medicine use among adult cancer
patients: a systematic review. Integr Cancer Ther. 4:274–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Che AJ, Zhang JY, Li CH, Chen XF, Hu ZD
and Chen XG: Separation and determination of active components in
Radix Salviae miltiorrhizae and its medicinal preparations
by nonaqueous capillary electrophoresis. J Sep Sci. 27:569–575.
2004.PubMed/NCBI
|
|
5
|
Zhou L, Zuo Z and Chow MS: Danshen: an
overview of its chemistry, pharmacology, pharmacokinetics and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su CC and Lin YH: Tanshinone IIA
downregulates the protein expression of ErbB-2 and upregulates
TNF-α in colon cancer cells in vitro and in vivo. Int J Mol Med.
22:847–851. 2008.PubMed/NCBI
|
|
7
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|
|
8
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
9
|
Cheng CY and Su CC: Tanshinone IIA may
inhibit the growth of small cell lung cancer H146 cells by
up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial
membrane potential. Mol Med Rep. 3:645–650. 2010.PubMed/NCBI
|
|
10
|
Cheng CY and Su CC: Tanshinone IIA
inhibits Hep-J5 cells by increasing calreticulin, Caspase-12 and
GADD153 protein expression. Int J Mol Med. 26:379–385.
2010.PubMed/NCBI
|
|
11
|
Fronza M, Murillo R, Œlusarczyk S, et al:
In vitro cytotoxic activity of abietane diterpenes from Peltodon
longipes as well as Salvia miltiorrhiza and Salvia
sahendica. Bioorg Med Chem. 19:4876–4881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chitpatima ST, Makrides S, Bandyopadhyay R
and Brawerman G: Nucleotide sequence of a major messenger RNA for a
21 kilodalton polypeptide that is under translational control in
mouse tumor cells. Nucleic Acids Res. 16:23501988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bommer UA, Lazaris-Karatzas A, De
Benedetti A, et al: Translational regulation of the mammalian
growth-related protein P23: involvement of eIF-4E. Cell Mol Biol
Res. 40:633–641. 1994.PubMed/NCBI
|
|
14
|
Yenofsky R, Cereghini S, Krowczynska A and
Brawerman G: Regulation of mRNA utilization in mouse
erythroleukemia cells induced to differentiate by exposure to
dimethyl sulfoxide. Mol Cell Biol. 3:1197–1203. 1983.PubMed/NCBI
|
|
15
|
Li F, Zhang D and Fujise K:
Characterization of fortilin, a novel antiapoptotic protein. J Biol
Chem. 276:47542–47549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang D, Li F, Weidner D, Mnjoyan ZH and
Fujise K: Physical and functional interaction between myeloid cell
leukemia 1 protein (MCL1) and Fortilin. The potential role of MCL1
as a fortilin chaperone. J Biol Chem. 277:37430–37438. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuynder M, Susini L, Prieur S, et al:
Biological models and genes of tumor reversion: cellular
reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci USA.
99:14976–14981. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graidist P, Phongdara A and Fujise K:
Antiapoptotic protein partners fortilin and MCL1 independently
protect cells from 5-fluorouracil-induced cytotoxicity. J Biol
Chem. 279:40868–40875. 2004. View Article : Google Scholar
|
|
19
|
Liu H, Peng HW, Cheng YS, Yuan HS and
Yang-Yen HF: Stabilization and enhancement of the antiapoptotic
activity of mcl-1 by TCTP. Mol Cell Biol. 25:3117–3126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Yang F, Xiong Z, et al: An
N-terminal region of translationally controlled tumor protein is
required for its antiapoptotic activity. Oncogene. 24:4778–4788.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Susini L, Besse S, Duflaut D, et al: CTP
protects from apoptotic cell death by antagonizing bax function.
Cell Death Differ. 15:1211–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Won SH, Lee HJ, Jeong SJ, et al:
Tanshinone IIA induces mitochondria dependent apoptosis in prostate
cancer cells in association with an inhibition of phosphoinositide
3-kinase/AKT pathway. Biol Pharm Bull. 33:1828–1834. 2010.
View Article : Google Scholar : PubMed/NCBI
|