Introduction
Ionizing radiation is an important clinical tool for
the treatment of cancer. However, it presents potential harmful
effects for human health, including acute radiation syndrome,
mutagenesis and carcinogenesis. To increase the effectiveness of
ionizing radiation it is critical to find a way to mitigate the
damaging effect of ionizing radiation on healthy tissues
surrounding the tumor. In addition to use with radiotherapy,
potential radioprotectors may also be useful for the protection of
radiation workers who are exposed to low or moderate levels of
radiation on a semi-frequent basis.
When tissues are irradiated with low linear energy
transfer (LET) radiation, for example X-rays or γ-rays, DNA is
primarily damaged indirectly through free radicals. The free
radicals are formed when the ionizing radiation interacts with
water within the cells. The free radicals that are formed are
highly reactive and easily interact with and damage DNA if they
form in a close proximity to the DNA itself. Indirect DNA damage
contributes to approximately two thirds of ionizing events during
low LET ionizing radiation (1).
There are two types of free radicals, short- and long-lived
radicals. Long-lived radicals are able to induce DNA lesions, for
example DNA base damage, however, do not cause serious lesions,
including DNA double-strand breaks. The short-lived radicals are
able to induce double-strand breaks and thus are the most damaging
and potentially harmful type of free radical (2). Since DNA damage in low LET radiation
mainly occurs indirectly, the majority of classical radiation
protectors, including dimethyl sulfoxide and WR-2721, are radical
scavengers that inhibit reactive oxygen species interacting with
DNA (3,4).
Natural flavonoids, such as quercetin and rutin, are
known antioxidants (5). Previous
studies have suggested that these flavonoids may protect cells and
mice from ultra-violet radiation and ionizing radiation (6,7).
These flavonoids are extremely insoluble in water, which has
prevented in depth studies into their potential use as
radioprotectors. In an effort to increase their water solubility,
modified flavonoids have been available, and one such flavonoid is
monoglucosyl-rutin (αG-rutin PS™) (8,9).
In the present study, the protective effect of
monoglucosyl-rutin against radiation was investigated in mammalian
Chinese hamster ovary (CHO) 10B2 cells. It was hypothesized that
irradiated cells treated with monoglucosyl-rutin would have an
increased survival rate when compared with the control cells, and
that monoglucosyl-rutin may be a potential free radical
scavenger.
Materials and methods
Cell culture
CHO10B2 cells were grown in α-minimum essential
medium (α-MEM; Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories, South Logan, UT, USA), antibiotic-antimycotic
(anti-anti; Invitrogen Life Technologies) in a humidified 5%
CO2 atmosphere at 37°C. Exponentially growing log phase
cells were used in the present study. Normal human fibroblast
cells, AG1521, were grown in α-MEM supplemented with 15% FBS and
anti-anti. Only cells with passages lower than 12 were used.
Contact inhibited G0/G1-phase cells were used for the
experiments.
Drug treatment
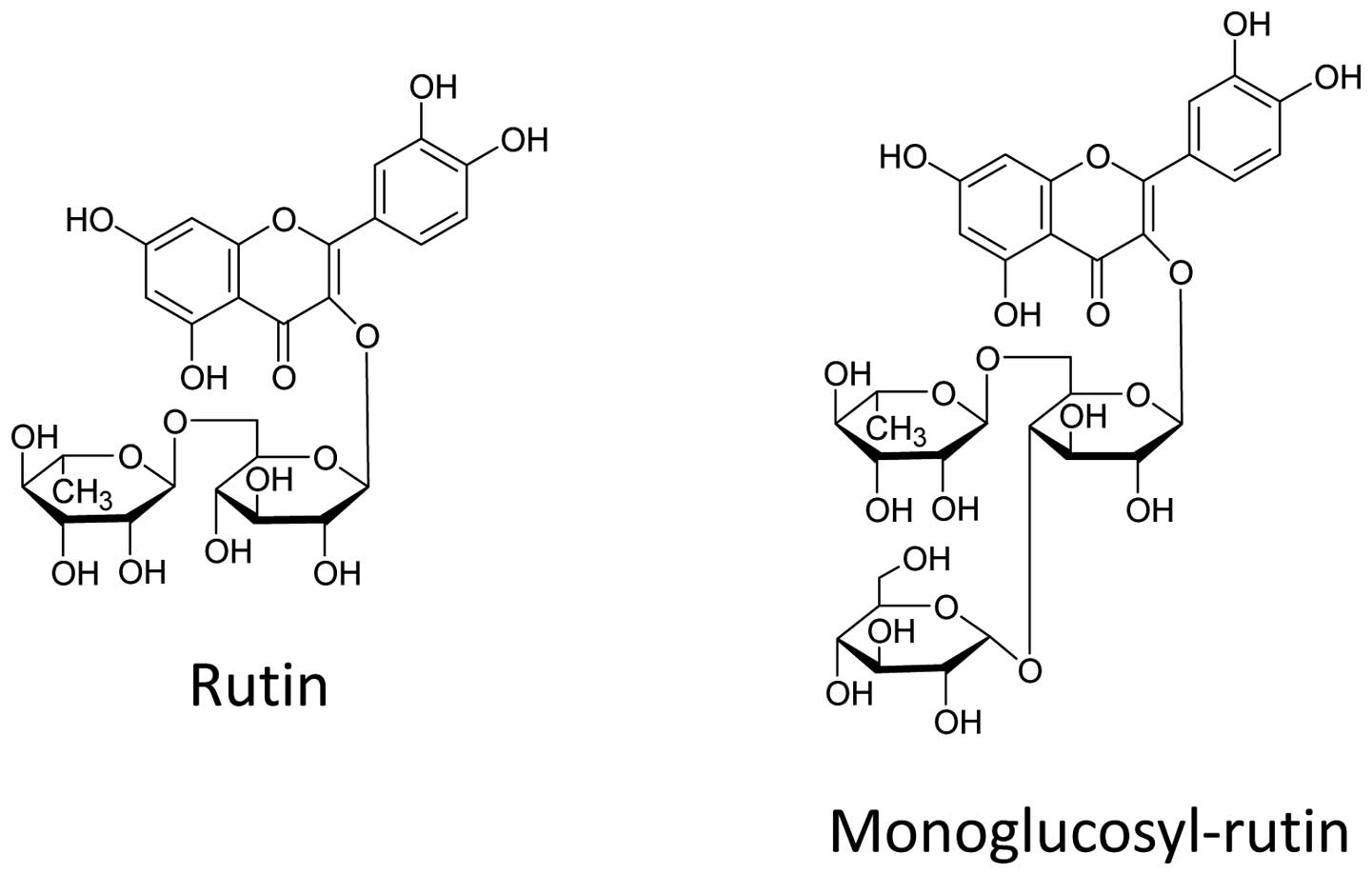
Monoglucosyl-rutin, an enzymatically modified form
of rutin (Fig. 1), was supplied by
Toyo Sugar Refining Co., Ltd. (Tokyo, Japan). Monoglucosyl-rutin
was dissolved in phosphate-buffered saline (PBS) and filtered for
sterilization. Monoglucosyl-rutin was freshly prepared for each
experiment and was added to the cell culture 1 h prior to the
beginning of the experiment.
Irradiation
Irradiation was performed using a J.L. Shepherd
Model Mark I-68 6000Ci 137Cs irradiator (J.L. Shepherd
and Associates, San Fernando, CA, USA) at room temperature and at a
dose rate of 250 cGy/min.
Growth inhibition assay
To measure the cell doubling time, CHO cells were
seeded at a density of 50,000 cells/P-60 cell culture dish with
various concentrations of monoglucosyl-rutin, and the number of
cells was counted twice a day (>8 h intervals) for 4 days. Cell
doubling time for each condition was calculated using GraphPad
Prism 6 software (GraphPad Software, San Diego, CA, USA). Three
independent experiments were conducted.
Cell survival assay
Cell survival was measured using a standard
clonogenic assay. CHO cells were seeded at a density designed to
yield ~100 viable colony-forming cells/P-60 cell culture dish
subsequent to treatment with a combination of monoglucosyl-rutin
and ionizing radiation. The colonies were scored 7–8 days after
treatment. The dishes were then treated with 100% ethanol to fix
colonies and stained with 0.1% crystal violet solution. The
colonies containing more than 50 cells were recorded as
reproductively viable surviving cells. Cell survival curves were
drawn using GraphPad Prism software with linear quadratic
regression. Three independent experiments were conducted.
Immunostaining for γH2AX foci
formation
The fixation and staining methods used for
immunocytochemistry were performed as previously described
(10). For immunostaining, AG1521
fibroblast cells were cultured on chamber slides and synchronized
to the G0/G1 phases by contact inhibition. Following 30 min of
treatment with monoglucosyl-rutin and radiation exposure at 37°C,
the cells were fixed in 4% paraformaldehyde for 15 min, washed in
PBS and then treated with 0.5% Triton X-100 for a further 10 min.
Non-specific binding sites were inhibited using PBS with 10% goat
serum at 4°C overnight. The chamber slides were then incubated with
mouse monoclonal anti-γ-H2AX antibody (Millipore, Billerica, MA,
USA) for 1 h, washed three times in PBS and incubated with Alexa
488 conjugated goat anti-mouse secondary antibody (Invitrogen Life
Technologies) for 1 h at 37°C. The cells were washed four times in
PBS and the nuclei were stained with Antifade Gold with DAPI
solution (Invitrogen Life Technologies). Images of the cells were
captured using a Z-stage motorized Zeiss Axioskop with Metamorph
system (Carl Zeiss AG, Jena, Germany). The z-stacked images were
stored and 50 cells from these were later scored in three
independent experiments.
Sister chromatid exchange (SCE)
assay
CHO cells were synchronized into G1-phase by mitotic
shake-off (11) and incubated for
2 h. Then, following treatment with monoglucosyl-rutin and
radiation exposure, the cells were incubated with 10 μM BrdU for
two rounds of cell replication. Colcemid was added and the cells
were harvested and treated with 75 mM KCl, fixed with
methanol:acetic acid (3:1) solution and placed onto slides. The
slides were stained with Hoechst 33258 for 30 min, immersed in PBS
and then exposed to a black light source at 55°C for 30 min. The
slides were subsequently treated with 2X saline sodium citrate
solution at 70°C for 30 min. Finally, the slides were stained with
filtered 10% Giemsa solution in Gurr solution for 5 min. Metaphase
images were obtained using an Olympus BX51 microscope equipped with
a Q-imaging Aqua Cooled CCD camera with Q-capture Pro software
(Olympus, Tokyo, Japan). The SCE frequency was scored for each
chromosome. At least 50 metaphase cells were scored for each three
independent experiments.
Statistical analysis
Statistical comparison of the mean values was
performed using a t-test with GraphPad Prism 6. P<0.05 was
considered to indicate a statistically significant difference.
Error bars indicate the standard error of the mean.
Results
Cellular toxicity of
monoglucosyl-rutin
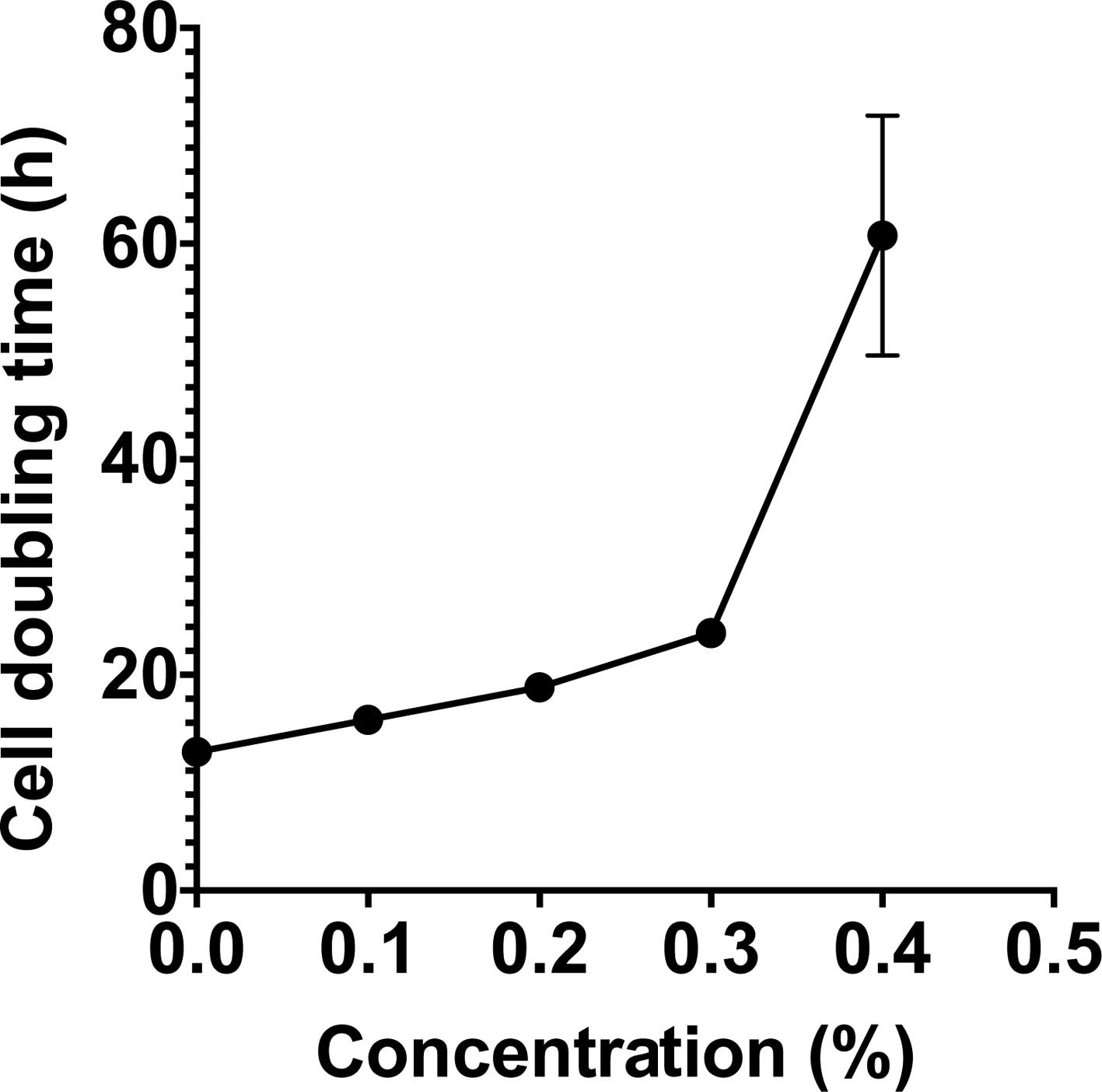
To assess the cellular toxicity of
monoglucosyl-rutin, the cell doubling time and plating efficiency
of CHO cells were investigated. To investigate cell doubling time,
CHO cells were treated with varying concentrations of
monoglucosyl-rutin during growth. The total cell numbers were
counted every 12 h to determine the cell doubling time for each
treatment condition. As shown in Fig.
2, unexposed CHO cells exhibited an average doubling time of
12.8 h. Monoglucosyl-rutin significantly increased cell doubling
time starting at concentrations as low as 0.1%. The IC50
for cells treated with monoglucosyl-rutin was 0.3% when the
doubling time increased to 23.9 h. In addition to cell doubling
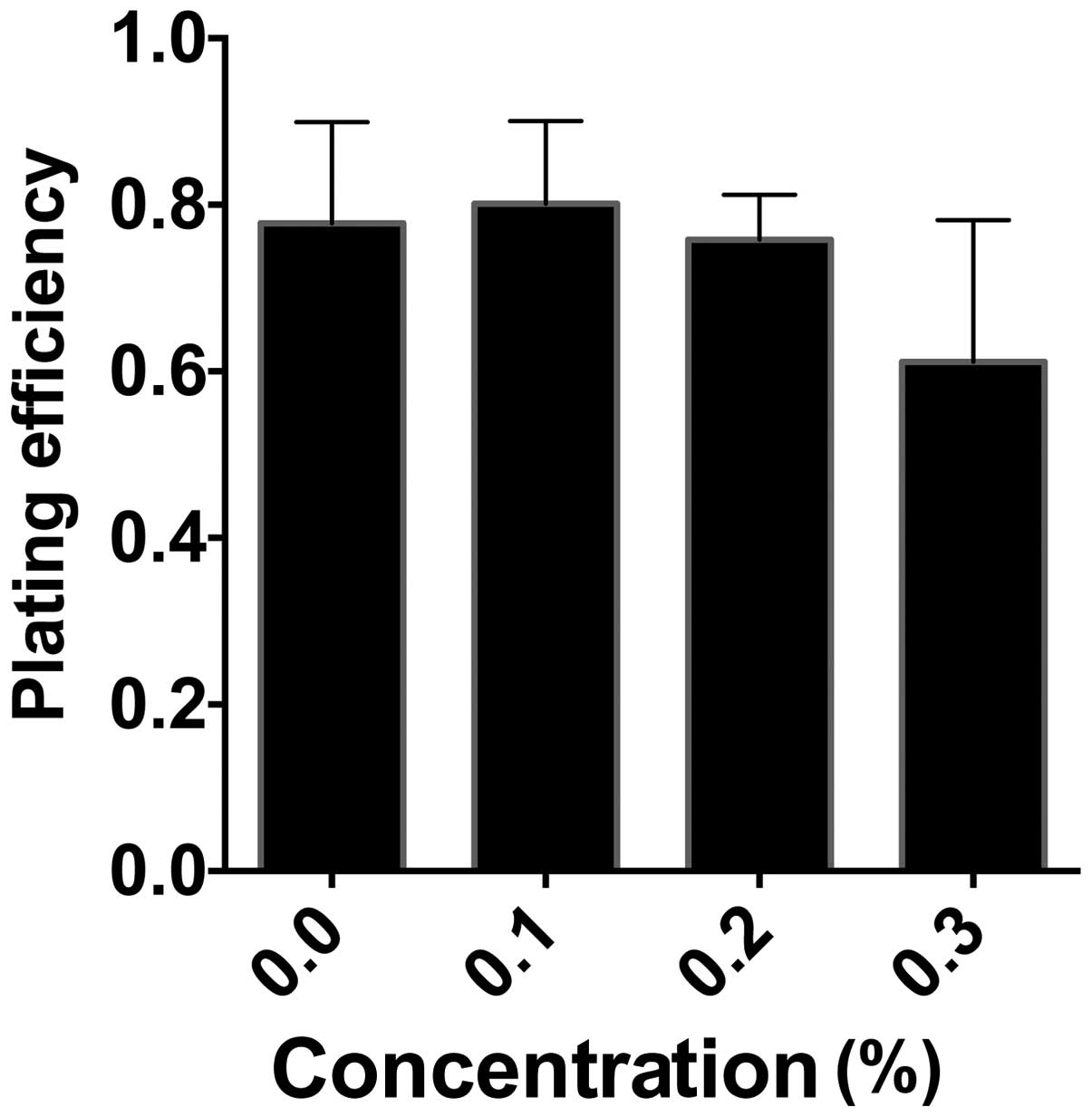
time, the effect of monoglucosyl-rutin on cell plating efficiency
was also investigated. CHO cells were treated with varying
concentrations of monoglucosyl-rutin for 1 h and 100 cells were
plated. Following seven days, the viable colonies were analyzed. As
shown in Fig. 3, the plating
efficiency of the treated cells was not significantly affected
until 0.3% monoglucosyl-rutin was applied and the plating
efficiency significantly decreased to 61%, compared with 77% in the
control cells.
Radioprotection in cell survival
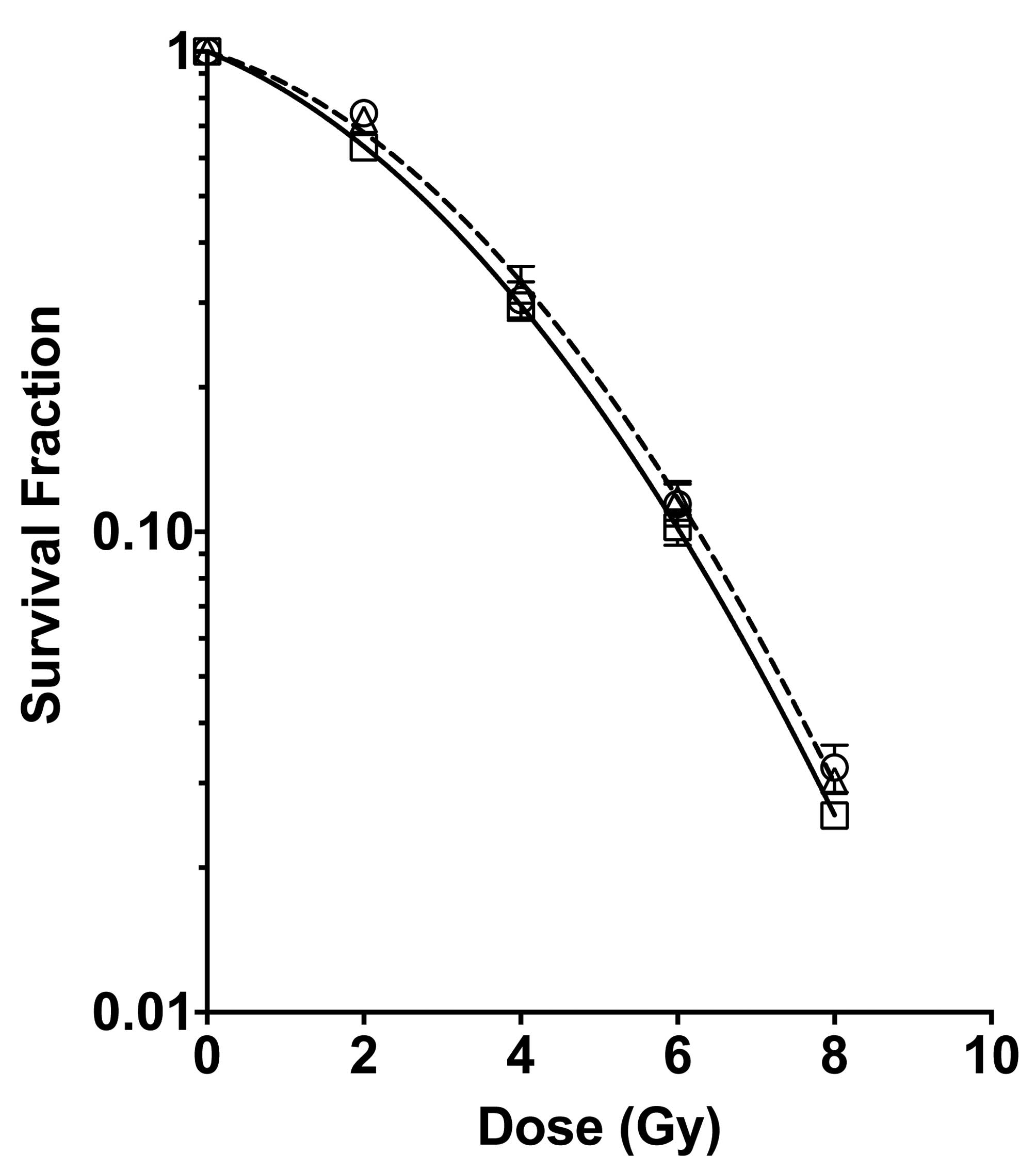
To assess the role of monoglucosyl-rutin as a
potential radioprotector, cell survival was investigated using a
colony formation assay. CHO cells were treated with 0.1 or 0.3%
monoglucosyl-rutin for 1 h and then exposed to various doses of
radiation. The irradiated cells were plated and the number of
surviving cells was determined. As shown in Fig. 4, the cells treated with 0.1%
monoglucosyl-rutin exhibited a statistically increased cell
viability at 2 Gy when compared with the control cells. The cells
treated with 0.3% monoglucosyl-rutin demonstrated a significant
increase in survival at doses greater than 2 Gy.
Radioprotection in DNA damage
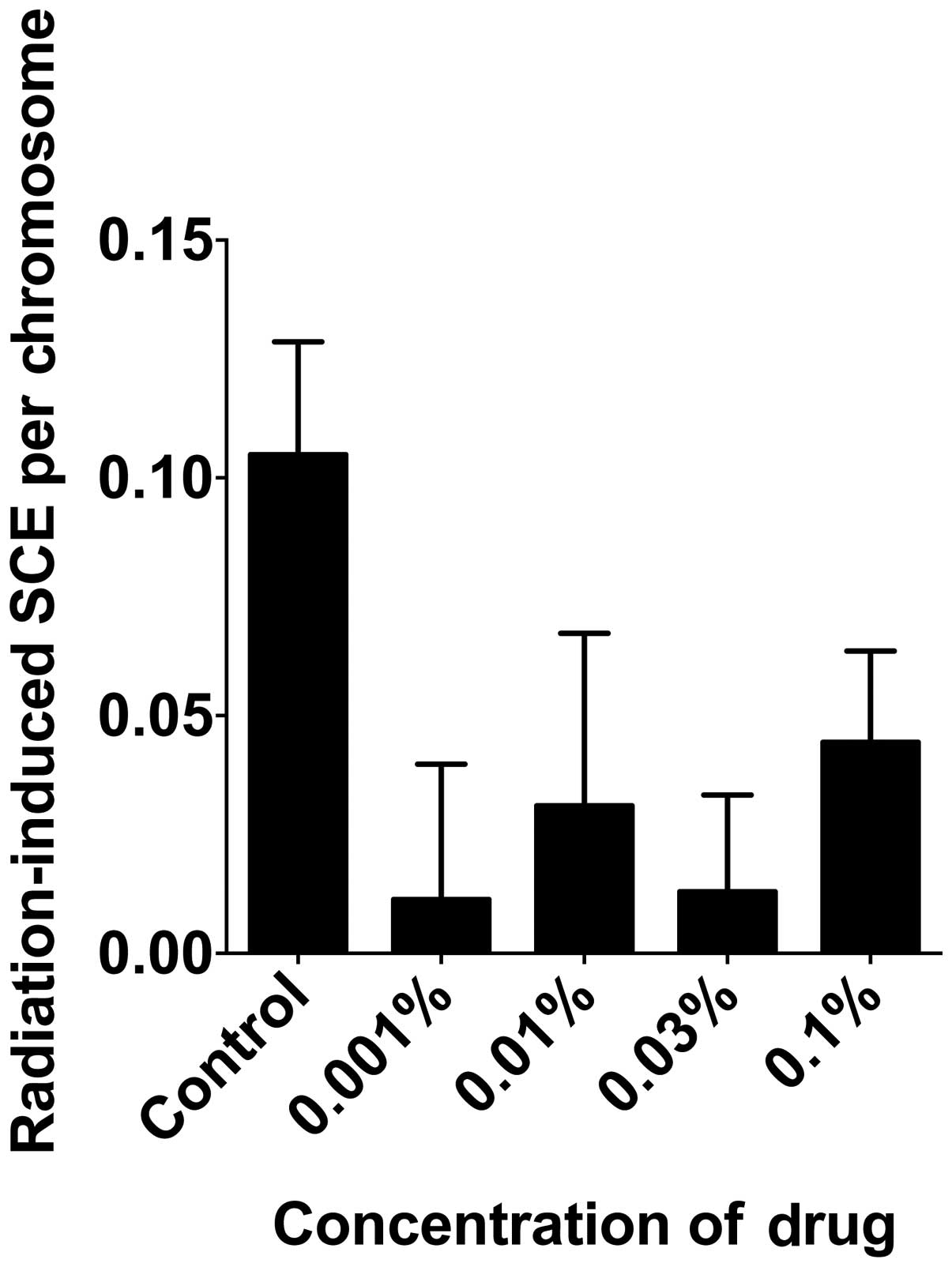
In order to determine the effect of
monoglucosyl-rutin on the prevention of radiation-induced DNA
damage, the number of induced SCEs and induced γH2AX foci were
analyzed. To determine the effect of monoglucosyl-rutin on the
prevention of DNA base damage, the number of 2 Gy γ-ray-induced
SCEs were analyzed per chromosome. As shown in Fig. 5, monoglucosyl-rutin significantly
decreased the number of SCEs per chromosome at all concentrations
compared with non-treated cells. Additionally, the effect of
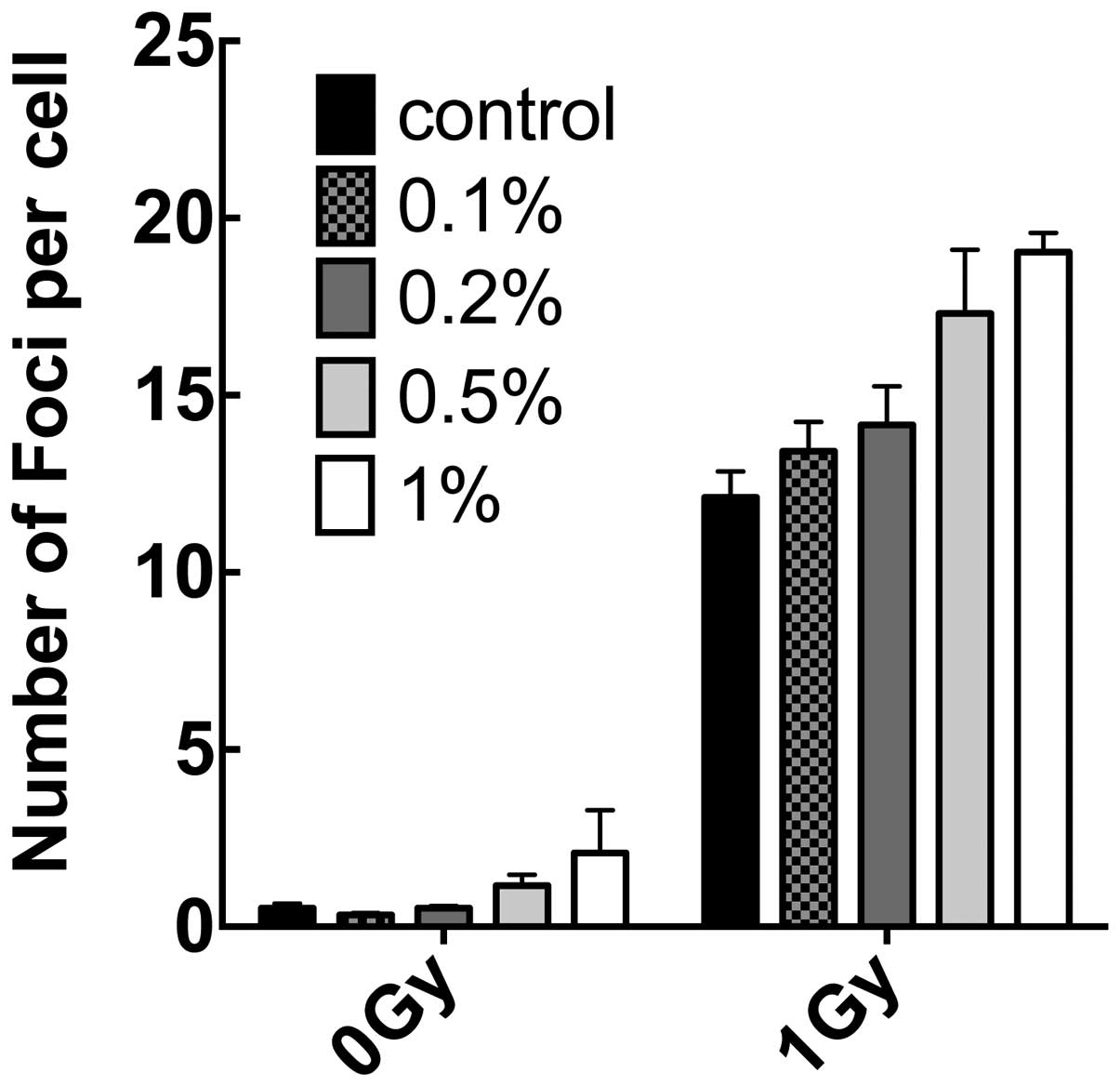
monoglucosyl-rutin on double-strand breaks was also investigated by
comparing the number of induced-γH2AX foci in treated and untreated
cells. As shown in Fig. 6, at
concentrations of 0.5 and 1%, monoglucosyl-rutin increased the
number of γH2AX foci in unexposed and exposed fibroblasts. At lower
concentrations used to determine cell survival and SCE, the
monoglucosyl-rutin-treated cells exhibited statistically similar
numbers of γH2AX foci with and without radiation.
Discussion
The present study demonstrated that
monoglucosyl-rutin exhibits a wide range of effects in mammalian
cells. Despite the ability of monoglucosyl-rutin to protect cells
from radiation at low concentrations, it was observed that at
elevated doses cells experienced an increase in baseline DNA
damage. These results are in accordance with previous studies
demonstrating that natural flavonoids have beneficial and negative
effects on cells (12). As
demonstrated in a previous study, several flavonoids have been used
to protect against radiation due to their ability to capture
radiation-induced radicals in vitro and in vivo
(7). However, it was also reported
that certain flavonoids function as DNA-dependent protein kinase
inhibitors (12).
Through colony formation assays it was demonstrated
that at a concentration of 0.3%, monoglucosyl-rutin increases cell
survival in cells exposed to 2 Gy. Concentrations of 0.1%
monoglucosyl-rutin, however, only increased cell survival in cells
exposed to 2 Gy but not 8 Gy. When the cells were analyzed for SCE
and γH2AX foci, it was observed that all doses of
monoglucosyl-rutin decreased the number of radiation-induced SCE
events, however, had no effect on the number of γH2AX foci formed.
These results suggest that monoglucosyl-rutin prevents DNA base
damage caused by ionizing radiation. This indicates that
monoglucosyl-rutin may inhibit long-lived radicals, however, not
short-lived radicals which cause the most harm to cells, thus only
a slight increase in cell survival was observed.
The limited effect of monoglucosyl-rutin on cell
survival may also be attributed to the size of the chemical, which
has a molecular weight (MW) of >700 Da. Compounds with a MW of
>500 Da tend to not be absorbed through the cell membrane
(13,14). This may potentially explain why
monoglucosyl-rutin was found to be effective at inhibiting
double-strand breaks in vitro in a previous study (15), however, demonstrated no effect
in vivo in the present study.
Finally, it was noted in the present study that
monoglucosyl-rutin had an effect on the plating efficiency and
doubling time of the treated cells. Cell doubling time and plating
efficiency were significantly affected at concentrations of
monoglucosyl-rutin ≥0.3%. This is in accordance with the data which
demonstrated that there was a statistical increase in γH2AX foci in
cells treated with 0.5% monoglucosyl-rutin. Despite
monoglucosyl-rutin being able to inhibit the formation of
radiation-induced SCEs, the drug itself causes DNA double-strand
breaks. These results demonstrated that there is a narrow window
between doses that are potentially beneficial and ones that cause
additional DNA damage to the cells.
In conclusion, the present study demonstrated that
monoglucosyl-rutin is able to decrease the number of
radiation-induced SCEs and increase cell survival at concentrations
that do not significantly increase cellular toxicity. This increase
in cell survival is possibly due to the ability of
monoglucosyl-rutin to inhibit the formation of long-lived radicals,
thus protecting against DNA base damage. At higher concentrations,
however, it was revealed that treatment with monoglucosyl-rutin
results in double-strand breaks, leading to increased cell doubling
time and a decrease in overall cell survival, potentially limiting
the use of monoglucosyl-rutin as a radioprotector. The present
study demonstrated that in vivo exposure to
monoglucosyl-rutin does not produce the same results as in
vitro experiments, and this is most likely due to the inability
of monoglucosyl-rutin to enter the cell owing to its large MW.
Acknowledgements
This study was partially supported by the Colorado
State University start up fund (TK) and the collaborative research
fund from Toyo Sugar Refining Co., Ltd. (MU).
References
|
1
|
Schulte-Frohlinde D: Comparison of
mechanisms for DNA strand break formation by the direct and
indirect effect of radiation. Basic Life Sci. 38:19–27.
1986.PubMed/NCBI
|
|
2
|
Koyama S, Kodama S, Suzuki K, Matsumoto T,
Miyazaki T and Watanabe M: Radiation-induced long-lived radicals
which cause mutation and transformation. Mutat Res. 421:45–54.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Howell RW, Goddu SM, Bishayee A and Rao
DV: Radioprotection against lethal damage caused by chronic
irradiation with radionuclides in vitro. Radiat Res. 150:391–399.
1998. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rasey JS, Nelson NJ, Mahler P, Anderson K,
Krohn KA and Menard T: Radioprotection of normal tissues against
gamma rays and cyclotron neutrons with WR-2721: LD50 studies and
35S-WR-2721 biodistribution. Radiat Res. 97:598–607. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alia M, Mateos R, Ramos S, Lecumberri E,
Bravo L and Goya L: Influence of quercetin and rutin on growth and
antioxidant defense system of a human hepatoma cell line (HepG2).
Eur J Nutr. 45:19–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choquenet B, Couteau C, Paparis E and
Coiffard LJ: Quercetin and rutin as potential sunscreen agents:
determination of efficacy by an in vitro method. J Nat Prod.
71:1117–1118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benavente-Garcia O, Castillo J, Lorente J
and Alcaraz M: Radioprotective effects in vivo of phenolics
extracted from Olea europaea L. leaves against X-ray-induced
chromosomal damage: comparative study versus several flavonoids and
sulfur-containing compounds. J Med Food. 5:125–135. 2002.PubMed/NCBI
|
|
8
|
Shimoi K, Yoshizumi K, Kido T, Usui Y and
Yumoto T: Absorption and urinary excretion of quercetin, rutin, and
alphaG-rutin, a water soluble flavonoid, in rats. J Agric Food
Chem. 51:2785–2789. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsunaga K, Yoshimi N, Shimoi K, et al:
Inhibitory effects of dietary monoglucosyl-rutin on
azoxymethane-induced colon carcinogenesis in rats. Asian Pac J
Cancer Prev. 1:211–216. 2000.PubMed/NCBI
|
|
10
|
Maeda J, Yurkon CR, Fujisawa H, et al:
Genomic instability and telomere fusion of canine osteosarcoma
cells. PLoS One. 7:e433552012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tobey RA and Ley KD: Regulation of
initiation of DNA synthesis in Chinese hamster cells. I. Production
of stable, reversible G1-arrested populations in suspension
culture. J Cell Biol. 46:151–157. 1970. View Article : Google Scholar
|
|
12
|
Izzard RA, Jackson SP and Smith GC:
Competitive and noncompetitive inhibition of the DNA-dependent
protein kinase. Cancer Res. 59:2581–2586. 1999.PubMed/NCBI
|
|
13
|
Camenisch G, Alsenz J, van de Waterbeemd H
and Folkers G: Estimation of permeability by passive diffusion
through Caco-2 cell monolayers using the drugs’ lipophilicity and
molecular weight. Eur J Pharm Sci. 6:317–324. 1998.PubMed/NCBI
|
|
14
|
Bos JD and Meinardi MM: The 500 Dalton
rule for the skin penetration of chemical compounds and drugs. Exp
Dermatol. 9:165–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshikawa Y, Mori T, Suzuki M, Imanaka T
and Yoshikawa K: Comparative study of kinetics on DNA double-strand
break induced by photo- and gamma-irradiation: Protective effect of
water-soluble flavonoids. Chem Phys Lett. 501:146–151. 2010.
View Article : Google Scholar
|