Introduction
Histone deacetylase (HDAC) inhibitors represent a
novel class of anticancer agents that catalyze the deacetylation of
histone and non-histone proteins and modulate the expression of
genes involved in multiple cellular processes, including
differentiation, apoptosis and autophagy (1–2).
In vitro studies have revealed that HDAC inhibitors, such as
suberoylanilide hydroxamic acid (SAHA), have a dual effect on
leukemic cells, triggering apoptosis at high concentrations
(3) and inducing differentiation
at low concentrations (4). In
addition to the modulation of gene transcription, HDAC inhibitors
have pleiotropic biological effects, which may be beneficial for
the destruction of acute myeloid leukemia (AML) cells. These
effects include the production of reactive oxygen species,
induction of oxidative damage to DNA and inactivation of HSP90
chaperone function (5,6). Hyperacetylation of HSP90 by HDAC
inhibitors leads to dysfunctional chaperone activity, resulting in
the degradation of leukemia-related client proteins, including
BCR-ABL (7). HDAC inhibitors may
also overcome the effects of leukemia fusion proteins, including
AML1-ETO, PML-RARα and MLL-CBP, on leukemogenesis by targeting the
HDAC complex and these fusion proteins (8,9).
These observations indicate that HDAC inhibitors represent
promising new agents for the treatment of AML. In a phase I
clinical trial, 7/31 patients with relapsed or refractory AML
exhibited a response to HDAC inhibitors, including 2 complete
remissions (CRs) and 2 CRs with incomplete blood count recovery
(10). However, these observations
were not consistent in a phase II study where only 1 CR was
observed among 37 patients (11).
Based on these results, more recent studies have focused on the
combination therapy of AML cells with SAHA and other antileukemia
drugs (12–16).
Homoharringtonine (HHT) is a natural alkaloid,
derived from various species of Cephalotaxus. HHT functions
as a protein synthesis inhibitor and has been found to induce
apoptosis in a variety of leukemic cells (17,18).
Early phase I trials in the United States have confirmed its
antileukemic activity; however, 4/16 patients who received daily
i.v. treatment (5–6 mg/m2/day) for 5 days exhibited
severe hypotension that resulted in cardiovascular collapse
(19). A phase II study of
low-dose continuous infusion HHT in AML demonstrated that there
were no serious cardiovascular complications when patients were
treated with an infusion of 2.5 mg/m2/day for 15–21 days
or 3.0 mg/m2/day for 15 days (20). In addition, combination therapy of
HHT and cytarabine (ara-C) in patients with late chronic-phase CML
resulted in 32% cytogenetic response and significantly improved
survival was reported compared with HHT alone (21). The combination regimen of HHT and
ara-C has been widely used in AML patients in China. For example,
elderly patients with AML were treated with HHT (2.0
mg/m2/day for 7 days) combined with low-dose ara-C. The
overall response rate was 56.5% and CR rate was 39.1% (22). Our pilot study (23) found that HHT combined with ara-C
and aclarubicin results in a CR rate of 83% in de novo AML
patients. The estimated 3-year overall survival is 53%.
Collectively, these results indicate that low-dose HHT is safe and
the combination therapy of HHT and other antileukemic agents is a
promising approach for treating AML.
In the present study, human AML Kasumi-1 and THP-1
cells were used to assess the role of HHT combined with SAHA in the
induction of cell death and studied the mechanisms of the
synergistic effect with attention to the death receptor pathway.
Low concentrations of HHT and SAHA were found to synergistically
induce higher levels of apoptosis in Kasumi-1 and THP-1 cells and
significantly inhibited the growth of leukemia xenografts in
vivo compared with each agent alone. In addition, the
combination upregulated expression of death receptor 4 (DR4)/DR5
and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
and the synergistic effect between HHT and SAHA was partially
blocked by a specific anti-TRAIL antibody. Together, these
observations provide a rationale for further clinical investigation
of this novel combination strategy in patients with AML.
Materials and methods
Cell lines and culture
The human AML cell line, Kasumi-1, was provided by
Professor SJ Chen (Shanghai Jiaotong University, Shanghai, China)
and THP-1 was purchased from the American Type Culture Collection
(Manassas, VA, USA). Cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (both Hyclone
Laboratories, Inc., Logan, UT, USA) and 1% L-Glutamine (Life
Technologies, Inc., Grand Island, NY, USA) at 37°C in a humidified
incubator containing 5% CO2. The study was approved by
the ethics committee of the First Affiliated Hospital, College of
Medicine, Zhejiang University (Hangzhou, China).
MTT assay
Effects on cell proliferation were examined by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma, St Louis, MO, USA).. In brief, cells were plated on
96-well plates at 1.0×105 cells/well, then treated with
HHT (Hangzhou Minsheng Pharmacy Factory, Hangzhou, China) and/or
SAHA (Binxinbio, Inc., Tianjin, China) at the indicated
concentrations for 24 h. Stock MTT solution (20 μl; 2.5 mg/ml) was
added to each well and cells were incubated at 37°C for an
additional 4 h. Following removal of the MTT solution in medium,
DMSO (200 μl) was added to each well and absorbance at 570 nm was
detected.
Apoptosis assay
Leukemic cells were treated with HHT and/or SAHA at
the indicated concentrations for 24 h and then washed with cold
PBS. Next, cells were co-stained with Annexin V-FITC and propidium
iodide (PI) using an apoptosis detection kit (Biouniquer, Suzhou,
China), according the manufacturer’s instructions. Cells were
analyzed with FACScan flow cytometer (Becton Dickinson, San Diego,
CA, USA). To detect chromatin condensation and nuclear
fragmentation, which are characteristics of apoptosis, cells were
plated on glass slides for fixation by 4% paraformaldehyde for 30
min at room temperature followed by three washes with PBS. Slides
were then incubated with Triton X-100 for 20 min and washed with
PBS. Next, slides were stained with Hoechst 33258 for 15 min in the
dark, washed 3 times with PBS and observed under a fluorescence
microscope (Olympus, Tokyo, Japan).
Western blot analysis
Cells from various conditions, following the
induction of apoptosis, were harvested and washed twice in PBS.
Whole cell extracts were prepared using a lysis buffer (Cell
Signaling Technology, Beverly, MA, USA), according to the
manufacturer’s instructions. Protein samples (equal protein/lane)
were subjected to 12% SDS-PAGE and transferred onto nitrocellulose
filters. Next, membranes were blocked in non-fat milk buffer and
the following primary antibodies were applied: caspase-3, -8 and
-9, poly (ADP-ribose) polymerase (PARP), histone-H3, acetylated
(Ac)-H3, Ac-H4, cytochrome c, Bid (all Cell Signaling Technology),
TRAIL (BD Pharmingen, San Diago, CA, USA), DR4 (Bioworld
Technology, Louis Park, MN, USA), DR5 (Millipore, Billerica, MA,
USA) and β-actin (housekeeping protein control; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The secondary antibody
was obtained from MultiSciences Biotech (Hangzhou, China). Blots
were visualized using enhanced chemiluminescence procedures
according to the manufacturer’s instructions.
Establishment of subcutaneous leukemia
xenografts and therapy
Severe combined immunodeficient (SCID) mice were
purchased from Shanghai Experimental Animal Center of the Chinese
Academy of Sciences (Shanghai, China) and were housed in The School
of Medicine, Zhejiang University (Hangzhou, China) under an
institute-approved animal protocol. For the subcutaneous leukemia
xenograft mouse model, 3 to 4-week-old female mice were inoculated
subcutaneously with 1×107 THP-1 cells (in 0.1 ml volume)
into the hind flanks. Tumor volume was measured and calculated
using the following formula: Volume=(length × width2)/2.
When tumor volumes reached ~100 mm3, mice were pooled
and randomly assigned to 4 groups (n=8/group): PBS (control),
intraperitoneal injection of SAHA (50 mg/kg−1) for 5
days, intraperitoneal injection of HHT (1 mg/kg−1) for 7
days and SAHA for 5 days combined with HHT for 7 days. One mouse
from each group was selected randomly and humanely sacrificed at
day 3 following treatment. Tumors were harvested and then processed
for a TUNEL assay using an In Situ Cell Death Detection kit
(Roche, Nutley, NJ, USA).
Statistical analysis
Student’s t-test was used to determine statistical
significance. Results of combination therapy were assessed by
calculating combination index (CI) values using CalcuSyn software
(Biosoft, Cambridge, UK). To assess tumor growth curves, xenograft
volumes were calculated as the mean ± SEM. In vivo survival
curves were estimated using the Kaplan-Meier method by the log-rank
test for pair-wise survival analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
HHT functions synergistically with SAHA
to inhibit AML cell growth
To improve the antileukemic efficacy of HHT in AML
cells, we aimed to identify an effective agent that enhanced the
cytotoxic effect of HHT. The effects of HHT and SAHA alone and HHT
plus SAHA on the growth of Kasumi-1 and THP-1 cells were analyzed.
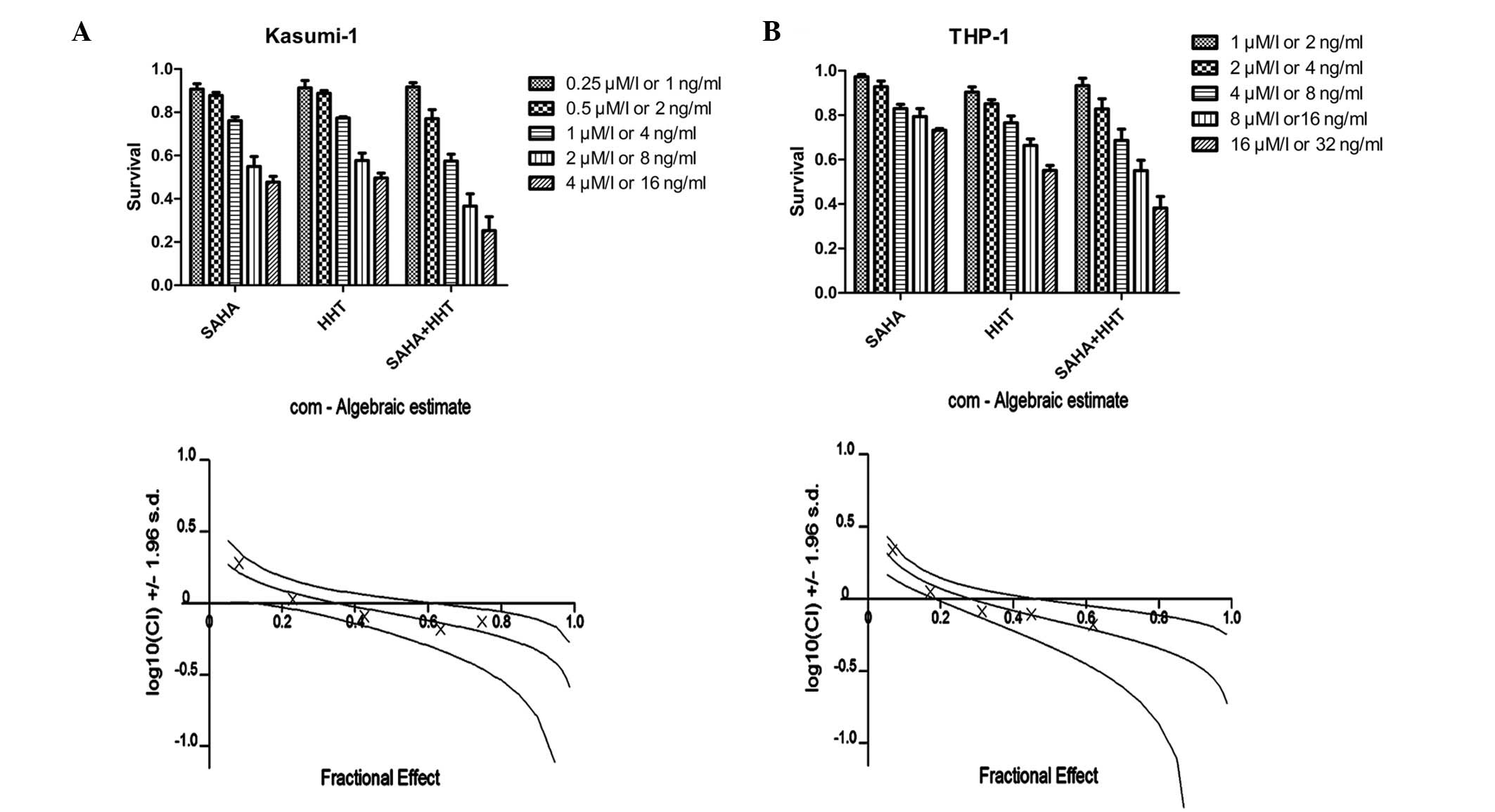
Following treatment with increasing doses of HHT or SAHA for 24 h,
Kasumi-1 cell viability was found to be significantly inhibited in
a dose-dependent manner (Fig. 1A).
Consistent effects were observed in THP-1 cells that were
relatively resistant to HHT and SAHA. When treated with HHT and
SAHA simultaneously, these cell lines revealed significantly
reduced cell viability compared with treatment alone with each
agent. CI analysis revealed that the CI for Kasumi-1 and THP-1 was
<1 when HHT and SAHA were used at lower concentrations (HHT,
4–16 ng/ml for Kasumi-1 and 8–32 ng/ml for THP-1; SAHA, 1–4 μM for
Kasumi-1 and 4–16 μM for THP-1). These results indicate a
synergistic effect between SAHA and HHT in the inhibition of AML
cell growth.
Treatment with HHT combined with SAHA
induces apoptosis in AML cells by activation of endogenous and
exogenous apoptotic pathways
To analyze the effect of HHT and SAHA on the
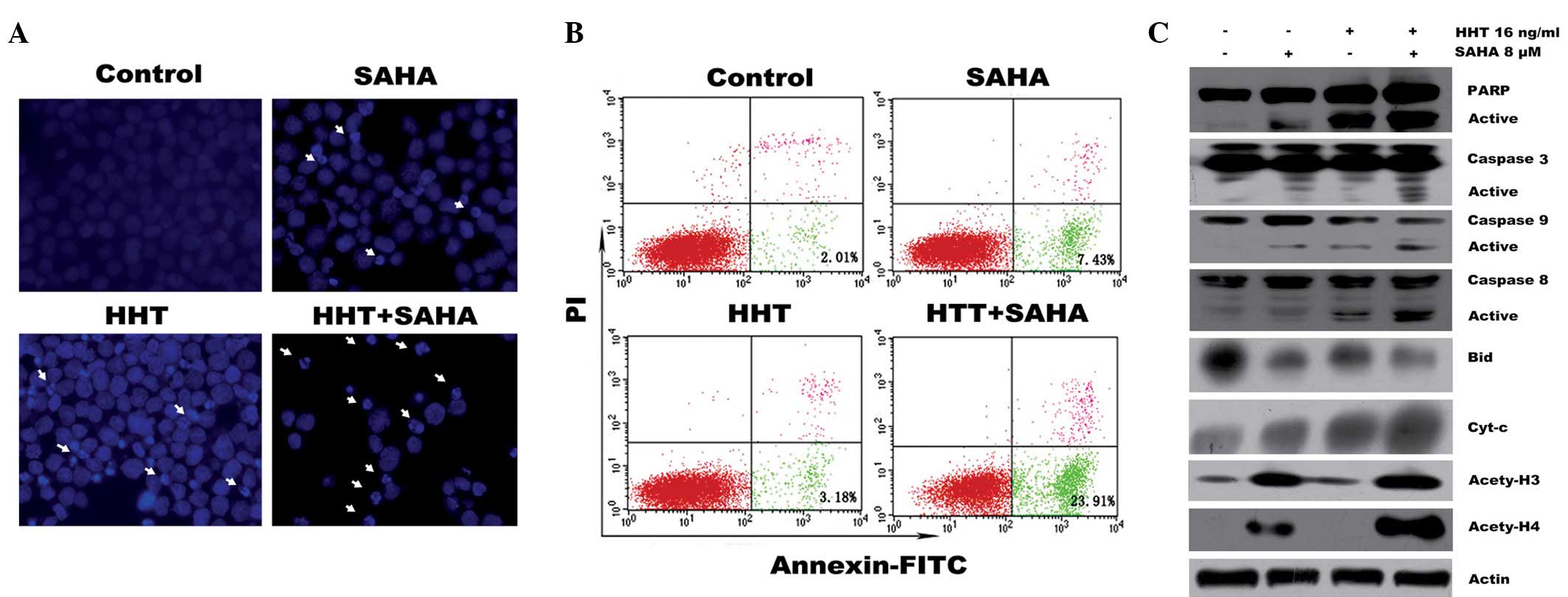
induction of apoptosis in AML cells, THP-1 cells were exposed for
24 h to 16 ng/ml HHT in the presence or absence of SAHA (8 μM).
Fig. 2A demonstrates that an
increased portion of apoptotic cells, characterized by concentrated
dense fluorescence and fragmented nuclei (apoptotic body), were
observed following co-treatment. Similarly, when these cells were
stained with Annexin V and PI and measured by flow cytometry, the
proportion of apoptotic cells (Annexin V+ and
PI− population) among cells treated with HHT and SAHA
simultaneously were found to be significantly increased compared
with cells treated with each agent alone (Fig. 2B). Next, key signaling molecules in
the apoptosis pathway were analyzed by western blot analysis. As
revealed in Fig. 2C, combined
treatment for 6 h resulted in significantly increased levels of
cleaved forms of caspase-8, -9 and -3 and PARP. In addition,
increased cytochrome c levels, indicative of decreased
mitochondrial membrane potential, and decreased levels of Bid were
observed in cells treated with HHT or SAHA alone and were further
enhanced following co-treatment. These results demonstrated that
SAHA enhanced the cytotoxicity of HHT against AML cells by
targeting the intrinsic and extrinsic caspase pathways, which may
represent one of the mechanisms by which HHT functions with SAHA to
synergistically induce apoptosis.
HHT and SAHA synergistically induce
apoptosis in AML cells via the TRAIL apoptotic pathway
To further investigate the underlying mechanism by
which HHT functions synergistically with SAHA to induce apoptosis
in AML cells, several signaling proteins involved in the apoptotic
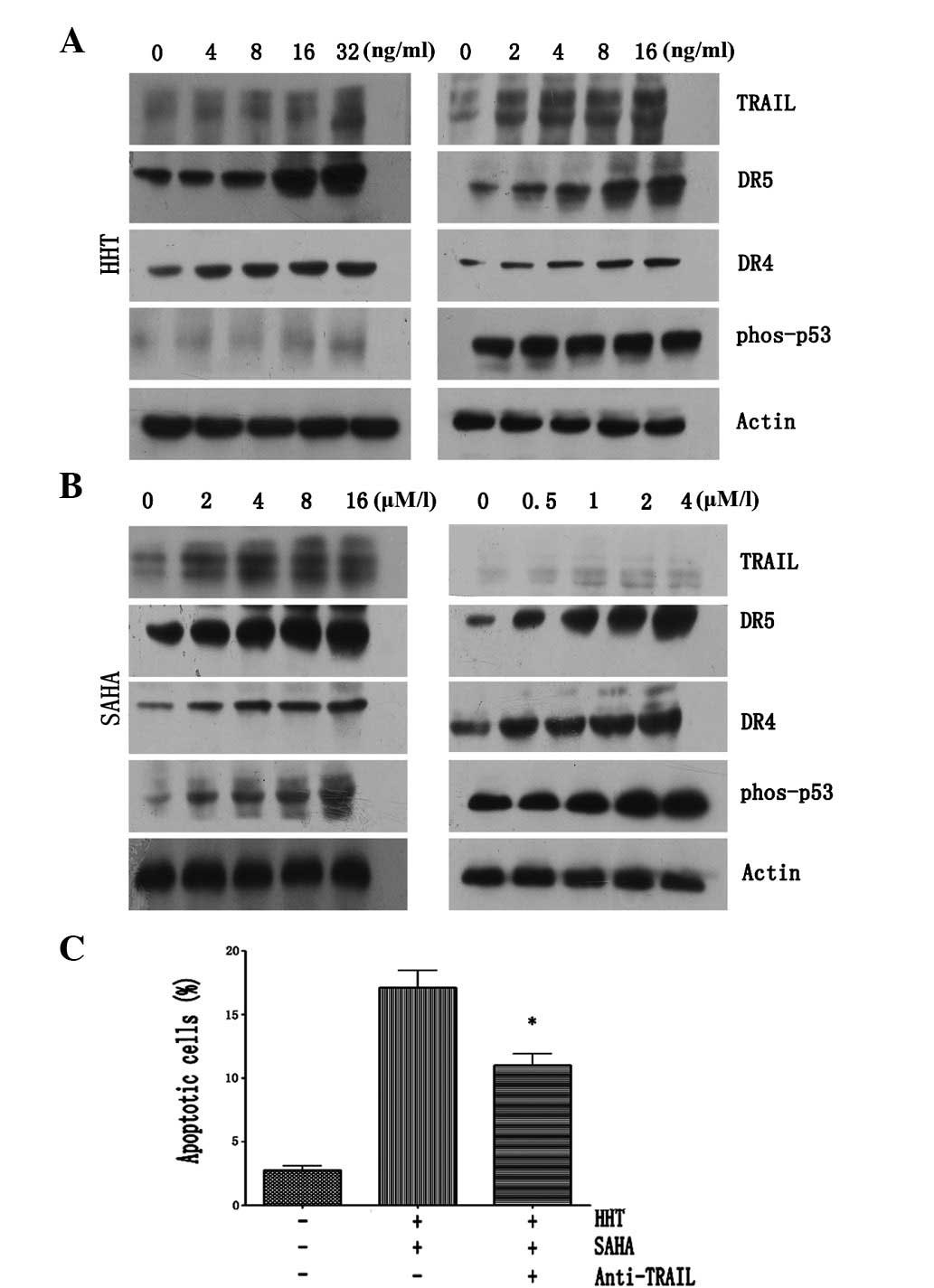
pathway were analyzed. As demonstrated in Fig. 3A, treatment with HHT for 6 h
upregulated the expression of TRAIL and DR5 in both cell lines in a
dose-dependent manner, while levels of DR4 and phos-p53 protein
expression were not altered. Consistent with a previous study
(24), significant upregulation of
DR5 and phos-p53 was identified to be induced by SAHA treatment.
However, marked upregulation of DR4 upon SAHA treatment was
observed in Kasumi-1 cells, but not in THP-1 cells (Fig. 3B), which may explain resistance to
SAHA. To confirm the role of the interaction between TRAIL and
DR4/DR5 in apoptosis induced by HHT plus SAHA, THP-1 cells were
incubated with a specific anti-TRAIL antibody for 1 h, followed by
co-treatment with HHT (8 ng/ml) and SAHA (4 μM) for 23 h. Next,
cells were co-stained with Annexin V/PI and analyzed by flow
cytometry. As revealed in Fig. 3C,
the proportion of apoptotic cells induced by HHT plus SAHA was
found to be significantly decreased (P<0.01) when cells were
co-cultured with the anti-TRAIL antibody, confirming that HHT
functions synergistically with SAHA to induce apoptosis in AML
cells in a TRAIL-dependent manner.
HHT and SAHA abrogate growth of
xenografted AML cells in SCID mice
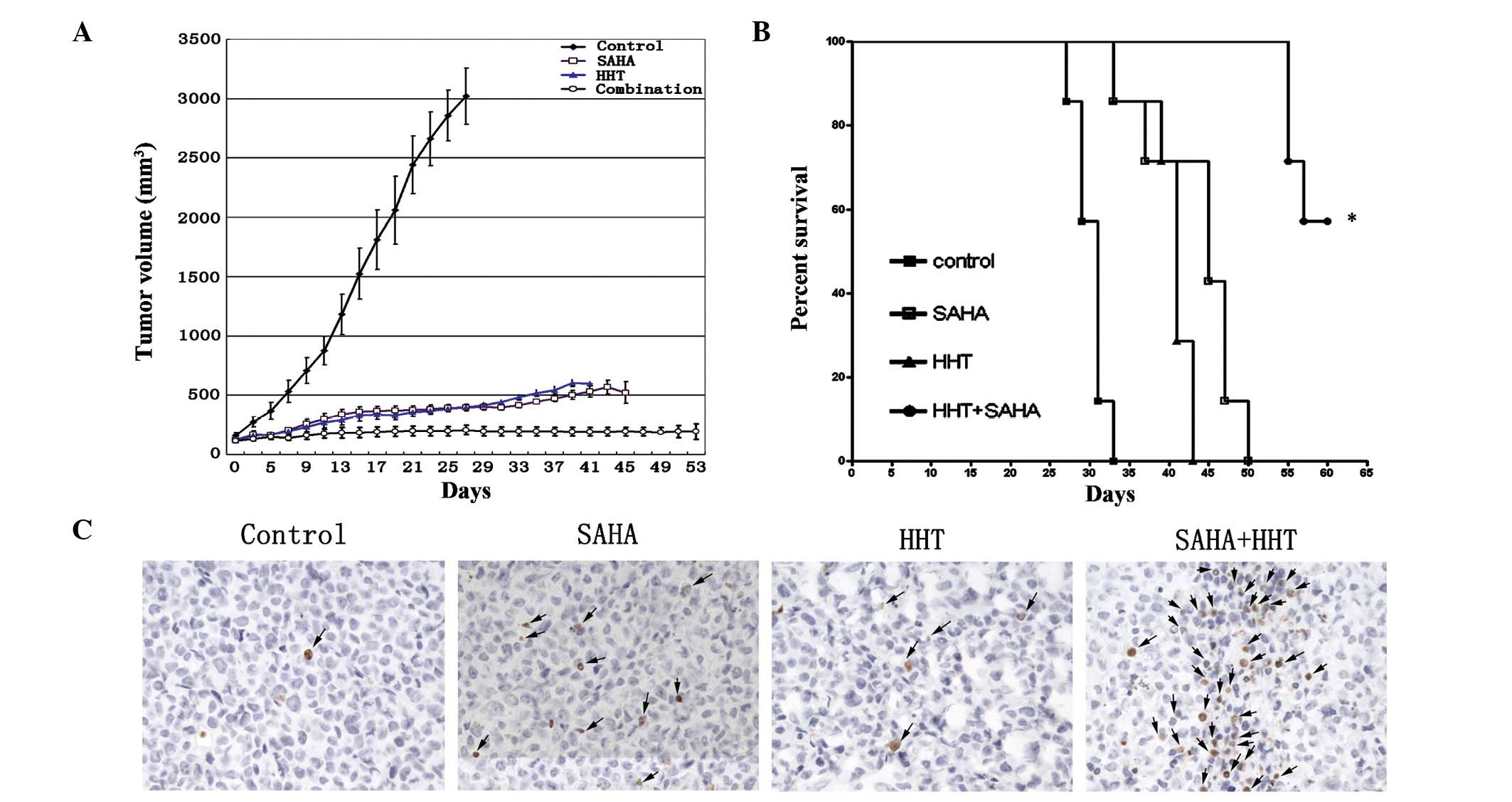
Human leukemia xenografts were established by
subcutaneously injecting THP-1 cells (1×107) into the
right flank of female SCID mice. Tumor size was measured regularly
following inoculation and mice (each group, n=8) bearing a tumor of
100–130 mm3 were randomized to receive treatment with
intraperitoneal injection of PBS, HHT, SAHA or two drugs. The
treatment regimen was as follows: monotherapy groups, injections of
50 mg/kg SAHA for 5 consecutive days or 1 mg/kg HHT for 7
consecutive days; and combination therapy group, injections of 50
mg/kg SAHA for 5 days and 1 mg/kg HHT for 7 days. When tumor
xenografts were established, tumor volume in the control group
markedly increased in a time-dependent manner and reached 3,102±238
mm3 on day 27. However, the volume of tumors in the
monotherapy group was only increased slightly (HHT, 602±28.8
mm3; SAHA, 521.1±91.6 mm3) and tumor growth
was inhibited further in mice receiving HHT and SAHA
simultaneously. In one case, a tumor xenograft in the combination
therapy group disappeared completely (Fig. 4A). In addition, mice in the
combination treatment group were found to survive for significantly
longer periods than mice in the other groups (Fig. 4B; P<0.0001). When tumor
xenografts were subjected to a TUNEL assay, tumors from the
combination therapy group revealed higher numbers of apoptotic
cells than the monotherapy group (Fig.
4C). These results further confirmed that the combination of
HHT and SAHA is effective in inhibiting the growth of AML
cell-derived tumors grafted in SCID mice via enhanced
apoptosis.
Discussion
HHT has demonstrated a marked efficacy in AML. In a
phase II trial, continuous infusion of HHT administered at a daily
dose of 5 mg/m2 led to CR in 7/43 patients with relapsed
AML (25). However, HHT is
associated with serious cardiovascular complications, including
hypotension at high doses and/or in short infusion schedules
(25). Previously, we demonstrated
that HHT (4 mg daily) combined with ara-C and aclarubicin resulted
in a high CR rate and longer survival in newly diagnosed non-M3 AML
patients (23). Additionally, a
Chinese study revealed that combination treatment with HHT (2
mg/m2/day) plus ara-C was an effective induction regimen
in elderly patients with de novo AML (22). There were no severe cardiovascular
complications observed in these clinical trials, indicating that
reducing drug dosage abrogates the occurrence of cardiotoxicity and
that HHT-based combination therapy is a promising therapeutic
approach to improve patient outcome.
In the present study, the effects of HHT combined
with SAHA on AML cells were investigated and a synergistic effect
between HHT and SAHA on Kasumi-1 and THP-1 cells was identified by
calculation of CI. The combination therapy also enhanced apoptosis
in THP-1 cells and was found to significantly inhibit the tumor
growth of leukemia xenografts in vivo compared with each
agent alone. These results demonstrate that synergistic
interactions occurred in both AML cell lines treated with low
concentrations (2–16 ng/ml) of HHT and clinically achievable doses
of SAHA (26), namely, 0.5–4 μM.
Previously, a pharmacokinetic study of semisynthetic HHT
demonstrated that the mean peak plasma and minimum concentrations
were 78 and 27.4 ng/ml, respectively, at day 5 following the final
subcutaneous injection of 3 mg/m2/day (27). These concentrations exceeded those
required to inhibit 50% of the growth of AML HL-60 cells in
vitro (20 ng/ml) (28).
Consistent pharmacokinetic parameters were observed in AML patients
treated with natural HHT (29).
Collectively, these observations indicate that co-administration of
HHT and SAHA results in a marked increase in antileukemic activity
and represents an attractive combination therapy for AML.
In the current study, mechanistic analyses in AML
cell lines indicated that the induction of apoptosis via activation
of intrinsic and extrinsic apoptotic pathways may contribute to the
potent synergism between HHT and SAHA. Notably, induction of
apoptosis was accompanied by upregulation of TRAIL expression
induced by HHT and increased expression of DR4/DR5 induced by SAHA.
SAHA has been previously demonstrated to upregulate DR4/DR5 in AML
HL-60 and U937 cells (24). HHT
also slightly enhanced expression of DR4/DR5. To the best of our
knowledge, this is the first study demonstrating that HHT treatment
leads to increased expression of TRAIL and DR5 in AML cells.
Previously, HHT has been revealed to induce apoptosis in various
types of leukemic cells, which was characterized by cytochrome c
release and activation of caspase (30). In AML cells, HHT leads to a
decrease in mitochondrial membrane potential (31), which is regulated by activation of
caspase-9 via cleaved Bid as a result of caspase-8 activation. In
the present study, enhanced activation of caspase-9 and increased
release of cytochrome c were observed, and may have been the result
of enhanced activation of the extrinsic apoptosis pathway induced
by upregulation of TRAIL and DR4/DR5.
It is well known that TRAIL has a marked apoptotic
effect in tumor cells but not in normal cells, thereby representing
a promising cancer therapeutic agent. By interacting with its
cognate cell receptors, DR4 and DR5, TRAIL activates the death
receptor-mediated apoptotic signaling (extrinsic) pathway,
resulting in activation of caspase-8 and additional downstream
caspases (32). In specific cells,
activation of caspase-8 leads to induction of the intrinsic
apoptotic pathway by Bid cleavage (32). Previous studies have confirmed that
primary AML cells are generally resistant to apoptosis induction by
TRAIL, which may be due to the expression of TRAIL decoy receptors
and downregulation of DR4 (33,34).
Min et al(35) investigated
DR4/DR5 expression in primary AML cells and found that 9/29 (31%)
patients were DR4−, whereas all patients were
DR5+. These studies and current observations demonstrate
that co-treatment with clinically relevant doses of HHT and SAHA
results in upregulation of the TRAIL pathway and enhanced cell
death, which was reduced by an anti-TRAIL antibody. Therefore, we
concluded that co-treatment with HHT and SAHA may be effective for
the treatment of AML by targeting the TRAIL pathway; however,
further clinical studies are required.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81070419 and
81200384), Zhejiang Provincial Natural Science Foundation of China
(no. R2090392) and Funds of Science Technology Department of
Zhejiang Province (nos. 2012C13021-2 and 2012C37103).
References
|
1
|
Taby R and Issa JP: Cancer epigenetics. CA
Cancer J Clin. 60:376–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao Y, Gao Z, Marks PA and Jiang X:
Apoptotic and autophagic cell death induced by histone deacetylase
inhibitors. Proc Natl Acad Sci USA. 101:18030–18035. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vrana JA, Decker RH, Johnson CR, et al:
Induction of apoptosis in U937 human leukemia cells by
suberoylanilide hydroxamic acid (SAHA) proceeds through pathways
that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but
independent of p53. Oncogene. 18:7016–7025. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richon VM, Emiliani S, Verdin E, et al: A
class of hybrid polar inducers of transformed cell differentiation
inhibits histone deacetylases. Proc Natl Acad Sci USA.
95:3003–3007. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosato RR, Almenara JA, Maggio SC, et al:
Role of histone deacetylase inhibitor-induced reactive oxygen
species and DNA damage in LAQ-824/fludarabine antileukemic
interactions. Mol Cancer Ther. 7:3285–3297. 2008. View Article : Google Scholar
|
|
6
|
Miller CP, Singh MM, Rivera-Del Valle N,
Manton CA and Chandra J: Therapeutic strategies to enhance the
anticancer efficacy of histone deacetylase inhibitors. J Biomed
Biotechnol. 2011:Jun 28–2011.(Epub ahead of print).
|
|
7
|
Bali P, Pranpat M, Bradner J, et al:
Inhibition of histone deacetylase 6 acetylates and disrupts the
chaperone function of heat shock protein 90: a novel basis for
antileukemia activity of histone deacetylase inhibitors. J Biol
Chem. 280:26729–26734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Redner RL, Wang J and Liu JM: Chromatin
remodeling and leukemia: new therapeutic paradigms. Blood.
94:417–428. 1999.PubMed/NCBI
|
|
9
|
Wang J, Saunthararajah Y, Redner RL and
Liu JM: Inhibitors of histone deacetylase relieve ETO-mediated
repression and induce differentiation of AML1-ETO leukemia cells.
Cancer Res. 59:2766–2769. 1999.PubMed/NCBI
|
|
10
|
Garcia-Manero G, Yang H, Bueso-Ramos C, et
al: Phase 1 study of the histone deacetylase inhibitor vorinostat
(suberoylanilide hydroxamic acid [SAHA]) in patients with advanced
leukemias and myelodysplastic syndromes. Blood. 111:1060–1066.
2008.
|
|
11
|
Schaefer EW, Loaiza-Bonilla A, Juckett M,
et al: Mayo P2C Phase II Consortium: A phase 2 study of vorinostat
in acute myeloid leukemia. Haematologica. 94:1375–1382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shiozawa K, Nakanishi T, Tan M, et al:
Preclinical studies of vorinostat (suberoylanilide hydroxamic acid)
combined with cytosine arabinoside and etoposide for treatment of
acute leukemias. Clin Cancer Res. 15:1698–1707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuendgen A, Bug G, Ottmann OG, et al:
Treatment of poor-risk myelodysplastic syndromes and acute myeloid
leukemia with a combination of 5-azacytidine and valproic acid.
Clin Epigenetics. 2:389–399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie D, Huang K, Yin S, et al:
Synergistic/additive interaction of valproic acid with bortezomib
on proliferation and apoptosis of acute myeloid leukemia cells.
Leuk Lymphoma. 53:2487–2495. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCormack E, Haaland I, Venås G, et al:
Synergistic induction of p53 mediated apoptosis by valproic acid
and nutlin-3 in acute myeloid leukemia. Leukemia. 26:910–917. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie C, Edwards H, Xu X, et al: Mechanisms
of synergistic antileukemic interactions between valproic acid and
cytarabine in pediatric acute myeloid leukemia. Clin Cancer Res.
16:5499–5510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen R, Guo L, Chen Y, Jiang Y, Wierda WG
and Plunkett W: Homoharringtonine reduced Mcl-1 expression and
induced apoptosis in chronic lymphocytic leukemia. Blood.
117:156–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou YJ, Qian WB and Jin J:
Homoharringtonine induces apoptosis and growth arrest in human
myeloma cells. Leuk Lymphoma. 48:1400–1406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Legha SS, Keating M, Picket S, Ajani JA,
Ewer M and Bodey GP: Phase I clinical investigation of
homoharringtonine. Cancer Treat Rep. 68:1085–1091. 1984.PubMed/NCBI
|
|
20
|
Kantarjian HM, Keating MJ, Walters RS,
Koller CA, McCredie KB and Freireich EJ: Phase II study of low-dose
continuous infusion homoharringtonine in refractory acute
myelogenous leukemia. Cancer. 63:813–817. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kantarjian HM, Talpaz M, Smith TL, et al:
Homoharringtonine and low-dose cytarabine in the management of late
chronic-phase chronic myelogenous leukemia. J Clin Oncol.
18:3513–3521. 2000.PubMed/NCBI
|
|
22
|
Wang J, Lü S, Yang J, et al: A
homoharringtonine-based induction regimen for the treatment of
elderly patients with acute myeloid leukemia: a single center
experience from China. J Hematol Oncol. 2009:Jul 30–2009.(Epub
ahead of print).
|
|
23
|
Jin J, Jiang DZ, Mai WY, et al:
Homoharringtonine in combination with cytarabine and aclarubicin
resulted in high complete remission rate after the first induction
therapy in patients with de novo acute myeloid leukemia. Leukemia.
20:1361–1367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shankar S, Singh TR, Fandy TE, Luetrakul
T, Ross DD and Srivastava RK: Interactive effects of histone
deacetylase inhibitors and TRAIL on apoptosis in human leukemia
cells: involvement of both death receptor and mitochondrial
pathways. Int J Mol Med. 16:1125–1138. 2005.
|
|
25
|
Feldman E, Arlin Z, Ahmed T, et al:
Homoharringtonine is safe and effective for patients with acute
myelogenous leukemia. Leukemia. 6:1185–1188. 1992.PubMed/NCBI
|
|
26
|
Kelly WK, O’Connor OA, Krug LM, et al:
Phase I study of an oral histone deacetylase inhibitor,
suberoylanilide hydroxamic acid, in patients with advanced cancer.
J Clin Oncol. 23:3923–3931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lévy V, Zohar S, Bardin C, et al: A phase
I dose-finding and pharmacokinetic study of subcutaneous
semisynthetic homoharringtonine (ssHHT) in patients with advanced
acute myeloid leukaemia. Br J Cancer. 95:253–259. 2006.PubMed/NCBI
|
|
28
|
Luo CY, Tang JY and Wang YP:
Homoharringtonine: a new treatment option for myeloid leukemia.
Hematology. 9:259–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan YP, Lee FW and Siu TS: Quantitation
of homoharringtonine in plasma by high-performance liquid
chromatography with amperometric detection. J Chromatogr.
496:155–166. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Z, Lin M, Wuchter C, et al: Apoptotic
response to homoharringtonine in human wt p53 leukemic cells is
independent of reactive oxygen species generation and implicates
Bax translocation, mitochondrial cytochrome c release and caspase
activation. Leukemia. 15:567–574. 2001. View Article : Google Scholar
|
|
31
|
Yin S, Wang R, Zhou F, Zhang H and Jing Y:
Bcl-xL is a dominant antiapoptotic protein that inhibits
homoharringtonine-induced apoptosis in leukemia cells. Mol
Pharmacol. 79:1072–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaufmann SH and Steensma DP: On the TRAIL
of a new therapy for leukemia. Leukemia. 19:2195–2202. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riccioni R, Pasquini L, Mariani G, et al:
TRAIL decoy receptors mediate resistance of acute myeloid leukemia
cells to TRAIL. Haematologica. 90:612–624. 2005.PubMed/NCBI
|
|
34
|
Jones DT, Ganeshaguru K, Mitchell WA, et
al: Cytotoxic drugs enhance the ex vivo sensitivity of malignant
cells from a subset of acute myeloid leukaemia patients to
apoptosis induction by tumour necrosis factor receptor-related
apoptosis-inducing ligand. Br J Haematol. 121:713–720. 2003.
View Article : Google Scholar
|
|
35
|
Min YJ, Lee JH, Choi SJ, et al: Prognostic
significance of Fas (CD95) and TRAIL receptors (DR4/DR5) expression
in acute myelogenous leukemia. Leuk Res. 28:359–365. 2004.
View Article : Google Scholar : PubMed/NCBI
|