Introduction
In the majority of developing countries, a severe
shortage of donor corneas has limited performance of keratoplasty.
Xenografts may provide a solution to this supply-demand disparity.
In view of the ethical issues and impracticalities associated with
the use of nonhuman primates, interest has focused on other
species, in particular the pig, as a suitable organ donor species
for humans. In addition to organ size and physiological
similarities to humans, pigs are highly inbred, making them
particularly amenable to genetic modifications that may improve
their ability to function as organ donors to humans (1).
A barrier to successful xenograft is hyperacute
rejection. In the case of the cornea, distinctive features exist
that partially exempt this tissue from this type of pathogenesis
(2). Previous studies (3) demonstrated that corneal xenografts
from WZS pigs were not hyperacutely rejected in the rhesus monkey
model. Immune rejection occurred approximately 15 days following
penetrating corneal xenotransplantation. The endothelium may be a
main target for rejection, due to evidence of cellular or chronic
graft rejection in this membrane region (3).
Induction of donor-specific immunological tolerance
would avoid graft rejection and side-effects associated with
chronic, non-specific immunosuppressive therapy. Establishment of
mixed chimerism (donor/host) by bone marrow transplantation (BMT)
is a reliable method to induce donor-specific tolerance (4–6).
Mixed chimerism refers to a state in which allogeneic cells coexist
with recipient cells. Together in the thymus, cells delete host-
and donor-reactive T cells, resulting in a peripheral T-cell
repertoire tolerant towards donor and host (7). Mixed hematopoietic chimeras exhibit
donor-specific transplantation tolerance and decreased
immunocompetence (8–12).
Lan et al demonstrated that mixed
hematopoietic chimerism mediates deletion of donor antigen-reactive
human thymocytes in the thymus and induces human T-cell tolerance
to porcine xenografts (1). WZS
pigs are suitable donor alternatives in corneal
xenotransplantation, due to high rates of inbreeding and high
homology between WZSP SLA and human HLA. However, it remains
unclear whether mixed chimerism, induced by donor BMT, is a
solution to the prevention of pig-rhesus xenograft rejection in
this model. To address this issue, we used the WZS pig-rhesus
xenotransplantation model to explore xenograft survival following
donor BMT.
Materials and methods
Animals
All animals in this study were used in accordance
with the ARVO Resolution on Use of Animals in Research. WZS pigs
(inbred for 14 generations; 3–4 months old; weight, 15–20 kg) were
purchased from the Institute of Animal Sciences (Chinese Academy of
Agricultural Sciences, Beijing, China). Rhesus monkeys (aged 3–5
years old) were purchased from the Academy of Military Medical
Sciences (Beijing, China). WZS pigs were used as donors and rhesus
monkeys as recipients. Only the right eye of each rhesus monkey
received the corneal xenotransplantation.
Donor preparation
Pig bone marrow cell preparation
Bone marrow cells were harvested from the ilium bone
of WZS pigs as previously described (6,7,13).
Marrow was filtered through a sterile nylon mesh and red cells were
lysed with Tris-NH4Cl. Bone marrow cells were
resuspended to produce a concentration of 1×108 cells/ml
(8–10,14,15).
Pig cornea preparation
WZS pigs were sacrificed and eyeballs were
enucleated and washed in saline (9). Eyeballs were immersed in sterile
saline solution containing 2000 U/ml tobramycin and streptomycin
for 20 min. The cornea was excised with Vannas scissors and
preserved in Optisol solution at 4°C for 3–5 days.
Groups
Group 1
Prior to bone marrow transplantation, 6 rhesus
monkeys received daily intravenous injection of cyclophosphamide
(CP; 15 mg/kg) for 2 days (16,17).
At day 3, recipients received bone marrow cells by intravenous
injection (2.5×108 cells/kg). In addition, between days
5–7 and 11–13, monkeys were injected intravenously with CP at the
same dosage described above. At day 14, orthotropic penetrating
corneal xenotransplantation was performed.
Group 2
Six rhesus monkeys received intravenous injection of
CP in the same way as group 1. No BMT was performed. Orthotropic
penetrating corneal xenotransplantation was also performed at day
14.
Orthotropic penetrating corneal
xenotransplantation
Rhesus monkeys were anesthetized with an
intramuscular injection of 10 mg/kg ketamine and an intravenous
injection of 25 mg/kg amobarbital. Donor grafts were excised by a
6.0 mm trephine and the graft bed was prepared using a 5.75 mm
trephine on the right eye. The donor graft was placed on the
recipient’s bed and sutured with 8 interrupted 10-0 nylon sutures
(Ethicon, Somerville, NJ, USA). The anterior chamber was restored
at the end of surgery with sterilized saline buffer. Antibiotic and
atropine ointment were applied and eyelids were closed with 5-0
nylon tarsorrhaphy. Eyelids remained closed for 48 h and were then
opened for clinical evaluation.
Assessment of xenograft survival
Following surgery, corneal xenografts were evaluated
by slit-lamp microscope every other day for 2 weeks, then twice a
week until the end of observation. Graft opacity, edema and
neovascularization were evaluated using a modified scoring system
as described previously (3). A
rejection index (RI) was based on the sum of the grades for
opacity, edema and neovascularization (Table I). Grafts with scores ≥6 were
considered to be rejected.
 | Table IGrades for opacity, edema and
neovascularization. |
Table I
Grades for opacity, edema and
neovascularization.
| Grades | Criteria |
|---|
| Opacity |
| 0 | Clear |
| 1 | Slight haze |
| 2 | Increased haze but
iris structure still clear |
| 3 | Advanced haze with
difficult view of iris structure |
| 4 | Severe opacity
without view of chamber structure |
| Edema |
| 0 | No stromal or
epithelial edema |
| 1 | Slight stromal
edema |
| 2 | Diffuse stromal
edema |
| 3 | Diffuse stromal edema
with epithelial microcystic edema |
| 4 | Bullous
keratopathy |
|
Neovascularization |
| 0 | No vessels |
| 1 | Vessels appearing in
the peripheral corneal bed |
| 2 | Vessels appearing in
the graft periphery |
| 3 | Vessels appearing in
the graft middle-periphery |
| 4 | Vessels extending to
the graft center |
Flow cytometry assay
Prior to surgery and at postoperative weeks 1, 2 and
4, peripheral blood samples from the rhesus monkeys were obtained.
Red blood cells were depleted from the samples by
Tris-NH4Cl and a mouse anti-pig MHC-I monoclonal
antibody was used to monitor the recovery of pig cell populations
in the monkeys by flow cytometry (Serotec, Oxford, UK).
FITC-conjugated goat anti-mouse IgG was set as the negative control
(Southern Biotech, Birmingham, AL, USA).
Histopathological analysis
At postoperative week 4, 2 rhesus monkeys/group were
sacrificed. Corneas were embedded in paraffin, sliced, stained with
hematoxylin and eosin and examined under a light microscope
(Olympus, Tokyo, Japan).
Mixed lymphocytes reaction (MLR) assay
Prior to surgery and at postoperative weeks 1, 2 and
4, peripheral blood of the rhesus monkeys was taken and lymphocytes
were used as reaction cells. RPMI-1640 culture medium was added to
the cells to prepare single-cell solutions at a concentration of
5×106 cells/ml. Cells were cultured in 96-well
flat-bottom tissue culture plates (200 μl/well) and stimulated with
200 μl concanavalin A (ConA; 5 μg/ml) or xenogeneic donor spleen
cells (radiated by cobalt-60 for 30 min, at a concentration of
5×106 cells/ml). Cultures were performed in triplicate,
including a blank RPMI-1640 control group in the absence of
mitogen. Following 72 h incubation at 37°C, with 5% CO2
in a humidified incubator, the cultures were pulsed with 10 μl
methyl-thiazolyl-tetrazolium (5 mg/ml). OD (optical density) values
were measured 5 h later, at a wavelength of 570 nm by an MRX
Microplate reader (Synateck Laboratories, Chantilly, VA, USA)
(18).
Immunoglobulin and complement assay (
19)
Time points and cell numbers were as described
above. Sera were separated by centrifugation at 3,000 rpm for 3 min
and suspensions were used to detect concentration of immunoglobulin
and complement with a Nephelometer (Beckman Coulter, Brea, CA, USA)
(19).
Statistical analysis
Data were presented as the mean ± SD and evaluated
using a two-tailed Mann-Whitney U-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Incidence and timing of graft
rejection
All 12 rhesus monkeys remained alive and healthy
during the follow-up period. Group 1, which had been injected with
CP and bone marrow cells, showed vomiting, inappetence and wilted
condition, returning to normal within 48 h. Group 2, which received
intravenous injection with CP only, had no symptoms of
sickness.
Mean survival time (MST) of the xenografts was
36.0±4.7 days in group 1 and 17.7±3.2 days in group 2 (Table II). A statistically significant
difference was identified between groups 1 and 2 (P<0.01).
 | Table IICorneal graft survival following
xenotransplantation. |
Table II
Corneal graft survival following
xenotransplantation.
| Groups | n | Survival time
(d) | MST (d) | Median (d) |
|---|
| 1 | 6 | 32, 42, 40, 34, 38,
30 | 36.0±4.7a | 36 |
| 2 | 6 | 12, 18, 16, 20, 20,
20 | 17.7±3.2 | 19 |
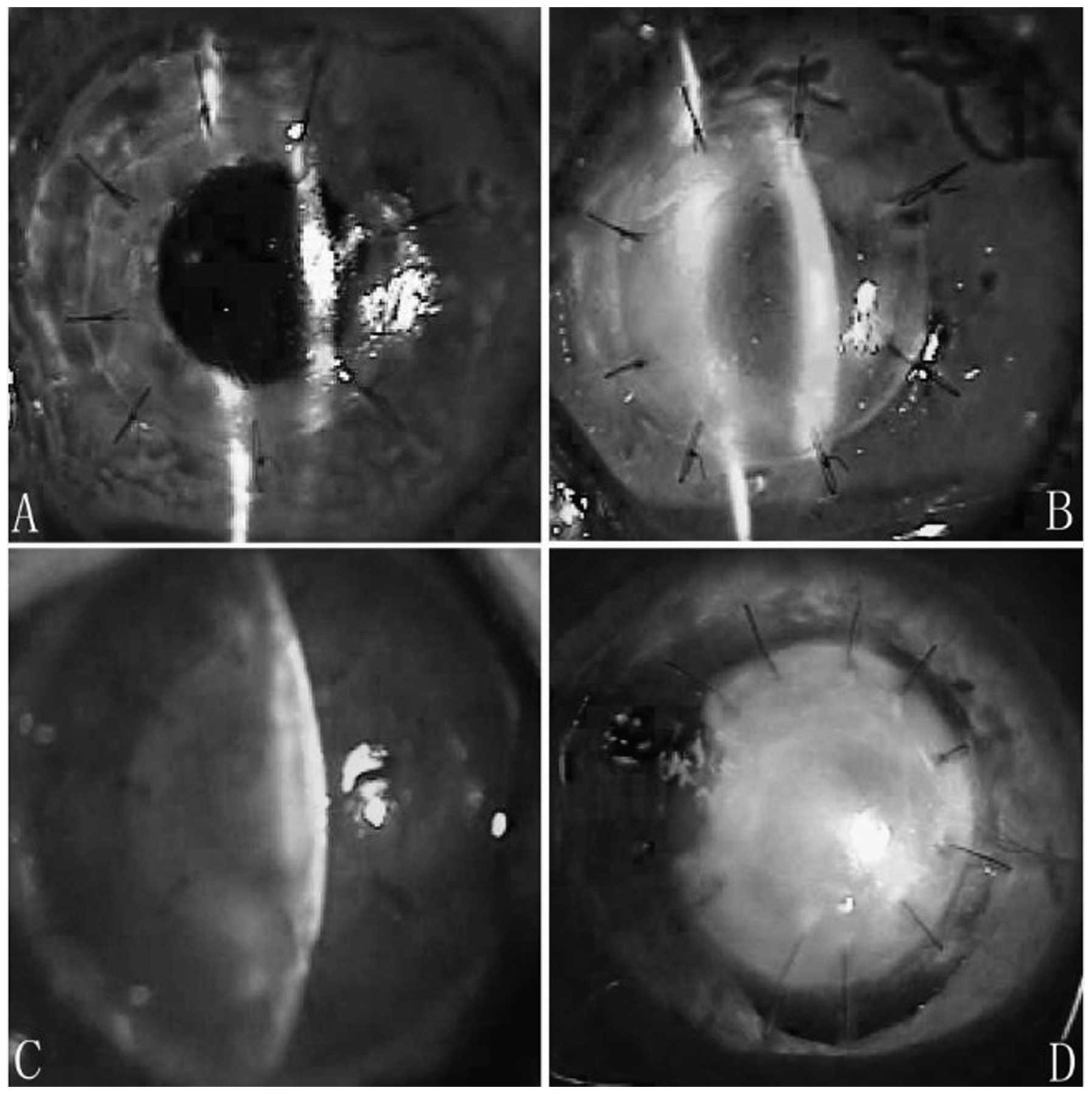
In group 1, corneal xenografts remained almost
transparent during postoperative week 1, although ciliary
congestion injection was noted. At postoperative day 20, xenografts
remained transparent. At postoperative day 40, xenografts revealed
slight diffuse inflammatory infiltration and stromal edema, but no
exudative membrane was observed in the anterior chamber and no
new-forming vessels appeared (Fig. 1A
and B). Infiltration and edema in the xenografts was
intensified at postoperative day 90, however, no neovascularization
and exudative membrane were observed in the anterior chamber and no
scarring was noted.
In group 2, corneal xenografts revealed mild edema
and exudative membrane was observed in the anterior chamber. No
infiltration in the stroma was observed during the initial
postsurgical period. At postoperative day 15, corneal edema
increased, stromal infiltration was observed and neovascularization
was noted in the peripheral corneal bed. At postoperative day 20,
xenografts demonstrated characteristics of immune rejection with
loss of graft clarity. Increased edema and new-forming vessels were
noted on the recipients’ bed. At postoperative day 40, xenografts
were filled with new-forming vessels and revealed complete opacity.
The pupil could not be viewed (Fig. 1C
and D). All grafts developed scarring 3 months following
surgery.
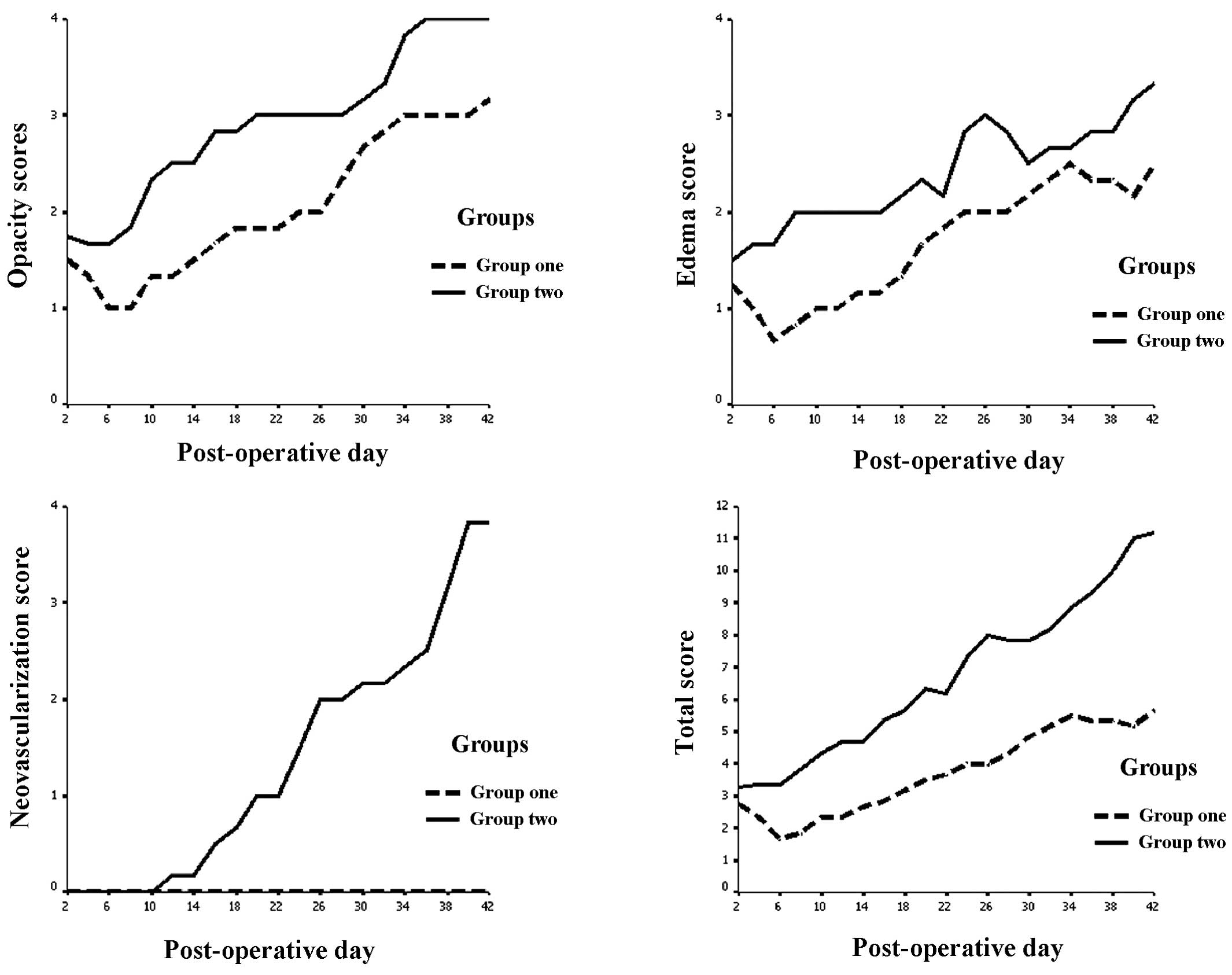
Following corneal xenotransplantation surgery, index
of opacity, edema and neovascularization increased with time.
However, at all time points, the index of group 1 was lower than
that of group 2 (Fig. 2).
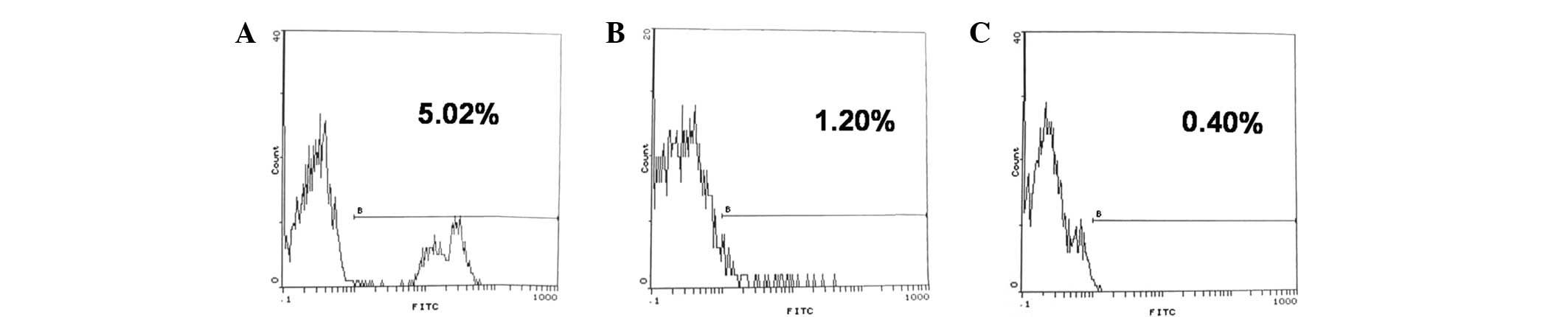
Chimerism formation
Rhesus monkeys that received CP and pig bone marrow
cell transplantation formed mixed chimerism. In postoperative week
1, the mean chimerism percentage was 5.20±1.02%. At postoperative
week 2, the mean chimerism percentage decreased to 1.20±0.03%,
decreasing further at postoperative week 4 to <1% (Fig. 3). However, in the non-BMT group, no
chimerism was detected. These results indicate that CP administered
in combination with BMT produces a transient mixed chimerism across
a fully xenogeneic barrier in the host.
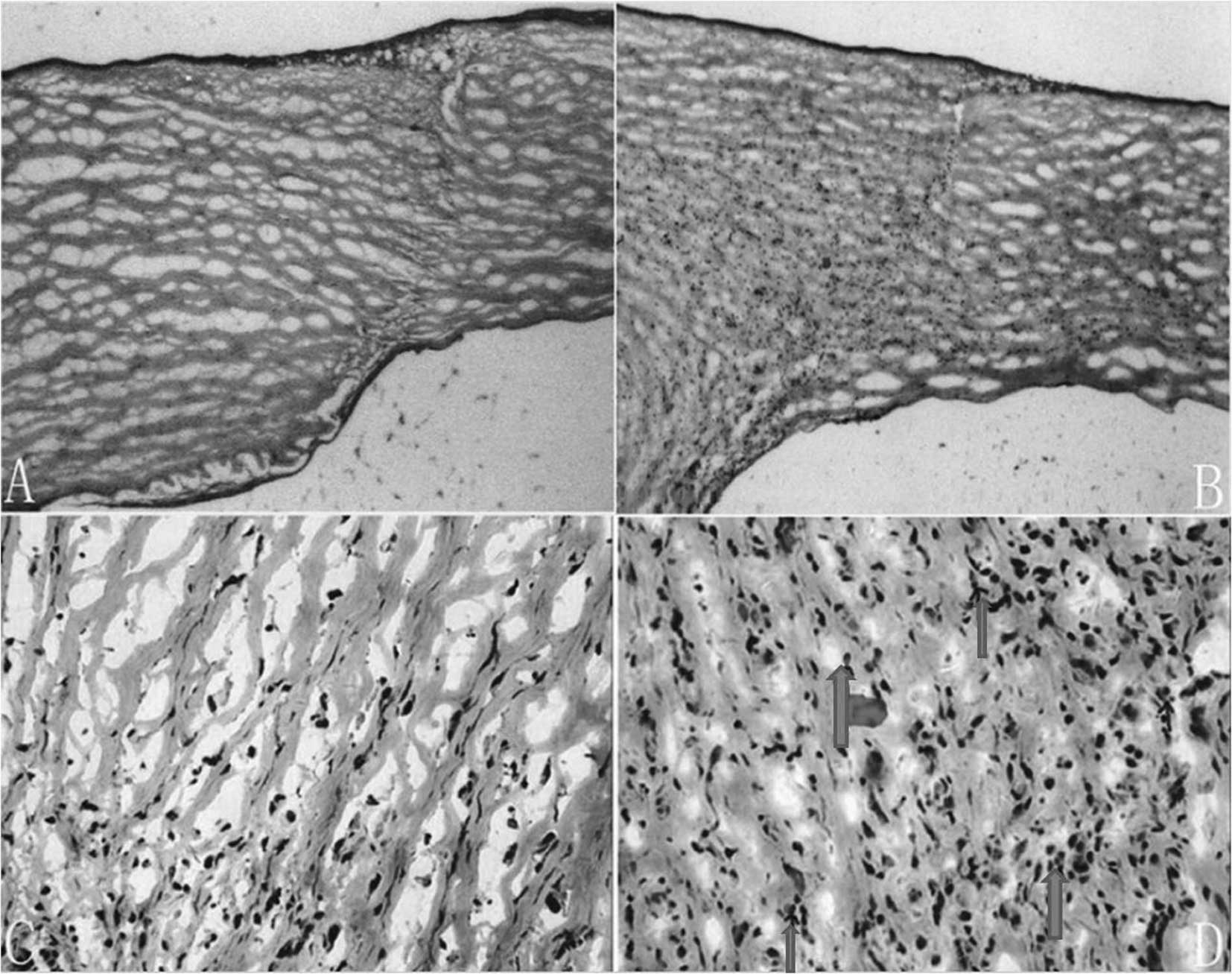
Histopathological staining
In group 1, low levels of inflammatory cells at the
intersection between the graft and bed were observed. The
epithelium and endothelium were intact. No eosinophil infiltration
was noted in the grafts (Fig. 4A and
C). However, in group 2, there was more inflammatory cell
infiltration in the stroma of the xenograft and scattered
eosinophil infiltration was observed (Fig. 4B and D).
MLR
Prior to CP injection, no significant difference was
found between the lymphocyte reaction ability of the rhesus
recipients in groups 1 and 2 to ConA and donor lymphocyte antigen
(P>0.05). Following CP and BMT, lymphocyte reaction ability was
identifed as significantly lower in group 1 compared with group 2.
The lowest value of 0.90±0.15 was obtained at postoperative week 2
and was identified as significantly reduced compared to group 2
(P<0.05; Table III).
 | Table IIIComparison of lymphocyte proliferation
capacity following BMT (n=4). |
Table III
Comparison of lymphocyte proliferation
capacity following BMT (n=4).
| Group 1 | Group 2 |
|---|
|
|
|
|---|
| Period | Negative control | ConA | Donor lymphocyte | Negative control | ConA | Donor lymphocyte |
|---|
| Before CP
injection | 0.02±0.00 | 1.22±0.27 | 1.18±0.27 | 0.01±0.00 | 1.22±0.25 | 1.17±0.14 |
| Postoperative week
1 | 0.02±0.01 | 0.98±0.27 | 0.91±0.43a | 0.02±0.01 | 1.12±0.38 | 1.10±0.16 |
| Postoperative week
2 | 0.02±0.01 | 0.95±0.48 | 0.90±0.15a | 0.01±0.00 | 1.24±0.42 | 1.23±0.21 |
| Postoperative week
3 | 0.01±0.00 | 1.13±0.32 | 1.06±0.24a | 0.02±0.00 | 1.19±0.27 | 1.19±0.38 |
Immunoglobulin and complement levels in
the serum
Concentration of IgA at postoperative week 2 was
100.85±65.74 mg/dl in group 1 and 126.30±61.17 mg/dl in group 2. A
statistically significant difference was found (P<0.05).
Concentration of complement C3 at postoperative week 2 was
123.75±13.89 mg/dl in group 1, compared with 160.50±28.41 mg/dl in
group 2, which was identified as a statistically significant
difference (P<0.05).
Discussion
Xenografts from pigs may provide a potential
solution to the severe shortage of donor cornea in developing
countries, including China. However, xenogeneic corneas are subject
to vigorous immune rejection, and the non-specific
immunosuppression that is required to overcome rejection negatively
affects recipients with dangerously low immune systems (3). Therefore, it is highly desirable to
eliminate immune response to xenografts, through the induction of
immune tolerance. Mixed chimerism has been proven to be a powerful
and reliable approach for tolerance induction across allogeneic and
closely related xenogeneic barriers (20,21).
In 1986, Cobbold and Waldmann (22) demonstrated that fully
MHC-mismatched marrow engraftment and specific tolerance was
achieved, by pretreating recipients with depleting doses of
anti-CD4 and anti-CD8 monoclonal antibodies combined with a
sublethal dose (6 Gy) of total body irradiation (TBI) (9). In a variety of non-myeloablative BMT
preparative regimens, CP was administered instead of TBI (17). With this protocol, long-lasting
mixed chimerism was induced in a series of HLA-matched donor BMT
recipients and in a small series of HLA-mismatched BMT recipients
(15). The mixed chimerism
approach has been successfully extended to xenogeneic models.
Mixed hematopoietic chimerism is associated with
tolerance to skin, islet cell, cardiac grafts and lung allograft.
However, donor-specific tolerance to corneal xenografts has never
been reported. Allocorneal graft rejection rarely occurs in
clinical BMT patients, therefore, BMT is highly favored for use in
high-risk patients, particularly those with immune system
dysfunction. A previous study demonstrated that steroid treatment
alone prolonged xenograft mean survival time (23). However, steroid treatment is
associated with reduced innate and acquired immune response,
including hyperacute immune response. Therefore, CP was utilized to
reduce activities of host lymphocyte cells and donor hematopoietic
cells with the aim to weaken donor-versus-host disease, while
retaining weak stimulation of donor cells.
Our data demonstrate that the donor chimerism was
detectable at postoperative week 1. Following this, donor chimerism
decreased and was not detectable 1 month after BMT. This was
correlated with xenograft survival at approximately 1 month. We
conclude that induction of prior mixed chimerism existence prolongs
corneal xenograft survival and xenograft survival was correlated
with the formation of chimerism in the recipient. A reduction in
MLR and lower levels of immunoglobulin and complement following
surgery also demonstrates that recipients were in lower
immunocompetence. CP treatment had no effect on graft survival,
consistent with previous data. CP has been revealed to suppress
lymphocyte cells reaction but not innate immune cells. We
hypothesize that innate immune cell-mediated xenograft rejection is
the main cause of graft failure.
Histopathology of the xenografts revealed a
reduction of inflammation cell infiltration in group 1 and no
eosinophil infiltration in the xenografts was observed. Observation
of eosinophil cells is characteristic of xenotransplantation
rejection and it has been demonstrated by Tanaka et al that
eosinophil-dependent xenograft rejection bears similarities to
immune elimination of parasites (24). However, this does not suggest that
eosinophil cells dominate the main population of cells involved in
corneal xenotransplantation. Lymphocytes, including CD4+
cells, antigen-presenting cells and neutrophils, remain the main
population involved in corneal xenotransplantation. Absence of
eosinophil infiltration in group 1 suggest that xenograft rejection
is inhibited by BMT.
In the MLR assay, a decreased response in group 1 to
donor lymphatic cells was observed, indicative of specific
immunosuppression. However, response to ConA was indicative of
non-specific immunosuppression. These MLR results require further
clarification, and may be explained by poorly controlled CP doses.
Further studies should utilize a third party mitogen to elucidate
the suppression response. There was reduction of IgA levels in
group 1, however, levels of IgG and IgM were not identified as
significantly different between the two groups (data not shown).
These results suggest that humoral immunity is also involved in
corneal xenotransplantation despite playing a minor role in the
immune response.
In general, preconditioning with CP and conventional
BMT induced immune suppression, prolonging corneal graft survival
in pig-monkey xenotransplantation. At present, chimerism only
exists in the recipient for a short time period. To sustain this
presence, future analysis may utilize suppressants, including CsA,
rapamycin and antibodies against T cells or costimulator cytokines
(CTLA4-Ag, CD40L). Staphylococcus enterotoxin B is a superantigen
used in a previous study (?) to induce immune tolerance in a high
risk rat corneal transplantation procedure. This tolerogen may
prove useful for induction of long-term chimerism formation. Future
studies are likely to involve modification of the treatment to
increase long-term chimerism and likelihood of genuine tolerance,
with the aim to produce a treatment suitable for clinical
application.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30801264 and 30471862), the New
Nova Program (no. 2007B050) and the National High Technology
Research and Development Program of China (863 Program; no.
2006AA02A131).
References
|
1
|
Lan P, Wang L, Diouf B, et al: Induction
of human T-cell tolerance to porcine xenoantigens through mixed
hematopoietic chimerism. Blood. 103:3964–3969. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ross JR, Howell DN and Sanfilippo FP:
Characteristics of corneal xenograft rejection in a discordant
species combination. Invest Ophthalmol Vis Sci. 34:2469–2476.
1993.PubMed/NCBI
|
|
3
|
Zhiqiang P, Cun S, Ying J, Ningli W and Li
W: WZS-pig is a potential donor alternative in corneal
xenotransplantation. Xenotransplantation. 14:603–611. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wekerle T and Sykes M: Mixed chimerism and
transplantation tolerance. Ann Rev Med. 52:353–370. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sachs DH: Mixed chimerism as an approach
to transplantation tolerance. Clin Immunol. 95:S63–S68. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wekerle T: Transplantation tolerance
induced by mixed chimerism. J Heart Lung Transplant. 20:816–823.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sykes M: Mixed chimerism and transplant
tolerance. Immunity. 14:417–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pham SM, Mitruka SN, Youm W, et al: Mixed
hematopoietic chimerism induces donor-specific tolerance for lung
allografts in rodents. Am J Respir Crit Care Med. 159:199–205.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang CA, Fuchimoto Y, Scheier-Dolberg R,
Murphy MC, Neville DM Jr and Sachs DH: Stable mixed chimerism and
tolerance using a nonmyeloablative preparative regimen in a large
animal model. J Clin Invest. 105:173–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abe M, Qi J, Sykes M and Yang YG: Mixed
chimerism induces donor-specific T cell tolerance across a highly
disparate xenogeneic barrier. Blood. 99:3823–3829. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beschorner WE, Sudan DL, Radio SJ, et al:
Heart xenograft survival with chimeric pig donors and modest immune
suppression. Ann Surg. 237:265–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami M, Ito H, Harada E, Enoki T,
Sykes M and Hamano K: Long-term survival of xenogeneic heart grafts
achieved by costimulatory blockade and transient mixed chimerism.
Transplantation. 82:275–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee PW, Cina RA, Randolph MA, et al:
Stable multilineage chimerism across full MHC barriers without
graft-versus-host disease following in utero bone marrow
transplantation in pigs. Exp Hematol. 33:371–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawai T, Cosimi AB, Wee SL, et al: Effect
of mixed hematopoietic chimerism on cardiac allograft survival in
cynomolgus monkeys. Transplantation. 73:1757–1764. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwai T, Tomita Y, Zhang QW, Shimizu I,
Nomoto K and Yasui H: Requirement of a higher degree of chimerism
for skin allograft tolerance in cyclophosphamide-induced tolerance.
Transplant Int. 17:795–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimikawa M, Sachs DH, Colvin RB,
Bartholomew A, Kawai T and Cosimi AB: Modifications of the
conditioning regimen for achieving mixed chimerism and
donor-specific tolerance in cynomolgus monkeys. Transplantation.
64:709–716. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sablinski T, Emery DW, Monroy R, et al:
Long-term discordant xenogeneic (porcine-to-primate) bone marrow
engraftment in a monkey treated with porcine-specific growth
factors. Transplantation. 67:972–977. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozlowski T, Shimizu A, Lambrigts D, et
al: Porcine kidney and heart transplantation in baboons undergoing
a tolerance induction regimen and antibody adsorption.
Transplantion. 67:18–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen D, Cao R, Guo H, et al: Pathogenesis
and pathology of delayed xenograft rejection in pig-to-rhesus
monkey cardiac transplantation. Transplant Proc. 36:2480–2482.
2004. View Article : Google Scholar
|
|
20
|
Yang YG: Application of xenogeneic stem
cells for induction of transplantation tolerance: present state and
future directions. Springer Semin Immunopathol. 26:187–200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Millan MT, Shizuru JA, Hoffmann P, et al:
Mixed chimerism and immunosuppressive drug withdrawal after
HLA-mismatched kidney and hematopoietic progenitor transplantation.
Transplantation. 73:1386–1391. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cobbold SP, Martin G, Qin S and Waldmann
H: Monoclonal antibodies to promote marrow engraftment and tissue
graft tolerance. Nature. 323:164–166. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li A, Pan Z, Jie Y, Sun Y, Luo F and Wang
L: Comparison of immunogenicity and porcine-to-rhesus lamellar
corneal xenografts survival between fresh preserved and dehydrated
porcine corneas. Xenotransplantation. 18:46–55. 2011. View Article : Google Scholar
|
|
24
|
Tanaka K, Yamagami S and Streilein JW:
Evidence that T-helper type 2 cell-derived cytokines and
eosinophils contribute to acute rejection of orthotopic corneal
xenografts in mice. Transplantation. 79:1317–1323. 2005. View Article : Google Scholar : PubMed/NCBI
|