Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
disease that mainly affects the human synovial membrane, cartilage
and bone. Approximately 1% of the population suffer from RA and it
is associated with significant morbidity and increased mortality.
Several studies have demonstrated that T lymphocytes, B
lymphocytes, mast cells and other immune cells are critical in the
pathogenesis and progression of inflammation. Several studies on
spontaneous animal models of arthritis and cell-cell interactions
have implicated T cells in driving synovial inflammation and joint
destruction (1–5), and Th1 cells are elevated as a
dominant cell in RA. T-bet is a specific Th1 transcriptional
factor, which contributes to the differentiation of Th1 and is
required for the generation of IFN-γ-producing Th1 cells (6–7).
Th17 cells are also important for the modulation of autoimmune
responses. IL-17 secreted by Th17 is critical in the pathogenesis
of RA and it is capable of promoting inflammation by inducing a
variety of proinflammatory mediators and inducing bone and
cartilage destruction (8–11). Recent studies have demonstrated
that T-bet has an effect on Th1 and Th17 cells (12) and is important in experimental
autoimmune encephalomyelitis and RA (13–14).
Collagen induced arthritis (CIA) is the most useful animal model
for studying RA in humans (15).
In the present study, we created a chicken type II CIA animal
model, established the T-bet shRNA recombinant plasmid
(p-T-shRNA) and examined its possible anti-inflammatory effect in
the CIA model by local injection of plasmid vectors.
Materials and methods
Animals and CIA model induction
Male, 8 to 10-week-old DBA/1LacJ mice (Shanghai SLAC
Laboratory Animal Co., Ltd., Shanghai, China) were used and 100 μg
of chicken type II collagen (CII; Sigma-Aldrich, St. Louis, MO,
USA) was injected intradermally at the base of the tail, which was
emulsified in equal volumes of Freund’s complete adjuvant (2 mg/ml;
Mycobacterium tuberculosis). The study was approved by the
Ethics Committee of Jiangsu University.
CII-immunized mice were divided into five groups and
then injected in the joint every 48 h with PBS buffer, pIRES-T-bet,
pIRES empty vector, p-T-shRNA or p-nonsense-shRNA, respectively,
from day 1 to 20. Starting at the first day following the first CII
immunization, the mice were examined for the development and
severity of arthritis until the 50th day. Disease severity was
scored on a scale from 0 to 4 by visual inspection of paws and the
score criteria were as follows: 0, no evidence of erythema and
swelling; 1, erythema and mild swelling confined to the mid-foot
(tarsals) or ankle joint; 2, erythema and mild swelling extended
from the ankle to the mid-foot; 3, erythema and moderate swelling
extended from the ankle to the metatarsal joints; 4, erythema and
severe swelling encompassed the ankle, foot and digits.
H&E and immunohistochemistry
staining
Mouse knee joints were fixed in 10% buffered
formalin in PBS overnight, decalcified in 10% formic acid in water
for 24 h, embedded in paraffin and sectioned at 5 μm thickness
prior to staining with H&E. Sections for immunochemistry
staining were immersed in 0.01 M, pH 6.0 citrate buffer (30 min) in
a water bath maintained at 100°C for antigen retrieval. Hydrogen
peroxide (3%) in methanol (30 min at room temperature) was used to
deactivate endogenous peroxidase. Sections were washed in PBS 3
times and 30 mg/ml BSA was added (30 min at room temperature) for
the closure of non-specific binding sites. Thereafter, the sections
were exposed to rabbit anti-T-bet antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), 1:200 diluted in 30
mg/ml BSA, at 4°C overnight in a wet box followed by staining with
a MaxVision™ kit (kit-5020; MaiXin Technology Co., Ltd., Shenzhen,
Guangdong, China), then stained with freshly prepared
diaminobenzidine solution and counterstained with hematoxylin.
Sections were dehydrated with a graded series of alcohol, vitrified
by dimethylbenzene and covered with neutral gum. Results were
observed and analyzed using a Leica fluorescence microscope and
image analysis system (Leica, Mannheim, Germany). Section
assessments were conducted by two blinded investigators for mean
inflammation, pannus formation, cartilage damage, bone damage and
T-bet expression.
Quantitative real-time PCR
Total RNA was isolated from mice splenocytes or
joint tissue with TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) and the joint was homogenated. T-bet, IFN-γ,
RORγt and IL-17 mRNA in splenocytes and synovial joints were
measured by quantitative real-time PCR.
Quantitative real-time PCR was performed with the
SYBR green 1 two-step qRT-PCR kit with ROX (Invitrogen Life
Technologies) according to the manufacturer’s instructions. The
threshold cycle (CT) of gene products was determined and set to the
log linear range of the amplification curve and kept constant.
Relative expression was calculated with normalization to β-actin
values. The sequences of the primers were designed to span at least
one intron. The primers used were as follows: T-bet sense,
5′-ATGCCAGGGAACCGCTTAT-3′ and antisense,
5′-CAGATGCGTACATGGACTCAAA-3′; IFN-γ sense,
5′-AAGCGTCATTGAATCACACC-3′ and antisense,
5′-CGAAATCAGCAGCGACTCCTTAG-3′; IL-17 sense,
5′-GGTCAACCTCCAAGTCTTTAACTC-3′ and antisense,
5′-TTAAAAATGCAAGTAAGTTTGCTG-3′; RORγt sense,
5′-GCTCCCGGCCTGGTCTGCTC-3′ and antisense,
5′-AGGTGGCGGGGTGGTTTCTGA-3′; FoxP3 sense,
5′-CCACCATATTCCAATACCTT-3′ and antisense,
5′-GCTGCTTGGACCCTGTTCT-3′.
Intracellular cytokine staining
CD4+ cells were isolated from peripheral
blood lymphocytes by magnetic-activated cell sorting (MACS) and the
purity of CD4+ cells was routinely 95%. To assess the
intracellular cytokine production, CD4+ lymphocytes were
incubated with ionomycin (500 ng/ml) and phorbol myristate acetate
(PMA; 50 ng/ml; Sigma-Aldrich) in the presence of monensin (1
μg/ml; eBioscience, San Diego, CA, USA). The cells were washed and
resuspended in 1% BSA in PBS. Following washing twice, cells were
fixed and permeabilized using Cytofix/Cytoperm solution (BD
Biosciences, Franklin Lakes, NJ, USA) for 20 min at 4°C, and
stained for FITC-IL-17 (BioLegend, San Diego, CA, USA) and PE-IFN-γ
(eBioscience) with specific Abs or isotype control according to the
manufacturer’s instructions. Data were acquired by flow cytometry
(FCM; BD Biosciences) and analyzed using Cell Quest software (BD
Biosciences).
Statistical analysis
Data were summarized as the mean ± SD. Means were
compared with the ANOVA where appropriate for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Locally enhanced expression of the T-bet
gene may promote the onset of arthritis at the joint
To determine whether T-bet acts as a driving
factor for CIA development, we injected recombinant plasmid
pIRES-T-bet into the hind ankle of CII-immunized mice every 48 h
for ten-doses, starting at day 1 following the first immunization,
and found an accelerated onset of arthritis (Fig. 1). The hind paw became red and
swollen more quickly following pIRES-T-bet injection.
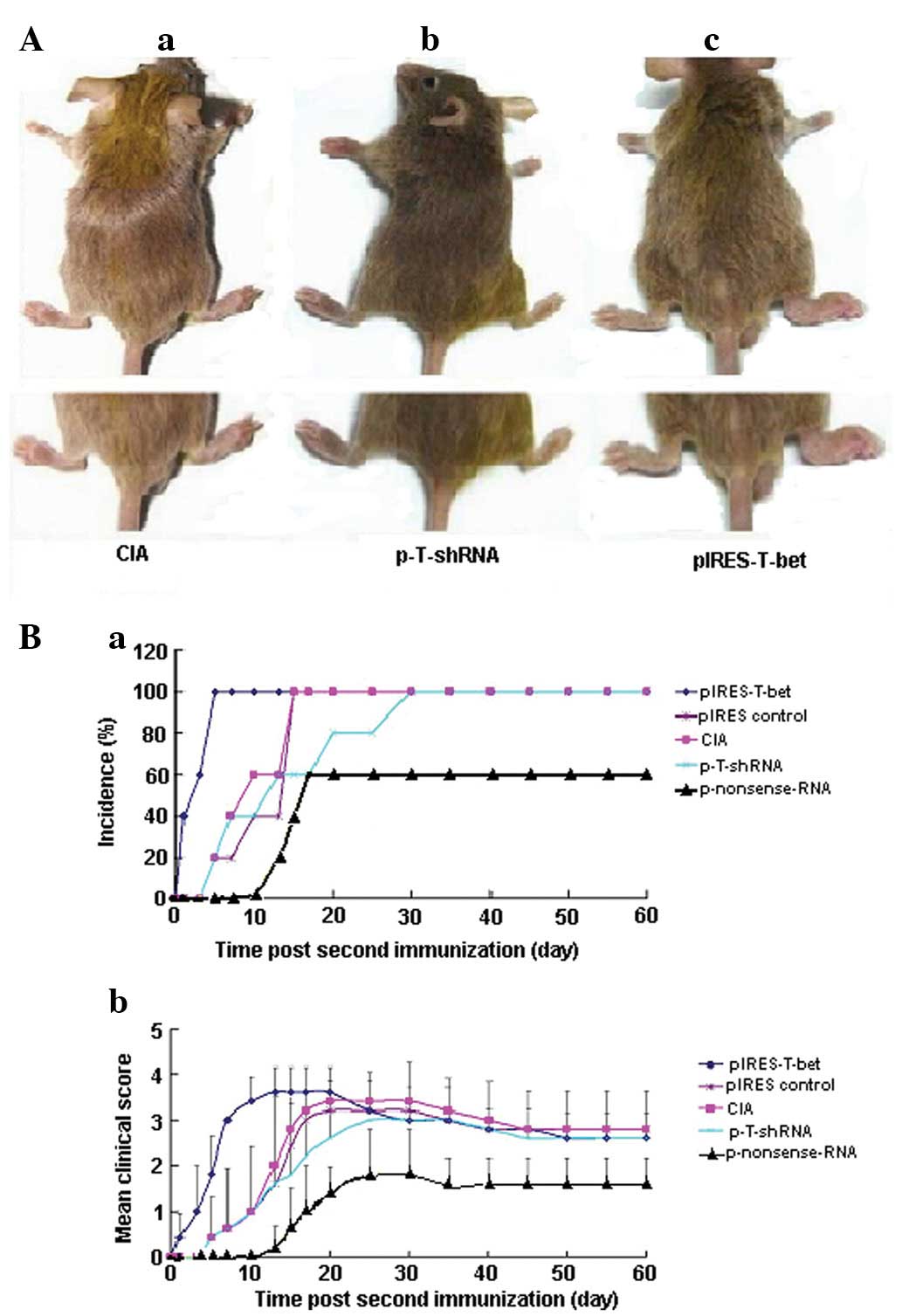 | Figure 1Effect of p-T-shRNA on the formation
of CIA. (A) Injection of p-T-shRNA was able to ameliorate
inflammation at the local joint, while pIRES-T-bet led to the
deterioration of the disease at the joint. (Aa) CIA blank control,
without treatment using p-T-shRNA or pIRES-T-bet; (Ab) the right
hind paw was administrated with p-T-shRNA and demonstrated that the
treated paw was thinner than the CIA blank control; (Ac) the right
hind paw, which was treated with pIRES-T-bet, was worse for
swelling. (B) The incidence and clinical score of arthritis in the
experimental mice following the second immunization with CII. (Ba)
The CIA incidence of mice from different groups: the arthritis
percentage in the mice injected with p-T-shRNA was 60% reduced,
however, in the pIRES-T-bet injected mice, the incidence of CIA was
100% (similar to the CIA blank control). The time of CIA onset was
~13 days in the p-T-shRNA group and 1 day in the pIRES-T-bet group.
(Bb) The inflammatory score. There was a distinct improvement in
the mice injected with p-T-shRNA compared with pIRES-T-bet or CIA
blank control, however there were no apparent differences between
pIRES-T-bet and the CIA blank control. CIA, collagen-induced
arthritis; p-T-shRNA, T-bet shRNA recombinant plasmid; pIRES-T-bet,
plasmid expressing T-bet; CII, type II collagen. |
Silencing the T-bet gene may induce
delayed onset of arthritis
The mice were administered with a single i.p.
injection of p-T-shRNA at the time of immunization to silence the
T-bet gene for long-lasting suppressive capacity on CIA
induction in vivo. The results demonstrated that the
expression of T-bet was effectively reduced in the joint
tissue of CIA mice treated with p-T-shRNA (Fig. 1A), the incidence rate of CIA was
significantly reduced and the onset of arthritis was markedly
delayed (Fig. 1B).
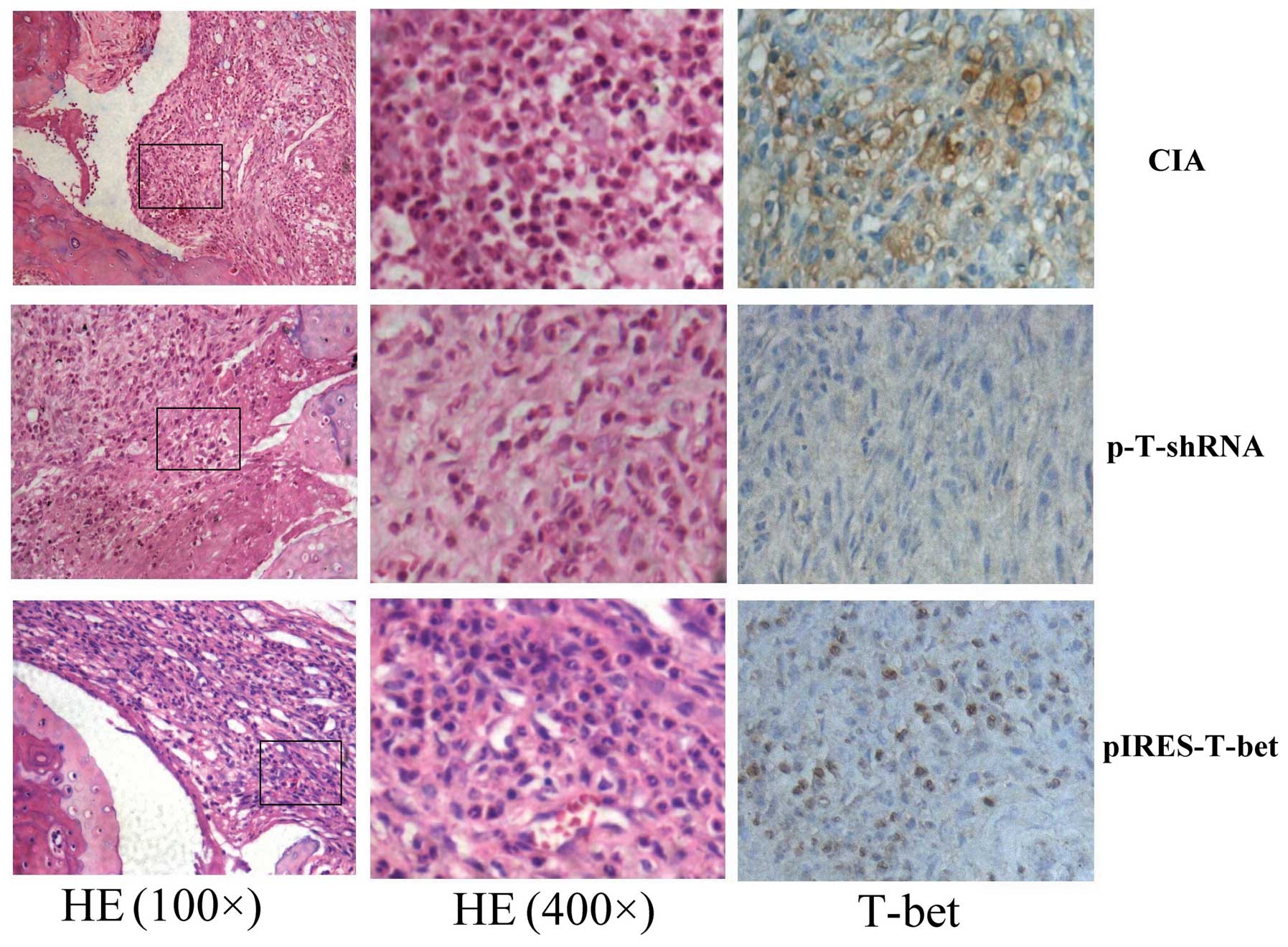
H&E and immunohistochemistry
staining
We compared the inflammatory score of three groups.
The infiltrated inflammatory cells and the level of T-bet
expression were different in different groups. The infiltrated
inflammatory cells in the pIRES-T-bet group were similar to the CIA
blank group, but higher than that in the p-T-shRNA group. The
immunohistochemistry staining result demonstrated that the
T-bet expression was higher in the pIRES-T-bet group than
that in the CIA blank group, however it was decreased in the
p-T-shRNA group compared with other groups. The T-bet
immunopositive products were subcellularly localized in the nuclei
(Fig. 2).
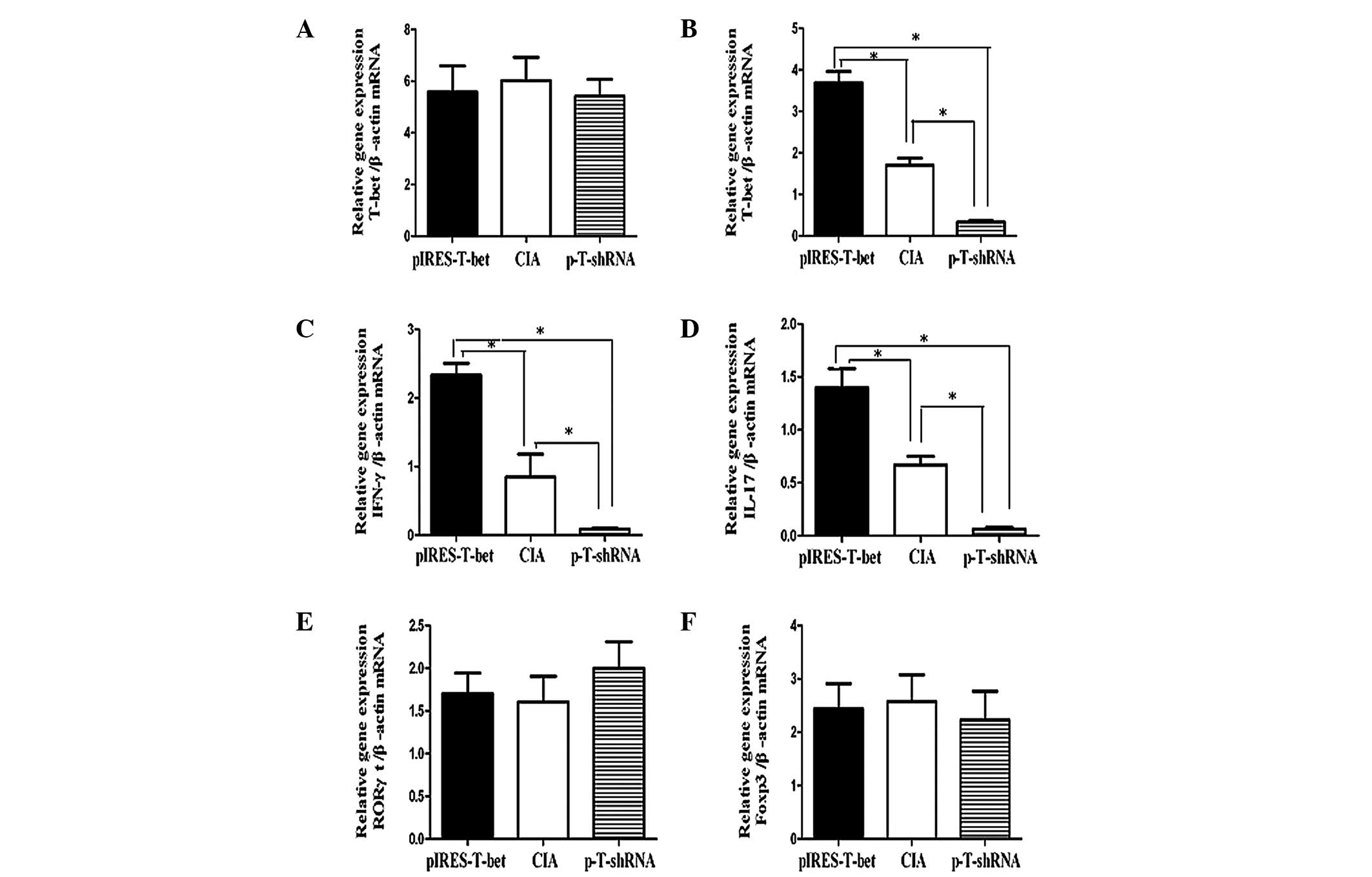
Expression of related inflammatory
factors in the spleen or local joint
We analyzed the levels of T-bet mRNA in the
spleen and local joints from different groups and the results
demonstrated that the T-bet expression was increased in the
limited joints following injection of plasmid pIRES-T-bet, while it
was downregulated by shRNA injection. There was no clear difference
in T-bet expression in the spleen among the CIA blank
control, pIRES-T-bet and p-T-shRNA groups. Our data indicated that
the changes in IFN-γ and IL-17 expression were consistent with the
level of T-bet mRNA, implying that T-bet was able to
affect the level of cytokines at a certain phase. The topical
application of pIRES-T-bet or p-T-shRNA on joint tissue was not
able to induce the reduction or increase of T-bet expression
in the splenocytes, however in the local joint, increased
T-bet expression was able to increase the level of IFN-γ and
IL-17, and reduced T-bet expression resulted in decreased
IFN-γ and IL-17 (Fig. 3).
Expression of intracellular IFN-γ or
IL-17 from popliteal lymph nodes
The popliteal lymph nodes were collected from
different groups, the CD4+ T cells were sorted and the
cells were exposed to PMA and ionomycin for 4 h, then stained with
IFN-γ-PE and IL-17-FITC for FCM analysis. The result was consistent
with that detected by qRT-PCR. FCM analysis for popliteal lymph
nodes, which correlated with the plasmid injection demonstrated,
that IL-17 and IFN-γ were upregulated in the mice injected with
pIRES-T-bet and downregulated in the p-T-shRNA group (Fig. 4).
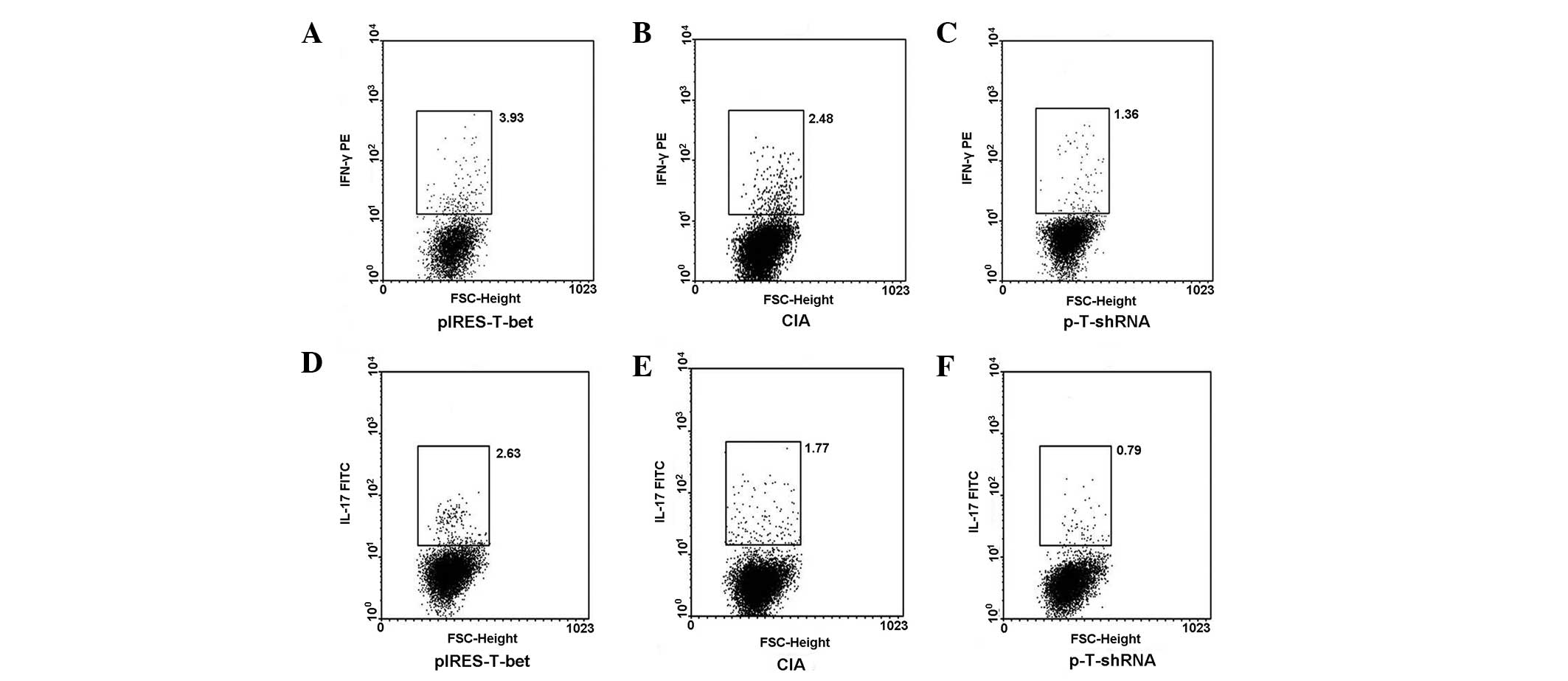 | Figure 4pIRES-T-bet or p-T-shRNA local
administration affected the expression of IFN-γ and IL-17 in PLN
cells. CD4+ cells were separated from PLN by MACS and
then the cultures were stimulated with PMA or ionomycin in the
presence of menosin for 4 h and stained with fluorescent antibodies
for IL-4 and IFN-γ analysis. (A–C) IFN-γ secreting cells
(IFN-γ+ cells) and (D–F) exhibit the levels of IL-17
secreting cells (IL-17+ cells) from three different
groups, respectively and the results correlate with that of the
qRT-PCR. The IFN-γ+ or IL-17+ cells were
higher in the pIRES-T-bet group than that in CIA blank control,
however it was less in the p-T-shRNA group than that in the CIA
blank control. p-T-shRNA, T-bet shRNA recombinant plasmid;
pIRES-T-bet, plasmid expressing T-bet; IFN-γ, interferon-γ; IL-17,
interleukin-17; IL-4, interleukin-4; CIA, collagen-induced
arthritis; PMA, phorbol myristate acetate; MACS, magnetic-activated
cell sorting; qRT-PCR, quantitative real-time PCR; PLN, peripheral
lymph node. |
Discussion
Recently, the study of RA has substantially
advanced. Genetic and environmental factors may lead to joint
destruction. RA is thought to be a disease of the immune system in
which self-tolerance breaks down (2,16)
and numerous studies have attempted to apply immunosuppression for
the treatment of RA. Immune cells, including T lymphocytes, B
lymphocytes and NK cells are all involved in the process of the
disease and dendritic cells are also important in the process
(17–19), as well as a number of cytokines,
chemokines, adhesion molecules and matrix metalloproteinases. These
molecules are controlled by a limited number of transcriptional
factors, including T-bet and NF-κB. Previous studies
have demonstrated that Th1 cells are the dominant cells in the
inflammatory reaction, including RA. T-bet is essential to
the development of T lymphocytes, directing the differentiation of
Th0 cells into Th1 or Th2 cells (1,6,20).
Numerous studies demonstrate that silencing T-bet may have
an apparent effect on autoreactive T cells and autoimmunity
(21–23).
The RNA interference technique has become a
convenient and easy way to knock down target genes (24–26).
We used the RNA interference technique to silence the target gene
T-bet in CIA mice in this experiment. Our results revealed a
previously unrecognized role of T-bet in promoting the
course of the disease and demonstrated that T-bet was a
critical factor in CIA development. We constructed the eukaryotic
plasmid carrying the T-bet gene (pIRES-T-bet) to enhance
T-bet expression and the small hairpin RNA (p-T-shRNA) to
silence T-bet expression in local joint tissue. Through
increasing or reducing the expression of T-bet in the right
hind paw in the experimental mice, we discovered it was effective
in reducing T-bet expression and hence ameliorated the
inflammatory condition at an early stage. The results also
indicated that, in the initial stage of CIA, silencing T-bet
was able to inhibit the expression of IFN-γ and IL-17. These
cytokines secreted from lymphocytes or joints produced a
significant effect on local inflammation. IFN-γ level was closely
associated with T-bet expression, while IL-17 was regulated
by several transcriptional factors, which may crosstalk with
T-bet. The effect of T-bet on the secretion and
function of IL-17 was not clear in the present study, although it
may be associated with the time point when pIRES-T-bet or p-T-shRNA
was injected. In the initial stage of joint inflammation, silencing
T-bet may relieve arthritis. Hence, we propose that RNA
interference to the T-bet gene may be developed as a
potential factor to prevent inflammation at an early stage in RA
patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 31270947, no. 81072453, no.
31170849), the Natural Science Foundation of Colleges and
Universities in Jiangsu Province and Innovation Fund for candidate
of doctor in Jiangsu Province (grant no. 09KJB310001 and
CXZZ11_0593, respectively).
References
|
1
|
Rodeghero R, Cao Y, Olalekan SA, Iwakua Y,
Glant TT and Finnegan A: Location of CD4+ T cell priming
regulates the differentiation of Th1 and Th17 cells and their
contribution to arthritis. J Immunol. 190:5423–5435.
2013.PubMed/NCBI
|
|
2
|
Lin GJ, Huang SH, Chen SJ, Wang CH, Chang
DM and Sytwu HK: Modulation by melatonin of the pathogenesis of
inflammatory autoimmune diseases. Int J Mol Sci. 14:11742–11766.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su Z, Shotorbani SS, Jiang X, Ma R, Shen
H, Kong F and Xu H: A method of experimental rheumatoid arthritis
induction using collagen type II isolated from chicken sternal
cartilage. Mol Med Rep. 8:113–117. 2013.PubMed/NCBI
|
|
4
|
Fox DA, Gizinski A, Morgan R and Lundy SK:
Cell-cell interactions in rheumatoid arthritis synovium. Rheum Dis
Clin North Am. 36:311–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chabas D, Baranzini SE, Mitchell D, et al:
The influence of the proinflammatory cytokine, osteopontin, on
autoimmune demyelinating disease. Science. 294:1731–1735. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szabo SJ, Kim ST, Costa GL, Zhang X,
Fathman CG and Glimcher LH: A novel transcription factor, T-bet,
directs Th1 lineage commitment. Cell. 100:655–669. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shier P, Hofstra CL, Ma XJ, Wu Y, Ngo K
and Fung-Leung WP: Tbt-1, a new T-box transcription factor induced
in activated Th1 and CD8+ T cells. Immunogenetics.
51:771–778. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang ES: Transcriptional regulation of T
helper 17 cell differentiation. Yonsei Med J. 51:484–491. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tesmer LA, Lundy SK, Sarkar S and Fox DA:
Th17 cells in human disease. Immunol Rev. 223:87–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shahrara S, Huang Q, Mandelin AM 2nd and
Pope RM: TH-17 cells in rheumatoid arthritis. Arthritis Res Ther.
10:R932008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stamp LK, James MJ and Cleland LG:
Interleukin-17: the missing link between T-cell accumulation and
effector cell actions in rheumatoid arthritis? Immunol Cell Biol.
82:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathur AN, Chang HC, Zisoulis DG, Kapur R,
Belladonna ML, Kansas GS and Kaplan MH: T-bet is a critical
determinant in the instability of the IL-17-secreting T-helper
phenotype. Blood. 108:1595–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Weiner J, Liu Y, Smith AJ, Huss
DJ, Winger R, Peng H, Cravens PD, Racke MK and Lovett-Racke AE:
T-bet is essential for encephalitogenicity of both Th1 and Th17
cells. J Exp Med. 206:1549–1564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gocke AR, Cravens PD, Ben LH, Hussain RZ,
Northrop SC, Racke MK and Lovett-Racke AE: T-bet regulates the fate
of Th1 and Th17 lymphocytes in autoimmunity. J Immunol.
178:1341–1348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ortmann RA and Shevach EM: Susceptibility
to collagen-induced arthritis: cytokine-mediated regulation. Clin
Immunol. 98:109–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scrivo R, Di Franco M, Spadaro A and
Valesini G: The immunology of rheumatoid arthritis. Ann NY Acad
Sci. 1108:312–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarkar S and Fox DA: Dendritic cells in
rheumatoid arthritis. Front Biosci. 10:656–665. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Modi S, Soejima M and Levesque MC: The
effect of targeted rheumatoid arthritis therapies on
anti-citrullinated protein autoantibody levels and B cell
responses. Clin Exp Immunol. 173:8–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Fathman JW, Lugo-Villarino G,
Scimone L, von Andrian U, Dorfman DM and Glimcher LH: Transcription
factor T-bet regulates inflammatory arthritis through its function
in dendritic cells. J Clin Invest. 116:414–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lametschwandtner G, Biedermann T,
Schwärzler C, Günther C, Kund J, Fassl S, Hinteregger S,
Carballido-Perrig N, Szabo SJ, Glimcher LH and Carballido JM:
Sustained T-bet expression confers polarized human Th2 cells with
Th1-like cytokine production and migratory capacities. J Allergy
Clin Immunol. 113:987–994. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lovett-Racke AE, Rocchini AE, Choy J,
Northrop SC, Hussain RZ, Ratts RB, Sikder D and Racke MK: Silencing
T-bet defines a critical role in the differentiation of
autoreactive T lymphocytes. Immunity. 21:719–731. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai Kwan Lam Q, King Hung Ko O, Zheng BJ
and Lu L: Local BAFF gene silencing suppresses Th17-cell generation
and ameliorates autoimmune arthritis. Proc Natl Acad Sci USA.
105:14993–14998. 2008.PubMed/NCBI
|
|
23
|
Bettelli E, Sullivan B, Szabo SJ, Sobel
RA, Glimcher LH and Kuchroo VK: Loss of T-bet, but not STAT1,
prevents the development of experimental autoimmune
encephalomyelitis. J Exp Med. 200:79–87. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Denli AM and Hannon GJ: RNAi: an
ever-growing puzzle. Trends Biochem Sci. 28:196–201. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Escobar T, Yu CR, Muljo SA and Egwuagu CE:
STAT3 activates miR-155 in Th17 cells and acts in concert to
promote experimental autoimmune uveitis. Invest Ophthalmol Vis Sci.
54:4017–4025. 2013. View Article : Google Scholar : PubMed/NCBI
|