Introduction
Soybean is widely used to produce various fermented
products, including cheonggukjang (CKJ), doenjang and gochujang in
Korea. Of these products, CKJ is predominantly produced by
fermentation with Bacillus subtilis for a short time. It
contains numerous enzymes, microorganisms and bioactive compounds
that are absent from unfermented soybean (1,2). In
particular, flavonoid glycosides are converted into aglycones by
hydrolysis, and a number of proteins are degraded into small
peptides and amino acids during fermentation (3,4).
CKJ exhibits diverse biological and pharmacological
activities, including anti-obesity, antidiabetic and
anti-inflammatory effects on human chronic diseases. In a study
involving human subjects, CKJ supplementation was shown to
significantly decrease visceral fat mass and apolipoprotein B/A1
levels (5). In another study,
C57BL/6J mice exhibiting diet-induced obesity showed improvements
in body weight, epididymal fat accumulation, and serum total
cholesterol and low-density lipoprotein cholesterol levels,
following CKJ treatment for 9 weeks (6). Furthermore, supplementation of CKJ
has been shown to significantly reduce blood glucose and
glycoslylated hemoglobin levels, as well as improve insulin
tolerance in C57BL/Ksj-db/db mice (a type II diabetic animal
model) (7,8). In addition, when the
anti-inflammatory activity of CKJ was investigated in the rat model
of type I hypersensitivity and arachidonic acid-induced ear edema,
CKJ treatment was demonstrated to decrease passive cutaneous
anaphylaxis (9). Moreover, ethanol
extracts of CKJ were shown to significantly increase the viability
of cultured mice spleen and thymus cells, by suppressing apoptotic
death (10). Although CKJ shows a
variety of therapeutic activities in human disease, its effect on
the sensitivity of body growth has not yet been investigated.
Therefore, in the present study, we investigated
whether oral administration of CKJ significantly improves body
growth via growth hormone (GH) secretion in young Sprague Dawley
(SD) rats. The data presented provide strong evidence that CKJ is a
potential candidate for the stimulation or enhancement of animal
growth from infancy to adulthood.
Materials and methods
Preparation of CKJ sample
CKJ was manufactured using the Pungwon soybean
strain that was kindly supplied by the National Institute of Crop
Science in Miryang, Korea. The B. subtilis was supplied by
the Applied and Environmental Microbiology Laboratory at Pusan
National University (Miryang, Korea). To prepare the CKJ, the
soybeans (100 g) were first washed, then soaked for 12 h with three
volumes of tap water at room temperature. The soybeans were then
treated with hot steam at 121°C for 30 min, before being allowed to
cool to 37°C. Once cool, the steamed soybeans were inoculated with
5% (w/w) B. subtilis (1×109 cells/ml), followed
by fermentation at 37°C for 48 h. Powder of fermented soybeans
(CKJ) was then prepared by freeze-drying, homogenization and
sifting. The final CKJ sample extract was stored at −75°C prior to
use.
Principle component analysis of CKJ
Freeze-dried CKJ powder (1 g) was subjected to
extraction with 10 ml of distilled water at 70°C for 2 h. The
sample was centrifuged (10 min, 102 × g) and the supernatant was
collected for analysis. The concentration of total phenolics in CKJ
was determined according to the Folin-Ciocalteu method (11). The sample (20 μl) was mixed with
100 μl of 0.2 N Folin-Ciocalteu reagent (Merck KGaA,
Darmstadt, Germany) for 5 min, and then combined with 300 μl of 20%
sodium carbonate. Following incubation at room temperature for 2 h,
the absorbance of the reaction mixture was measured at 765 nm.
Gallic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as a
standard to produce the calibration curve. Total phenolic content
was expressed as gallic acid equivalents (mg) per gram of CKJ
powder. The level of total flavonoids in CKJ was determined
according to the method of Meda et al(12). The sample (200 μl) was added to
test tubes containing 60 μl of 5% potassium nitrite, 600 μl of
distilled water and 60 μl of 10% aluminum chloride. Following
incubation at 25°C for 5 min, the absorbance of the reaction
mixture was measured at 510 nm. The total flavonoid content was
determined using a standard curve, with quercetin as a standard,
and was expressed as quercetin equivalents (mg) per gram of CKJ
powder. Furthermore, the total protein content was determined by
the Bradford method (13), using
bovine serum albumin as a standard. Total sugar content was
determined according to the method of Dubois et al(14) with the modifications indicated
below. The samples (0.5 ml) and the 5% (w/v) phenol solution (0.5
ml) were added to screw cap tubes, which were capped and
vortex-stirred. Then 2.5 ml of concentrated sulfuric acid was added
and mixed by vortexing. After incubation for 20 min at room
temperature, the absorbance was measured at 490 nm using a
spectrophotometer (BioSpec-mini; Shimadzu Corp., Kyoto, Japan).
High-performance liquid chromatography
(HPLC) analysis of daidzein and genistein concentration
The concentration of daidzein and genistein in CKJ
was analyzed, using a previously described method (15). Standard samples of 98% daidzein and
99% genistein were purchased from Sigma-Aldrich. The aqueous
extracts of CKJ were mixed with 50% methanol and then this mixture
was filtered with a 0.2-μm membrane filter (Wayers Co., Milford,
MA, USA), before HPLC injection. The daidzein and genistein
concentration was analyzed by the iLC 3000 HPLC system (Interface
Engineering, Seoul, Korea) equipped with a Corona®
CAD® Detector (ESA Biosciences, Inc., Chelmsford, MA,
USA). The chromatographic separation was performed on a CAPCELL PAK
MG C18 (4.6×250 mm; particle size, 5 μm; Shiseido Co. Ltd., Tokyo,
Japan). The mobile phase consisted of solvent A (deionized water)
and solvent B (acetonitrile), using the gradient elution program:
0–25 min, 30–90% of solvent B and 25–40 min, 90% of solvent B. A
flow rate of 1.0 ml/min was used for the sample analysis. The
nebulizer gas was compressed nitrogen. The gas flow rate and gas
pressure were maintained at 1.53 l/min and 35±2 psi, respectively.
The output signal of the detector was recorded using Clarity™
chromatography software (DataApex, Prague, Czech Republic).
Care and use of animals
Four-week-old female SD rats (SamTacho, Osan, Korea)
were bred at the Pusan National University-Laboratory Animal
Resources Center (Korea Food and Drug Administration accredited;
registration no., 231). The SD rats received an ad libitum
diet of standard irradiated chow (Purina Mills, Seoungnam, Korea).
The rats were maintained in specific pathogen-free conditions under
a strict 12-h light/dark cycle (lights on at 6:00 a.m. and off at
6:00 p.m.), at a temperature of 23±2°C, with 50±10% relative
humidity, according to the Guide for Laboratory Animals (Institute
for Laboratory Animal Research, Washington, DC, USA). Animal
experiment protocols were carefully reviewed and approved in
accordance with the ethical and scientific care procedures of the
Pusan National University-Institutional Animal Care and Use
Committee (approval no. PNU-2012-0006).
Experimental design and detection of
body/organ weights
A total of 15 four-week-old female SD rats were
divided randomly into three groups, with five rats per group. The
first [low concentration of CKJ (L-CKJ)-treated] and second [high
concentration of CKJ (H-CKJ)-treated] groups of rats received 50
and 100 mg/kg body weight/day of CKJ extract diluted in water,
respectively, via oral administration. The third group
(vehicle-treated) received a daily administration of a comparable
volume of water. The body weights of the rats were measured using
an electronic balance (Mettler Toledo, Greifensee, Switzerland) at
the indicated time points during the experimental period. At 10
days after the CKJ treatment, all animals were sacrificed using
CO2 gas and the weights of five organs collected from SD
rats were also measured using the same method as that used to
detect the body weight. Furthermore, bone and tissue samples were
acquired and stored in Eppendorf tubes at −70°C until assay.
Western blotting
Protein prepared from liver and muscle tissues of
vehicle-, L-CKJ- and H-CKJ-treated rats were separated by 4–20%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 3 h,
following which, the resolved proteins were transferred to
nitrocellulose membranes for 2 h at 40 V. Each membrane was
incubated separately with the following primary antibodies
overnight at 4°C: Anti-GH receptor (ab78426; Abcam, Cambridge, UK),
anti-Erk (sc-94; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), anti-pErk (sc-7383; Santa Cruz Biotechnology, Inc.), anti-Akt
(#9272; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-pAkt (#4058; Cell Signaling Technology, Inc.), and anti-actin
(A5316; Sigma-Aldrich). The membranes were then washed with washing
buffer (137 mM NaCl, 2.7 mM KCl, 10 mM
Na2HPO4, 2 mM KH2PO4
and 0.05% Tween 20) and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (Zymed Laboratories, Inc.,
South San Francisco, CA, USA) at a 1:1,000 dilution, at room
temperature for 2 h. Membrane blots were developed using a
Chemiluminescence Reagent Plus kit (Pfizer, New York, NY, USA).
Histological analysis and observation by
optical microscopy
Femur bones collected from the SD rats were fixed
with 10% formalin, for at least two days at room temperature. The
fixed femur bones were then demineralized for four days in 15%
formic acid-prepared deionized water, embedded in paraffin wax and
sectioned into 4-μm slices. Bone sections were subsequently stained
with hematoxylin and eosin (Sigma-Aldrich) and observed by optical
microscopy. The thickness of the epiphyseal growth plate was
measured at three points using the Leica Application Suite [Leica
Microsystems (Schweiz) AG, Heerbrugg, Switzerland].
Quantification of GH by enzyme-linked
immunosorbent assay (ELISA)
The concentration of GH in sera collected from
vehicle- and CKJ-treated rats was determined by following the
ultra-sensitive assay procedure, using reagents in the Growth
Hormone Rat ELISA kit (KRC5311; Invitrogen Life Technologies,
Carlsbad, CA, USA). The sera and standards were incubated in
antibody-coated plates for 2 h at room temperature on a plate
shaker at 51–61 × g. The wells were then washed six times
using a PV100 automatic plate washer (Hoefer Inc., Holliston, MA,
USA). HRP conjugates were subsequently added to all wells, followed
by incubation for 30 min at room temperature on the shaker. The
reaction was terminated by the addition of 50 μl of stop solution.
Color alterations in the wells were read using a VMax®
microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 450
nm.
Statistical analysis
The significance of differences between the vehicle-
and CKJ-treated groups in SD rats was analyzed using one-way ANOVA
(SPSS for Windows, Release 10.10, Standard Version; SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. Values are presented as the
mean ± standard deviation.
Results
Concentration of key components in
CKJ
To identify changes in the functional components of
CKJ, the concentration of several important components, including
proteins, total flavonoids and total phenolic compounds, were
measured using a number of traditional methods. The levels of these
three aforementioned components significantly increased following
fermentation of the soybean (Table
I). However, no significant alteration in the total sugar
concentration was detected. In addition, the levels of two key
components that were correlated with the putative beneficial
effects for animal growth, were measured by HPLC analysis. The
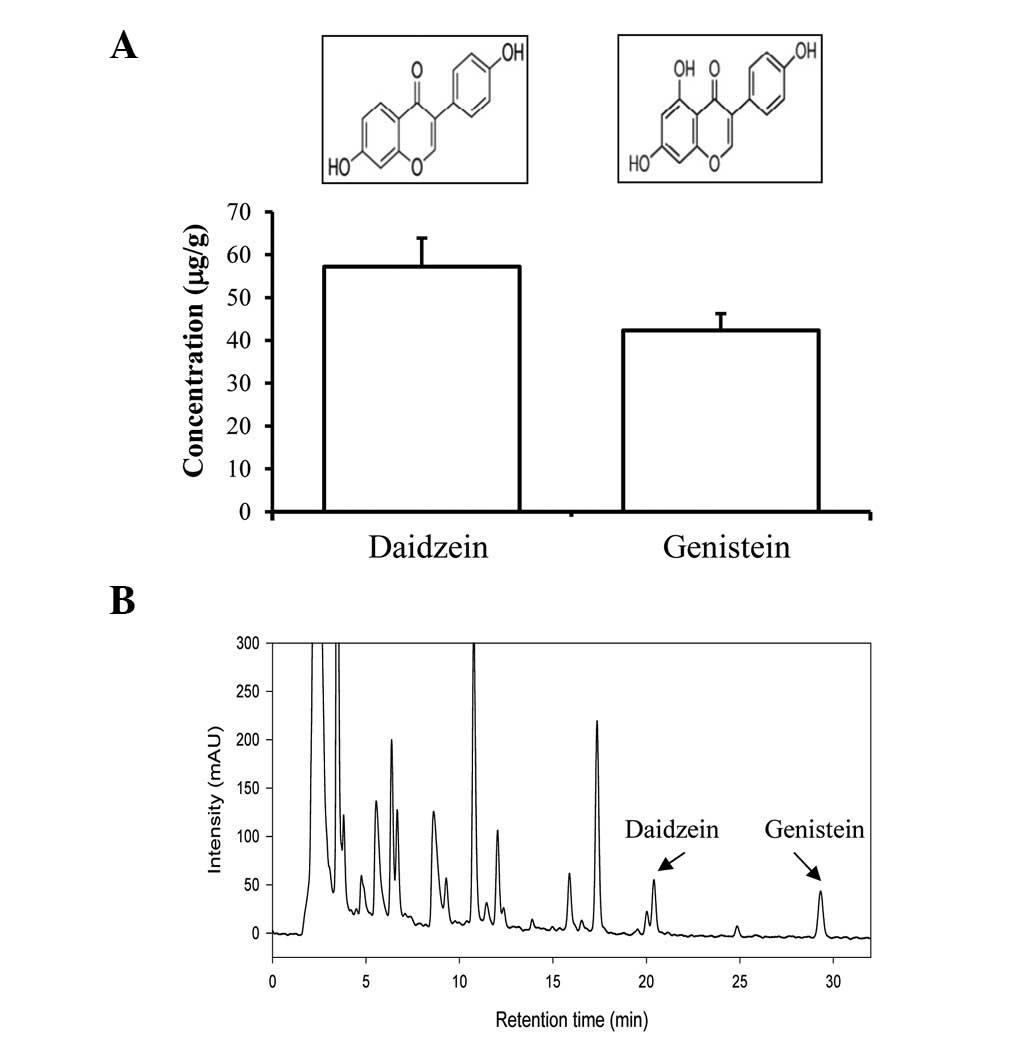
concentration of daidzein was detected as 57.19 μg/g, while the
concentration of genistein was detected as 42.31 μg/g (Fig. 1). Therefore, the aforementioned
results indicate that the CKJ used in this study contained high
concentrations of protein, total flavonoids, total phenolic
compounds, daidzein and genistein.
 | Table IContent of key components in CKJ. |
Table I
Content of key components in CKJ.
| Components | Non-fermented soybean
(mg/g) | Fermented soybean
(cheonggukjang) (mg/g) |
|---|
| Protein | 489.060 |
565.030±12.141a |
| Total sugar | 5.141 | 5.252±0.721 |
| Total flavonoid | 0.595 | 0.761±0.094a |
| Total phenolic
compound | 8.927 | 9.861±0.885a |
Effect of CKJ on total body and organ
weights
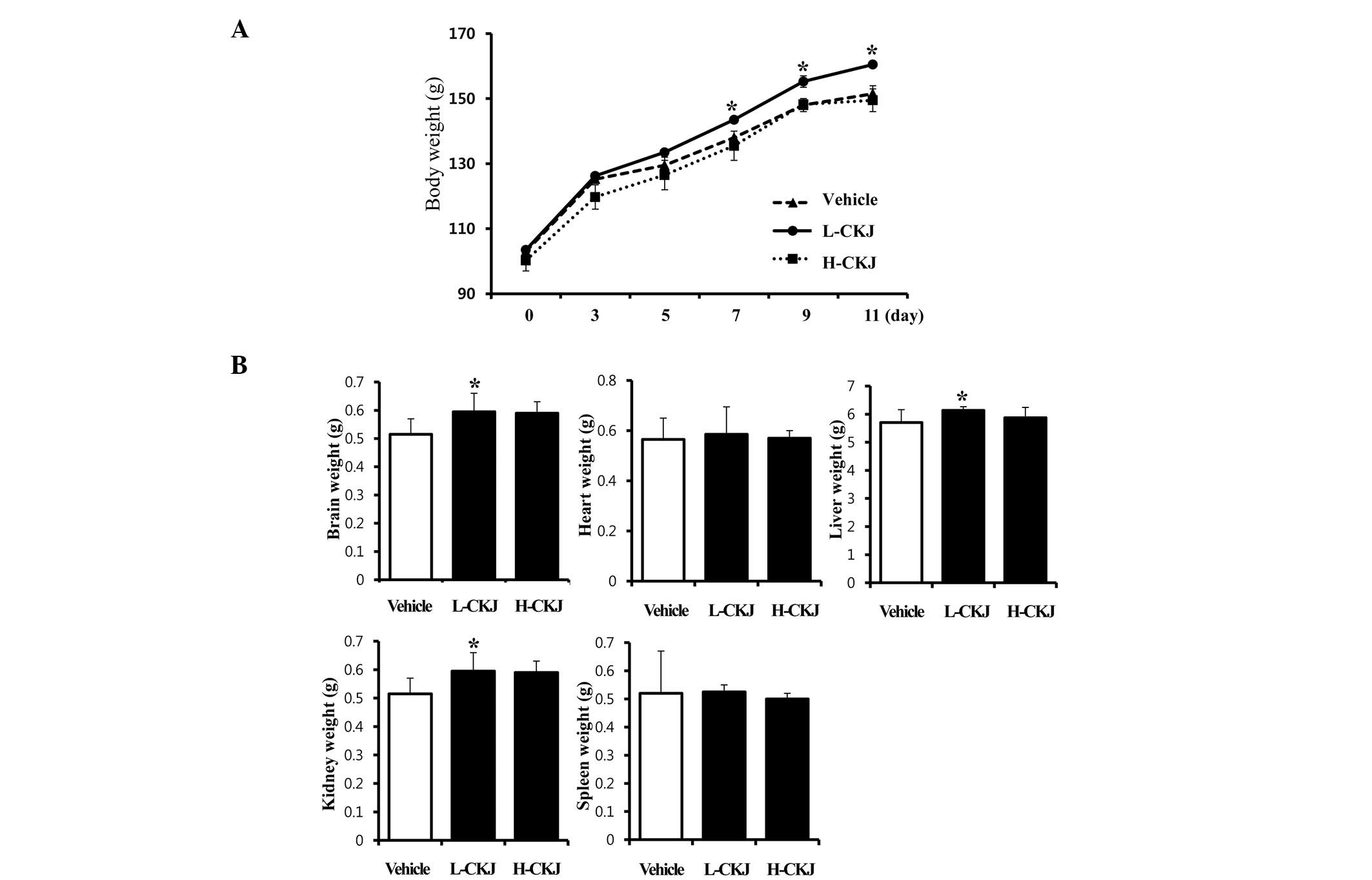
In order to determine the short-term effects of CKJ
on animal growth, alterations of total body and organ weight in
young SD rats were detected following CKJ administration for 10
days. The body weights of L-CKJ-treated rats were significantly
higher compared with those of vehicle-treated SD rats from days
seven to 11. However, in the H-CKJ-treated group, an increase in
body weight was detected only on day three, and its level returned
to that of the vehicle-treated group from day five (Fig. 2A).
Furthermore, of the five important organs, the
weights of the brain, liver and kidney significantly increased in
the L-CKJ-treated group compared with those in the vehicle-treated
group. However, heart and spleen weights were maintained at
constant levels in the L-CKJ- and H-CKJ-treated groups (Fig. 2B). These results suggest that the
L-CKJ induced significant increases in total body and organ weights
in young SD rats.
Effect of CKJ on the weight and length of
the femur, and thickness of the epiphyseal growth plate
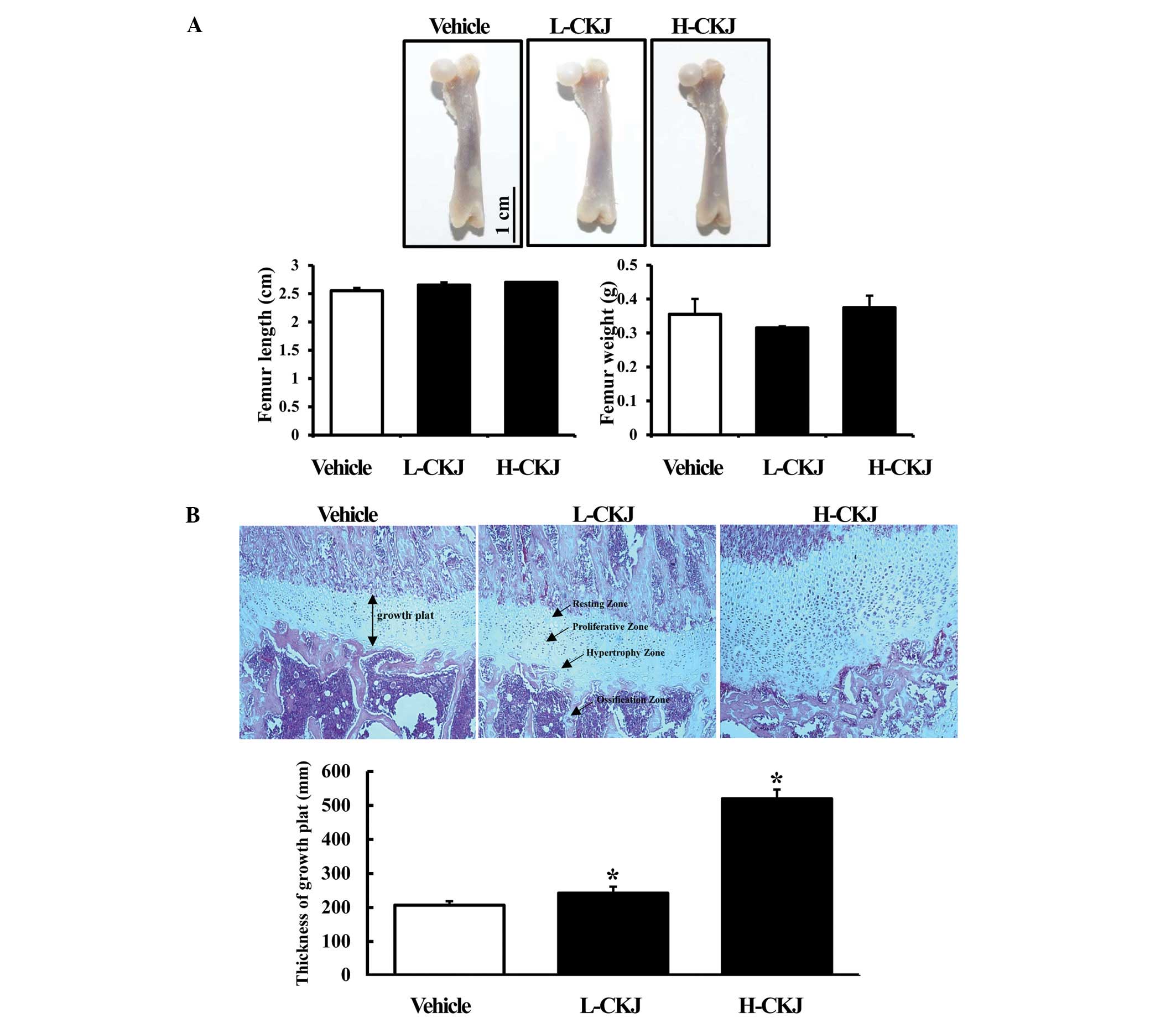
To investigate the effect of short-term CKJ
treatment on skeletal growth, femur weight and length were measured
in young SD rats treated with two different concentrations of CKJ.
The femur is the most proximal bone of the leg in tetrapod
vertebrates capable of walking or jumping (16). Therefore, we selected the femur as
a skeletal growth indicator and investigated its characteristics in
relation to the growth of young rats. Notably, significant
alterations in the femur weight and length were not detected in SD
rats treated with L-CKJ or H-CKJ for 10 days (Fig. 3A). However, a marked difference was
observed in the epiphyseal growth plate of the distal femur. In the
H-CKJ-treated group, the thickness of the epiphyseal growth plate
significantly increased compared with that of the vehicle-treated
group. The number of cells in the proliferation and hypertrophy
zone of the growth plate significantly increased in this group.
Furthermore, the thickness of the epiphyseal growth plate in the
L-CKJ-treated group was marginally enhanced compared with that of
the H-CKJ-treated group (Fig. 3B).
The aforementioned results suggest that short-term CKJ treatment
stimulated the growth of epiphyseal plate in young SD rats,
although there was no direct effect on femur weight and length.
Effect of CKJ on GH secretion from the
pituitary gland
To investigate the effects of short-term CKJ
treatment on GH secretion ability, alteration of the GH
concentration was examined in young SD male rats following CKJ
treatment for 10 days. The GH concentration in the blood serum
significantly increased in the L-CKJ-treated group compared with
that of the vehicle-treated group. However, the H-CKJ-treated group
showed a 37% lower GH level compared with that of the L-CKJ-treated
rats, although its level remained markedly higher compared with
that of the vehicle-treated group (Fig. 4A). These results show that the
L-CKJ treatment induced GH secretion from the pituitary gland.
Effect of CKJ on GH sensitivity in the
liver and muscle
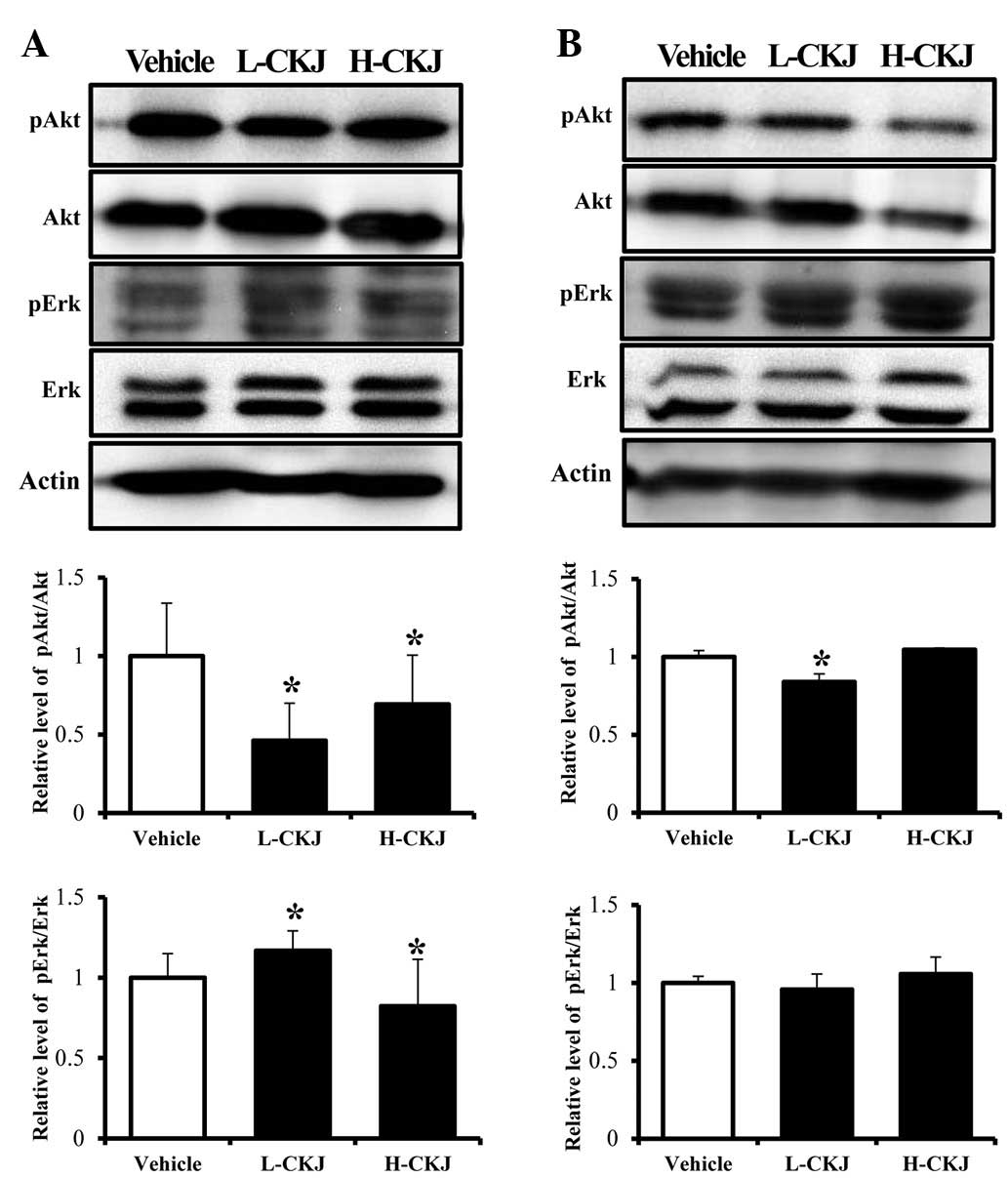
To test whether the CKJ treatment has an effect on
GH sensitivity in two target organs, the expression levels of the
GH receptor and downstream proteins were analyzed in the liver and
muscle of SD rats. The liver tissue showed a dose-dependent
response to the CKJ treatment in terms of GH receptor expression,
although the H-CKJ-treated group showed significantly higher GH
receptor expression compared to the L-CKJ-treated group. However,
the muscle tissue did not show any response to the CKJ treatment
(Fig. 4B). Of the downstream
signaling proteins in the GH receptor pathway, Akt and Erk were
selected as key target proteins for the investigation of GH
sensitivity. In the liver, the phosphorylation level of Akt
significantly decreased in both the L-CKJ- and H-CKJ-treated groups
compared with that of the vehicle-treated group. However, in the
muscle, its level was lower only in the L-CKJ-treated group and
there was no change in Akt phosphorylation in the H-CKJ-treated
group. Furthermore, the expression levels of pAkt and Akt
simultaneously decreased in a CKJ concentration-dependent manner.
The phosphorylation pattern of Erk differed from that of Akt. In
the liver tissue, the phosphorylation level of Erk marginally
increased in the L-CKJ-treated group, whereas it slightly decreased
in the H-CKJ-treated group. In the muscle tissue, the
phosphorylation level of Erk was dependent on the CKJ concentration
(Fig. 5). These results indicate
that the CKJ treatment differentially regulated the response to GH
in liver and muscle.
Discussion
Fermented soybean includes different types of
isoflavones, such as daidzein, genistein, enistein and glycetin.
Their functions are closely associated with lowering the risk of
breast and prostate cancer, the risk of cardiovascular disease and
improving bone health (1).
Generally, the concentration of daidzein and genistein, which was
analyzed in this study, was found to be significantly increased in
fermented soybean (data not shown). In particular, the
concentration of the two isoflavones in the 60% ethanol extract of
the fermented soybean was ~10-fold greater than that from the
non-fermented soybean (15).
However, in a study using 80% ethanol extracts of fermented
soybean, the concentration of daidzein was markedly increased
compared with that of the non-fermented soybean, yet genistein was
undetected in the two forms of the soybean (10). In the present study, using aqueous
extracts of the CKJ, the results were in agreement with the
aforementioned study, although the rate of increase varied.
Therefore, we suggest that the high concentration of daidzein and
genistein may be a key contributory factor in the bone growth of
CKJ-treated SD rats.
The synthesis and secretion of GH in somatotroph
cells of the anterior pituitary gland may be induced by several
natural products (17,18). For instance, administration of
yeast extract for four weeks was shown to increase the body weight
and GH secretion of young SD male rats (19). The bioassay-guided fraction of MeOH
extract from the fenugreek (Trigonella foenum-graecum
L.) seed stimulates the release of GH from rat pituitary cells. In
particular, fenugreek saponin I and dioscin have been demonstrated
to induce marked increases in GH levels, compared with other
compounds (20). In the case of
Glycyrrhizae radix, its MeOH extract and n-hexane fraction
induce GH secretion by up to 1.89- and 4.59-fold, respectively,
compared with the basal level (21). Recently, GH stimulation has
received attention as a treatment for overcoming short stature
(20,21). In an effort to identify medicinal
foods that have a beneficial effect on human growth, the effect of
CKJ on GH secretion in SD rats was investigated. As shown in
Fig. 4, the results suggest that
the CKJ was able to stimulate GH release from the pituitary
gland.
Furthermore, GH receptor exists in two forms: The
full-length membrane-bound human receptor and the GH-binding
protein. After GH binds to its receptor, dimerization of the GH
receptor is followed by phosphorylation of JAK2 and the GH receptor
in cells. The signal induced downstream of the GH-GH receptor is
transferred into the nucleus, where transcription factors,
including signal transducers and activators of transcription,
regulate the expression of the GH and GH receptor (22). Moreover, the GH-GH receptor complex
is known to activate the PI3K pathway via JAK2 phosphorylation of
IRSs (23). In this downstream
pathway, Akt/PKB are important in numerous cell processes,
including proliferation, survival and metabolism (24). Following direct injection of GH
into mice, it was observed that phosphorylation of Akt decreases
slightly in the liver, whereas it increases in muscle tissue
(23). The results of the present
study using liver tissue are in agreement with previous results
(23). However, the results of the
current study using muscle differ in that Akt phosphorylation
decreased or remained unchanged in the CKJ-treated groups.
In addition, GH has been demonstrated to control
cell proliferation, differentiation and migration through the
regulation of mitogen-activated protein kinases, which are
activated by several mitogens and growth factors (25). A previous study demonstrated a
lower level of Erk phosphorylation in the liver of rats following
GH treatment (23). However, in
the present study, the phosphorylation level of Erk significantly
increased in the two organs. Therefore, these results differ from
previous results, in which the phosphorylation level of Erk
decreased in the liver and remained unchanged in the muscle of
GH-treated mice (23). We
therefore suggest that this difference may be attributable to the
various compositions of CKJ.
Bone growth may be induced by several natural
compounds and extracts. The Buguzhi extract applied to the collagen
matrix has the effect of stimulating new bone formation in the
parietal bone of New Zealand white rabbits (26). Fetal bone growth may be stimulated
by maternal administration of Cissus quadrangularis
petroleum ether extract during pregnancy (27). Additionally, SD male rats fed yeast
extract exhibit increased tibial bone and femur bone growth
(19). This study investigated the
effects of fermented soybean extract on bone growth in SD rats. The
results differed greatly from those of previous studies. The CKJ
treatment did not induce an increase in femur weight or length,
although thickness of the growth plate markedly increased. Such a
significant difference in femur bone growth may be attributed to
the dose and period of the CKJ administration, or the extract
composition.
In conclusion, these results suggest that treatment
with CKJ, containing enhanced flavonoid and phenolic compounds, for
a short time period improves GH sensitivity in the epiphyseal
growth plate, liver and muscle of SD rats through the upregulation
of GH secretion.
Acknowledgements
This study was supported by grants to Dr Dae Youn
Hwang from the Korea Institute of Planning Evaluation for
Technology of Food, Agriculture, Forestry and Fisheries
(111030-3).
References
|
1
|
Lee JJ, Lee DS and Kim HB: Fermentation
patterns of chungkookjang and Kanjang by Bacillus
licheniformis B1. Kor J Microbiol. 35:296–301. 1999.
|
|
2
|
Su CL, Wu CJ, Chen FN, Wang BJ, Shen SR
and Won SJ: Supernatant of bacterial fermented soybean induces
apoptosis of human hepatocellular carcinoma Hep 3B cells via
activation of caspase 8 and mitochondria. Food Chem Toxicol.
45:303–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakajima N, Nozaki N, Ishihara K, Ishikawa
A and Tsuji H: Analysis of isoflavone content in tempeh, a
fermented soybean, and preparation of a new isoflavone-enriched
tempeh. J Biosci Bioeng. 100:685–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH
and Park S: The isoflavonoid aglycone-rich fractions of
Chungkukjang, fermented unsalted soybean, enhance insulin signaling
and peroxisome proliferation-activated receptor-gamma activity in
vitro. Biofactors. 26:245–258. 2006. View Article : Google Scholar
|
|
5
|
Baek HI, Kim SR, Yang JA, Kim MG, Chae SW
and Cha YS: Effect of chungkookjang supplementation on obesity and
atherosclerotic indices in overweight/obese subjects: a 12-week,
randomized, double-blind, placebo-controlled clinical trial. J Med
Food. 14:532–537. 2011. View Article : Google Scholar
|
|
6
|
Soh JR, Kwon DY and Cha YS: Hepatic gene
expression profiles are altered by dietary unsalted Korean
fermented soybean (chongkukjang) consumption in mice with
diet-induced obesity. J Nutr Metab. March 9–2011.(Epub ahead of
print). View Article : Google Scholar
|
|
7
|
Kim DJ, Jeong YJ, Kwon JH, Moon KD, Kim
HJ, Jeon SM, Lee MK, Park YB and Choi MS: Beneficial effect of
chungkukjang on regulating blood glucose and pancreatic β-cell
functions in C75BL/Ksj-db/db mice. J Med Food. 11:215–223.
2008.PubMed/NCBI
|
|
8
|
Kwon DY, Daily JW III, Kim HJ and Park S:
Antidiabetic effects of fermented soybean products on type 2
diabetes. Nutr Res. 30:1–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi YH, Lim H, Heo MY, Kwon DY and Kim
HP: Anti-imflammatory activity of the ethanol extract of
Chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J
Med Food. 11:539–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HB, Lee HS, Kim SJ, Yoo HJ, Hwang JS,
Chen G and Youn HJ: Ethanol extract of fermented soybean,
Chungkookjang, inhibits the apoptosis of mouse spleen, and thymus
cells. J Microbiol. 45:256–261. 2007.PubMed/NCBI
|
|
11
|
Singleton VL, Orthofer R and
Lamuela-Raventos RM: Analysis of total phenols and other oxidation
substrates and antioxidants by means of Folin-Ciocalteu reagent.
Methods Enzymol. 299:152–178. 1999. View Article : Google Scholar
|
|
12
|
Meda A, Lamien CE, Romito M, Millogo J and
Nacoulma OG: Determination of the total phenolic, flavonoid and
proline contents in Burkina Fasan honey, as well as their radical
scavenging activity. Food Chem. 91:571–577. 2005. View Article : Google Scholar
|
|
13
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dubois M, Gilles KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Biochem. 28:350–356. 1956.
|
|
15
|
Zheng J, Jin Y and Row KH: Analysis of
isoflavones from Korean and Chinese soybean and processed products
by HPLC. J Kor Chem Soc. 49:349–354. 2005. View Article : Google Scholar
|
|
16
|
Nareliya R and Kumar V: Biomechanical
analysis of human femur bone. Int J Eng Sci Tech. 3:3090–3094.
2011.
|
|
17
|
Daniels ME: Lilly’s Humatrope experience.
Nat Biotechnol. 10:792–812. 1992.
|
|
18
|
Powers M: Performance-enhancing drugs.
Principles of Pharmacology for Athletic Trainers. Houglum JE and
Harrelson GL: 2nd edition. SLACK Incorporated; Thorofare, NJ: pp.
331–332. 2005
|
|
19
|
Kim JM, Kim SY, Jung EY, Bae SH and Suh
HJ: Yeast hydrolysate induces longitudinal bone growth and growth
hormone release in rats. Phytother Res. 23:731–736. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shim SH, Lee EJ, Kim JS, Kang SS, Ha H,
Lee HY, Kim C, Lee JH and Son KH: Rat growth-hormone release
stimulators from fenugreek seeds. Chem Biodivers. 5:1753–1761.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HY, Jung DY, Ha H, Kang SS, Kim JS and
Kim C: Induction of growth hormone release by glycyrrhizae
radix on rat. J Biochem Mol Biol. 40:979–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Postel-Vinay MC and Finidori J: Growth
hormone receptor: structure and signal transduction. Eur J
Endocrinol. 133:654–659. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miquet JG, Muñoz MC, Giani JF, González L,
Dominici FP, Bartke A, Turyn D and Sotelo AI: Ames dwarf
(Prop1(df)/Prop1(df)) mice display increased sensitivity of the
major GH-signaling pathways in liver and skeletal muscle. Growth
Horm IGF Res. 20:118–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishimoto S and Nishida E: MAPK
signalling: ERK5 versus ERK1/2. EMBO Rep. 7:782–786. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong RW and Rabie AB: Effect of Buguzhi
(Psoralea corylifolia fruit) extract on bone formation.
Phytother Res. 24(Suppl 2): S155–S160. 2010.
|
|
27
|
Potu BK, Rao MS, Kutty NG, Bhat KM,
Chamallamudi MR and Nayak SR: Petroleum ether extract of Cissus
quadrangularis (LINN) stimulates the growth of fetal bone
during intra uterine developmental period: a morphometric analysis.
Clinics (Sao Paulo). 63:815–820. 2008.
|