Introduction
Cytochrome P450 (CYP450) enzymes constitute a
superfamily of membrane-bound heme proteins that catalyse the
oxidative metabolism of a variety of endogenous compounds,
including steroids, fatty acids, and prostaglandins (1), and exogenous compounds, including
drugs, carcinogens, agrochemicals (2–4), and
environmental pollutants (5–7).
Seven human isoforms of CYP (CYP1A2, -2C9, -2C18, -2C19, -2D6, -2E1
and -3A4) metabolize >90% of drugs currently used in the clinic
(8). Alternatively, certain drugs
are able to modify the expression and activity of CYP450 enzymes,
thereby changing drug efficacy and pharmacokinetics. Various in
vitro models have been used to assess CYP induction, including
precision-cut liver slices (9,10),
primary hepatocytes (11,12), and reporter gene constructs
(13,14). In particular, HepG2 cells retain
the expression of all human CYPs (15) and are frequently used to evaluate
the effects of xenobiotics on CYP450 expression. Additionally, the
cocktail method is the most predominant technique used for studies
on CYP450 conducted on human liver microsomes (HLMs).
Medicinal plants have been used to treat numerous
types of diseases for thousands of years in China. In particular,
the use of medicinal plants combined with conventional medicine is
common in the treatment of chronic diseases, cancers, immunological
disorders and infectious diseases. However, in contrast to the
popular presumption that ‘natural is safe’, medicinal plants may
have significant side effects. Additionally, there is an increasing
concern for the risk of drug-drug interactions when medicinal
plants are administered concomitantly with conventional medicines
(16–19).
Glycyrrhiza uralensis (G. uralensis),
also known as Chinese licorice, is a Traditional Chinese Medicine
(TCM) that has been used in the clinic for centuries. Its functions
include regulating drug properties, improving spleen function and
blood circulation and reducing cough. The main bioactive components
of G. uralensis are triterpene saponins and various types of
flavonoids, including glycyrrhetinic acid (GA), glycyrrhizic acid
(GL), liquiritigenin (L), isoliquiritigenin (IL), liquiritin (LG)
and licochalcone A (LA). These components possess various
biological activities, including antiulcer, anti-inflammatory,
anti-allergic, antichrombotic, antidiabetic, hepatoprogenic and
neuroprotective activities (20,21).
Results of previous studies have shown that G.
uralensis or its components activate the nuclear receptor PXR,
inducing CYP3A and affecting lidocaine pharmacokinetics (22), and inhibit CYP2E1 expression when
administered prior to carbon tetrachloride, exerting a protective
effect on liver injury (23).
However, the specific CYP450 isoforms involved and the effects of
specific G. uralensis components have yet to be identified.
Thus, G. uralensis has not currently been adopted for common
use in clinical practice.
The present study aimed to investigate the effects
of the main bioactive constituents of G. uralensis on the
expression and activity of CYP isoforms.
Materials and methods
Chemicals
GA and LG were obtained from the National Institute
for the Control of Pharmaceutical and Biological Products (Beijing,
China). GL, L, IL, and LA were purchased from Shanghai Tauto
Biotech (Shanghai, China). All the above constituents of G.
uralensis were dissolved in DMSO (MP Biomedicals, Strasbourg,
France). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT, Sigma-Aldrich Co., St. Louis, MO, USA) was dissolved
in PBS (pH 7.4). Melatonin, dextromethorphan, coumarin and
omeprazole are purchased from Wako (Osaka, Japan). Tolbutamide,
chlozoxazone, phenacetin, amodiaquine, 6-hydroxymelatonin, and
dextrophan were purchased from Sigma-Aldrich. Desmethylomeprazole,
6-hydroxychlozoxazone, 4-hydroxytolbutamide, sulfone omeprazole,
and 5-hydroxyomeprazole were purchased from International
Laboratory (San Francisco, CA, USA).
Cell culture and cytotoxicity assay
Human hepatoma HepG2 cells were obtained from the
Chinese Academy of Sciences Committee Type Culture Collection Cell
Bank/CAS Shanghai Institute for Biologic Science Cell Resource
Center (Shanghai, China) and maintained in Williams’ Medium E
supplemented with 10% fetal calf serum, 100 IU/ml penicillin G, and
100 μg/ml streptomycin. The cells were maintained in
25-cm2 flasks at 37°C in a humidified incubator with 5%
CO2.
The cytotoxic effects of the compounds on HepG2
cells were evaluated by measuring the metabolic activity using the
MTT assay. Cells were grown in 96-well plates (~1×104
cells/well) for 24 h. The medium was replaced with fresh medium
containing serial dilutions of the test compound (0–100 μM). For
all treatments, the final concentration of DMSO did not exceed
0.01% (v/v). Following incubation for 24 h, the medium was removed,
20 μl MTT (5 mg/ml in PBS) was added to each well, and the cells
were incubated for an additional 4 h at 37°C at 5% CO2.
The supernatant was then removed, and 150 μl DMSO was added to each
well. The absorbance at 490 nm was recorded using a BIO-TEK
spectrophotometer.
RNA extraction, cDNA synthesis, and
quantitative polymerase chain reaction (qPCR)
HepG2 cells were seeded in 12-well plates and
cultured until they reached 70–80% confluence. The medium was then
replaced with fresh medium containing 25 or 50 μM of different test
compounds dissolved in DMSO. Following 24 h of incubation, total
RNA was isolated using TRIzol according to the manufacturer’s
instructions (Takara Bio Inc., Otsu, Japan) and was quantified by
measuring the absorbance at 260/280 nm. First-strand cDNA synthesis
was performed using the High-Capacity cDNA Reverse Transcription
kit (Applied Biosystems, USA) according to the manufacturer’s
instructions. Briefly, 2 μg total RNA from each sample was added to
a mixture of 2.0 μl 10X RT buffer, 0.8 μl 25X dNTP mix (100 mM),
1.0 μl MultiScribe reverse transcriptase, 1.0 μl Universal RT
primer (20 μM 5′-aagc cgagacgacgacagactttttttttttttttttttttVVN-3′)
and RNase-free water. The reaction mixture (20 μl) was incubated at
25°C for 10 min, 37°C for 120 min, 85°C for 5 min and then cooled
to 4°C for the final step. For all experiments, cDNA was diluted to
a concentration equivalent to 20 ng/ml RNA. Synthesized cDNA was
stored at −20°C.
qPCR was performed using the Bio-Rad IQ5 system
(Bio-Rad, Hercules, CA, USA). PCR assays were performed in 96-well
optical reaction plates. To avoid the impact of DNA contamination,
primer pairs were designed to span an intron-exon boundary.
Reaction mixtures (25 μl) contained 0.1 μl each (stock
concentration, 10 μM) of the forward and reverse primers (final
concentration of each primer, 40 nM; Table I), 12.5 μl Power SYBR-Green
Universal Master Mix (Applied Biosystems, Beijing, China), 10.3 μl
nuclease-free water and 2 μl cDNA solution. The reaction conditions
were as follows: Initiation at 95°C for 10 min, followed by 50
cycles of denaturation at 95°C for 15 sec and annealing/extension
at 60°C for 1 min, followed by a dissociation step. Expression
levels were normalized to the expression of the housekeeping gene,
GAPDH, and relative expression was calculated using the
2−ΔΔCt method (24).
Data are presented as the fold change relative to GAPDH
expression.
 | Table IPrimers used for the analysis of gene
expression by qPCR. |
Table I
Primers used for the analysis of gene
expression by qPCR.
| Gene | Primers | Amplicon size
(bp) |
|---|
| GADPH | Forward:
TCTCCCCTCCTCACAGTTGC | |
| Reverse:
AAGCCGAGACGACGACAGAC | 143 |
| CYP1A2 | Forward:
AGCTTCTCCTGGCCTCTGC | |
| Reverse:
GGACTTTTCAGGCCTTTGGG | 88 |
| CYP2D6 | Forward:
CGCATCCCTAAGGGAACGA | |
| Reverse:
TCCCAGACGGCCTCATCCT | 68 |
| CYP2E1 | Forward:
GCAAGAGATGCCCTACATGGA | |
| Reverse:
GGGCACGAGGGTGATGAA | 64 |
| CYP3A4 | Forward:
CAGGAGGAAATTGATGCAGTTTT | |
| Reverse:
GTCAAGATACTCCATCTGTAGCACAGT | 78 |
HLM metabolic assay
HLMs were obtained from iPhase Pharmaceutical
Services (Beijing, China). Microsomes were incubated with 500 μg/ml
human microsomal protein in 0.1 M potassium phosphate buffer (pH
7.4), 1 mM nicotine amide dinucleotide phosphate (NADPH), and
substrates at the indicated concentrations. Substrates and their
final concentration for incubation were as follows: Melatonin (6
μM), coumarin (4 μM), tolbutamide (30 μM), omeprazole (20 μM),
dextromethorphan (0.6 μM) and chlozoxazone (30 μM). The first
dilution of the substrate was produced in appropriate solvents,
i.e., methanol (melatonin, coumarin, omeprazole), water
(dextromethorphan), DMSO (tolbutamide) or 60 mM potassium hydroxide
solution (chlozoxazone), and subsequent dilutions were produced in
0.1 M phosphate buffer (pH 7.4). The final amount of solvent in the
incubation mixture was <0.05% (v/v). The final concentration of
each compound tested (i.e., GL, GA, L, IL, LG and LA), as well as
the mixture containing all the compounds at the same ratio (GC),
was 25 μM. The reaction mixture, at a final volume of 250 μl, was
pre-incubated for 2 min at 37°C in a water bath prior to initiation
of the reaction by the addition of NADPH. Following incubation for
20 min, the reaction was terminated by the addition of 100 μl
acetonitrile containing 0.5 μM phenacetin as an internal standard
for each drug metabolite. The sample was then cooled in an ice bath
to precipitate the protein. The mixture was vortexed and
centrifuged at 14000 × g for 10 min. The supernatant was analyzed
using ultra performance liquid chromatography time of flight mass
spectrometry (UPLC/TOF-MS).
Cocktail assay
For the cocktail assay, we used liquid
chromatography-tandem mass spectrometry (LC-MS/MS) analysis. The
analytical column was a Waters ACQUITY UPLC BEH C18 column (1.7 μm,
2.1×5 mm; Waters, Milford, MA, USA) with a C18 1.7 μm VanGuard 3/PK
2.1×5 mm pre-column (Waters). The flow rate was 0.35 ml/min, and
the column temperature was 30°C. The eluents used were (A) aqueous
1% formic acid and 10 mM ammonium acetate and (B) methanol. The
gradient elution was performed with 5% eluent A from 0 to 4 min and
80% eluent A from 4 to 4.5 min.
Of the compounds in the cocktail substrate (i.e.,
melatonin, coumarin, omeprazole, dextromethorphan, tolbutamide and
chlozoxazone), only the metabolite of chlozoxazone was able to be
detected in negative ion mode, while the remaining metabolites were
detected using the positive ion electrospray mode. The data were
collected on a Micromass oa-Q-Tof (Waters) equipped with an
electrospray ionization source. Basic parameter conditions included
capillary, sample cone, and extraction cone voltages of 3500, 30,
and 2 V, respectively; desolvation and nebulization N2
flows of 450 and 45 l/h, respectively; and desolvation and source
temperatures of 350 and 120°C, respectively. The mass spectrometer
and UPLC systems were operated using Micromass MassLynx4.1
software. Chromatographic traces of protonated metabolites of
CYP-specific substrates were extracted from total ion
chromatograms. The masses of metabolites extracted at various
retention times are shown in Table
II.
 | Table IISubstrates, CYP-specific model
reactions and substrate-extracted ions in the cocktail. |
Table II
Substrates, CYP-specific model
reactions and substrate-extracted ions in the cocktail.
| Substrate | CYP | Reaction | Metabolite | Extracted ion
(m/z) |
|---|
| Melatonin | 1A2 |
6-Hydroxylation | 6-OH-MEL | 249 |
| Coumarin | 2A6 |
7-Hydroxylation | 7-OH-COU | 163 |
| Amodiaquine | 2C8 | Desethylation | deEt-AMO | 328 |
| Tolbutamide | 2C9 |
Methylhydroxylation | OH-TOL | 287 |
| Omeprazole | 2C19 |
5-Hydroxylation | OH-OME | 362 |
| 2C19 | Demethylation | Dem-OME | 332 |
| 3A4 | Sulfoxidation |
SO2-OME | 362 |
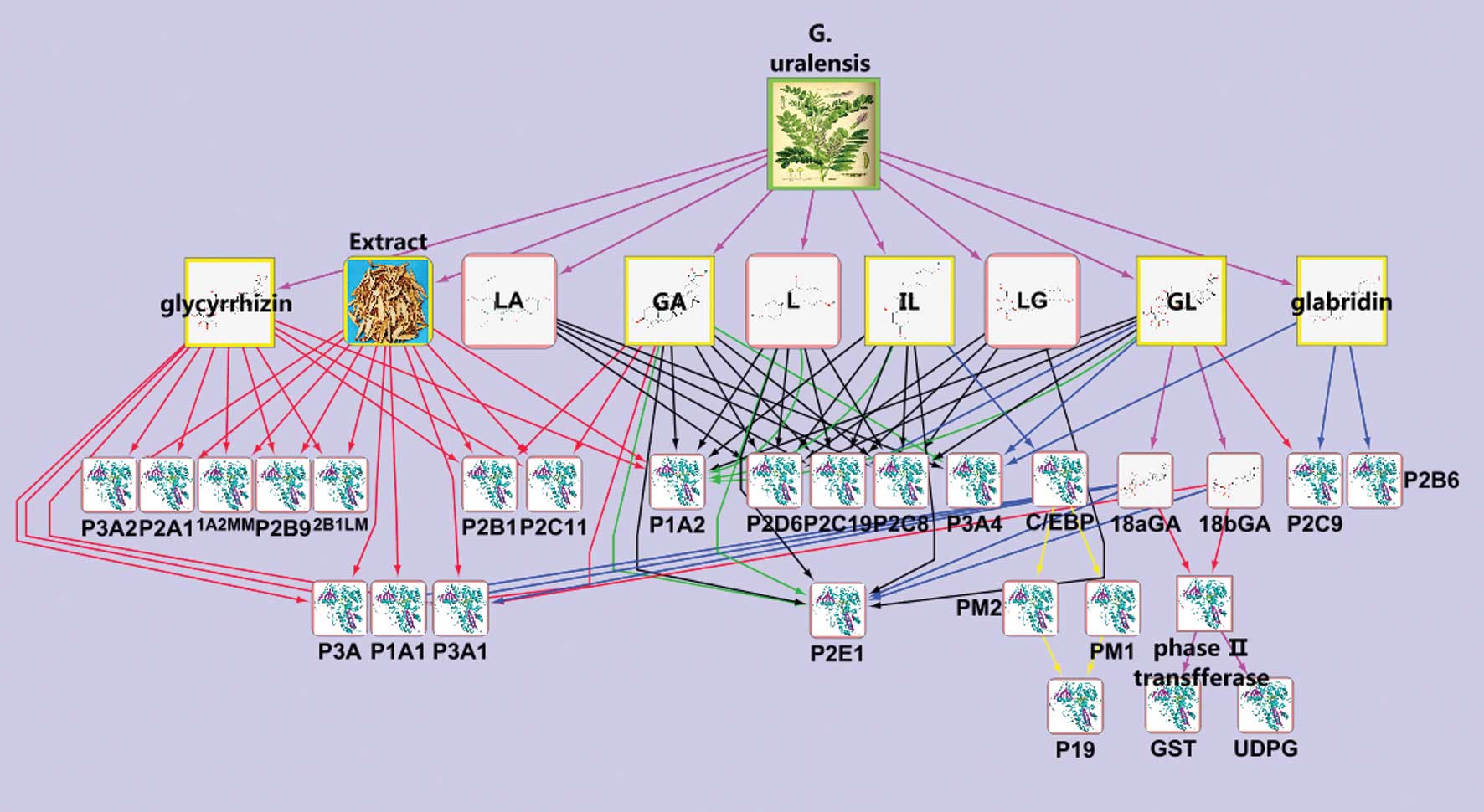
Network of influence on metabolic enzymes
of G. uralensis
In recent years, numerous studies (25–27)
have shown that the different bioactive substances found in G.
uralensis are metabolized by different CYP450 isoforms; at the
same time, they are able to inhibit or activate the various
isoforms of CYP450 to different degrees. In order to obtain a
comprehensive understanding of the influence of G. uralensis
on metabolic enzymes, a bioinformatics network of the influence of
G. uralensis on metabolic enzymes through the PubMed
literature database was created using Cytoscape software (28). The 47 documents selected through
keyword retrieval from the PubMed database were imported to the
Agilent Literature Search module of the Cytoscape software. A
network figure was automatically generated by Cytoscape, and nodes
and edges of the network were modified manually according to
detailed information from the study abstracts. From this, a new
network of the influence of G. uralensis on metabolic
enzymes was produced, and a macroscopic overview of the effect of
G. uralensis on metabolic enzymes was obtained.
Statistical analysis
Each experiment was performed twice. One-way
analysis of variance (ANOVA) was used to analyze differences
between samples, and P<0.05 was considered to indicate
statistically significant differences. The data for activity of the
CYP450 enzyme were presented as the fold change compared with the
control sample.
Results
Effect of components of G. uralensis on
HepG2 cell viability
To determine whether GL, GA, L, IL, LG, LA and GC
were cytotoxic, cell viability assays were performed. HepG2 cells
were treated with serial dilutions (0–50 μM) of GL, GA, L, IL, LG,
LA or GC for 24 h, and the cell survival rate was evaluated. Cell
viability was not affected by any of the compounds at
concentrations <50 μM when compared with the control cells
(Fig. 1). Therefore,
concentrations of 50 and 25 μM were used for all experiments on
HepG2 cells.
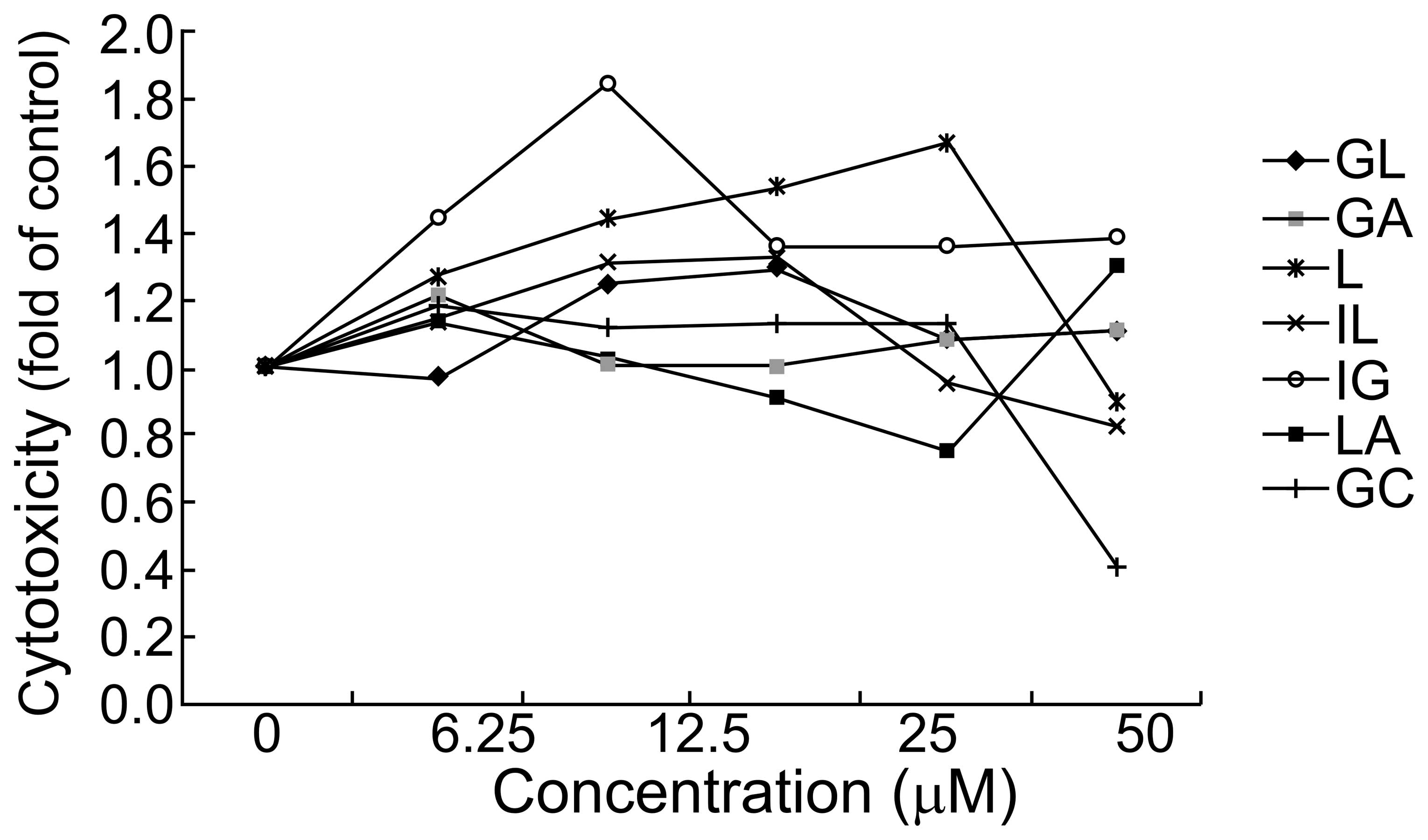 | Figure 1Effects of GL, GA, L, IL, LG, LA and
GC on HepG2 cell viability. HepG2 cell viability was tested by MTT
assay following incubation with 0–50 μM GL, GA, L, IL, LG, LA and
GC, respectively, for 24 h. GA, glycyrrhetinic acid; GL,
glycyrrhizic acid; L, liquiritigenin; IL, isoliquiritigenin; LG,
liquiritin; LA, licochalcone A; GC, equimolar mixture of GA, GL, L,
IL, LG and LA. |
Expression of CYP1A2 mRNA following
treatment with components of G. uralensis
The CYP450 superfamily of drug metabolism enzymes
has a vital role in metabolizing most drugs. CYP1A2 accounts for
>10% of hepatic CYP enzymes and is known to be regulated by the
aryhydrocarbon receptor (AhR). Additionally, CYP1A2 is involved in
the metabolism of numerous steroid hormones and procarcinogens. The
present study assessed whether GL, GA, L, IL, L, LA and GC modulate
CYP1A2 expression. HepG2 cells were treated with the
above-mentioned compounds at 25 and 50 μM, respectively, for 24 h,
and CYP1A2 mRNA levels were analyzed using qPCR. No significant
difference was observed following treatment with LA (Fig. 2). By contrast, treatment with 25 μM
GL, L or IL induced a significant increase in CYP1A2 expression
compared with the controls. However, when the cells were treated
with 50 μM GL, L or IL, CYP1A2 mRNA levels were significantly
down-regulated. GA inhibited CYP1A2 expression in a dose-dependent
manner; however, the inhibitory effects of LG and GC were not
dose-dependent. The results indicated that the latter compounds may
interact with each other to modulate CYP1A2 expression.
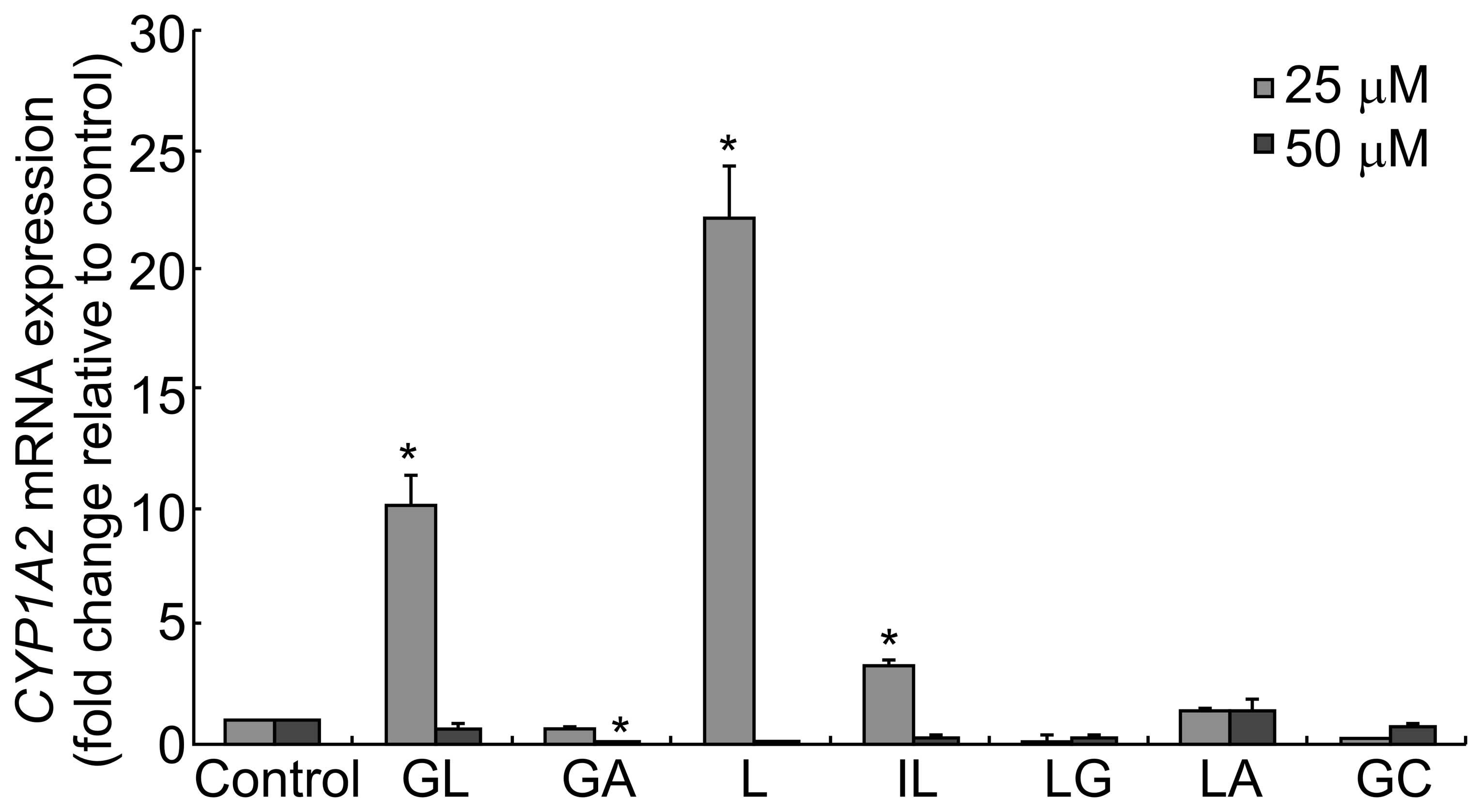 | Figure 2CYP1A2 mRNA levels following
treatment with the various compounds. HepG2 cells were treated with
25 or 50 μM GL, GA, L, IL, LG, LA or GC for 24 h. The relative
expression of CYP1A2 mRNA was assessed using qPCR. Fold change
values were determined by normalizing to GADPH expression, and
values were expressed as the fold change relative to the control.
Data are presented as the mean ± standard deviation
(*P<0.05 versus control). qPCR, quantitative
polymerase chain reaction; CYP, cytochrome P; GA, glycyrrhetinic
acid; GL, glycyrrhizic acid; L, liquiritigenin; IL,
isoliquiritigenin; LG, liquiritin; LA, licochalcone A; GC,
equimolar mixture of GA, GL, L, IL, LG and LA. |
Expression of CYP2D6 mRNA following
treatment with G. uralensis
The effects of the components of G. uralensis
on CYP2D6 expression were investigated. CYP2D6 is known for
being polymorphic, and 30% of CYP-metabolized drugs are metabolized
by CYP2D6. The results showed that GL, GA, L, IL, LG and LA
significantly inhibited the expression of CYP2D6 transcripts
(Fig. 3). However, following
treatment with GC, a mixture of all the compounds, the expression
of CYP2D6 was increased. This may be due to interactions
between the different components of the mixture.
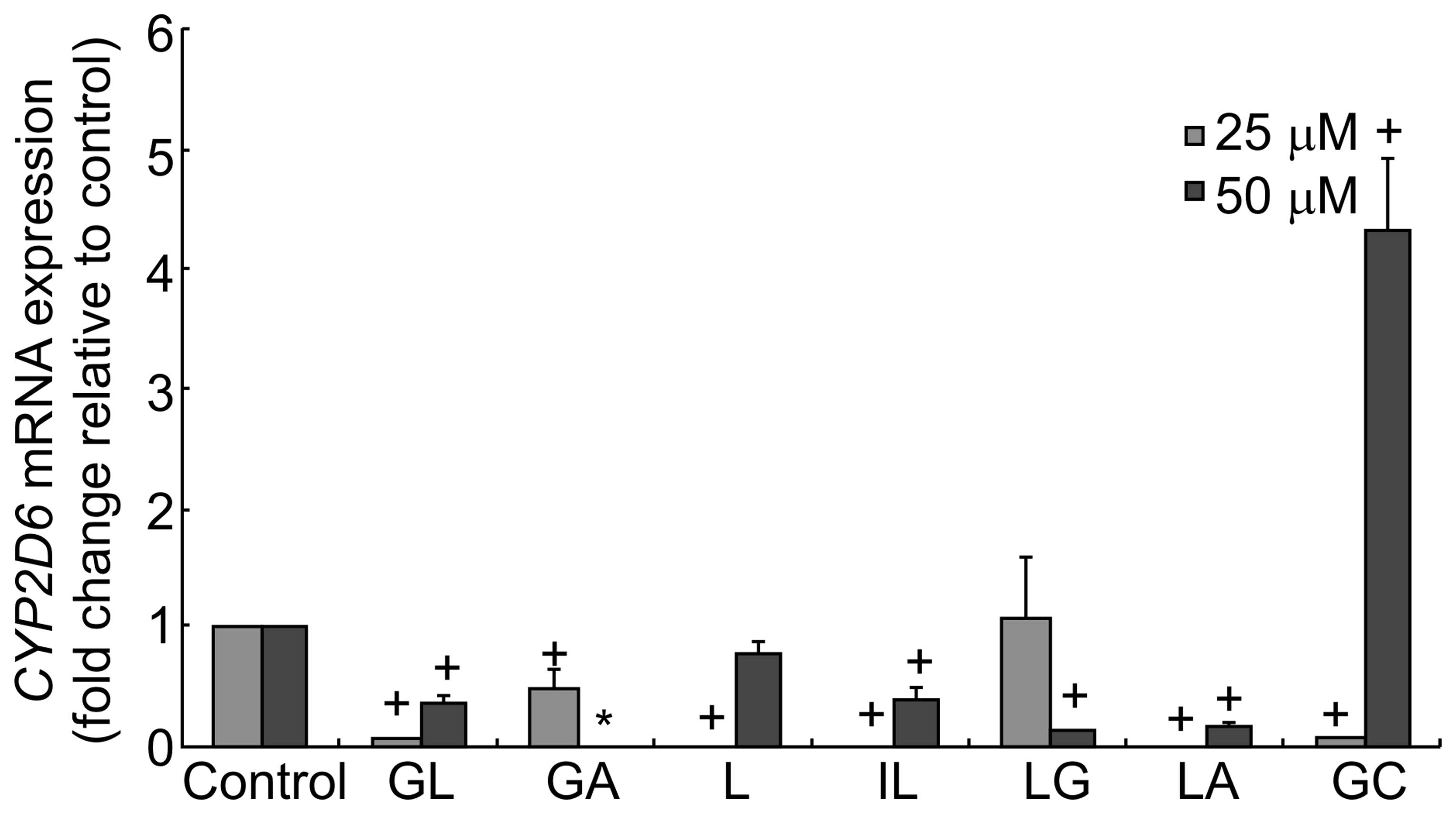 | Figure 3CYP2D6 mRNA levels following
treatment with G. uralensis-derived compounds. HepG2 cells were
treated with 25 or 50 μM of GL, GA, L, IL, LG, LA or GC for 24 h.
The relative expression of CYP2D6 mRNA was assessed using qPCR.
Fold change values were determined by normalizing to GADPH
expression, and values were expressed as the fold change relative
to the control. Data are presented as the mean ± standard deviation
(*P<0.05 versus control). CYP, cytochrome P; GA,
glycyrrhetinic acid; GL, glycyrrhizic acid; L, liquiritigenin; IL,
isoliquiritigenin; LG, liquiritin; LA, licochalcone A; GC,
equimolar mixture of GA, GL, L, IL, LG and LA. |
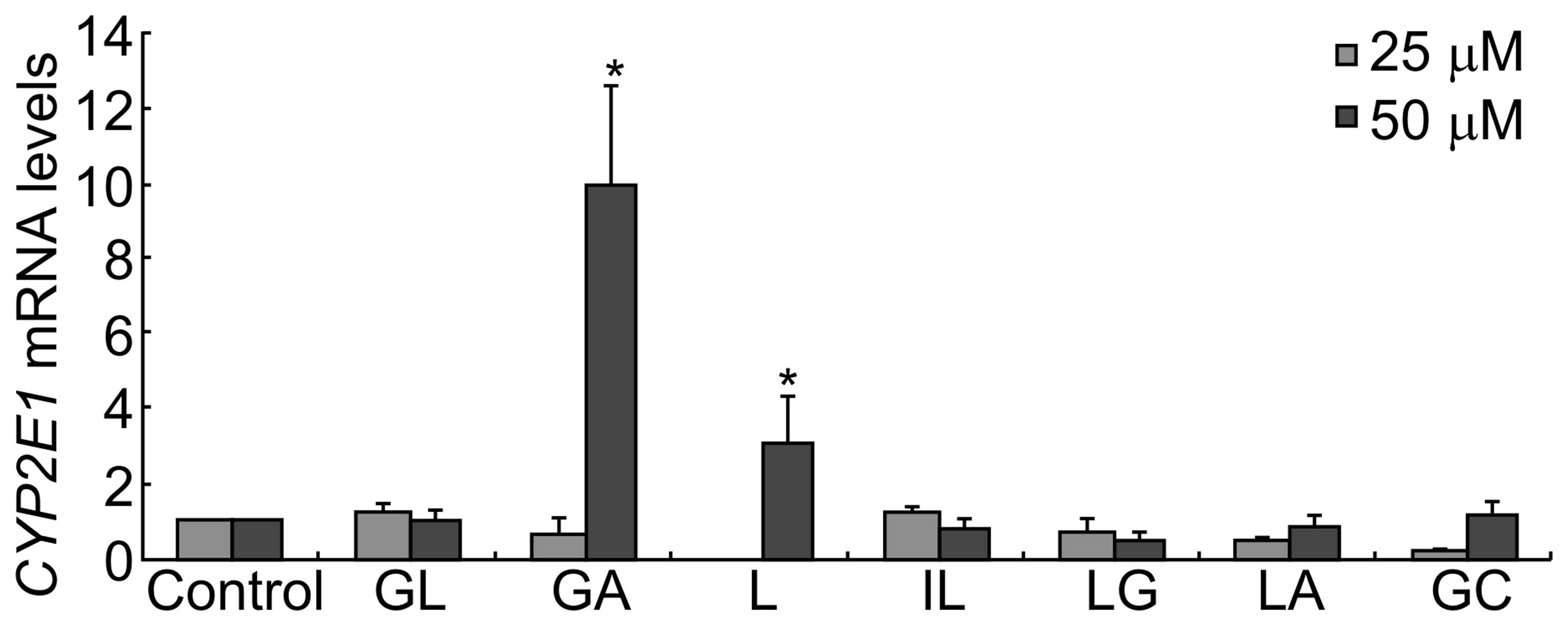
Expression of CYP2E1 mRNA following
treatment with G. uralensis-derived compounds
Expression of CYP2E1 mRNA did not differ
significantly following treatment with GL. However, exposure to 25
μM GA, L or GC inhibited the expression of CYP2E1
transcripts, while 50 μM GA, L or GC significantly increased the
expression of CYP2E1.
Previous studies showed that the hepatoprotective
effect of these compounds may be due to their ability to block the
bioactivation of carbon tetrachloride by inhibiting P450 2E1
activity and expression (29–31).
The present study showed that GL did not inhibit CYP2E1
expression (Fig. 4). However, IL
and LG inhibited the expression of CYP2E1 transcripts in a
dose-dependent manner. GA and L inhibited the expression of
CYP2E1 transcripts when used at a low concentration (25 μM),
which is consistent with the study by Jeong et al (32), which showed that GA was able to
inhibit the expression and activity of P450 2E1 and had protective
effects against carbon tetrachloride-induced hepatotoxicity;
however, they significantly increased CYP2E1 expression at a
high concentration (50 μM). However, when HepG2 cells were treated
with a mixture of the six bioactive constituents of G.
uralensis (25 μM), the expression of CYP2E1 was slightly
inhibited.
Expression of CYP3A4 mRNA following
treatment with G. uralensis-derived compounds
CYP3A4, the major isoform of the CYP3A subfamily in
humans, is the most important drug-metabolizing enzyme. As shown in
Fig. 5, GL and GA increased the
expression of CYP3A4 at a concentration of 50 μM, and GL,
GA, L, IL, LG LA and GC modulated the expression of CYP3A4
in a dose-dependent manner.
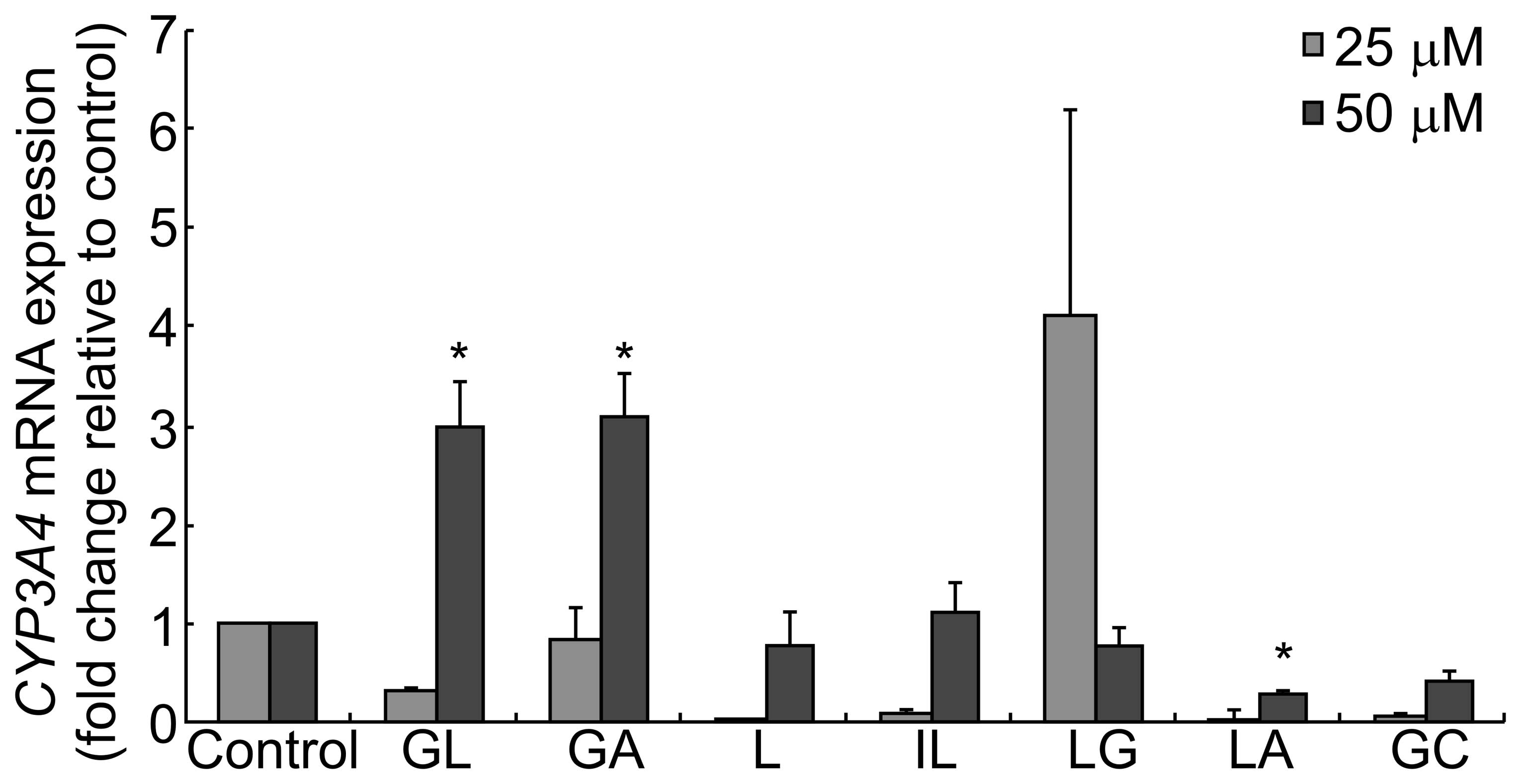 | Figure 5CYP3A4 mRNA levels following
treatment with compounds derived from G. uralensis. HepG2 cells
were treated with 25 or 50 μM GL, GA, L, IL, LG, LA or GC for 24 h.
The relative expression of CYP3A4 mRNA was determined by qPCR. Fold
change values were determined by normalizing to GAPDH expression,
and values were expressed as the fold change relative to the
control. Data are presented as the mean ± standard deviation
(*P<0.05 versus control). qPCR, quantitative
polymerase chain reaction; CYP, cytochrome P; GA, glycyrrhetinic
acid; GL, glycyrrhizic acid; L, liquiritigenin; IL,
isoliquiritigenin; LG, liquiritin; LA, licochalcone A; GC,
equimolar mixture of GA, GL, L, IL, LG and LA. |
CYP450 activity in HLMs following
treatment with compounds derived from G. uralensis
Based on preliminary experiments in HLMs (33), appropriate probe substrates were
selected as follows: Melatonin (CYP1A2), coumarin (CYP2A6),
amodiaquine (CYP2C8), tolbutamide (CYP2C9), omeprazole (CYP1C19 and
CYP3A4), dextromethorphan (CYP2D6) and chlozoxazone (CYP2E1).
Cocktail activity assays were then performed in cultures of HLMs to
assess all seven P450 enzymes simultaneously following treatment
with the bioactive compounds from G. uralensis for 20 min.
Compared with the control sample, IL and GC strongly inhibited the
activity of CYP2C8 and all the compounds derived from G.
uralensis as well as their mixture (GC) significantly inhibited
the activity of CYP2C19 (Fig. 6).
LG showed the highest activity, inducing a 3-fold increase in the
expression of the CYP2D6 enzyme.
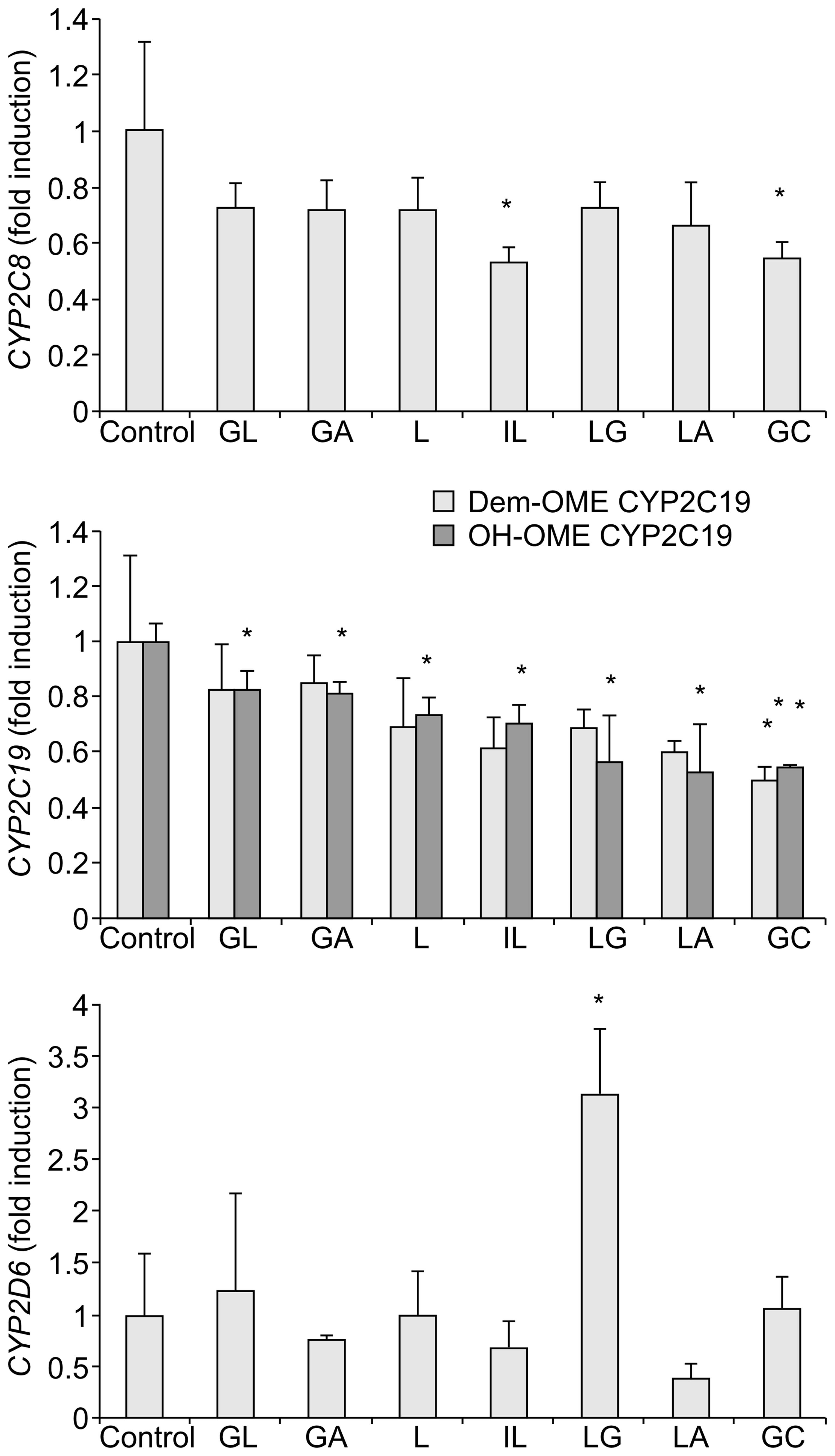 | Figure 6Effects of 25 μM GL, GA, L, IL, LG,
LA, and GC on the activities of CYP450 enzymes. Enzyme activities
were measured by treatment of the cultures with the substrate
cocktail and subsequent determination of the metabolites by
LC-MS/MS. Results are shown as the mean ± standard deviation
(*P<0.05 versus control). Analytical measurements
were performed in duplicate. LC-MS/MS, liquid chromatography-tandem
mass spectrometry; CYP, cytochrome P; GA, glycyrrhetinic acid; GL,
glycyrrhizic acid; L, liquiritigenin; IL, isoliquiritigenin; LG,
liquiritin; LA, licochalcone A; GC, equimolar mixture of GA, GL, L,
IL, LG and LA. |
When detecting the metabolites of certain probes
following treatment of HLMs with the bioactive compounds from G.
uralensis, the activity of CYP1A2, -2A6, -2C9, -2E1, and -3A4
were not able to be determined.
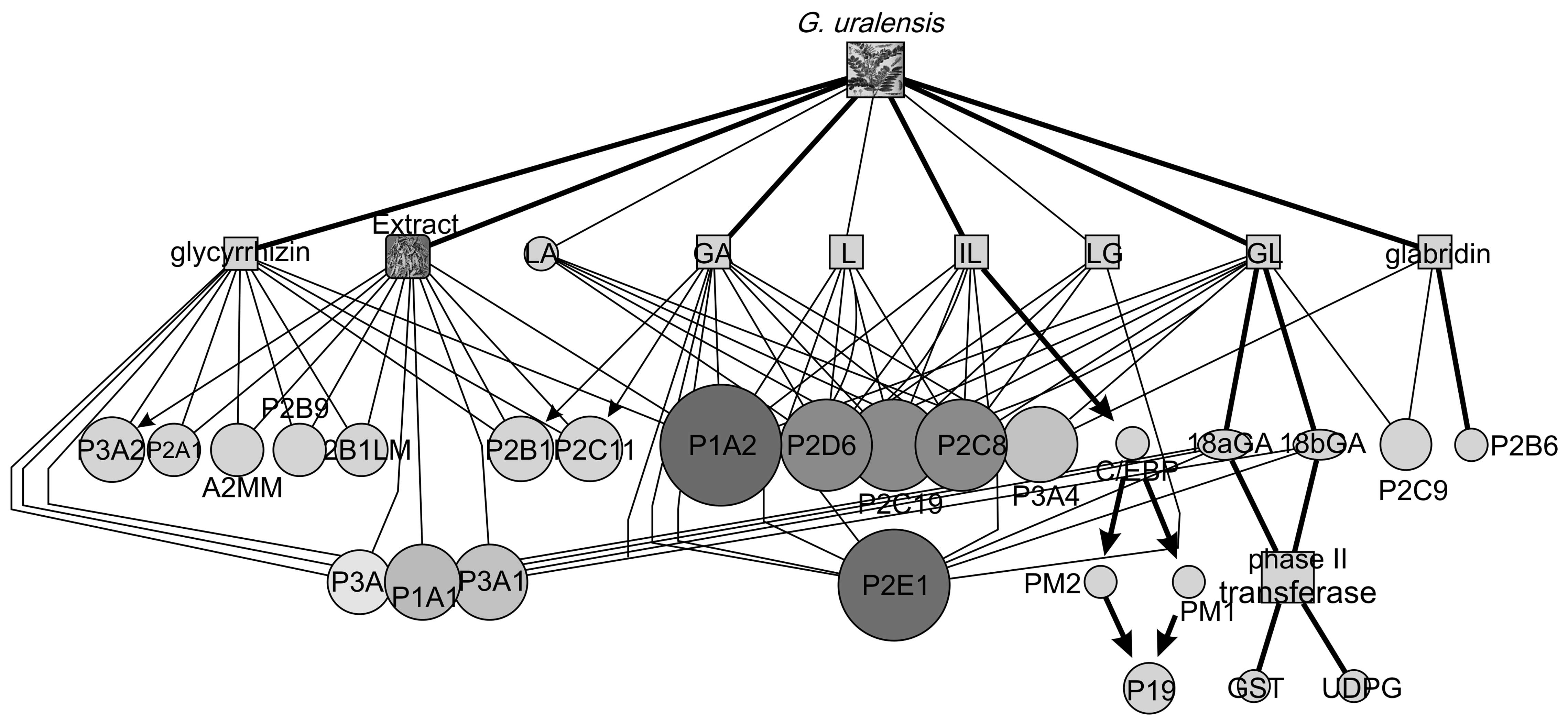
Network of influence of bioactive
compounds from G. uralensis on metabolic enzymes
The overview of the network of influences of G.
uralensis-derived compounds on metabolic enzymes is shown in
Fig. 7; this network was created
through integration of data found in the literature and the results
of the present study. The network was then analysed using the
network analysis module in Cytoscape (Fig. 8). This analysis provided additional
evidence that the compounds GL, GA, L, and IL, derived from G.
uralensis, had effects on metabolic enzymes, particularly
CYP450 family enzymes, and CYP1A, -2D, -2C, -2E, -3A, and -2A were
most susceptible to regulation by the bioactive components in G.
uralensis.
Discussion
The present study aimed to reveal the correlation
between G. uralensis and CYP450 enzymes. Consequently, the
effect of the major bioactive constituents of G. uralensis
on the expression of CYP isoforms in HepG2 cells was assessed using
qPCR and the effect of the compounds on the activity of certain
CYP450-associated enzymes in human liver microsomes was assessed
using the cocktail assay.
CYPP450s constitute a superfamily of membrane-bound
heme proteins that catalyze the oxidative metabolism of a variety
of endogenous compounds and environmental pollutants. Seven human
isoforms of CYP are responsible for metabolizing >90% of drugs
currently used in the clinic (8).
Alternatively, certain drugs are able to modify the expression and
activity of CYP450 enzymes, thereby changing drug efficacy and
pharmacokinetics. Therefore, it is highly important to study the
significance of CYP enzymes in the regulation of drug efficacy.
The current medicinal applications of G.
uralensis include regulating drug properties, improving spleen
function and blood circulation as well as reducing cough.
Regulating the activity of other drugs is the unique
pharmacological trait of G. uralensis and is documented as
an important concept in TCM. It is the most widely used medicinal
plant in TCM that is co-administered with other medicines mainly
due to its function of regulating the activity of drugs. With the
wide use of G. uralensis, it is becoming increasingly
important to be cautious about the interactions between G.
uralensis and other drugs to avoid reduction in efficacy and
increased side effects of co-administered drugs. However, studies
in this field are rare. Previous studies (34) have shown that G. uralensis
or its components were able to affect the activity of certain
isoforms of CYP450 and pharmacokinetics of drugs. However, the
specific isoforms of CYP450 involved and the effects of specific
components of G. uralensis remain to be elucidated.
Consistent with the results of the present study, it
has been reported that aqueous extracts of G. uralensis are
able to activate the nuclear receptor PXR, and activated PXR is
known to induce CYP3A expression (22,35).
Additionally, it has been demonstrated that GA significantly
inhibits the activity of CYP3A4 in HLMs (36,37).
The signalling mechanisms mediating these processes may be diverse.
Furthermore, CYP3A4 is regulated by several nuclear receptors,
including the constitutive androstane receptor (CAR), PXR and the
glucocorticoid receptor (GR). It has been reported that L is able
to inhibit CYP3A4 in vitro (38), which is consistent with the results
of the present study.
LG and LA are bioactive constituents of G.
uralensis. These two compounds have various biological
activities, including antioxidative, anti-inflammatory and
anticarcinogenic activities. However, it remains unknown how these
two compounds modulate the expression of CYP450 enzymes. The
present study is, to the best of our knowledge, the first study to
report the effects of LG and LA on the expression of CYP in HepG2
cells. It was demonstrated that LG inhibited the expression of
CYP1A2 and CYP2E1 and induced the expression of
CYP2D6 and CYP3A4 HepG2 cells at a concentration of
25 μM. Moreover, these compounds inhibited the expression of
CYP2D6 and CYP3A4 at a higher concentration (50 μM).
The results showed that LA effectively inhibited the expression of
CYP2D6, CYP2E1, and CYP3A4, but did not affect
CYP1A2 expression.
The results demonstrating the effects of these
compounds on CYP2D6 activity were similar to the qPCR data showing
the effects of the compounds on CYP2D6 gene expression.
Moreover, the cocktail assay allowed for determination of the
activity of CYP2C8 and CYP2C19, which are expressed at very low
levels in HepG2 cells. Of note, the data for the two latter enzymes
were highly distinctive from the results of the gene expression
analysis as well as the gene expression and cocktail results in the
present study, which may be due to the system used in these
experiments and the concentrations of compounds used to treat the
cells. Moreover, certain compounds may have induced or inhibited
the expression of genes and affected the activity of proteins
through diverse pathways.
Through the overview of the bioinformatics network
of the influence of G. uralensis-derived compounds on
metabolic enzymes, increasing evidence has shown that the compounds
GA, IL, GL, and L, derived from G. uralensis, had effects on
metabolic enzymes, particularly CYP450 family enzymes, and CYP1A,
-2D, -2C, -2E, -3A and -2A were most susceptible to regulation by
the bioactive components of G. uralensis. This showed the
major targets among the CYP450 enzymes in regard to the modulation
of drug functions and properties by G. uralensis.
Due to the complexity of the components of G.
uralensis and pathophysiology, it is very difficult to fully
elucidate the mechanisms of the modulation of the activity of drugs
exclusively from the aspect of metabolic enzymes. Thus, the present
study was incomplete, and a metabolomics study could be conducted
to explain the mechanism from the aspect of endogenous
metabolites.
In conclusion, the present study has demonstrated
that the bioactive constituents of G. uralensis
differentially modulated the expression of CYP enzymes. Moreover, a
combination of gene expression studies using qPCR and activity
assays for CYP450 in HLMs using cocktail probes and UPLC-MS/MS
analysis allowed for correlation between Chinese Herbal Medicine
and CYP450 enzymes. The findings of the present study are useful
and may aid physicians to avoid risks and side effects caused by
interactions between G. uralensis extracts and conventional
drugs metabolized by these enzymes. The present study also sheds
light on the mechanism by which G. uralensis modulates the
properties of other drugs in the context of metabolism by CYP450
expression.
Acknowledgements
The authors thank Professor Gengfu Chen, School of
Basic Courses, Guangdong Pharmaceutical University, for his
guidance in the experiments. The present study was supported by the
National Natural Science Foundation of China (no. 30801543).
References
|
1
|
Nelson DR, Koymans L, Kamataki T, et al:
P450 superfamily: update on new sequences, gene mapping, accession
numbers and nomenclature. Pharmacogenetics. 6:1–42. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodgson E and Rose RL: The importance of
cytochrome P450 2B6 in the human metabolism of environmental
chemicals. Pharmacol Ther. 113:420–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodgson E and Rose RL: Metabolic
interactions of agrochemicals in humans. Pest Manag Sci.
64:617–621. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimazu S, Inui H and Ohkawa H:
Phytomonitoring and phytoremediation of agrochemical and related
compounds based on recombinant cytochrome P450s and aryl
hydrocarbon receptors (AhRs). J Agric Food Chem. 59:2870–2875.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naiman K, Frei E and Stiborova M:
Identification of rat cytochromes P450 metabolizing
N-(2-methoxyphenyl)hydroxylamine, a human metabolite of the
environmental pollutants and carcinogens o-anisidine and
o-nitroanisole. Neuro Endocrinol Lett. 31(Suppl 2): 36–45.
2010.
|
|
6
|
Uppstad H, Øvrebø S, Haugen A and Mollerup
S: Importance of CYP1A1 and CYP2B1 in bioactivation of
benzo[a]pyrene in human lung cell lines. Toxicol Lett. 192:221–228.
2010.
|
|
7
|
Sanada N, Gotoh Y, Shimazawa R, Klinge CM
and Kizu R: Repression of activated aryl hydrocarbon
receptor-induced transcriptional activation by
5α-dihydrotestosterone in human prostate cancer LNCaP and human
breast cancer T47D cells. J Pharmacol Sci. 109:380–387.
2009.PubMed/NCBI
|
|
8
|
Park BK: Cytochrome P450 enzymes in the
heart. Lancet. 355:945–946. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui X, Thomas A, Han Y, et al:
Quantitative PCR assay for cytochromes P450 2B and 3A induction in
rat precision-cut liver slices: correlation study with induction in
vivo. J Pharmacol Toxicol Methods. 52:234–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martignoni M, de Kanter R, Grossi P,
Saturno G, Barbaria E and Monshouwer M: An in vivo and in vitro
comparison of CYP gene induction in mice using liver slices and
quantitative RT-PCR. Toxicol In Vitro. 20:125–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng Y, Bi HC, Zhao LZ, et al: Induction
of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides
in human and rat primary hepatocytes. Drug Metab Lett. 2:60–66.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lobo ED, Bergstrom RF, Reddy S, et al: In
vitro and in vivo evaluations of cytochrome P450 1A2 interaction
with duloxetine. Clin Pharmacokinet. 47:191–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sparfel L, Payen L, Gilot D, et al:
Pregnane X receptor-dependent and -independent effects of
2-acetylaminofluorene on cytochrome P450 3A23 expression and liver
cell proliferation. Biochem Biophys Res Commun. 300:278–284. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Volotinen M, Mäenpää J, Kankuri E, et al:
Expression of cytochrome P450 (CYP) enzymes in human nonpigmented
ciliary epithelial cells: induction of CYP1B1 expression by TCDD.
Invest Ophthalmol Vis Sci. 50:3099–3105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Westerink WM and Schoonen WG: Cytochrome
P450 enzyme levels in HepG2 cells and cryopreserved primary human
hepatocytes and their induction in HepG2 cells. Toxicol In Vitro.
21:1581–1591. 2007. View Article : Google Scholar
|
|
16
|
Saxena A, Tripathi KP, Roy S, Khan F and
Sharma A: Pharmacovigilance: effects of herbal components on human
drugs interactions involving cytochrome P450. Bioinformation.
3:198–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kennedy DA and Seely D: Clinically based
evidence of drug-herb interactions: a systematic review. Expert
Opin Drug Saf. 9:79–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang XX, Hu ZP, Duan W, Zhu YZ and Zhou
SF: Drug-herb interactions: eliminating toxicity with hard drug
design. Curr Pharm Des. 12:4649–4664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borrelli F and Izzo AA: Herb-drug
interactions with St John’s Wort (Hypericum perforatum): an update
on clinical observations. AAPS J. 11:710–727. 2009.
|
|
20
|
Zhang Q and Ye M: Chemical analysis of the
Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A.
1216:1954–1969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asl MN and Hosseinzadeh H: Review of
pharmacological effects of Glycyrrhiza sp and its bioactive
compounds. Phytother Res. 22:709–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang J, Song X, Zhu M and Zhang J: Study
on the pharmacokinetics drug-drug interaction potential of
Glycyrrhiza uralensis, a traditional Chinese medicine, with
lidocaine in rats. Phytother Res. 23:603–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma T, Huang C, Zong G, Zha D, Meng X, Li J
and Tang W: Hepatoprotective effects of geniposide in a rat model
of nonalcoholic steatohepatitis. J Pharm Pharmacol. 63:587–593.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao K, Ding M, Cao H and Cao ZX: In-vitro
metabolism of glycyrrhetinic acid by human and rat liver microsomes
and its interactions with six CYP substrates. J Pharm Pharmacol.
64:1445–1451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu K, Chen F and Li C: Absorption,
disposition, and pharmacokinetics of saponins from Chinese
medicinal herbs: what do we know and what do we need to know more?
Curr Drug Metab. 13:577–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Xiao J, Peng ZH and Chen Y: In
vitro metabolism of glycyrrhetic acid by human cytochrome P450. Yao
Xue Xue Bao. 46:81–87. 2011.PubMed/NCBI
|
|
28
|
Smoot M, Ono K, Ruscheinski J, Wang PL and
Ideker T: Cytoscape 2.8: new features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fuhr U: Induction of drug metabolizing
enzymes: pharmacokinetic and toxicological consequence in humans.
Clin Pharmacokinet. 38:493–504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quan J, Yin X and Xu H: Boschniakia
rossica prevents the carbon tetrachloride-induced hepatoxicity in
rat. Exp Toxicol Pathol. 63:53–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Waluga M and Hartleb M: Alcoholic liver
disease. Wiad Lek. 56:61–70. 2003.
|
|
32
|
Jeong HG, You HJ, Park SJ, Moon AR, Chung
YC, Kang SK and Chun HK: Hepatoprotective effects of 18
beta-glycyrrhetinic acid on carbon tetrachloride-induced liver
injury: inhibition of cytochrome P450 2E1 expression. Pharmacol
Res. 46:221–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alden PG, Plumb RS, Jones MD, Rainville PD
and Shave D: A rapid ultra-performance liquid chromatography/tandem
mass spectrometric methodology for the in vitro analysis of Pooled
and Cocktail cytochrome P450 assays. Rapid Commun Mass Spectrom.
24:147–154. 2010. View Article : Google Scholar
|
|
34
|
Paolini M, Barillari J, Broccoli M,
Pozzetti L, Perocco P and Cantelli-Forti G: Effect of liquorice and
glycyrrhizin on rat liver carcinogen metabolizing enzymes. Cancer
Lett. 145:35–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mu Y, Zhang J, Zhang S, et al: Traditional
Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan
Cao (Glycyrrhiza uralensis Fisch) activate pregnane x receptor and
increase warfarin clearance in rats. J Pharmacol Exp Ther.
316:1369–1377. 2006. View Article : Google Scholar
|
|
36
|
Li HY, Xu W, Su J, Zhang X, Hu LW and
Zhang WD: In vitro and in vivo inhibitory effects of glycyrrhetinic
acid on cytochrome P450 3A activity. Pharmacology. 86:287–292.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu L, Xiao J, Peng ZH and Chen Y: In
vitro metabolism of glycyrrhetic acid by human cytochrome P450. Yao
Xue Xue Bao. 46:81–87. 2011.PubMed/NCBI
|
|
38
|
Tsukamoto S, Aburatani M, Yoshida T,
Yamashita Y, El-Beih AA and Ohta T: CYP3A4 inhibitors isolated from
Licorice. Biol Pharm Bull. 28:2000–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|