Introduction
Alzheimer's disease (AD) is the most common
dementing illness globally. By 2050, one person in every 85 is
predicted to suffer from this disease (1). Neurodegenerative diseases may cause
memory loss, impaired cognitive function and eventually, death.
Pathological features include the impairment of axonal and
dendritic transport, swelling of axons, dendrites and varicosities
and breakage. Early experiments revealed that the maintenance of
normal neurite transport and the morphology of neurons are vital
for the biological functions of neurons (2). Our study casts light on the mechanism
of neuronal development and provides a novel and valuable
possibility for therapeutic intervention in AD.
Gene modulation, particularly post-transcriptional
gene regulation, acts as a pivotal regulator in brain development
(3). MicroRNAs (miRNAs) are a type
of non-coding small RNA mediating gene silencing, and are 21- to
23-nucleotides long. Thousands of miRNAs have been identified in
mammals, a number of which are expressed spatially and temporally
in the process of brain development (4). Significant progress has been made in
identifying targets of miRNAs. More than 60% of all mammalian mRNAs
are tightly controlled by miRNAs. miRNAs are part of signaling
networks serving as an important mediator of crosstalk between
signaling pathways, coordinating their activity (4,5).
miRNA biogenesis pathways are widely recognized as regulators in
the development of the central nervous system (CNS). For instance,
miR-124, which accounts for 25–48% of all brain-specific miRNAs,
has been studied extensively (6).
miR-9 is specifically and abundantly expressed in the neurogenic
regions of the brain (7). miR-124
and miR-128 are prone to appear in neurons, whereas miR-23 is
expressed in astrocytes. miR-132 has been reported to modulate
neuronal morphogenesis via suppressing the expression of the
GTPase-activating protein p250GAP (8). miR-134 has been reported to regulate
the development of neurite spines through repressing expression of
the Limk1 protein kinase (9).
During brain development, the expression of miR-128 is upregulated,
and ectopic expression of miR-128 may promote neural
differentiation and augment the average dendritic spine length of
neural stem cells (NSCs) (10).
Accumulating evidence supports the crucial roles of miRNAs in
neuronal development. Our current study demonstrated that the
expression of miR-26a in neurons is higher than that in NSCs;
however, the function of miR-26a in neurons has not been validated
experimentally. In addition, the correlation between miR-26a,
synaptic plasticity and the growth of neurites has not yet been
documented. Therefore, the present study focused on the function of
miR-26a in neuronal outgrowth.
Materials and methods
Cell culture and transfection
All procedures were performed in accordance with a
protocol approved by the Institutional Animal Care and Use
Committee (Chinese Academy of Science). Neonatal rat cortical
neurons and NSCs were obtained from the cortex of male
Sprague-Dawley rats (purchased from the Animal Center of Chinese
Academy of Science, Beijing, China), as described previously
(11). Neurons were plated on
poly-D-lysine (10 μg/ml) and laminin (5 μg/ml; Sigma-Aldrich, St.
Louis, MO, USA)-coated glass. The electroporation of primary
cortical neurons was conducted using a rat neuron nucleofector kit
(Amaxa Biosystems, Gaithersburg, MD, USA) according to the
manufacturer's instructions, and the neurons were cultured in
neurobasal media supplemented with factor B27, 2 mM L-glutamine,
0.06 mg/ml cysteine, 1 mM sodium pyruvate, penicillin and
streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C and 5%
CO2.
Expression vectors
The phosphatase and tensin homolog (PTEN) and its
short hairpin RNA (shRNA) were amplified and cloned into pCAG-EGFP.
The primers were as follows: PTEN forward,
5′-CGGGATCCGACATGACAGCCATCATCAAAG-3′ and reverse,
5′-CCGCTCGAGTCAGACTTTTGTAATTTGTGTATG-3′; shRNA forward,
5′-GGCGCUAUGUGUAUUAUUATT-3′ and reverse,
5′-UAAUAAUACACAUAGCGCCTT-3′; and control miRNA sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′. The sequences for the miR-26a mimics
were as follows: sense, 5′-UUCAAGUAAUCCAGGAUAGGCU-3′ and
anti-sense, 5′-CCUAUCCUGGAUUACUUGAAUU-3′; miR-26a inhibitor,
5′-AGCCUAUCCUGGAUUACUUGAA-3′ and control,
5′-CAGUACUUUUGUGUAGUACAA-3′.
Cell viability analysis
The viability of transfected neurons was determined
by a CCK assay kit (Dojindo, Kumamoto, Japan). Transfected neurons
were seeded at a density of 5×103 cells per well into
96-well plates. The CCK-8 solution was diluted with neuronal media
(1:10), and 100 μl of the resulting solution was added to each well
and incubated for 2 h at 37°C. The absorbance was determined at 450
nm by a microplate reader (BioTek, Winooski, VT, USA).
Western blot analysis
For western blot analysis, cells were first washed
with cold PBS 3 times and then lysed in RIPA buffer (Solarbio,
Beijing, China). Protein lysates (30 μg) were electrophoresed on
SDS-PAGE gels and transferred to PVDF membranes (Millipore,
Billerica, MA, USA). The membranes were blocked for 1 h at room
temperature (RT) with 5% milk protein and 0.05% Tween 20 in PBS.
Mouse monoclonal anti-PTEN IgG and mouse monoclonal anti-GADPH
(Cell Signaling Technology, Inc., Danvers, MA, USA) were used
according to the manufacturer's instructions. The membranes were
washed 3 times with PBS for 10 min and then incubated for 1 h at RT
with the anti-mouse infrared dye-conjugated secondary antibodies.
After washing 3 times, the bands and band intensity were analyzed
using an Odyssey instrument (LI-COR, USA).
Reverse transcription and quantitative
(q)PCR
Total RNA was isolated from cells using TRIzol
(Invitrogen) according to the manufacturer's instructions. For
miR-26a, total RNA (50 ng) was transcribed into cDNA using a TaqMan
MicroRNA Reverse Transcription kit (Applied Biosystems, Carlsbad,
CA, USA) according to the manufacturer's instructions. qPCR was
performed with a TaqMan MicroRNA Assay Kit (Applied Biosystems).
The expression of U6 was used as a control, and the fold-change was
calculated by the 2−ΔΔCt method. The amplification and
detection of specific products were performed using the ABI Prism
7500 system (Applied Biosystems).
Luciferase reporter assay
The 3′ untranslated region (3′-UTR) sequence of rat
PTEN mRNA containing the miR-26a binding site was amplified and
cloned into a pMIR-REPORT luciferase reporter vector, known as
pMIR-PTEN-3′UTR. The vector of PTEN 3′UTR with the miR-26a binding
site was co-transfected separately with miR-26a, miRNA control or
miR-26a inhibitor into primary neurons. After 24 h, the luciferase
assay was performed according to the manufacturer's
instructions.
Immunocytochemistry and quantitation of
neuronal morphology
Cortical neurons were fixed with 4% paraformaldehyde
at RT for 30 min. Neurons were blocked (1 h at RT) in 5% BSA in PBS
with 0.01% Triton X-100 and probed with neuronal class III
β-tubulin (1:1,000; Sigma-Aldrich) and DAPI (2 μg/ml;
Sigma-Aldrich). The cells were then analyzed under a Zeiss 780
confocal microscope (Carl Zeiss AG, Oberkochen, Germany). Images of
neurons were traced and quantified by the NeuronJ program (12).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Variance analysis between the groups was performed
by one-way ANOVA, and significant differences between the groups
were analyzed by Dunnett's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analysis was performed using SPSS 16.0 statistical
software (SPSS, Inc., Chicago, IL, USA).
Results
miR-26a is highly expressed in mature
neurons
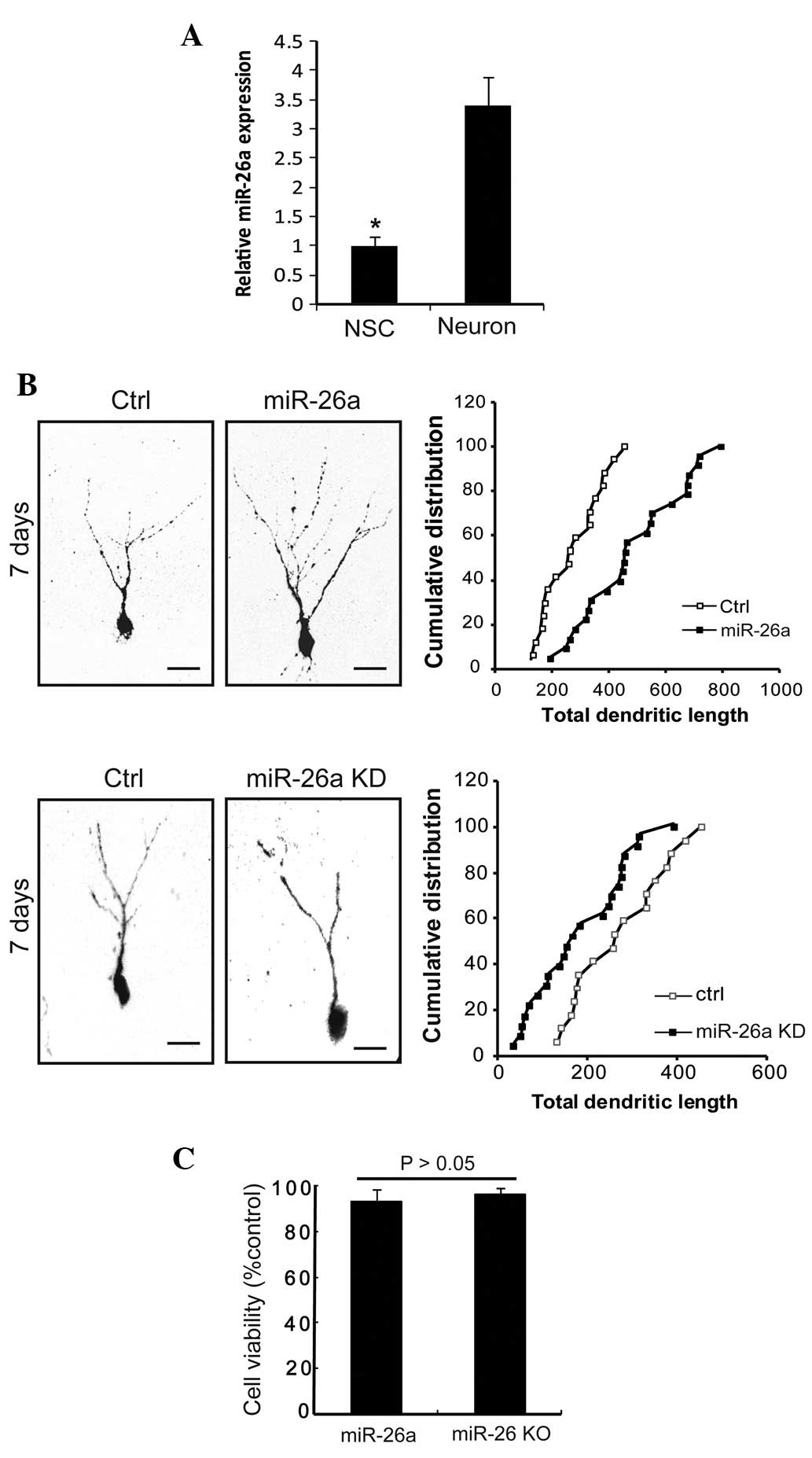
miR-26a has exhibited a consistent pattern of
expression in in vivo and in vitro models (12). To identify whether miR-26a is
involved in the development of neurons, qPCR analysis was performed
to characterize its expression. The expression of miR-26a in
neurons was markedly increased by 3.4-fold compared with NSCs
(Fig. 1A). Thus, the expression
level of miR-26a was higher in neurons than in NSCs. These data
suggested a marked correlation between neuronal development and the
miR-26a expression level, indicating that miR-26a may play a role
in the development of neurons.
Expression of miR-26a progressively
promotes the growth of neurites
Considering the expression of miR-26a in primary
neurons, we examined whether miR-26a plays a role in regulating the
growth of neurites. With the upregulation of miR-26a, neurite
morphology significantly changed. Morphometric analysis revealed
that miR-26a-transfected neurons had higher neurite numbers and the
average total length was greater than that of control
miRNA-transfected neurons (Fig.
1B). In addition, transfection of miR-26a inhibitor (miR-26a
knockdown, KD) induced a marked decrease in the distribution of
neurites and total neurite length (Fig. 1B). Growth curves of the two groups
were generated and it was found that the neurite branches were more
pronounced in the miR-26a overexpression (OE) group (Fig. 1B). To further examine whether this
phenotype was associated with the viability of neurons, neuronal
apoptosis was measured by a CCK assay, and it was shown that
miR-26a (OE) and miR-26a (KD) did not affect the survival of
neurons (94.9±4.3 vs. 96.1±2.8%; Fig.
1C). Taken together, our data suggested that miR-26a promotes
the growth of neurites and does not induce cell death.
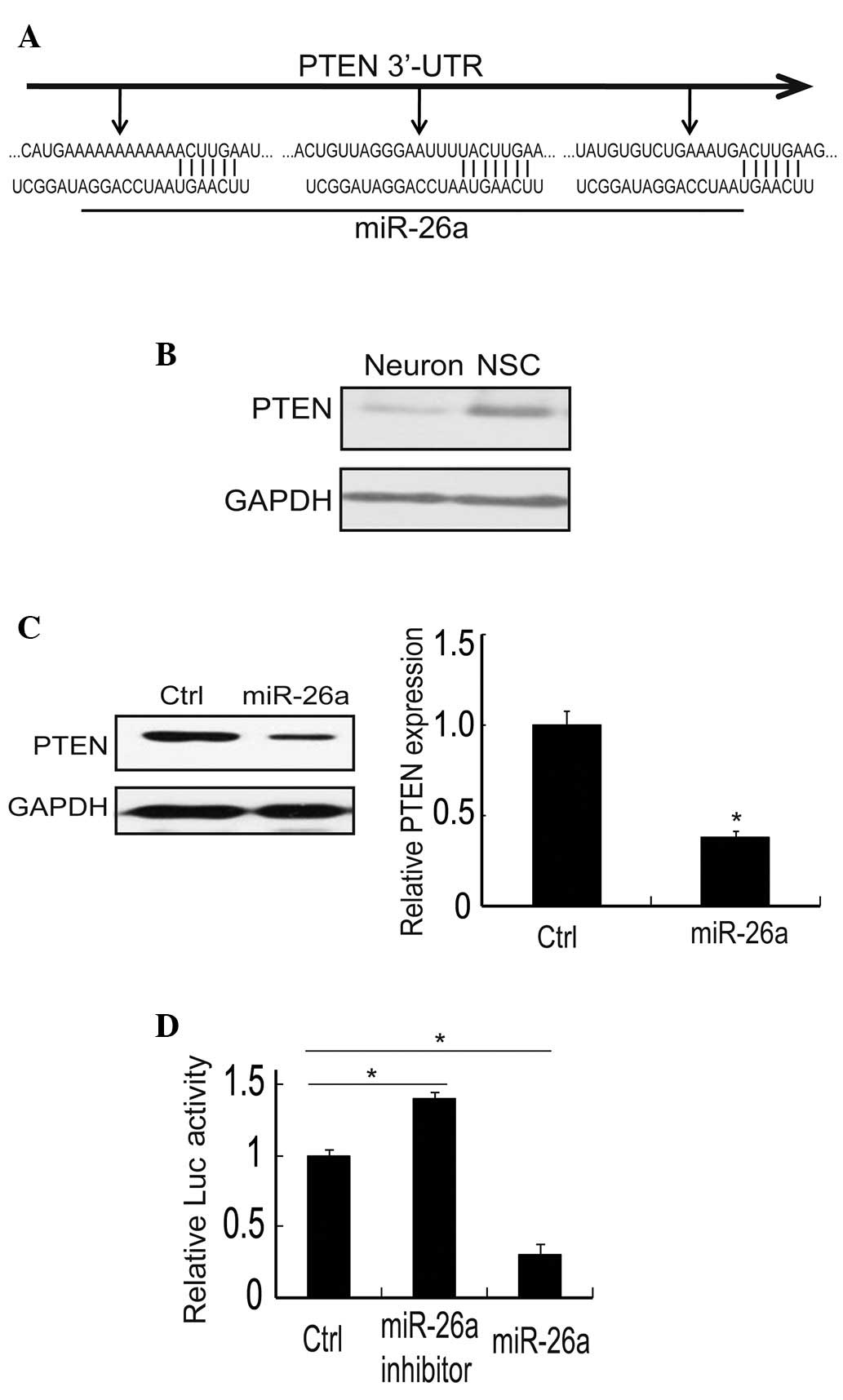
PTEN is a direct target of miR-26a in
neurons
A number of genes have been reported to be the
target of miR-26a. To gain insight into the mechanisms by which
miR-26a exerts its biological functions (13–15),
we explored predictive targets of miR-26a through the TargetScan
bioinformatics algorithm (www.targetscan.org). Result revealed that the 3′-UTR
of PTEN has three potential binding sites, which are highly
evolutionarily conserved (Fig.
2A). Due to miRNA modulation, the correspondent mRNA expression
and protein level may change (6).
Our data showed that the protein expression of PTEN is higher in
NSCs than in neurons (Fig. 2B). On
further study, the expression of PTEN protein was specifically
reduced in neurons transfected with miR-26a mimics, and the dose of
endogenous PTEN was reduced to 38.3% (Fig. 2C). Therefore, these results
indicated that there was a close correlation between PTEN and
miR-26a. A luciferase reporter gene assay was performed in order to
determine whether PTEN is a direct target of miR-26a. Firstly, the
3′-UTR of PTEN with one binding site was cloned into the
pMIR-REPORT luciferase vector as a positive control. This plasmid
was then co-transfected with miR-26a mimic, its control miR-control
and miR-26a inhibitor into primary neurons. The results indicated
that luciferase activity was markedly decreased in the miR-26a
group (31±4.2%), whereas it did not change with miR-control and was
increased in the miR-26a inhibitor group (139±2.1%; Fig. 2D). Taken together, these data
revealed that miR-26a suppressed the expression of PTEN and that
PTEN is a direct target of miR-26a.
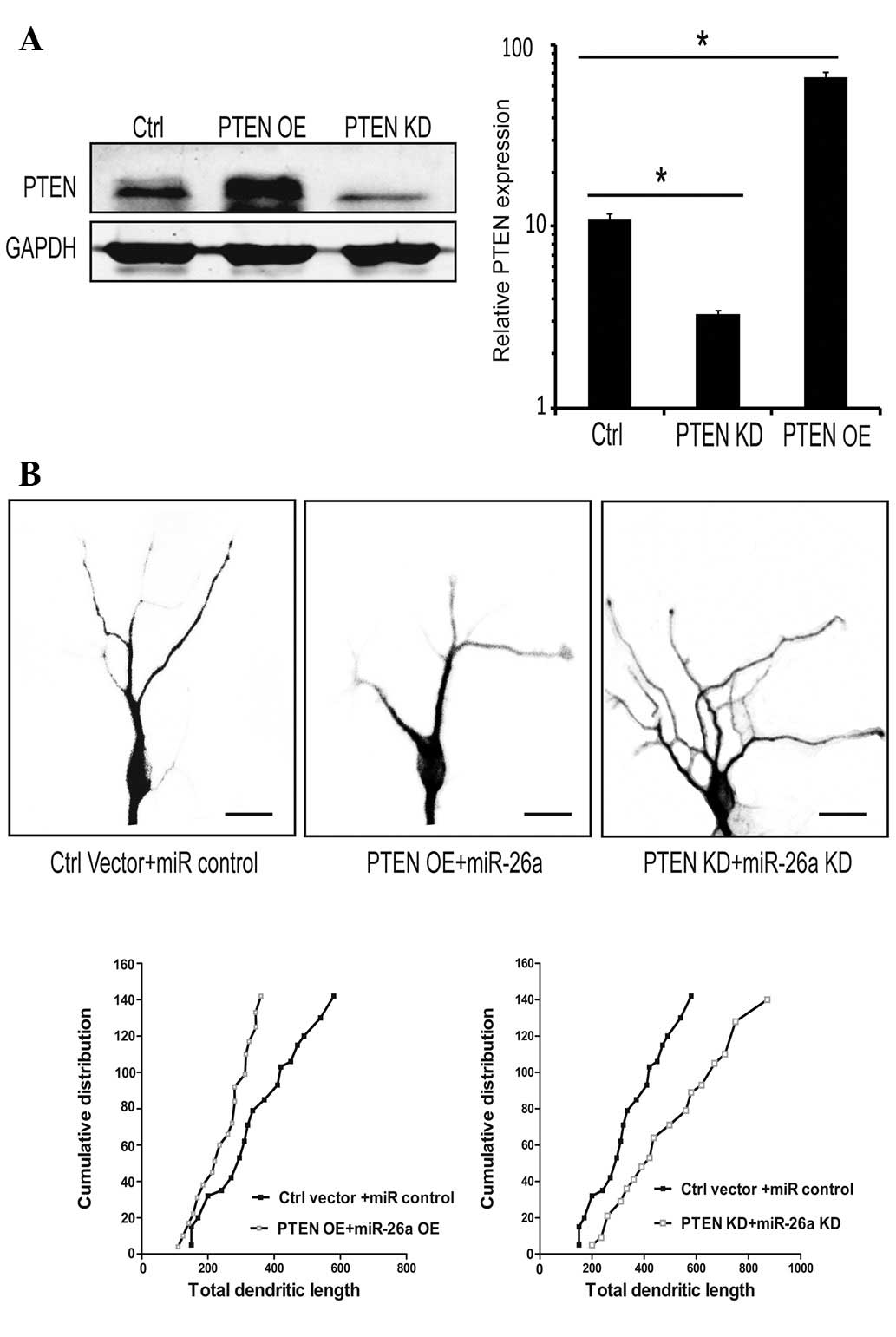
PTEN mediates the effect of miR-26a on
neurite outgrowth
miR-26a was shown to promote neurite outgrowth and
PTEN is involved in this process. To further investigate the effect
of PTEN on the growth of neurites, the efficiency of PTEN
overexpression (PTEN OE) and its knockdown (PTEN KD) were examined
(Fig. 3A). The results showed that
neurons transfected with PTEN generated an opposite result compared
with neurons transfected with miR-26a; the growth of neurites was
largely suppressed. By contrast, transfection with the shRNA of
PTEN enhanced neurite outgrowth, and the number and distribution of
neurites was significantly increased (Fig. 3B). The overexpression of PTEN
abolished the enhancement of neurite growth by miR-26a. PTEN
knockdown rescued the growth defect of neurons induced by treatment
with miR-26a inhibitor. The number and length of neurites was
attenuated and suppressed by PTEN overexpression. Therefore, we
concluded that PTEN is an important molecule in this signaling
pathway, which has marked biological control over the development
of the neuron, particularly neuronal morphogenesis.
Discussion
With the advance of therapeutics, the
biodemographics of the world is likely to change, since the number
of elderly people is constantly increasing. The number of patients
with AD and related dementias will also increase significantly from
current levels (16,17). The cognitive impairment of patients
seriously affects their quality of life (1); therefore, finding a method to cure or
prevent AD and other dementias is important. Current research has
provided us with more information on the basic biology of AD and
this will make the prevention of AD possible (1).
AD is the most common neurodegenerative disease.
Axonopathy and dendropathy is a subset of AD. It has been
demonstrated that the normal link between neurons in the cortex is
critical for the function of the brain (18,19).
Numerous miRNAs may also be involved in this process, including
miR9, miR124, miR155 and miR132. Together, these molecules maintain
the normal development of the CNS (8,20–22).
Our study showed that the expression of miR-26a was lower in NSCs
than in neurons, and lower in dysfunctional neurons. Furthermore,
we identified that miR-26a promotes the growth of neurites and
increases the number and length of the neurite branch. These
results indicate that miR-26a may be capable of ameliorating
cognitive impairment, although this requires further demonstration
in in vivo experiments. The pathway of miR-26a is considered
to be different depending on the cell and tissue types. A number of
previous studies have shown that PTEN is a target of miR-26a
(23,24). However, none of these studies
involved neurons. Our study revealed that PTEN is important in
neuronal development and that miR-26a affects the morphology of
neurons. It is conceivable that there may be other additional
targets regulated by miR-26a. This process also requires the
coordinated function of several genes, including the genes
associated with cytoskeletal reorganization (25). Further study is required to explore
the underlying mechanisms and the details of signaling pathways
involved in this process (26–28).
A conditional mouse model may provide more details on the precise
role of miR-26a in neurite outgrowth and neuronal maturation. These
results and future work may provide a novel insight into the
mechanism of neuronal dysfunction and may be beneficial for the
prevention of AD.
miR-26a was found to be capable of regulating
neurite outgrowth via the repression of PTEN expression. The
overexpression of miR-26a may induce the growth of neurites, and
this is beneficial to the function of impaired neurons. This
finding may prove useful for the treatment of patients with AD.
Acknowledgements
This work was sponsored by the National Science
Foundation of China (NSFC; no. 3087221).
References
|
1
|
Carrillo MC, Brashear HR, Logovinsky V, et
al: Can we prevent Alzheimer's disease? Secondary “prevention”
trials in Alzheimer's disease. Alzheimers Dement. 9:123–131.
2013.
|
|
2
|
McKhann G, Drachman D, Folstein M, et al:
Clinical diagnosis of Alzheimer's disease. Neurology.
34:9391984.
|
|
3
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010. View
Article : Google Scholar
|
|
5
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deo M, Yu JY, Chung KH, Tippens M and
Turner DL: Detection of mammalian microRNA expression by in situ
hybridization with RNA oligonucleotides. Dev Dyn. 235:2538–2548.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vo N, Klein ME, Varlamova O, et al: A
cAMP-response element binding protein-induced microRNA regulates
neuronal morphogenesis. Proc Natl Acad Sci USA. 102:16426–16431.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim LP, Lau NC, Garrett-Engele P, et al:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruno IG, Karam R, Huang L, et al:
Identification of a microRNA that activates gene expression by
repressing nonsense-mediated RNA decay. Mol Cell. 42:500–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikolic M, Chou MM, Lu W, Mayer BJ and
Tsai LH: The p35/Cdk5 kinase is a neuron-specific Rac effector that
inhibits Pak1 activity. Nature. 395:194–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meijering E and Jacob M: Design and
validation of a tool for neurite tracing and analysis in
fluorescence microscopy images. Cytometry A. 58:167–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YF, Zhang AR, Zhang BC, et al:
miR-26a regulates cell cycle and anoikis of human esophageal
adenocarcinoma cells through Rb1-E2F1 signaling pathway. Mol Biol
Rep. 40:1711–1720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miko E, Czimmerer Z, Csánky E, et al:
Differentially expressed microRNAs in small cell lung cancer. Exp
Lung Res. 35:646–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Wu X, Liu B, et al: miR-26a
enhances metastasis potential of lung cancer cells via AKT pathway
by targeting PTEN. Biochim Biophys Acta. 1822:1692–1704. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim H, Huang W, Jiang X, et al:
Integrative genome analysis reveals an oncomiR/oncogene cluster
regulating glioblastoma survivorship. Proc Natl Acad Sci USA.
107:2183–2188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaupel JW: Biodemography of human ageing.
Nature. 464:536–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graves AB, Larson EB, Edland SD, Bowen JD,
et al: Prevalence of dementia and its subtypes in the Japanese
American population of King County, Washington state. The Kame
Project. Am J Epidemiol. 144:760–771. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moceri VM, Kukull WA, Emanuel I, van Belle
G and Larson EB: Early-life risk factors and the development of
Alzheimer's disease. Neurology. 54:415–420. 2000. View Article : Google Scholar
|
|
20
|
Cheng LC, Pastrana E, Tavazoie M and
Doetsch F: miR-124 regulates adult neurogenesis in the
subventricular zone stem cell niche. Nat Neurosci. 12:399–408.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao C, Sun G, Li S and Shi Y: A feedback
regulatory loop involving microrna-9 and nuclear receptor tlx in
neural stem cell fate determination. Nat Struct Mol Biol.
16:365–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunand-Sauthier I, Santiago-Raber ML,
Capponi L, et al: Silencing of c-Fos expression by microRNA-155 is
critical for dendritic cell maturation and function. Blood.
117:4490–4500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lobnig BM, Krömeke O, Optenhostert-Porst C
and Wolf OT: Hippocampal volume and cognitive performance in
long-standing type 1 diabetic patients without macrovascular
complications. Diabet Med. 23:32–39. 2006.
|
|
24
|
Zhang B, Liu XX, He JR, et al:
Pathologically decreased miR-26a antagonizes apoptosis and
facilitates carcinogenesis by targeting MTDH and EZH2 in breast
cancer. Carcinogenesis. 32:2–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alajez NM, Shi W, Hui AB, et al: Enhancer
of Zeste homolog 2 (EZH2) is overexpressed in recurrent
nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and
miR-98. Cell Death Dis. 1:e852010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuhn TB, Meberg PJ, Brown MD, et al:
Regulating actin dynamics in neuronal growth cones by ADF/cofilin
and rho family GTPases. J Neurobiol. 44:126–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacobson C, Schnapp B and Banker GA: A
change in the selective translocation of the Kinesin-1 motor domain
marks the initial specification of the axon. Neuron. 49:797–804.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang H, Guo W, Liang X and Rao Y: Both
the establishment and the maintenance of neuronal polarity require
active mechanisms: critical roles of GSK-3beta and its upstream
regulators. Cell. 120:123–135. 2005.PubMed/NCBI
|