Introduction
The increased lifespan of human beings has led to
the development of novel therapeutic strategies aiming to preserve
aged tissue, which range from the replacement of lost or injured
tissues to regeneration of the damage tissue (1). Regenerating alveolar bone as an
alternative therapy for periodontal regeneration has been
increasingly used in dentistry. A deficient alveolar ridge not only
fails to provide sufficient support and retention for teeth or
dentures, but also hinders dental implant placement (2). The finding of stem cells was a
medical breakthrough and led to the novel field of medicine
entitled regenerative medicine (3). Postnatal stem cells have been
isolated from various tissues, including dental tissue (4). Seo et al suggested that the
human periodontal ligament (PDL) contains a population of postnatal
multipotent stem cells (5). Stem
cells require specific microenvironments for survival (6), thus, in theory periodontal ligament
stem cells (PDLSCs) are the most direct and reliable source for
periodontal tissue regeneration. Although PDLSCs are easy to
separate and purify, the number of cells required for bone
regeneration is unachievable in a short period of time. After
several passages, it is difficult to induce the differentiation of
cells that are prone to aging and changes in phenotype (7,8).
Thus, an effective way to rapidly amplify PDLSCs is a current focus
of numerous studies.
Growth factors stimulate the differentiation of
mesenchymal cells into osteoblasts and accelerate the osteogenesis
of these cells. The growth factors that are known to be associated
with bone formation are bone morphogenetic protein (BMP),
transforming growth factor (TGF), platelet-derived growth factor
(PDGF), insulin-like growth factor (IGF), and epidermal growth
factor (EGF) (9–11). When these growth factors are used
alone, there are often certain shortcomings. For example, when
using BMP-2 alone, it has a short half-life and is susceptible to
proteolytic degradation. When the concentration is too high, it may
lead to tissue edema, an increased inflammatory response, and
prevention of novel bone formation (12–14).
In addition, the human body is a complex biological environment;
thus, stem cell osteogenic differentiation is not regulated by one
growth factor alone. Therefore, it is essential to identify an
autologous source and effective cytokine or growth factor group in
stem cell osteogenic induction studies. Platelet-rich plasma (PRP)
(15), a platelet concentrate
product, contains numerous growth factors. Previously, it was
demonstrated that the PRP may induce proliferation, but inhibits
the differentiation of PSLSCs (16). The application of PRP has also been
controversial as the addition of dissimilar thrombin and
anticoagulant during preparation may pose risks of immune rejection
and transmission of infectious diseases. The platelet-rich fibrin
(PRF), the second generation of platelet concentrate products
(17), exhibits the same
properties as PRP with the advantages of an osteogenic ability, a
simple preparation process, and lack of added biological agents, as
it is produced from autologus blood. Concentrated growth factors
(CGF) were developed by Sacco in 2006. It is produced by a
centrifuge device (Medifuge Silfradent srl, Italy), similar to the
production of PRF (18). The
different centrifugation speed permits the isolation of fibrin
matrix that is markedly larger, denser and richer in growth factors
as compared to PRF. In theory, CGFs appear to exhibit superior
clinical and biotechnological application potential (19); however, there are few studies
supporting this.
The aim of this study was to analyze the in
vitro biological effects of CGFs on the proliferation and
differentiation of canine PDLSCs, and to investigate whether this
effect occurs in a dose-dependent manner.
Materials and methods
Isolation of beagle PDLSCs and
preliminary identification
One healthy beagle dog (18 months old, 14.5 kg,
male) was supplied by the Laboratory Center of the Second Military
Medical University of the Chinese PLA (No. SCXK-Shanghai
2012–0003). The entire experimental procedure was in accordance
with the Regulations of the Administration of Affairs Concerning
Experimental Animals formulated by the Ministry of Science and
Technology of China. Beagle PDLSCs were isolated and cultured as
described previously (4,20,21).
Briefly, periodontal ligament tissues were scraped from the
intermediate 1/3 of the root. Collagenase type I (3 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) and dispase II (4 mg/ml; Roche,
Basel, Switzerland) were added at a 1:1 mixture and digested for 1
h at 37ºC. The mixture was passed through a 70 μm cell sieve (BD
Biosciences Franklin Lakes, NJ, USA), using α-minimal essential
medium (MEM) containing 15% fetal bovine serum (FBS) to adjust the
cell density to 1×104/ml. The cells were then incubated
in a 5% CO2 atmosphere at 37ºC. Following expansion, the
STRO-1+ cells (Biolegend, San Diego, CA, USA) were
separated by flow cytometry. The cells were then inoculated on
α-MEM growth medium (Gibco-BRL, Carlsbad, CA, USA) containing 20%
FBS (Gibco), 2 mM L-glutamine, 100 μM L-ascorbate-2-phosphate, 1 mM
sodium pyruvate, 50 U/ml penicillin and 50 μg/ml streptomycin
(21). The logarithmic growth
phase cells underwent osteogenic, chondrogenic and adipogenic
multi-induction experiments, and the induced cells were stained on
day 21 with Alizarin Red S (Sigma-Aldrich), Alcian blue
(Sigma-Aldrich) and Oil Red O (Sigma-Aldrich) accordingly.
CGF preparation
It was essential to use cultured PDLSCs and CGFs
from the same donor. Under sterilized conditions, venous blood was
drawn from the beagle forearm, divided into sterile Vacuette tubes
(1 and 3 ml samples in each tube, respectively) without
anticoagulants and immediately placed in a centrifuge for
centrifugation. The built-in program was: 30″ acceleration, 2′ 408
× g, 4′ 323 × g, 4′ 408 × g, 3′ 3,000 rpm, and 36″ deceleration and
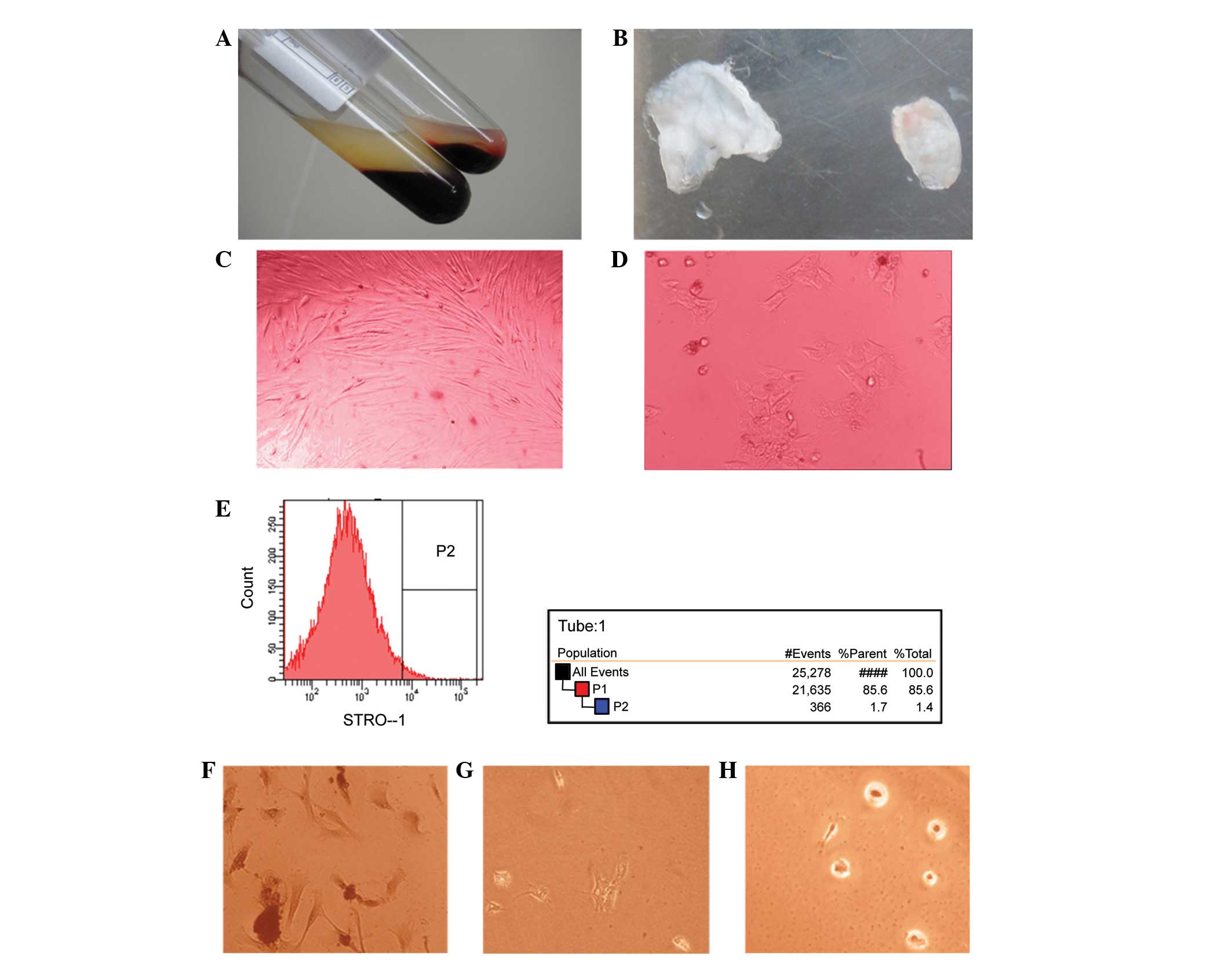
stop. The fresh whole blood was divided into three layers (Fig. 1A). The intermediate filament
protein gel layer was collected and pressed onto membranous film
(MF200, Wisdom, Beijing, China; Fig.
1B).
Experimental groups
There were 6 groups used in the present study, the
standard group (0 CGF), standard group + 1 CGFs (prepared with 1 ml
fresh whole blood), standard group + 3 CGFs (prepared with 3 ml
whole blood), osteogensis induced fluid (0 CGFs), osteogensis
induced fluid + 1 CGF and osteogensis induced fluid + 3 CGFs. CGFs
were added on the first day and experimental check points were at
days 3 (D3), 7, 14 and 21 following inoculation.
The standard culture medium was composed of α-MEM
growth medium as described previously. The osteogenic medium
(21) was composed of α-MEM
supplemented with 10% FBS, 2 mM L-glutamine, 100 μM
L-ascorbate-2-phosphate, 10−7 M dexamethasone, 1.8 mM
inorganic phosphate (KH2PO4), 50 U/ml
penicillin and 50 μg/ml streptomycin.
Cell growth and proliferation
analysis
PDLSC proliferation increased following CGF
treatment and was analyzed by cell counting and an MTT assay. The
absorbance value of each well was measured at 490 nm by the
microtiter analyzer (Multiskan FC; Thermo, Vantaa Finland).
According to the measured OD values, with time as the x-axis and
absorbance value as the y-axis, the cell growth curve was
plotted.
Osteoinduction assessment
The mineralized nodules were counted by Alizarin Red
S (Sigma-Aldrich, St. Louis, MO, USA) staining and mineralized
nodule formation was observed under the microscope (BX43; Olympus,
Tokyo, Japan).
Alkaline phosphatase (ALP) activity detection was
conducted using a commercialized reagent cartridge (?, USA) at each
experimental check point and was analyzed in the microtiter
analyzer at OD 405 nm. The ALP activity was expressed as nU/cell
and mU/plate.
qPCR was conducted using TRIzol reagent to extract
RNA, and using a reverse transcription kit (Takara, Tokyo, Japan)
to synthesize cDNA. Primers are listed in Table I. A two-step amplification response
procedure was selected with specifications of 95ºC for 120 sec,
95ºC for 15 sec and 61ºC for 40 sec for 40 cycles.
 | Table IList of primers. |
Table I
List of primers.
| Gene | Sequences
(5′-3′) |
|---|
| BSP | F:
CGATTTCCAGTTCAGAGCAGTAGT
R: CAGCGTCGGATTCATCTTCAT |
| Collagen | I F:
TGGGGCAAGACAGTGATCG
R: GGAGGGAGTTTACAGGAAGCAG |
| OCN | F:
GCTGTGGCCGCACTCTGC
R: AGAGTGGGGCTGGCCGCTC |
| GAPDH | F:
AAGGTCGGAGTCAACGGATTT
R: GGTTCACGCCCATCACAAA |
For the western blot analysis, RIPA lysis buffer was
added, homogenated and centrifuged and the protein supernatant was
collected for sodium dodecyl sulphate-polyacrylamide gel
electrophoresis. The proteins were transferred to nitrocellulose
membranes, sealed and incubated with primary [BSP (Abcam,
Cambridge, MA, USA), Col-I (Abcam) and OCN (Abcam)] and secondary
antibodies (ZSGB-Bio, Beijing, China). The membranes were washed
and immunoblot chemiluminescence detection reagents (enhanced
chemiluminescence, ECL), were used for exposure. Glyceraldehyde
3-phosphate dehydrogenase protein served as a system internal
reference and stripe gray values were measured. The results were
compared based on the differences in the expression of
purpose/internal reference ratio.
For immunohistochemical detection, cell climbing
film was produced, sealed and incubated with primary and secondary
antibodies. DAB, chromogenic and hematoxylin staining was
conducted, and the samples were sealed and observed under the
microscope.
Statistical analysis
Cell count, ALP activity, mineralized nodule numbers
and the positive expression rate of immunohistochemical staining
were presented as the mean ± SE and compared with the control
group.
For the MTT assay, the survival capacity of the
control group was set as 100%. The experimental groups were
calculated as percentages of the control group. P<0.05 was
considered to indicate a statistically significant difference.
Relative quantification of qPCR results were
obtained using the comparative Ct method. According to the equation
Fold = 2−ΔΔCt, the differences in the relative
expression of the target gene of the experimental and control
groups were calculated.
Western blot analysis results of three experiments
were compared with the protein expression differences by scanning
the striped gray value.
Results
Cell culture, activity and
proliferation
Flow cytometry was used to screen PDLSCs (Fig. 1D) from PDLCs (Fig. 1C); the separation rate was 1.7%
(Fig. 1E). To assess the
differentiation capacity of PDLSCs, the separated PDLSCs were
divided into osteogenic, chondrogenic and adipogenic induction
groups. The cells were then stained by Alizarin Red S (Fig. 1F), Alcian blue (Fig. 1G) and Oil Red O (Fig. 1H). The results showed a positive
reaction which indicated that the PDLSCs exhibited stem
cell-specific differentiation ability.
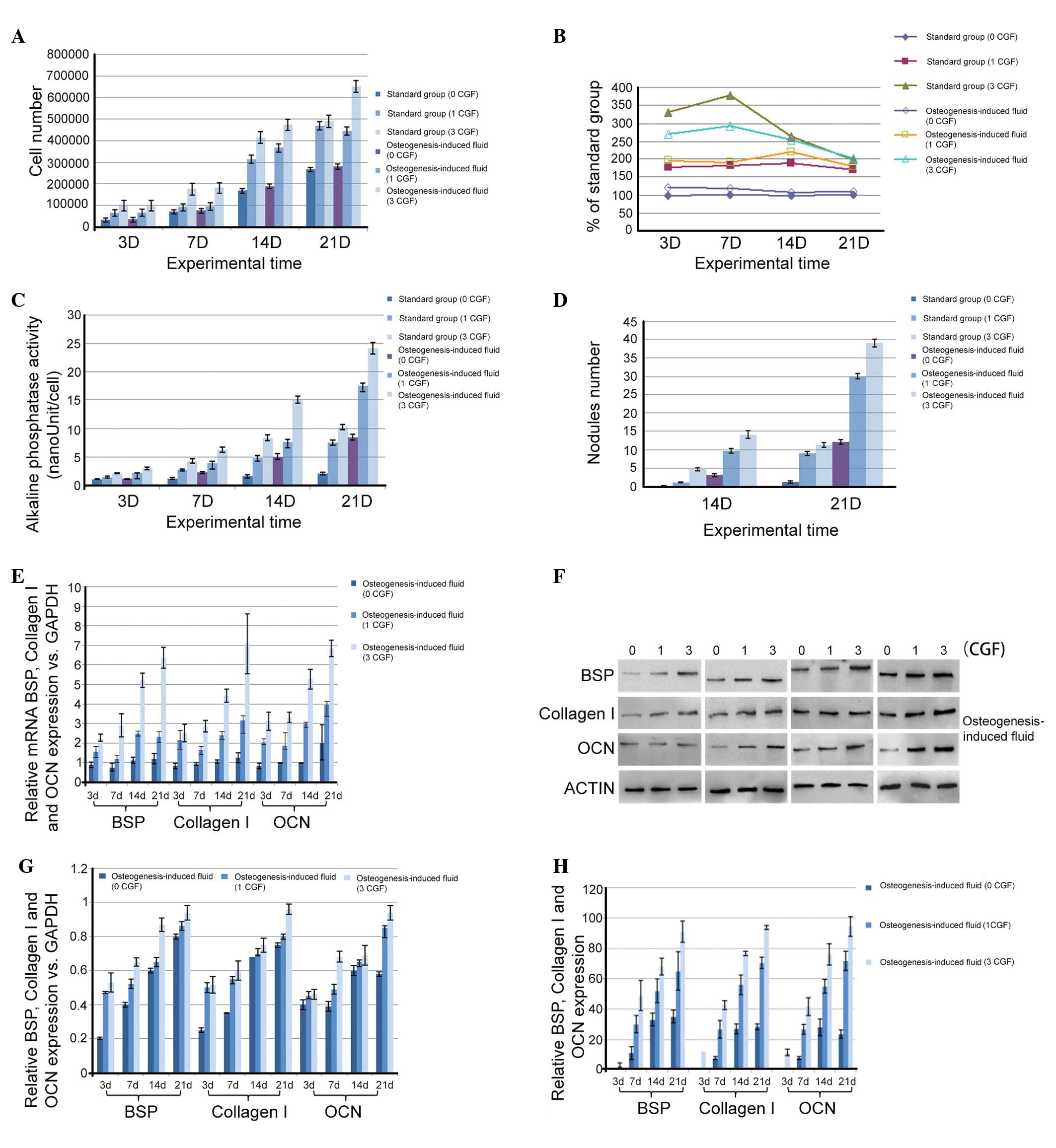
Fig. 2A shows the
proliferation effects of CGFs on PDLSCs. CGFs were identified to
increase PDLSC proliferation in a time- and dose-dependent manner
(P<0.05), and a significant increase appeared at 14 day. From
the MTT assay, in standard culture conditions, the stimulation of
proliferation ranged from 178 to 189% with 1 CGF and from 198 to
330% with 3 CGF (Fig. 2B). The
stimulation level was stable with 1 CGF, but the cell activity
peaked on day 7 with 3 CGF. The cultures with 3 CGF were more
strongly stimulated compared with those with 1 CGF (P<0.05) at
least up to 14 days. In the osteogenic medium, the stimulation of
proliferation ranged between 178 and 196% with 1 CGF and between
201 and 292% with 3 CGF (Fig. 2B).
The stimulation level was stable with 1 and 3 CGF. In addition, it
was observed that the cultures with 3 CGF were more strongly
stimulated than those with 1 CGF (P<0.05) throughout the
experimental period.
Osteoinduction
ALP activity detection and mineralized nodule counts
were performed to determine the effect of CGFs on the induction of
osteogenic differentiation of PDLSCs cells. ALP activity was
detected on days 3, 7, 14 and 21, and mineralized nodule counts
were conducted on days 14 and 21. In the presence of 1 or 3 CGFs,
the ALP activities (Fig. 2C) and
the number of mineralized nodules (Fig. 2D) were significantly higher
(P<0.05) than the values of the respective control groups, and
stimulation of differentiation was significantly higher (P<0.05)
with 3 CGF than with 1 CGF, regardless of the culture
conditions.
The experiments performed showed that the CGFs
exhibits a significant positive effect on PDLSC cell morphology due
to osteogenic induction and differentiation. To the best of our
knowledge, no previous study has investigated this event at the
molecular level. In the present study, qPCR analysis was used to
detect the expression of bone sialoprotein 2 (BSP), collagen I and
osteocalcin (OCN) of different groups at each experimental check
point during osteogenic induction. In Fig. 2E, the mRNA expression level of BSP
showed an increasing trend along with the increasing quantity of
CGF added (P<0.05). In addition, as the culture time increased,
BSP mRNA expression was increased, however, it showed no
significant increase for the first 7 days (P>0.05) and then
significantly increased on day 14 (P<0.05). OCN and collagen I
mRNA expression also showed the same increasing trend following CGF
addition.
Furthermore, western blot analysis was used to
analyze cell growth on the protein level. As shown in Fig. 2F, CGFs were observed to upregulate
BSP protein expression in PDLSCs during the culture period
(P<0.05). The quantitative measurement is shown in Fig. 2G. BSP protein expression in cells
cultured in osteogenic medium was significantly increased with 3
CGF (P<0.05) on day 7 and 21 compared with 1 CGF. Collagen I
protein expression levels also increased in a time- and
dose-dependent manner, and peaked on day 14 (P<0.05). The OCN
protein expression levels increased with time and peaked at day 21
(P<0.05).
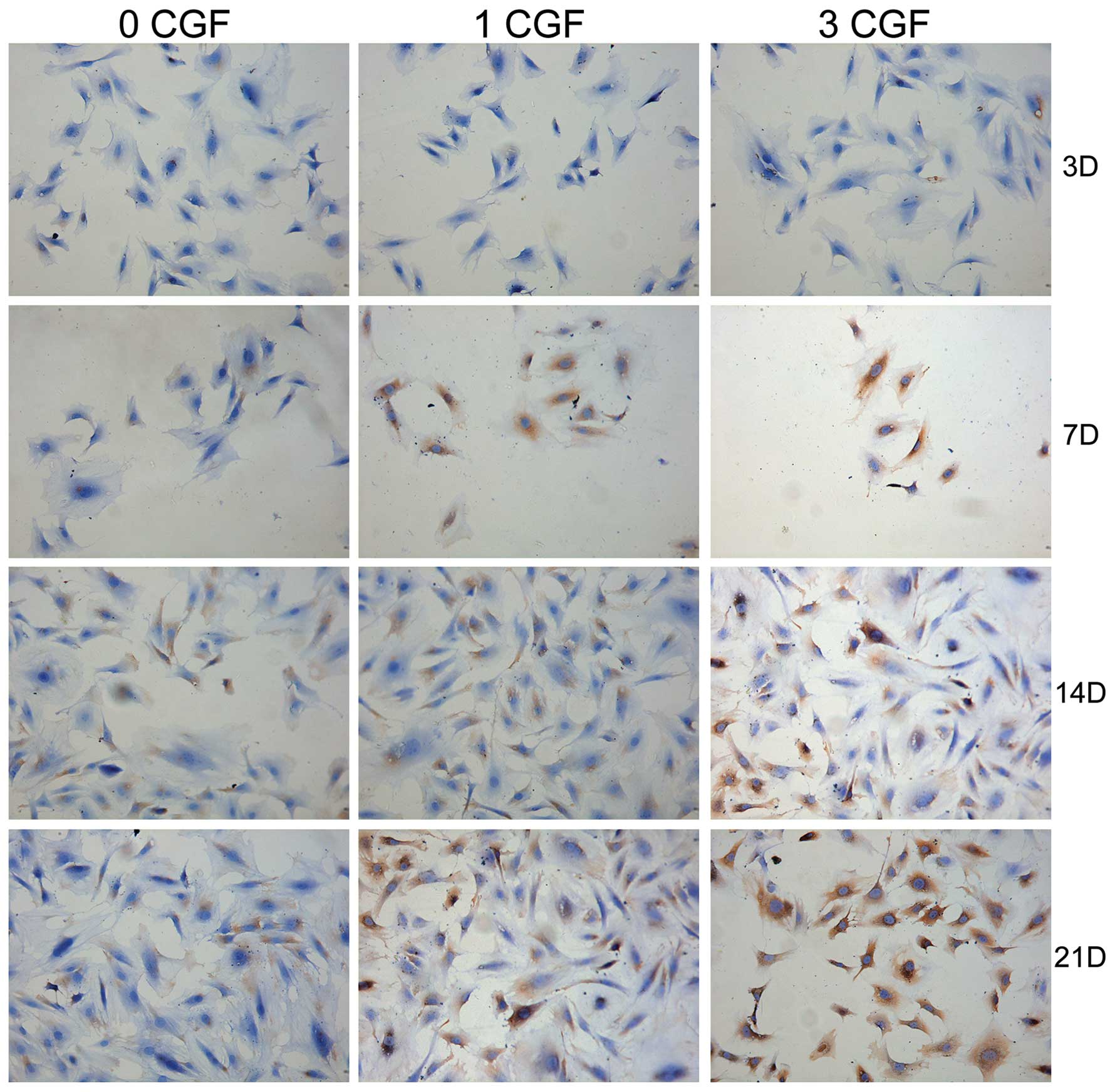
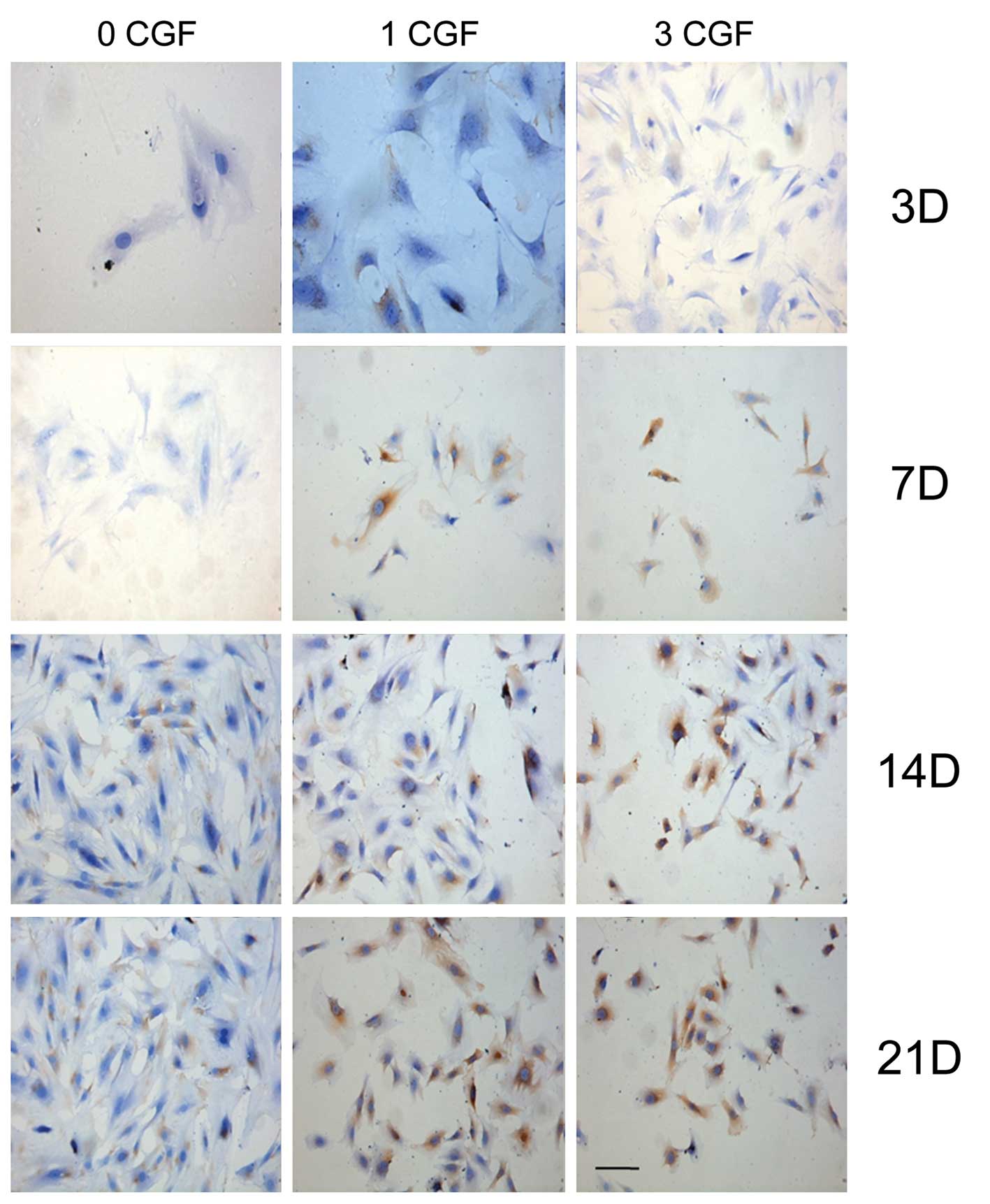
Furthermore, immunohistochemical analysis of the
flag factor BSP, collagen I and OCN protein expression was also
performed during the osteogenic induction process at different
experimental check points in all the experimental groups. As shown
in Figs. 3–5, BSP, collagen I and OCN-positive
expression, respectively, occurred at day 7 when co-cultured with
CGF; however, the control group showed a positive expression at day
14. The results indicated that BSP, collagen I and OCN-positive
expression increased (Fig. 2H) in
a time- and dose-dependent manner (P<0.05).
Discussion
Adult stem cells are specific to adult tissues and
organs. Under normal circumstances, the majority of the cells are
in a resting state; however, damage to body tissues stimulates
differentiation of the stem cells in the tissue. The stem cells
differentiate into the damaged tissue cells and are involved in
tissue repair and reconstruction. Therefore, in theory PDLSCs are
the most direct and reliable seed cells for periodontal tissue
regeneration. There is no ideal method for the isolation of adult
stem cells. Generally, stem cells are separated and identified
based on their colony-forming ability and specific biological and
physical characteristics. As no specific markers have been
identified for PDLSCs, they are predominanly identified by STRO-1,
CD146 and other mesenchymal stem cell marker proteins and are
isolated by the immunomagnetic bead separation method or flow
cytometry. These methods are the same for bone marrow stem cells
and dental pulp stem cells, which have similar organizational
structure and embryonic origin to PDLSCs (21). As an increasing number of PDLSC
studies have been conducted, STRO-1, CD146 and other mesenchymal
stem cell surface markers and certain perivascular cell surface
markers, such as CD105, CD106 and CD166, have been widely
recognized. The STRO-1 antibody immunomagnetic or the flow
cytometry separation methods are commonly known to be the most
effective separation methods for PDLSCs (4,20–23).
In the present study, STRO-1+ cells were separated by
flow cytometry and preliminary identification indicated that these
cells possess high self-renewal capacity and multi-lineage
differentiation potential.
Similar to PRF, the CGFs were only centrifuged once
to avoid the loss of fibrinogen. The CGFs formed free modification
gelatinous fiber blocks, which were convenient for operation and
application. In the present study, CGFs were able to significantly
stimulate PDLSC proliferation in a dose-dependent manner in
vitro, in standard or differentiation medium. The mechanism
responsible for the cell proliferation by CGFs may be explained as
follows: CGFs are rich in a variety of growth factors, such as
TGF-β, PDGF-AB, VEGF and IGF-I (24). These growth factors while
functioning on their own (25–29),
are also synergistic and create close contact tissue repair
regulatory systems (24,30,31).
Unlike PRP, CGFs do not dissolve rapidly following application,
instead, the strong fibrin gel in the matrix addition is slowly
remodeled in a similar manner to a natural blood clot. Thus, CGFs
prolonged the duration of growth factor action, which is conducive
for the growth factor synergy (32–34),
and enhances cell proliferation and osteogenic differentiation.
Previous studies have demonstrated that the PRP may induce
proliferation, but inhibits differentiation (16), and certain studies have indicated
that high concentrations of PRP may also exhibit an inhibitory
effect on the cultured cells (35). In the present study, this was not
observed. To avoid any bias related to immune incompatibility, in
the present study, CGF was obtained from the same donor as for the
PDLSCs. CGFs exhibited no cytotoxic effect on the PDLSCs at the two
doses tested similar to PRF (36).
CGF also significantly promoted the proliferation of PDLSCs, and
exhibited a dose-dependent effect on the activation and
differentiation of the stem cells.
With the aid of the osteogenic inductive medium,
PDLSCs develop osteogenically. The cells undergo multiple
independent stages of differentiation in order to evolve into
osteoblasts. The process includes: The conversion phrase, the
proliferation phase, cell aggregation secretory phase and
extracellular matrix calcification period (37). Throughout the differentiation
process, the alkaline phosphatase activity, matrix mineralization,
osteocalcin and collagen I are all specific markers for stem cell
osteogenic differentiation. Osteoblasts synthesize different
products in different stages. Collagen I is produced in the cell
proliferation phase, cell aggregation secretory phase synthesizes
alkaline phosphatase, and osteocalcin is produced in the
extracellular matrix calcification phase (38). Thus, in the present study, the
indicators that determine osteoblast differentiation of PDLSCs were
mineralized nodule formation, alkaline phosphatase activity, type I
collagen synthesis and specific protein expression, such as BSP and
OCN. In the present study, ALP activity and mineralized nodule
count were elevated by CGF in a time- and dose-dependent manner.
qPCR, western blot analysis and immunohistochemical results were
used to determine that CGF mediated the osteogenic differentiation
of PDLSCs and the results show that CGF accelerates osteogenesis
during the differentiation process. Similar the results have been
demonstrated previously (39);
thus, CGF may contribute to the differentiation of PDLSCs.
In conclusion, CGF significantly promotes the
proliferation of PDLSCs and has a dose-dependent effect. In
standard culture medium, CGF induces PDLSC osteogenic
differentiation. In conditioned medium, CGFs significantly
accelerate the osteogenesis transformation process of PDLSCs. CGF
mediated culture medium either directly induces transformation or
accelerates transformation in a dose-dependent manner. Future
studies are required to identify the optimal induction dose of CGF,
to determine the mechanism underlying the dose-dependent trend and
to reproduce the results in in vivo studies. The present
study has therefore provided an experimental basis for further
PDLSCs clinical applications and studies.
Acknowledgements
This study was supported by a research grant (no.
09411955100) from Tongji University to Professor Zuolin Wang, the
2010 Shanghai Committee of Science and Technology of China (grant
no. 10XD1404500) and National Natural Science Foundation of China
(grant no. 81271110).
References
|
1
|
Hench LL: Biomaterials: a forecast for the
future. Biomaterials. 19:1419–1423. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davo R, Malevez C and Rojas J: Immediate
function in the atrophic maxilla using zygoma implants: a
preliminary study. J Prosthet Dent. 97(Suppl 6): S44–S51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrovic V and Stefanovic V: Dental tissue
- new source for stem cells. Scientific World Journal. 9:1167–1177.
2009.PubMed/NCBI
|
|
4
|
Gronthos S, Brahim J, Li W, et al: Stem
cell properties of human dental pulp stem cells. J Dent Res.
81:531–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo BM, Miura M, Gronthos S, et al:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radtke S and Horn PA: Cells, niche, fate:
meeting report on the 6th International Meeting of the Stem Cell
Network North Rhine Westphalia. Cell Reprogram. 13:381–384.
2011.PubMed/NCBI
|
|
7
|
Forte A, Galderisi U, Cipollaro M and
Cascino A: Mesenchymal stem cells: a good candidate for restenosis
therapy? Curr Vasc Pharmacol. 7:381–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deryugina EI and Müller-Sieburg CE:
Stromal cells in long-term cultures: keys to the elucidation of
hematopoietic development? Crit Rev Immunol. 13:115–150.
1993.PubMed/NCBI
|
|
9
|
Fini M, Giavaresi G, Torricelli P, et al:
Osteoporosis and biomaterial osteointegration. Biomed Pharmacother.
58:487–493. 2004. View Article : Google Scholar
|
|
10
|
Davies JE: Mechanisms of endosseous
integration. Int J Prosthodont. 11:391–401. 1998.PubMed/NCBI
|
|
11
|
Meyer U, Joos U, Mythili J, et al:
Ultrastructural characterization of the implant/bone interface of
immediately loaded dental implants. Biomaterials. 25:1959–1967.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shields LB, Raque GH, Glassman SD, et al:
Adverse effects associated with high-dose recombinant human bone
morphogenetic protein-2 use in anterior cervical spine fusion.
Spine (Phila Pa 1976). 31:542–547. 2006. View Article : Google Scholar
|
|
13
|
Alanay A, Chen C, Lee S, et al: The
adjunctive effect of a binding peptide on bone morphogenetic
protein enhanced bone healing in a rodent model of spinal fusion.
Spine (Phila Pa 1976). 33:1709–1713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glassman SD, Carreon LY, Campbell MJ, et
al: The perioperative cost of Infuse bone graft in posterolateral
lumbar spine fusion. Spine J. 8:443–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Assoian RK, Grotendorst GR, Miller DM and
Sporn MB: Cellular transformation by coordinated action of three
peptide growth factors from human platelets. Nature. 309:804–806.
1984. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vogel JP, Szalay K, Geiger F, Kramer M,
Richter W and Kasten P: Platelet-rich plasma improves expansion of
human mesenchymal stem cells and retains differentiation capacity
and in vivo bone formation in calcium phosphate ceramics.
Platelets. 17:462–469. 2006. View Article : Google Scholar
|
|
17
|
Choukroun J, Adda F, Schoeffler C and
Vervelle A: Une opportunité en paro-implantologie: le PRF.
Implantodontie. 42:55–62. 2001.
|
|
18
|
Corigliano M, Sacco L and Baldoni E: CGF-
una proposta terapeutica per la medicina rigenerativa. Odontoiatria
- no 1 anno XXIX - Maggio. 1:69–81. 2010.
|
|
19
|
Sohn DS, Heo JU, Kwak DH, et al: Bone
regeneration in the maxillary sinus using an autologous fibrin-rich
block with concentrated growth factors alone. Implant Dent.
20:389–395. 2011.PubMed/NCBI
|
|
20
|
He H, Yu J, Cao J, et al: Biocompatibility
and osteogenic capacity of periodontal ligament stem cells on
nHAC/PLA and HA/TCP scaffolds. J Biomater Sci Polym Ed. Jun
16–2010.(Epub ahead of print).
|
|
21
|
Mrozik K, Gronthos S, Shi S and Bartold
PM: A method to isolate, purify, and characterize human periodontal
ligament stem cells. Methods Mol Biol. 666:269–284. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coura GS, Garcez RC, de Aguiar CB,
Alvarez-Silva M, Magini RS and Trentin AG: Human periodontal
ligament: a niche of neural crest stem cells. J Periodontal Res.
43:531–536. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee UL, Jeon SH, Park JY and Choung PH:
Effect of platelet-rich plasma on dental stem cells derived from
human impacted third molars. Regen Med. 6:67–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodella LF, Favero G, Boninsegna R, et al:
Growth factors, CD34 positive cells, and fibrin network analysis in
concentrated growth factors fraction. Microsc Res Tech. 74:772–777.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Jiang S and Hantash BM:
Transforming growth factor beta1 induces osteogenic differentiation
of murine bone marrow stromal cells. Tissue Eng Part A. 16:725–733.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeiter S, Lezuo P and Ito K: Effect of TGF
beta1, BMP-2 and hydraulic pressure on chondrogenic differentiation
of bovine bone marrow mesenchymal stromal cells. Biorheology.
46:45–55. 2009.PubMed/NCBI
|
|
27
|
Wirz S, Dietrich M, Flanagan TC, et al:
Influence of platelet-derived growth factor-AB on tissue
development in autologous platelet-rich plasma gels. Tissue Eng
Part A. 17:1891–1899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakano N, Nakai Y, Seo TB, et al:
Characterization of conditioned medium of cultured bone marrow
stromal cells. Neurosci Lett. 483:57–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang Y, Wang LP, Du FL, Liu WJ and Ren GL:
Effects of insulin-like growth factor I on alveolar bone remodeling
in diabetic rats. J Periodontal Res. 48:144–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sunitha Raja V and Munirathnam Naidu E:
Platelet-rich fibrin: evolution of a second-generation platelet
concentrate. Indian J Dent Res. 19:42–46. 2008.PubMed/NCBI
|
|
31
|
Dohan DM, Choukroun J, Diss A, et al:
Platelet-rich fibrin (PRF): a second-generation platelet
concentrate. Part II: platelet-related biologic features. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 101:e45–e50. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang YH, Jeon SH, Park JY, et al:
Platelet-rich fibrin is a Bioscaffold and reservoir of growth
factors for tissue regeneration. Tissue Eng Part A. 17:349–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dohan Ehrenfest DM, de Peppo GM, Doglioli
P and Sammartino G: Slow release of growth factors and
thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold
standard to achieve for all surgical platelet concentrates
technologies. Growth Factors. 27:63–69. 2009.PubMed/NCBI
|
|
34
|
Dohan Ehrenfest DM, Rasmusson L and
Albrektsson T: Classification of platelet concentrates: from pure
platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin
(L-PRF). Trends Biotechnol. 27:158–167. 2009.PubMed/NCBI
|
|
35
|
Graziani F, Ivanovski S, Cei S, Ducci F,
Tonetti M and Gabriele M: The in vitro effect of different PRP
concentrations on osteoblasts and fibroblasts. Clin Oral Implants
Res. 17:212–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dohan Ehrenfest DM, Doglioli P, de Peppo
GM, Del Corso M and Charrier JB: Choukroun’s platelet-rich fibrin
(PRF) stimulates in vitro proliferation and differentiation of
human oral bone mesenchymal stem cell in a dose-dependent way. Arch
Oral Biol. 55:185–194. 2010.
|
|
37
|
Yoshikawa T, Nakajima H, Takakura Y and
Nonomura A: Osteogenesis with cryopreserved marrow mesenchymal
cells. Tissue Eng. 11:152–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu G, Shu C, Cui L, Liu W and Cao Y:
Tissue-engineered bone formation with cryopreserved human bone
marrow mesenchymal stem cells. Cryobiology. 56:209–215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang FM, Yang SF, Zhao JH and Chang YC:
Platelet-rich fibrin increases proliferation and differentiation of
human dental pulp cells. J Endod. 36:1628–1632. 2010. View Article : Google Scholar : PubMed/NCBI
|