Introduction
Staphylococcus aureus (S. aureus) is one of
the most common bacterial infections worldwide, and is a common
pathogen that is encountered clinically by obstetricians,
gynecologists and neonatologists (1–3). It
is carried by 25–35% of the population as residential bacterial
flora, which may cause a wide range of diseases (including
arthritis, osteomyelitis, dermatitis and mastitis), as well as
bacteremia, abscesses, toxemia and sepsis. These suppurative
infections are associated with tissue destruction and cell death
(4). Multiple virulence factors
are required for S. aureus to induce apoptosis in infected
cells, among which staphylococcal enterotoxin B (SEB) and α-toxin
are important. α-toxin, a 34-kDa pore-forming protein, is essential
for the modulation of S. aureus-induced cytotoxicity in
Jurkat T lymphocytes, human peripheral blood lymphocytes and
monocytes (5). In addition,
α-toxin is capable of inducing the apoptosis of epithelial
(6) and endothelial (7,8)
cells. SEB is not only an exotoxin associated with fatal toxic
shock syndrome (9), but also a
superantigen with the capacity to strongly activate oligoclonal
populations of T lymphocytes, which express antigen receptors with
homologous β chain variable regions (Vβ families) (10). A number of studies have
demonstrated that SEB activates the pathways controlling the
process of apoptosis in a variety of cell types (11,12).
As SEB and α-toxin are simultaneously produced by S. aureus
and are involved in disease pathogenesis, it is necessary to
investigate whether SEB and α-toxin are able to induce the
apoptosis of the same cells.
Apoptosis, or programmed cell death, is primarily
mediated by a family of intracellular cysteine proteases, termed
caspases (13). Caspases are
synthesized as inactive proenzymes and are proteolytically
processed to form an active complex. Caspases may be divided into
initiator and effector caspases, according to their structure and
order. Initiator caspases, including caspase-8 and -9, exert
regulatory roles. Upon binding to signal transducing molecules,
they activate downstream effector caspases, such as caspase-3,
which cleave various cellular substrates, thereby inducing the
death of the cell (14,15). The activation of caspases is
achieved via two principal signaling pathways, namely the extrinsic
and intrinsic death pathways (14). The extrinsic death pathway involves
the ligation of death receptors (CD95/Fas/APO-1 and TNF
receptor-1), resulting in the recruitment of the adapter molecule,
Fas-activated death domain (FADD), and pro-caspase-8 into a
death-inducing signaling complex. By contrast, the intrinsic death
pathway is initiated at the mitochondrion by the release of
cytochrome c, a process that is inhibited by anti-apoptotic
proteins of the Bcl-2 family. When released, cytosolic cytochrome
c binds to dATP, and apoptosis-activating factor-1 binds to
pro-caspase-9, to form the apoptosome. Upon the formation of this
death-inducing signaling complex, pro-caspase-8 and -9 are
autoproteolytically processed, respectively, resulting in the
activation of downstream caspases. In this study, we utilized
ECV304 cells, which exhibit certain endothelial characteristics and
are advantageous for the study of receptor pharmacology and
cytochemistry (16). The cells
were used to investigate whether SEB and α-toxin were able to
induce the apoptosis of ECV304 cells, and whether this occurred via
similar mechanisms.
Materials and methods
Reagents
SEB and α-toxin were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Polyclonal anti-human TNF-α was purchased
from PeproTech, Inc. (Rocky Hill, NJ, USA). Caspase-3 inhibitory
peptide [z-N-acetyl-Asp-Glu-Val-Asp-aminomethyl-coumarin
(DEVD)-fluoromethyl ketone (FMK)], caspase-8 inhibitory peptide
[z-N-acetyl-Ile-Glu-Thr-Asp (IETD)-FMK], the Caspase-3 Colorimetric
Assay kit and the Caspase-8 Colorimetric Assay kit were purchased
from BioVision (Mountain View, CA, USA). The human TNF-α Legend
Max™ ELISA kit was obtained from BioLegend, Inc. (San Diego, CA,
USA), and the Annexin V-FITC staining kit was purchased from
Pharmingen (Heidelberg, Germany).
Cell lines and culture
ECV304 cells (Cell Lines Service, Gmbh, Eppelheim,
Germany) were maintained in RPMI-1640 medium (Gibco-BRL, Carlsbad,
CA, USA) with 2 mM L-glutamine, adjusted to contain 1.5 mg/ml
sodium bicarbonate, and supplemented with 0.1 mg/ml heparin, 10%
heat-inactivated fetal bovine serum (FBS), 50 μg/ml streptomycin
and 50 μg/ml penicillin at 37°C in a humidified atmosphere with 5%
CO2. Assays were performed in RPMI-1640 medium that was
not supplemented with 10% FBS. In certain experiments, the
RPMI-1640 medium was supplemented with various inhibitors,
antibodies and/or different concentrations of SEB or α-toxin.
Treatment of cultured cells with SEB or
α-toxin, antibodies and various inhibitors
Cultured cells were counted and plated at a density
of 1×105/ml in 6-well tissue culture plates. When the
cells had adhered to the plates and the medium was changed to
serum-free RPMI-1640 medium, different concentrations of SEB or
α-toxin were added to the cells for 8 h. Soluble culture
supernatants and cell pellets were collected following
centrifugation at 400 × g for 10 min. The level of TNF-α in the
culture supernatants was measured using the ELISA kit, according to
the manufacturer’s instructions. The levels of apoptosis and
caspase activity were measured in the cell pellets. When antibody
or caspase inhibitors were used, anti-human TNF-α, z-DEVD-FMK or
z-IETD-FMK was added to the cultured cells, resulting in final
concentrations of 20 μg/l, 2 mM and 2 mM, respectively. Cell
pellets were also collected in order to detect apoptosis by flow
cytometry. Cells cultured without SEB or α-toxin were used as
controls in all assays.
Detection of apoptosis by flow cytometric
analysis
To monitor the apoptosis-related changes in the
plasma membrane, phosphatidylserine was detected on the surface of
the plasma membrane using annexin V, and the membrane permeability
was assessed using propidium iodide (PI) (17), according to the instructions
provided by the manufacturer of the Annexin V staining kit.
Briefly, 1×105/ml cells were exposed to medium alone
(that did not contain FBS) or various inhibitors, antibodies,
and/or different concentrations of SEB or α-toxin. The cells were
then washed and re-suspended in 0.1 ml staining solution [2%
annexin V-FITC and 2% PI-phycoerythrin (PE), by volume, in HEPES
buffer], and incubated for 15 min in the dark at room temperature.
The cells were then analyzed by flow cytometry on a
fluorescence-activated cell sorting system (FACSCalibur, Becton
Dickinson, Heidelberg, Germany) using CellQuest analysis software
(BD Biosciences). For each determination, a minimum of 50,000 cells
were analyzed. Live cells were not stained with PI or annexin V.
Early apoptotic cells were defined as those stained with annexin V,
but not with PI, and late apoptotic or necrotic cells were defined
as those stained with both reagents.
Colorimetric determination of caspase-3
and -8 activity
The activity of the caspases was determined using
Caspase Colorimetric Assay kits according to the manufacturer’s
instructions. Briefly, cell lysates were incubated for 2 h at 37°C
with 5 μl of the 4 mM substrates [DEVD-p-nitroanilide
(pNA) for caspase-3, or IETD-pNA for caspase-8] in a
50-μl reaction buffer, containing 50 mM HEPES (pH 7.4), 100 mM
NaCl, 10% sucrose, 0.1% CHAPS and 10 mM DTT. Based on the
chromophore pNA following cleavage from the labeled
substrate (DEVD-pNA or IETD-pNA), the pNA
light emission was quantified using a spectrophotometer at 405 nm.
The comparison between the absorbance of pNA in an apoptotic
sample and in an uninduced control enabled the determination of the
fold-increase in caspase activity.
Statistical analysis
The results are presented as the mean ± SEM. To
assess the significance of differences within experiments, a Tukey
b test in a one-way analysis of variance (ANOVA) was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Induction of apoptosis in ECV304 cells
exposed to SEB or α-toxin
Flow cytometry analysis of FITC-annexin V-labeled
cells stained with PI enabled host cells in the early and late
stages of apoptosis to be distinguished. Following the incubation
of the ECV304 cells with different concentrations of SEB or α-toxin
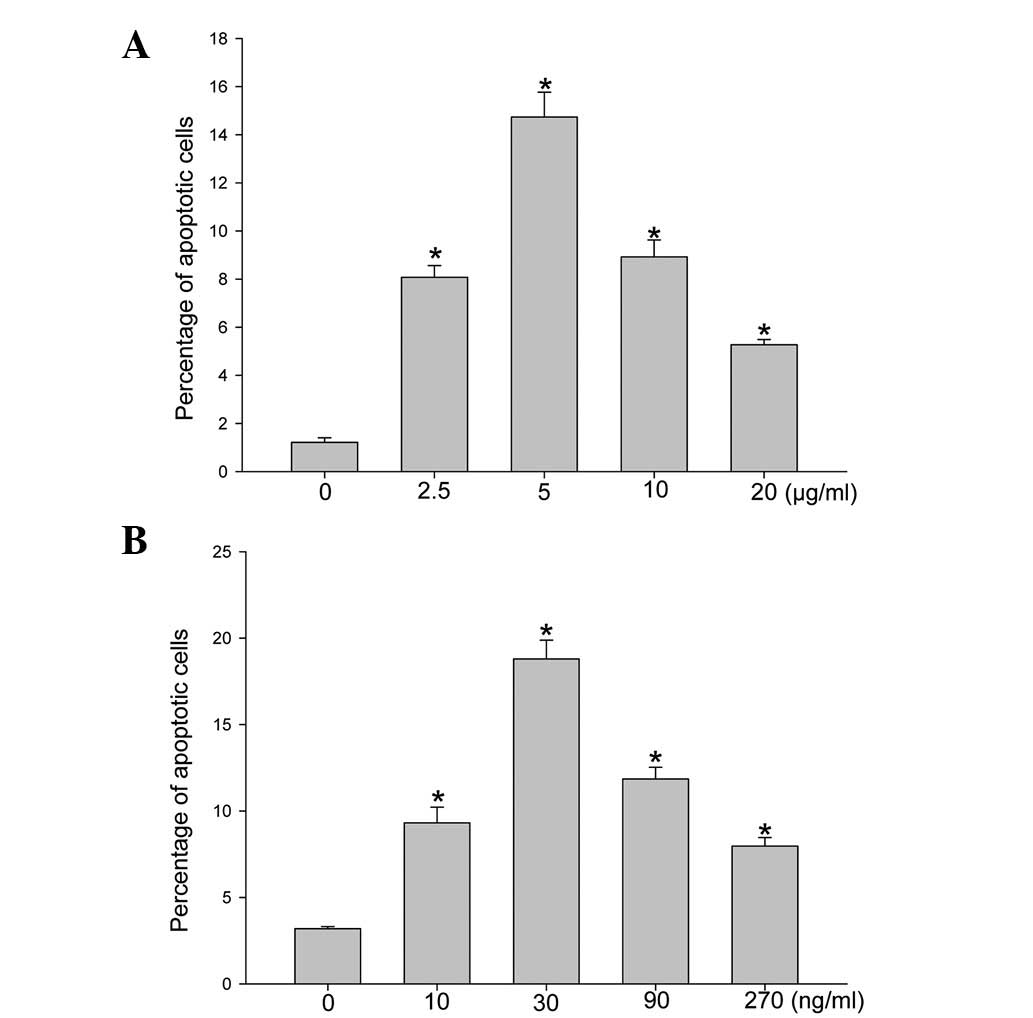
for 8 h, SEB (Fig. 1A) and α-toxin
(Fig. 1B) were demonstrated to
induce the apoptosis of ECV304 cells in a dose-dependent manner.
The level of apoptotic cells reached a peak at 5 μg/ml SEB and 30
ng/ml α-toxin; hence, these concentrations were then utilized when
investigating the level of apoptosis over 8 h in the incubated
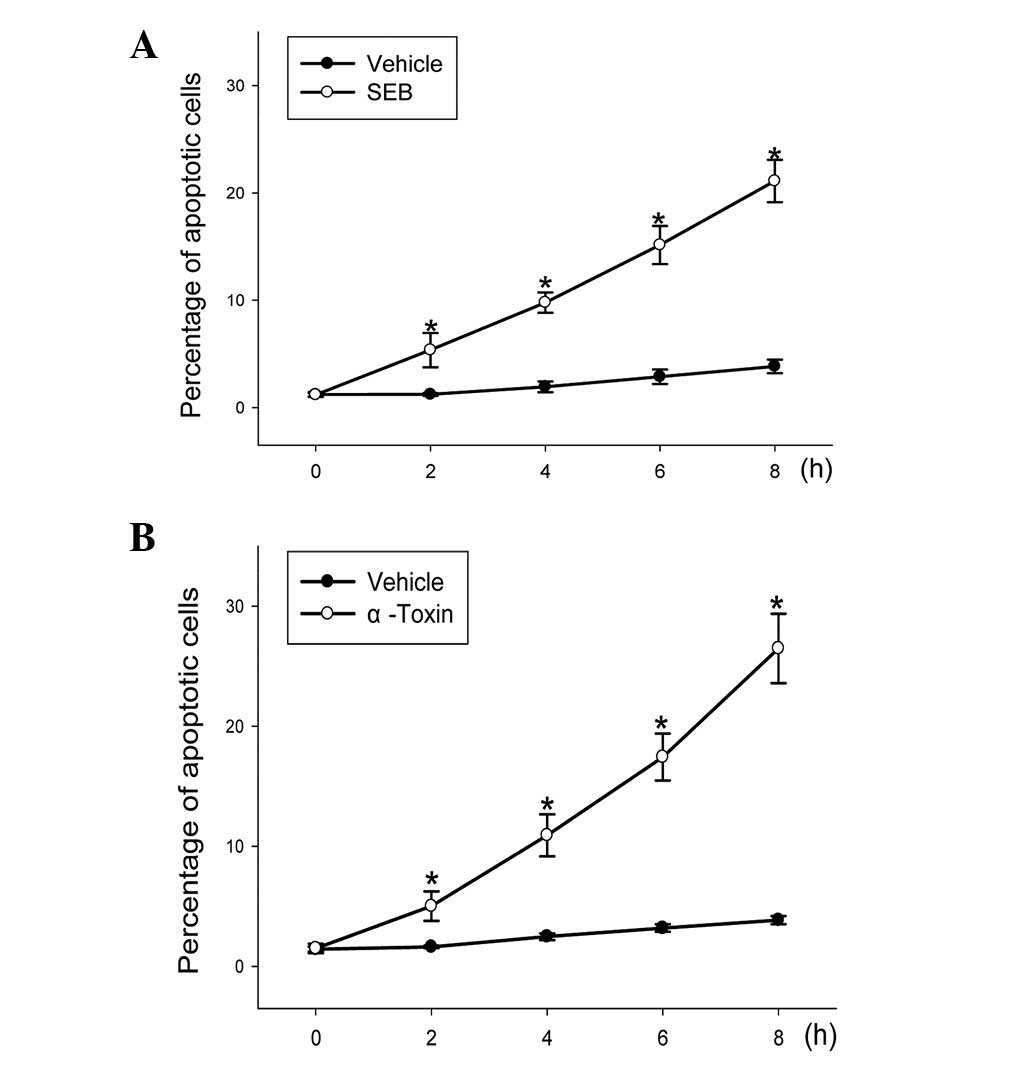
ECV304 cells. When the ECV304 cells were incubated with 5 μg/ml SEB
or 30 ng/ml α-toxin for different lengths of time, the level of
apoptotic cells represented a statistically significant (P<0.05)
time-dependent increase (Fig.
2).
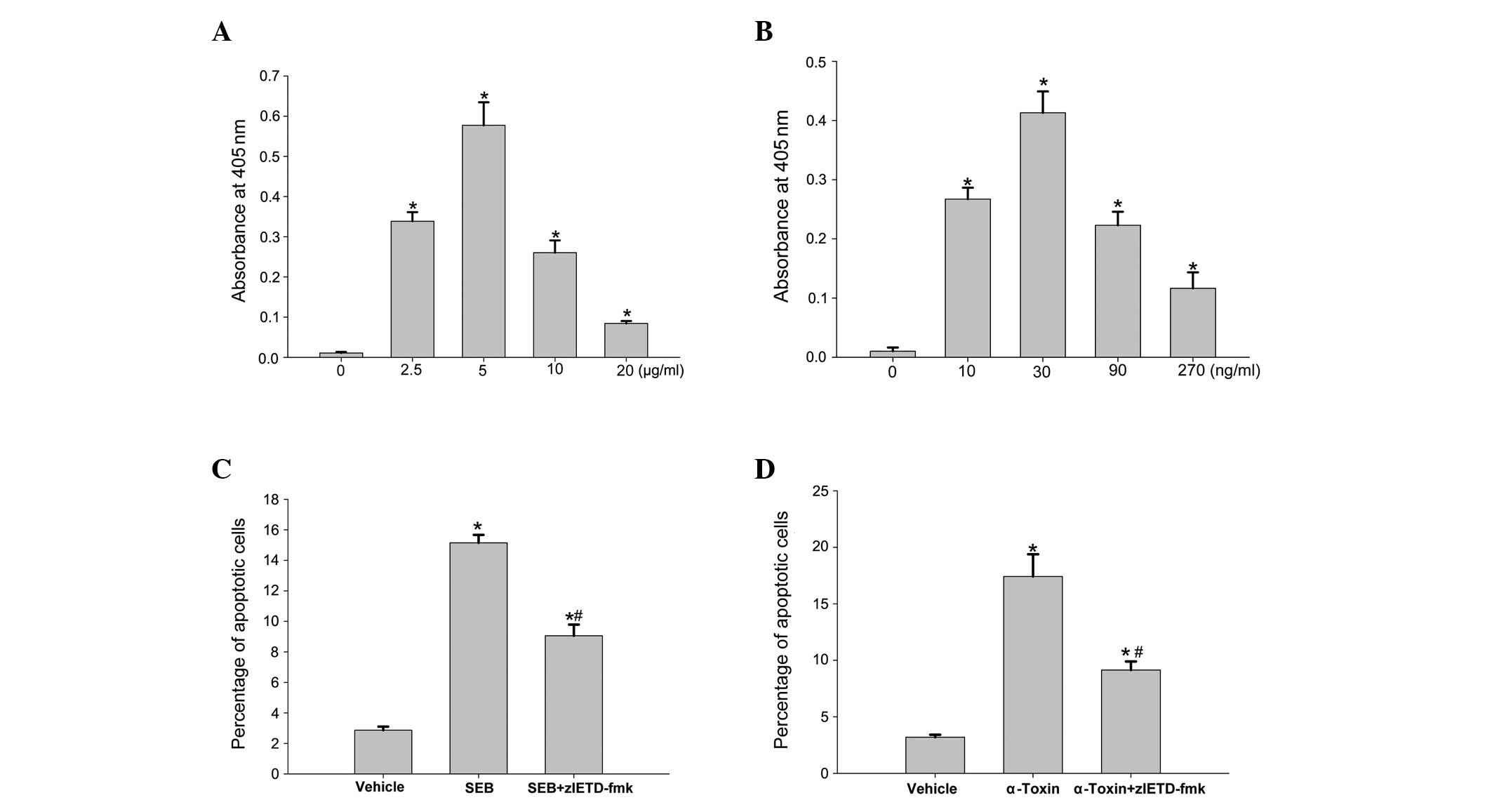
Cell apoptosis induced by SEB or α-toxin
is partially mediated by TNF-α
Apoptosis is achieved via two principal signaling
pathways, the extrinsic and intrinsic death pathways (14,15).
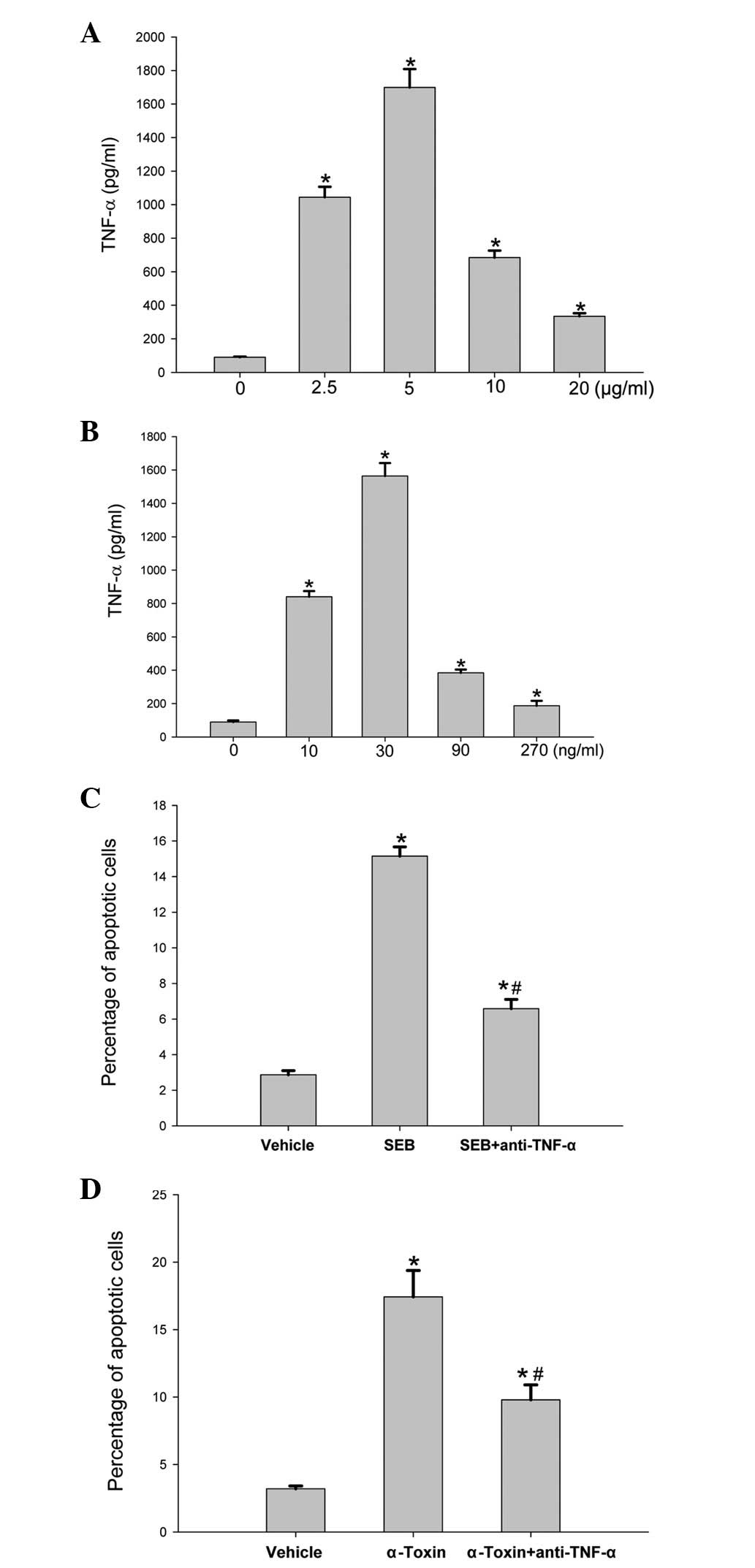
It was predicted that TNF-α may be involved in the induction of
cell death. To test the hypothesis, we measured the level of TNF-α
production and the effect of TNF-α antibody on cell apoptosis
induced by SEB or α-toxin. Following an 8-h incubation of the
ECV304 cells with different concentrations of SEB or α-toxin, the
level of TNF-α production was significantly higher than that of the
control cells (Fig. 3A and B). The
addition of an anti-TNF-α antibody to the incubated ECV304 cells
prior to the addition of SEB or α-toxin significantly decreased the
rates of cell apoptosis induced by SEB or α-toxin (Fig. 3C and D); however, the rates of
apoptosis remained higher than those of the control cells.
SEB and α-toxin activate caspase-3 and -8
in ECV304 cells
Apoptosis is predominantly mediated by activation of
the caspase cascade, resulting in the cleavage of several death
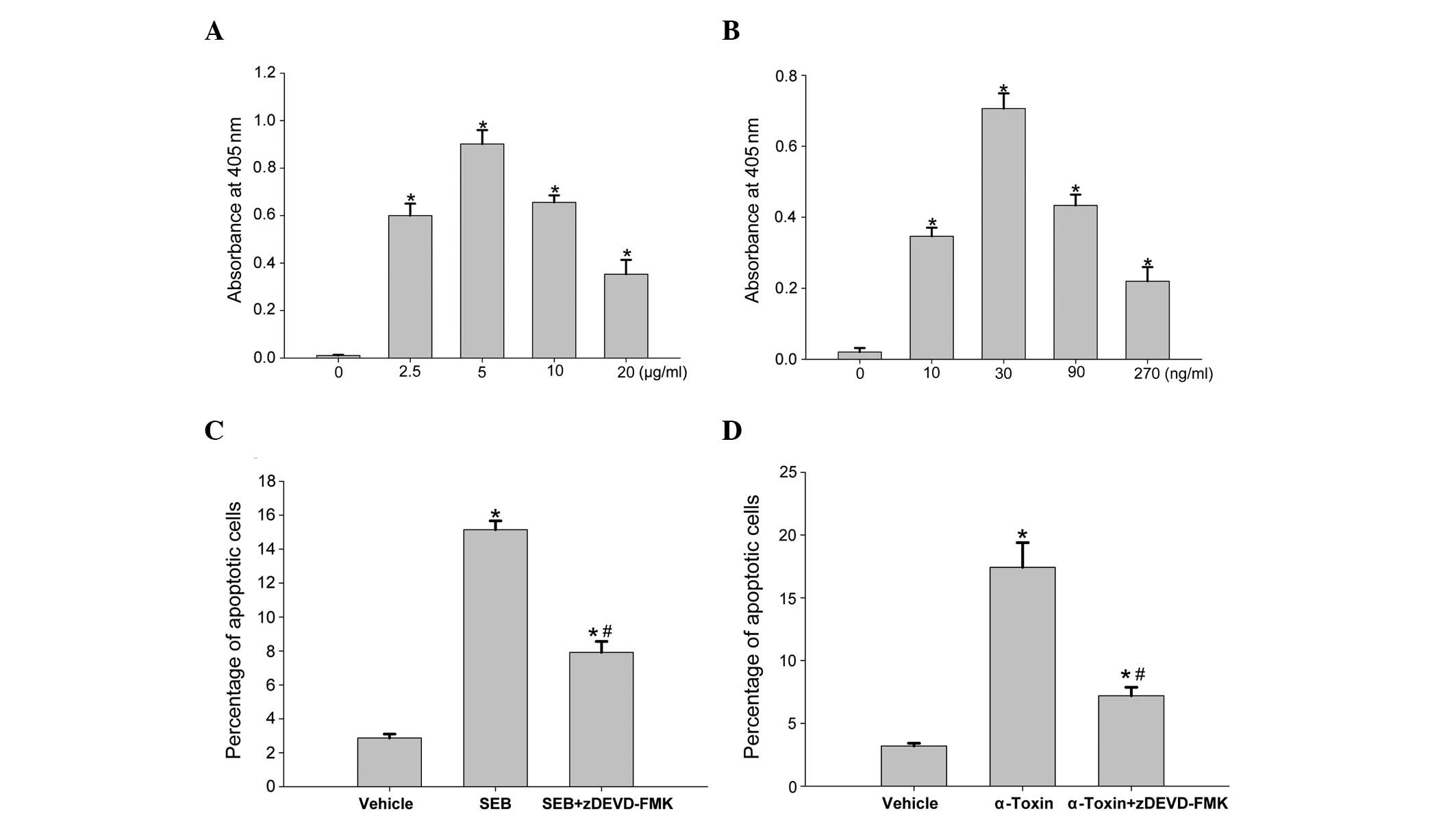
substrates. To examine the cell death pathway induced by SEB or
α-toxin in more detail, we incubated ECV304 cells with SEB or
α-toxin, and analyzed the cell extracts for caspase-3 and -8
activity, as determined by the ability to cleave DEVD-pNA or
IETD-pNA (the labeled substrates), respectively. Following 8
h of incubation, the addition of SEB and α-toxin resulted in a
dose-dependent increase in caspase-3 (Fig. 4A and B) and -8 (Fig. 5A and B) activity in the ECV304
cells. Similar to the rates of apoptotis induced by SEB and
α-toxin, caspase-3 and -8 activation also peaked at 5 μg/ml SEB and
30 ng/ml α-toxin, respectively. Following the addition of caspase-3
inhibitory peptide (z-DEVD-FMK) or caspase-8 inhibitory peptide
(z-IETD-FMK) to the incubated ECV304 cells, prior to the addition
of SEB or α-toxin, the rates of cell death were significantly
decreased compared with those observed with SEB or α-toxin alone.
However, compared with the control cells (Figs. 4C and D, and 5C and D), the rates of apoptosis remained
higher in the cells with z-IETD-FMK and z-DEVD-FMK. These results
suggested that the inhibitors exerted a significant inhibitory
effect on the subsequent induction of apoptosis and activation of
caspase-3/8, which was partially mediated by SEB and α-toxin.
Discussion
S. aureus is a human commensal bacteria and
also a predominant cause of community and nosocomial infection
(2,3,18).
SEB and α-toxin produced by S. aureus are important in
disease pathogenesis. Apoptosis is a major cause of cell death and
tissue destruction in S. aureus infection (19). In this study, we identified that
SEB and α-toxin were able to induce the apoptosis of ECV304 cells
in a dose- and time-dependent manner. This is concordant with other
studies demonstrating that SEB was capable of inducing the
apoptosis of human Jurkat cells (11), and α-toxin was able to induce the
apoptosis of human peripheral blood lymphocytes, monocytes
(5), and epithelial (6) and endothelial (7,8)
cells.
As SEB and α-toxin induce the apoptosis of ECV304
cells, it may be beneficial to understand the mechanism by which
this occurs. Apoptosis is primarily mediated by a family of
intracellular cysteine proteases, termed caspases (13,20).
Caspase activation is achieved via two principal signaling
pathways, the extrinsic and intrinsic death pathways (14,15).
In this study, it was revealed that α-toxin stimulated the
expression of TNF-α in the supernatants of ECV304 cells, which was
consistent with a previous study demonstrating that the expression
of TNF-α was upregulated in epithelial (6) and peripheral blood mononuclear
(21) cells treated by S.
aureus α-toxin. In addition, the removal of TNF-α with a
neutralizing antibody significantly decreased α-toxin-induced cell
death, but did not completely inhibit apoptosis. These findings
suggested that TNF-α contributes to α-toxin-induced apoptosis
through the extrinsic death pathway. In addition, we demonstrated
that ECV304 cell apoptosis induced by SEB shared similar mechanisms
to apoptosis induced by α-toxin. To the best of our knowledge, this
is the first study indicating that SEB may partially induce the
apoptosis of ECV304 cells through the extrinsic death pathway.
Since SEB and α-toxin initiated cell apoptosis by
TNF-α binding to the TNF-α receptors in the host, downstream
caspase pathways were also investigated. A small number of studies
have demonstrated that S. aureus and α-toxin are able to
activate both the effector caspase-3 and the two key initiator
caspases, caspase-8 and -9 (22,23).
Concordant with these studies, the results of the present study
demonstrated that α-toxin induced the activation of caspase-3 and
-8. In addition, the inhibition of either caspase-3 or -8 by their
inhibitors significantly decreased, but did not completely block,
the α-toxin-induced cell death. The downstream caspase pathways of
ECV304 cell apoptosis induced by SEB were also investigated. It was
identified that SEB and α-toxin had a similar mechanism of inducing
the activation of caspase-3 and caspase-8. These results
demonstrated that caspase-8, of the extrinsic death pathway,
partially mediates the apoptosis of ECV304 cells induced by SEB and
α-toxin.
In conclusion, SEB and α-toxin induced the apoptosis
of ECV304 cells in a dose- and time-dependent manner. TNF-α
expression induced by SEB and α-toxin contributed to mediating the
death of the ECV304 cells, by the activation of caspase-3 and -8.
These results suggested that SEB and α-toxin induce ECV304 cell
apoptosis via similar mechanisms, partially mediated by the
extrinsic death pathway of TNF-α and caspase-8. This study has
provided insights into the synergistic pathogenicity of SEB and
α-toxin during S. aureus infection.
Acknowledgements
The authors would like to thank Dr Bai-qing Li,
Department of Immunology, Bengbu Medical College (Bengbu, China)
for assistance in the FACS analysis. This study was supported by
grants from the National Science Foundation of China (grant no.
81070506).
References
|
1
|
Alonzo F 3rd, Kozhaya L, Rawlings SA, et
al: CCR5 is a receptor for Staphylococcus aureus leukotoxin
ED. Nature. 493:51–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewis SS, Moehring RW, Anderson DJ, Sexton
DJ and Chen LF: Increasing rates of methicillin-resistant
Staphylococcus aureus in academic hospitals: a result of
active surveillance? Infect Control Hosp Epidemiol. 34:105–106.
2013.PubMed/NCBI
|
|
3
|
Sax H, Posfay-Barbe K, Harbarth S, et al:
Control of a cluster of community-associated, methicillin-resistant
Staphylococcus aureus in neonatology. J Hosp Infect.
63:93–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alexander EH and Hudson MC: Factors
influencing the internalization of Staphylococcus aureus and
impacts on the course of infection in humans. Appl Microbiol
Biotechnol. 56:361–366. 2001.PubMed/NCBI
|
|
5
|
Essmann F, Bantel H, Totzke G, et al:
Staphylococcus aureus alpha-toxin-induced cell death:
predominant necrosis despite apoptotic caspase activation. Cell
Death Differ. 10:1260–1272. 2003. View Article : Google Scholar
|
|
6
|
Liang X and Ji Y: Involvement of
alpha5beta1-integrin and TNF-alpha in Staphylococcus aureus
alpha-toxin-induced death of epithelial cells. Cell Microbiol.
9:1809–1821. 2007.PubMed/NCBI
|
|
7
|
Menzies BE and Kourteva I:
Staphylococcus aureus α-toxin induces apoptosis in
endothelial cells. FEMS Immunol Med Microbiol. 29:39–45. 2000.
|
|
8
|
Haslinger-Löffler B, Kahl BC, Grundmeier
M, et al: Multiple virulence factors are required for
Staphylococcus aureus-induced apoptosis in endothelial
cells. Cell Microbiol. 7:1087–1097. 2005.
|
|
9
|
Karauzum H, Chen G, Abaandou L, et al:
Synthetic human monoclonal antibodies toward staphylococcal
enterotoxin B (SEB) protective against toxic shock syndrome. J Biol
Chem. 287:25203–25215. 2012. View Article : Google Scholar
|
|
10
|
Bi S, Das R, Zelazowska E, et al: The
cellular and molecular immune response of the weanling piglet to
staphylococcal enterotoxin B. Exp Biol Med (Maywood).
234:1305–1315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higgs BW, Dileo J, Chang WE, et al:
Modeling the effects of a Staphylococcal Enterotoxin B (SEB) on the
apoptosis pathway. BMC Microbiol. 6:482006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kedzierska A, Kaszuba-Zwoińska J,
Słodowska-Hajduk Z, et al: SEB-induced T cell apoptosis in atopic
patients - correlation to clinical status and skin colonization by
Staphylococcus aureus. Arch Immunol Ther Exp (Warsz).
53:63–70. 2005.PubMed/NCBI
|
|
13
|
Los M, Wesselborg S and Schulze-Osthoff K:
The role of caspases in development, immunity, and apoptotic signal
transduction: lessons from knockout mice. Immunity. 10:629–639.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schulze-Osthoff K, Ferrari D, Los M,
Wesselborg S and Peter ME: Apoptosis signaling by death receptors.
Eur J Biochem. 254:439–459. 1998. View Article : Google Scholar
|
|
15
|
Li H and Yuan J: Deciphering the pathways
of life and death. Curr Opin Cell Biol. 11:261–266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown J, Reading SJ, Jones S, et al:
Critical evaluation of ECV304 as a human endothelial cell model
defined by genetic analysis and functional responses: a comparison
with the human bladder cancer derived epithelial cell line T24/83.
Lab Invest. 80:37–45. 2000. View Article : Google Scholar
|
|
17
|
Koopman G, Reutelingsperger CP, Kuijten
GA, Keehnen RM, Pals ST and Van Oers MH: Annexin V for flow
cytometric detection of phosphatidylserine expression on B-cells
undergoing apoptosis. Blood. 84:1415–1420. 1994.PubMed/NCBI
|
|
18
|
McKinnell JA, Huang SS, Eells SJ, Cui E
and Miller LG: Quantifying the impact of extranasal testing of body
sites for methicillin-resistant Staphylococcus aureus
colonization at the time of hospital or intensive care unit
admission. Infect Control Hosp Epidemiol. 34:161–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Menzies BE and Kourteva I: Internalization
of Staphylococcus aureus by endothelial cells induces
apoptosis. Infect Immun. 66:5994–5998. 1998.
|
|
20
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
21
|
Haslinger B, Strangfeld K, Peters G,
Schulze-Osthoff K and Sinha B: Staphylococcus aureus
alpha-toxin induces apoptosis in peripheral blood mononuclear
cells: role of endogenous tumor necrosis factor-alpha and the
mitochondrial death pathway. Cell Microbiol. 5:729–741. 2003.
View Article : Google Scholar
|
|
22
|
Bantel H, Sinha B, Domschke W, Peters G,
Schulze-Osthoff K and Janicke RU: alpha-Toxin is a mediator of
Staphylococcus aureus-induced cell death and activates
caspases via the intrinsic death pathway independently of death
receptor signaling. J Cell Biol. 155:637–647. 2001.PubMed/NCBI
|
|
23
|
Wesson CA, Deringer J, Liou LE, Bayles KW,
Bohach GA and Trumble WR: Apoptosis induced by Staphylococcus
aureus in epithelial cells utilizes a mechanism involving
caspases 8 and 3. Infect Immun. 68:2998–3001. 2000.PubMed/NCBI
|