Introduction
Successful chemotherapy of pulmonary and
extrapulmonary tuberculosis (TB) requires the prolonged
administration of at least three anti-TB drugs (1). Prolonged drug therapy is required to
eliminate persistent bacilli, which are small populations of
metabolically inactive microorganisms. The recommended standard
treatment for adult respiratory TB is a regimen of isoniazid (INH),
rifampicin (RIF) and pyrazinamide (PYR) for two months, followed by
four months of INH and RIF. Ethambutol (ETH) and streptomycin are
also commonly added to this regimen (1).
Tuberculosis is one of the most common causes of
mortality from curable infectious diseases. The World Health
Organization (WHO) estimated that the incidence of TB was 0.13%
worldwide in 2010 (2).
Osteo-articular TB (OATB) accounts for ~2–3% of all TB cases, and
~35% of extra-pulmonary TB cases (3). TB of the spine is potentially the
most damaging form of OATB and is responsible for ~50% of OATB
cases (3). Furthermore, spinal
infection is hematogenous or postoperative (4).
The cytotoxic effects of certain antibiotics on
intervertebral disc (IVD) cells were investigated in animals and
humans (5,6). The penetration and distribution of
antibiotics into avascular IVDs is significantly dependent on the
charge of the antibiotics (7). The
concentration of cephazolin, a drug administered intravenously
during spinal surgery, reached a maximum concentration in the serum
in <10 min, and in the IVDs it reached a concentration 15 times
lower than that in the serum in <60 min (8). Although studies have been conducted
concerning the serum concentrations of anti-TB drugs, there are no
studies that describe the penetration of these drugs into IVDs.
Liver injury, skin reactions, gastrointestinal and
neurological disorders have frequently been observed as adverse
effects of anti-TB treatment (9).
Hepatotoxicity has been identified to be a common adverse reaction
to RIF, and peripheral neuropathy affecting muscles, joints and
limbs in anti-TB drug-treated patients has been linked to
neurotoxicity (10). In addition,
INH has been connected to an increased risk of developing hepatitis
and INH has been demonstrated to compete with vitamin B6 in its
action as a cofactor in the synthesis of synaptic neurotransmitters
(11). In anti-TB treatment using
ETH, optic neuritis and retrobulbar neuritis were common toxic
effects as well as pruritus, joint pain, gastrointestinal problems
and hepatotoxicity (12).
Dose-related hepatotoxicity and gastrointestinal imbalance were
also adverse effects resulting from treatment with PYR (11). As anti-TB drugs are also applied in
the treatment of OATB, they are required to reach infected IVDs.
The effect of anti-TB drugs on IVD cells, particularly nucleus
pulposus (NP) cells, has not yet been observed in terms of cell
viability, gene expression or glycosaminoglycans (GAGs)
concentration.
There are clear morphological and physiological
differences between articular cartilage and NP tissues, suggesting
that there are differences in their cellular phenotypes; however,
COL1A1, COL2A1, ACAN, SOX9 and
TGFB1, the key marker genes, were observed in the two
tissues. The extracellular matrix of the NP is ≤80% hydrated, as a
result of large quantities of the aggregating proteoglycan,
consisting of aggrecan protein encoded by ACAN. This
proteoglycan is entangled in a variably orientated scaffold of type
II collagen fibers encoded by COL2A1(13). Sox9 encoded by SOX9, is
involved in chondrocyte differentiation and maintenance of the
chondrocytic phenotype (14).
Transforming growth factor β1 (TGF-β1, encoded by TGFB1) is
a member of the family of cytokines acting through the Smad protein
pathway and regulates chondrogenesis (15). There have been no attempts to
characterize the expression of these key chondrogenic genes in NP
cells when treated with anti-TB drugs. Thus, this study aimed to
demonstrate the impact of anti-TB drugs on IVDs as the incidence of
tuberculosis is increasing in countries with AIDS epidemics
(2), thus it is important to fully
understand the effects of the anti-TB drugs.
NP cells were observed in the present study, as
damage to this susceptible section of the IVDs by anti-TB drugs may
be irreversible. In this study, by the incubation of human NP cells
with INH, RIF, ETH and PYR, the following hypotheses were
investigated: i) the expression of COL1A1, COL2A1,
ACAN, SOX9 and TGFB1 chondrocyte marker genes
in NP cells may be sensitive to treatment with anti-TB drugs; and
ii) the transcriptional activity of the genes encoding matrix
protein in the cultured cells may monitor functional changes during
anti-TB therapy. Additionally, anti-TB drugs may influence NP cell
viability or GAG synthesis. This study aimed to determine whether
anti-TB drugs resulted in adverse side effects in NP cells.
Subjects and methods
Subjects
Human NP cells were collected (using an anterior
approach) from 12 patients undergoing treatment to correct
thoraco-lumbar or lumbar scoliosis during the routine preparation
of the site for anterior spodylodesis. All patients were treated in
Poznań Medical University Hospital of Pediatric Orthopaedics and
Traumatology and were recruited into the study consecutively.
The following eligibility criteria was adopted: i)
10–19 years of age; ii) adolescent idiopathic scoliosis (AIS); iii)
a Cobb angle of >40 degrees; and iv) scoliosis correction from
an anterior approach with routine removal of an IVD for preparation
of the site for anterior spodylodesis. NP cells were extracted from
non-degenerative IVDs.
Patients were excluded from the study if they had
taken painkillers, antibiotics or steroids prior to hospital
admission; had undergone previous surgery in the spinal area; or
had exhibited indications of TB infection.
Patients who fulfilled the inclusion criteria
received in-depth information concerning the aim of the study and
were assured anonymity. Informed consent from the legal guardians
of each patient was obtained prior to requesting permission from
the patients to obtain the NP cells. The mean age of the patients
was 16±2.3 years (range, 14–19). The Ethics Committee of Poznań
University of Medical Sciences (Poznań, Poland; approval no.
838/09) approved the design of this study and confirmed its
accordance with universal ethical principles.
Cell culture
Non-degenerate IVD tissue was dissected
predominantly from Th12 to L3 vertebrae (in one case lumbar tissue
was dissected from L1 to L4) to separate the NP from the annulus
fibrosus (AF) tissue. The NP was enzymatically digested overnight
at 37°C with 0.02% collagenase type II (Sigma, St. Louis, MO, USA)
in a serum-free medium containing an antibiotic antimycotic
solution (100 units penicillin, 100 μg streptomycin and 25 ng
amphotericin B per milliliter; Sigma). The digested tissue
suspension was filtered through a sterile nylon fabric to remove
the remaining tissue debris. Cells were centrifuged at 300 × g for
5 min, seeded onto a tissue culture flask and cultured at 37°C in
5% CO2/95% air, in (1:1 v/v) Dulbecco’s modified Eagle’s
medium/Nutrient F-12 Ham (DMEM/F-12; Sigma) supplemented with 10%
fetal bovine serum (FBS; Sigma) and an antibiotic antimycotic
solution (Sigma). For gene expression experiments, 150,000 cells
were placed in each well of 6-well plates; while for cell viability
experiments, 25,000 cells were placed in each well of 24-well
plates. The activity of caspase-3 and -7 was determined on 96-well
plates with 5,000 cells/well. For GAG assays 200,000 cells were
placed in each well of 6-well plates. The following concentrations
of test substances were used: 2.5 ng/ml TGF-β1 (Promega
Corporation, Madison, WI, USA), 5 μg/ml INH, 10 μg/ml RIF, 2 μg/ml
ETH and 5 μg/ml PYR (Sigma).
Gene expression
Following the 24-h incubation of NP cells with test
substances, total RNA was extracted from the cultured cells using
TRItidy G, according to the manufacturer’s instructions (Applichem
GmbH, Darmstadt, Germany). Total RNA (1 μg) was reverse-transcribed
using the Superscript Reverse Transcriptase kit (Invitrogen Life
Technologies, Carlsbad, CA, USA). Oligo(dT)15-primed
cDNAs were amplified by quantitative PCR (qPCR) using the primers
listed in Table I and the Light
Cycler FastStart DNA Master SYBR-Green I kit (Roche Diagnostics
GmbH, Mannheim, Germany). Crossing-point values were calculated
automatically, based on the second derivative algorithm, and the
results were analyzed by the relative expression method.
HMBS and MRPL19 were used as reference genes.
 | Table IPrimers used in qPCR. |
Table I
Primers used in qPCR.
| Gene | Primer
sequence | Amplicon length
(bp) | GenBank Accession
nos. |
|---|
| ACAN |
5′-ACCAGACTGTCAGATACCCC-3′
5′-CATAAAAGACCTCACCCTCC-3′ | 156 | NM_001135 |
| SOX9 |
5′-GAAGAACGGGCAGGCGGA-3′
5′-TTTGGGGGTGGTGGGTGG-3′ | 181 | NM_000346 |
| COL2A1 |
5′-ACCAGGACCAAAGGGACA-3′
5′-GCAGCAAAGTTTCCACCA-3′ | 246 | NM_033150 |
| COL1A1 |
5′-GAAGGGACACAGAGGTTTCAG-3′
5′-TTCCACGAGCACCAGCAG-3′ | 179 | NM_000088 |
| TGFB1 |
5′-GAAACCCACAACGAAATC-3′
5′-AATTTCCCCTCCACGGCT-3′ | 300 | NM_000660 |
| MRPL19 |
5′-TCCAACCGCCGCCGAAAC-3′
5′-AACACGAAGAATACTTCCAACA-3′ | 197 | NM_014763.3 |
| HMBS
(PBGD) |
5′-CCCTGGAGAAGAATGAAGTG-3′
5′-TCCCCGAATACTCCTGAA-3′ | 254 | NM_000190 |
Cell viability and caspase activity
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT)-based Cell Growth Determination kit (Sigma) was used for cell
viability analyses. Following 24, 48 or 192 h of stimulation with
test substances, the cells in 24-well plates were incubated in
serum-free DMEM/F-12 with the addition of MTT (0.5 mg/ml).
Following incubation for 4 h, the cell culture medium was removed
and solvent was added (1 mM hydrochloric acid in isopropanol
anhydride; POCH S.A., Gliwice, Poland). Following gentle mixing,
analysis was performed using the Stat-Fax 2100 spectrophotometer
(Awareness Technology Inc., Palm City, FL, USA) at a wavelength of
630 nm (background absorbance was measured at a wavelength of 405
nm).
Caspase activity was analyzed by a Caspase-Glo 3/7
assay (Promega Corporation). Following cell stimulation,
Caspase-Glo 3/7 Reagent was added (culture medium: Caspase Glo 3/7
reagent, 4:1) to cells in a 96-well plate. Subsequent to incubation
for 1 h, luminescence was measured in the TD20/20 luminometer
(Turner Designs Inc., Sunnyvale, CA, USA).
GAG level in NP cells
NP cells were grown in culture media with the
addition of the test substances and digested with papain after 192
h. Subsequently, Blyscan Sulfated Glycosaminoglycan assays
(Biocolor Ltd., Carrickfergus, UK) were performed according to the
manufacturer’s instructions.
Statistical analysis
For qPCR data, crossing-point values were
transformed into relative quantities by the instrument software
(LightCycler Software 4.05; Roche Diagnostics GmbH). Gene specific
standard curves were applied to calculate the relative levels of
respective transcript upon Cp values. The mean values of the
target-to-reference ratios from at least three independent
experiments were presented as the mean ±SEM. Controls were
normalized to one.
P-values for all gene expressions were calculated
and P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed on data
transformed into log10 values with the use of MS Excel
and GraphPad InStat software (La Jolla, CA, USA) applying the
Student Neuman-Keuls post hoc analysis of variance.
Results
Gene expression
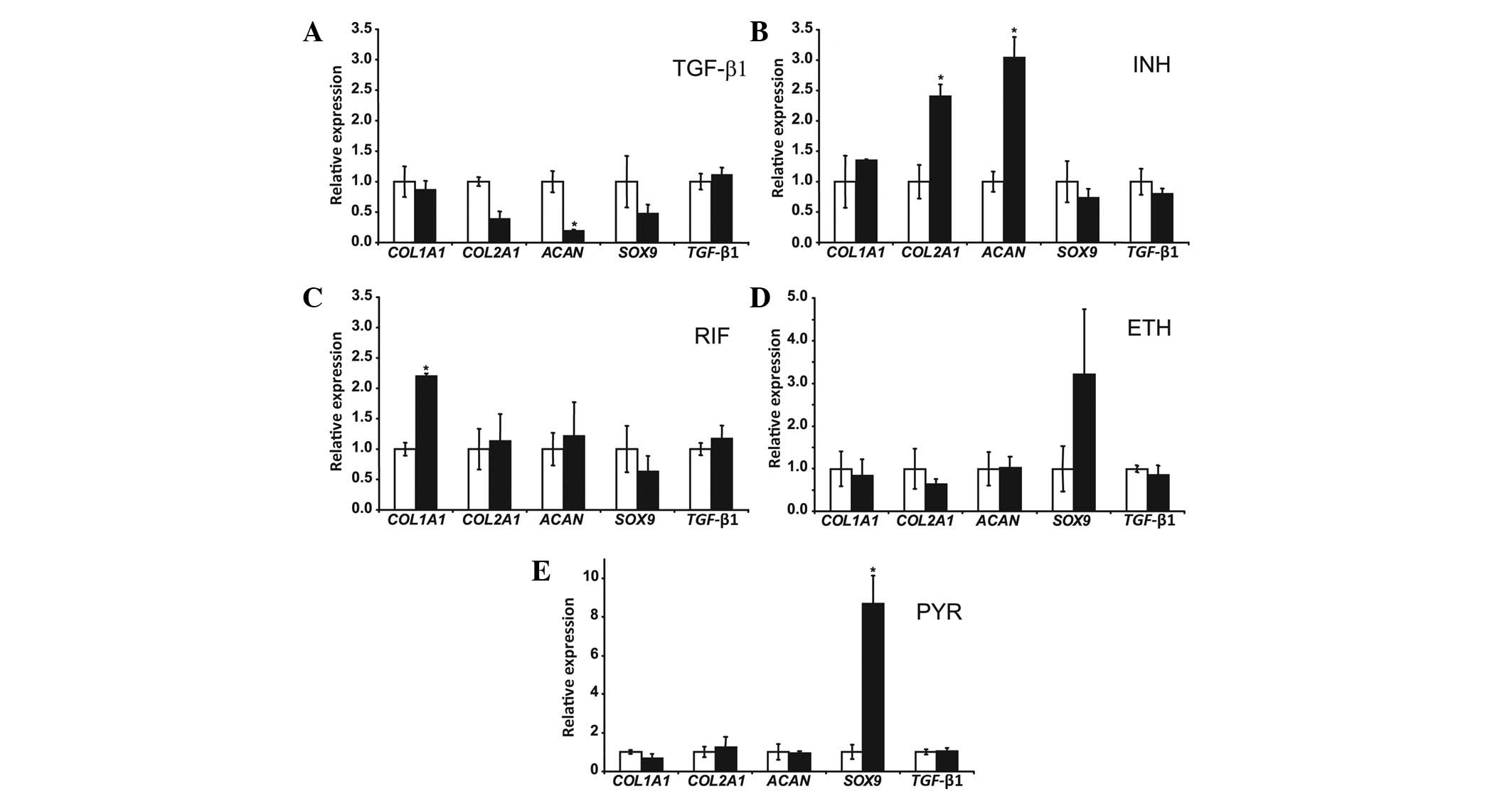
Fig. 1 demonstrates
that treatment of NP cells with 2.5 ng/ml TGF-β1 for 24 h resulted
in an 80% decrease in ACAN mRNA levels (P=0.003); while
COL1A1, COL2A1, SOX9 and TGFB1 mRNA
levels were not significantly changed. The NP cells were treated
with 5 μg/ml INH for 24 h, resulting in a 2.4-fold increase in
COL2A1 mRNA levels (P=0.025) and a 3-fold increase in
ACAN mRNA levels (P=0.045); while those of COL1A1,
SOX9 and TGFB1 were not significantly affected.
Treatment of NP cells with 10 μg/ml RIF for 24 h increased
COL1A1 mRNA levels 2.2-fold (P=0.010) but did not result in
changes in COL2A1, ACAN, SOX9 and TGFB1
mRNA levels. Treatment of NP cells with 2 μg/ml ETH for 24 h did
not change the COL1A1, COL2A1, ACAN,
SOX9 and TGFB1 mRNA levels. Treatment of NP cells
with 5 μg/ml PYR for 24 hours resulted in an 8.7-fold increase in
SOX9 mRNA levels (P=0.017). COL1A1, COL2A1,
ACAN and TGFB1 mRNA levels were not significantly
changed by PYR.
Cell viability
NP cells were treated with 2.5 ng/ml TGF-β1 and
anti-TB drugs to determine whether these substances induced changes
in the cell viability. Fig. 2
demonstrates that following 192 h of treatment with 2.5 ng/ml,
TGF-β1 decreased the viability of NP cells by 30%. The same length
of treatment with 5 μg/ml INH led to a 15% decrease in the
viability of NP cells. Treatment of NP cells with 10 μg/ml RIF for
192 h diminished cell viability by >30%. Treatment with ETH (2
μg/ml) for 24 h increased the viability of NP cells 1.3-fold;
however, PYR did not significantly alter NP cell viability. The
caspase-3 and -7 activity assay did not demonstrate an increase in
activity in cells treated by anti-TB drugs (data not shown).
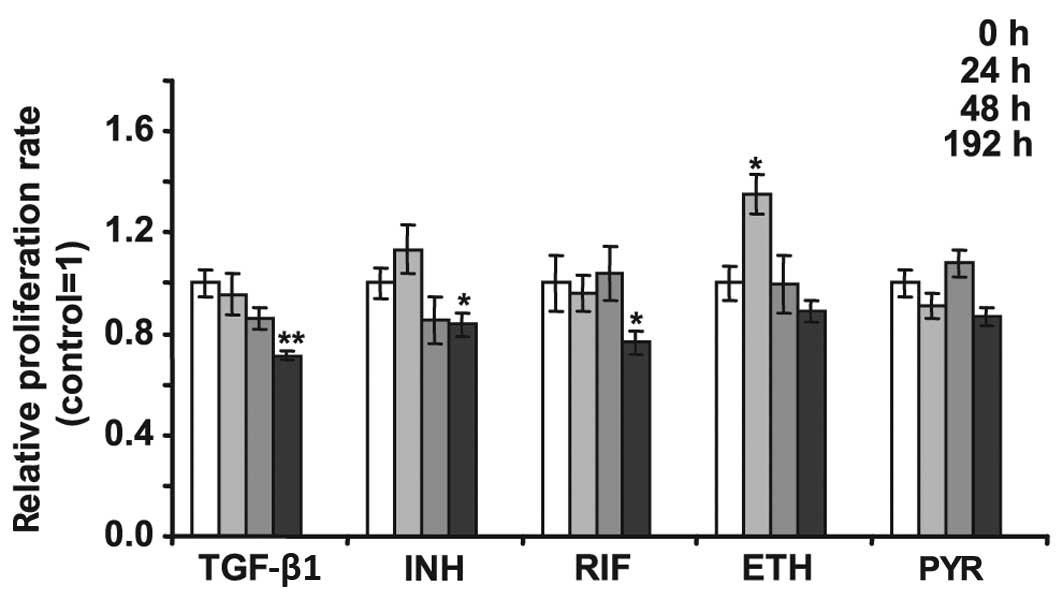 | Figure 2Viability test of NP cells treated
24, 48 and 192 h with 2.5 ng/ml TGF-β1, 5 μg/ml INH, 10 μg/ml RIF,
2 μg/ml ETH and 5 μg/ml PYR. Untreated control equals 1 (open
bars). Cells were grown in culture media with addition of test
substances and an MTT assay was performed at the indicated time.
Error bars represent ± SEM. *P<0.05 and
**P<0.01. NP, nucleus polposus; TGF-β1, transforming
growth factor-β1; INH, isoniazid; RIF, rifampicin; ETH, Ethambutol;
PYR, pyrazinamide; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
GAG level in NP cells
NP cells were treated for 192 h with 2.5 ng/ml
TGF-β1, 5 μg/ml INH, 10 μg/ml RIF, 2 μg/ml ETH and 5 μg/ml PYR. A
Sulfated Glycosaminoglycan assay (Blyscan) was subsequently
performed. TGF-β1 led to a 1.7-fold increase in GAGs production;
however, treatment with anti-TB drugs did not result in a
significant change in GAG content (Fig
3).
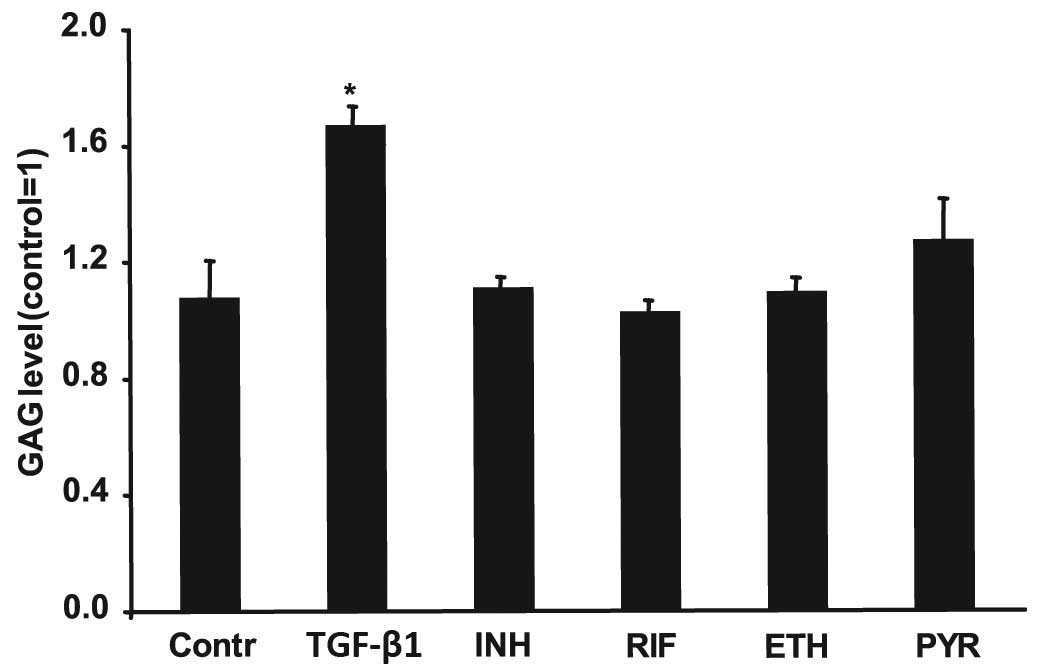 | Figure 3GAG level in NP cells. The NP cells
were treated for 192 h with 2.5 ng/ml TGF-β1, 5 μg/ml INH, 10 μg/ml
RIF, 2 μg/ml ETH and 5 μg/ml PYR. After 192 h, the NP cells were
digested with papain. Subsequently, a Sulfated Glycosaminoglycan
assay was performed to analyze the GAG content. Data are shown as a
fold of the untreated control value. Error bars represent ± SEM.
*P<0.05. GAG, glycoseaminoglycan; NP, nucleus
polposus; TGF-β1, transforming growth factor-β1; INH, isoniazid;
RIF, rifampicin; ETH, Ethambutol; PYR, pyrazinamide. |
Discussion
The present study demonstrated the specific side
effects of anti-TB drugs on human NP cell gene expression,
viability and GAG synthesis. The toxicity of anti-TB drugs (INH,
RIF, ETH and PYR) has been studied in hepatocytes and other types
of cells, but no effects have been demonstrated in cells from the
human articular system, particularly in human IVD cells. In the
present study the side effects of the anti-TB drugs were divided
into three categories: Stimulating chondrocyte marker genes and
decreasing cell viability (INH and RIF), stimulating chondrocyte
marker genes and non-regulating viability (PYR) and drug
non-stimulating chondrocyte marker genes with observed viability
effects (ETH).
Prior to investigating the impact of INH, RIF, ETH
and PYR on NP cells, the identity of the cultured primary cells
obtained during surgery was validated by the expression of the
following chondrocyte marker genes: COL1A1, COL2A1,
SOX9, ACAN and TGFB1. In addition, TGF-β1 was
also used, as its impact on chondrocyte marker gene expression in
NP cells has been determined previously (16). In the present study, the expression
of ACAN in NP cells decreased with the addition of 2.5 ng/ml
TGF-β1, which was concordant with previous observations concerning
the effect of 10 ng/ml TGF-β1 on ACAN expression in
adolescent and adult NP cells (17). However, in similar conditions, in a
previous study, 72 h of treatment with 10 ng/ml TGF-β1 led to an
increase in ACAN expression and COL1A1, COL2A1
and SOX9 mRNA levels were constant (18). The presence of 1 ng/ml TGF-β1 in
platelet-rich plasma induced an increase in ACAN,
COL2A1 and SOX9 expression (15). Moreover, the effect of TGF-β1 on NP
cells was limited at 1 ng/ml and 2 ng/ml, whereas concentrations of
≤1 ng/ml TGF-β1 increased the viability of NP cells but a
concentration of ≥2 ng/ml TGF-β exhibited no effect (15). In the present study, TGF-β1 led to
a decrease in NP cell viability after 192 h and this
antiproliferative effect of the growth factor confirms its
involvement in cell growth. Although TGF-β1 is an inhibitor of cell
proliferation in the majority of cell types and has been shown to
stimulate the growth of human nasal septal chondrocytes (19), it exhibited no visible effect on
the growth of NP cells or stimulated NP cells (18,20).
It was hypothesized that TGF-β1 requires a proportional quantity of
other growth factors to be present in platelet-rich plasma to
stimulate ACAN, COL2A1 and SOX9 as well as
promote cell viability. It appears that the effects of TGF-β1 on
chondrocytes are dependent upon the culture system to which it is
added; therefore results may differ between studies (18).
To determine whether apoptosis may be responsible
for the TGF-β1 effect, the activity of caspase-3 and -7 were
analyzed. Caspases-3 and -7 belong to the group of caspases termed
executioner caspases and exist within the cytosol as inactive
zymogen dimers that are activated by initiator caspases-8 and -9
(21). As no changes were observed
in the caspase-3 and -7 activity it was concluded that apoptosis
was not responsible for the decrease in NP cell viability in the
presence of TGF-β1 (data not shown).
Proteoglycan aggregates are commonly located in the
structure of cartilage and IVDs. GAGs attach covalently to
proteins, such as aggrecan, form proteoglycans. In the present
study, similar to the study by Yang et al(17), the level of GAGs was increased by
the addition of TGF-β1 in NP cells. As TGF-β1 induced a
simultaneous decrease in the expression of the ACAN gene, it was
concluded that the entire effect of TGF-β1 on proteoglycan content
in the extracellular matrix of NP remains unknown. This data
implies that the proteoglycans, due to the action of TGF β1, may
contain less ACAN protein which is highly glycosylated (22). The present data are consistent with
the observation that TGF-β1, via Smad proteins, stimulates the
expression of glucuronosyl transferase I (GlcAT-I), an important
enzyme in the GAG biosynthesis pathway (23). Although NP cells from adolescents
with idiopathic scoliosis may reveal some degenerative changes, we
demonstrated that our NP cells were not degenerated as disc
degeneration eliminates the regulation of GAGs synthesis by TGF-β1
(17,23)
NP cells that expressed marker genes have been used
to investigate the effect of anti-TB drugs. The INH concentration
in the plasma following oral administration of a 700-mg dose has
been demonstrated to reach 3–5 μg/ml in 6 h (24). In the present study, NP cells were
incubated with 5 μg/ml INH for 24 h, resulting in increased
ACAN expression, which was the opposite effect to that of
TGF-β1. Moreover, INH stimulated COL2A1 gene expression,
which may result in the biosynthesis of extracellular matrix. The
mechanism of these anti-aging effects of INH remains to be
elucidated, as it is not clear whether the drug itself, or its
metabolites (such as hydrazine), act on COL2A1 and
ACAN gene expression (1).
It has previously been suggested that the predominant toxic effect
of INH is a result of its metabolites, monoacetyl hydrazine and
hydrazine, as well as related compounds, which induce changes in
the expression of numerous genes in the rat liver (1,6,25,26).
We suggest that a similar mechanism may be associated with the
antiproliferative effect of INH on NP cells; however, this requires
further investigation, as caspase-3 and -7 were not activated (data
not shown) and the synthesis of GAGs was not affected by treatment
with INH.
In a study by Boman (27), the RIF concentration in the plasma
decreased to 4 μg/ml in 6 h following oral administration of a
10-mg/kg dose, and the peak serum concentration was 8 g/ml. Similar
to the results of the present study, RIF was previously observed to
inhibit the growth of different types of cancer cells and
osteoblast cells in vitro(28–30).
The predominant elimination pathway is deacetylation into desacetyl
rifampicin, while a 3-formyl rifampicin is produced separately by
hydrolysis (20,28,29).
RIF acts through the pregnane X receptor (PXR) and mediates the
induction of the CYP2C9, CYP3A, CYP2 and
MDR1 genes (1,28,31).
In the current study, RIF stimulated COL1A1 expression though a
typical PXR motif, TGAACT, is not present in the 1000bp located
upstream of the transcription start site of COL1A1.
Therefore, the mechanism of COL1A1 regulation by RIF remains
to be elucidated. In the NP cells of aged rabbits, COL1A1
was upregulated; thus, it was concluded that RIF may promote the
aging of NP cells in patients treated with this drug (32). Observations of the
antiproliferative effect of RIF on NP cells without a concomitant
increase in caspase-3 and -7 activities requires additional
investigation.
Following the administration of a single 25 mg/kg
dose, the mean maximum concentration of ETH in serum has been
demonstrated to reach 4.5 μg/ml (33–35).
In the present study, of all the tested anti-TB drugs, ETH had the
least effect on gene expression and was the only drug to stimulate
cell viability after 24 h. This anti-aging property of ETH
subsequent to short-term incubation was not observed after 192 h.
The co-administration of therapeutic doses of ETH with other
anti-TB drugs led to a marked decrease in male rat fertilizing
capacity and fertility, and an increase in pre- and
post-implantation embryo lethality (36). As the most serious potential
adverse effect of ETH is ocular toxicity, manifested by optic or
retrobulbar neuritis (7), the
safety of ETH treatment in NP cells is required to be determined
for longer therapeutic periods.
PYR achieved a maximum concentration of 49 μg/ml in
the blood after 2 h (34). PYR is
converted to pyrazinoic acid and further oxidized to
5-hydroxypyrazinoic acid by xanthine oxidase (37). Pyrazinoic acid inhibits translation
in Mycobacterium tuberculosis; however, the mechanism of the
influence of PYR on eukaryotic cells is not known (38). In this study, 5 μg/ml PYR induced
the expression of SOX9, a transcription factor that is key
in chondrogenesis (39). The same
concentration of PYR did not affect cell viability, caspase-3 and
-7 activity, and GAG synthesis. It was concluded that PYR exerted a
moderate anti-aging effect on NP cells. PYR may be beneficial in
IVD regeneration and may be used to prevent the loss of SOX9
expression in degenerated IVD cells. It may also prevent a decrease
in COL2A1 and ACAN expression, as SOX9 is a
common transcription factor for the genes (14,40).
Prolonged treatment with PYR is required to determine whether
COL2A1 and ACAN expression increases.
We propose that INH and PYR may have a dual role in
anti-TB therapy; they decrease Mycobacterium levels, as well
as stimulate the expression of genes encoding the extracellular
matrix proteins (COL2A1 and ACAN) and the SOX9
protein. It was demonstrated that 192 h of treatment with INH or
RIF resulted in cytotoxicity, indicated by a decrease in the number
of NP cells. These results are similar to those obtained by
Hoelscher et al(5) in
annulus fibrosus cells, in which antibiotics (cefazolin and
cefamandole) used in spinal surgery led to lower cell
proliferation. The results, albeit on a small number of samples,
suggested that the anti-TB drugs generated certain side effects on
NP cells in vitro. To confirm these results, the collection
of data following prolonged treatment with anti-TB drugs is
required, as antimycobacterial therapy lasts for several months.
Application of TGF-β1 and anti-TB drugs revealed opposite effects
on the expression of key chondrocyte genes and therefore should be
investigated in combination. To the best of our knowledge, this is
the first study to demonstrate that anti-TB drugs may be harmful to
articular cells. The potential adverse effects of anti-TB drugs may
be of importance to clinicians who use anti-TB drug for the routine
chemotherapy of mycobacterial infections. In addition, the
vertebral column of patients suffering pulmonary or extra-pulmonary
mycobacterial infection, treated with INH, RIF, ETH or PYR, should
be monitored periodically.
Acknowledgements
This study was supported by a Polish Ministry of
Science and Higher Education grant (grant no. N N 403600538). The
authors would like to thank to Ms. Beata Raczak and Ms. Bogumila
Ratajczak for their help in the preparation of this paper.
References
|
1
|
Tostmann A, Boeree MJ, Aarnoutse RE, de
Lange WC, van der Ven AJ and Dekhuijzen R: Antituberculosis
drug-induced hepatotoxicity: concise up-to-date review. J
Gastroenterol Hepatol. 23:192–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
WHO. Global tuberculosis control.
http://www.who.int/tb/publications/global_report/2011/gtbr11_main.pdf.
Accessed October 9, 2013
|
|
3
|
Donald PR: The chemotherapy of
osteo-articular tuberculosis with recommendations for treatment of
children. J Infect. 62:411–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hadjipavlou AG, Mader JT, Necessary JT and
Muffoletto AJ: Hematogenous pyogenic spinal infections and their
surgical management. Spine (Phila Pa 1976). 25:1668–1679. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoelscher GL, Gruber HE, Coldham G,
Grigsby JH and Hanley EN Jr: Effects of very high antibiotic
concentrations on human intervertebral disc cell proliferation,
viability, and metabolism in vitro. Spine (Phila Pa 1976).
25:1871–1877. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schaberg T, Rebhan K and Lode H: Risk
factors for side-effects of isoniazid, rifampin and pyrazinamide in
patients hospitalized for pulmonary tuberculosis. Eur Respir J.
9:2026–2030. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riley LH III, Banovac K, Martinez OV and
Eismont FJ: Tissue distribution of antibiotics in the
intervertebral disc. Spine (Phila Pa 1976). 19:2619–2625. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walters R, Moore R and Fraser R:
Penetration of cephazolin in human lumbar intervertebral disc.
Spine (Phila Pa 1976). 31:567–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Black M, Mitchell JR, Zimmerman HJ, Ishak
KG and Epler GR: Isoniazid-associated hepatitis in 114 patients.
Gastroenterology. 69:289–302. 1975.PubMed/NCBI
|
|
10
|
Aristoff PA, Garcia GA, Kirchhoff PD and
Hollis Showalter HD: Rifamycins - obstacles and opportunities.
Tuberculosis (Edinb). 90:94–118. 2010. View Article : Google Scholar
|
|
11
|
Frydenberg AR and Graham SM: Toxicity of
first-line drugs for treatment of tuberculosis in children: review.
Trop Med Int Health. 14:1329–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Griffith DE, Brown-Elliott BA, Shepherd S,
McLarty J, Griffith L and Wallace RJ Jr: Ethambutol ocular toxicity
in treatment regimens for Mycobacterium avium complex lung disease.
Am J Respir Crit Care Med. 172:250–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sive JI, Baird P, Jeziorsk M, Watkins A,
Hoyland JA and Freemont AJ: Expression of chondrocyte markers by
cells of normal and degenerate intervertebral discs. Mol Pathol.
55:91–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gruber HE, Norton HJ, Ingram JA and Hanley
EN Jr: The SOX9 transcription factor in the human disc: decreased
immunolocalization with age and disc degeneration. Spine (Phila Pa
1976). 30:625–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen WH, Lo WC, Lee JJ, et al:
Tissue-engineered intervertebral disc and chondrogenesis using
human nucleus pulposus regulated through TGF-beta1 in platelet-rich
plasma. J Cell Physiol. 209:744–754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freyria AM and Mallein-Gerin F:
Chondrocytes or adult stem cells for cartilage repair: the
indisputable role of growth factors. Injury. 43:259–265. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SH, Lin CC, Hu MH, Shih TT, Sun YH
and Lin FH: Influence of age-related degeneration on regenerative
potential of human nucleus pulposus cells. J Orthop Res.
28:379–383. 2010.PubMed/NCBI
|
|
18
|
Yang SH, Wu CC, Shih TT, Sun YH and Lin
FH: In vitro study on interaction between human nucleus pulposus
cells and mesenchymal stem cells through paracrine stimulation.
Spine (Phila Pa 1976). 33:1951–1957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bujía J, Sittinger M, Wilmes E and Hammer
C: Effect of growth factors on cell proliferation by human nasal
septal chondrocytes cultured in monolayer. Acta Otolaryngol.
114:539–543. 1994.PubMed/NCBI
|
|
20
|
Zhang R, Ruan D and Zhang C: Effects of
TGF-beta1 and IGF-1 on proliferation of human nucleus pulposus
cells in medium with different serum concentrations. J Orthop Surg
Res. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007.PubMed/NCBI
|
|
23
|
Wu Q, Wang J, Skubutyte R, et al: SMAD3
controls β-1, 3-glucuronosyltransferase 1 expression in nucleus
pulposus cells: implications of dysregulated expression in disc
disease. Arthritis Rheum. 64:3324–3333. 2012.
|
|
24
|
Boxenbaum HG, Berkersky I, Mattaliano V
and Kaplan SA: Plasma and salivary concentrations of isoniazid in
man: preliminary findings in two slow acetylator subjects. J
Pharmacokinet Biopharm. 3:443–456. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klenø TG, Kiehr B, Baunsgaard D and
Sidelmann UG: Combination of ‘omics’ data to investigate the
mechanism(s) of hydrazine-induced hepatotoxicity in rats and to
identify potential biomarkers. Biomarkers. 9:116–138. 2004.
|
|
26
|
Knowles SR, Uetrecht J and Shear NH:
Idiosyncratic drug reactions: the reactive metabolite syndromes.
Lancet. 356:1587–1591. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boman G: Serum concentration and half-life
of rifampicin after simultaneous oral administration of
aminosalicylic acid or isoniazid. Eur J Clin Pharmacol. 7:217–225.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holdiness MR: Clinical pharmacokinetics of
the antituberculosis drugs. Clin Pharmacokinet. 9:511–544. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trnka L, Mison P and Staflova S:
Interaction aspects of antimycobacterial drugs in the chemotherapy
of tuberculosis. II The role of rifampicin and other drugs in the
dependent or independent action of drug associations in vitro.
Chemotherapy. 20:82–91. 1974.PubMed/NCBI
|
|
30
|
Westphal JF, Vetter D and Brogard JM:
Hepatic side-effects of antibiotics. J Antimicrob Chemother.
33:387–401. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuang W, Jia Z, Feng H, et al: The
mechanism of the G0/G1 cell cycle phase arrest induced by
activation of PXR in human cells. Biomed Pharmacother. 65:467–473.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clouet J, Pot-Vaucel M, Grimandi G, et al:
Characterization of the age-dependent intervertebral disc changes
in rabbit by correlation between MRI, histology and gene
expression. BMC Musculoskelet Disord. 12:1472011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Conte JE Jr, Golden JA, Kipps J, Lin ET
and Zurlinden E: Effects of AIDS and gender on steady-state plasma
and intrapulmonary ethambutol concentrations. Antimicrob Agents
Chemother. 45:2891–2896. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McIlleron H, Wash P, Burger A, Norman J,
Folb PI and Smith P: Determinants of rifampin, isoniazid,
pyrazinamide, and ethambutol pharmacokinetics in a cohort of
tuberculosis patients. Antimicrob Agents Chemother. 50:1170–1177.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peloquin CA, Bulpitt AE, Jaresko GS,
Jelliffe RW, Childs JM and Nix DE: Pharmacokinetics of ethambutol
under fasting conditions, with food, and with antacids. Antimicrob
Agents Chemother. 43:568–572. 1999.PubMed/NCBI
|
|
36
|
Shayakhmetova GM, Bondarenko LB and
Kovalenko VM: Damage of testicular cell macromolecules and
reproductive capacity of male rats following co-administration of
ethambutol, rifampicin, isoniazid and pyrazinamide. Interdiscip
Toxicol. 5:9–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ellard GA and Haslam RM: Observations on
the reduction of the renal elimination of urate in man caused by
the administration of pyrazinamide. Tubercle. 57:97–103. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi W, Zhang X, Jiang X, et al:
Pyrazinamide inhibits trans-translation in Mycobacterium
tuberculosis. Science. 333:1630–1632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Watts HG and Lifeso RM: Tuberculosis of
bones and joints. J Bone Joint Surg Am. 78:288–298. 1996.PubMed/NCBI
|
|
40
|
Hu G, Codina M and Fisher S: Multiple
enhancers associated with ACAN suggest highly redundant
transcriptional regulation in cartilage. Matrix Biol. 31:328–337.
2012. View Article : Google Scholar : PubMed/NCBI
|