Introduction
Type 2 diabetes mellitus (T2DM) is a complex and
heterogeneous disorder affecting >220 million individuals
worldwide; this is projected to reach 366 million by 2030 (1). Despite the number of individuals
affected by the disease, the therapeutic strategies that are
currently available for T2DM are limited. These strategies involve
insulin and four main classes of oral antidiabetic agents that act
to i) stimulate the secretion of insulin by the pancreas, such as
sulfonylureas, rapid-acting secretagogues/insulinotropic agents,
glipizide, glibenclamide and rapaglinide; ii) reduce the production
of glucose by the liver, including biguanides (metformin); iii)
delay the digestion and absorption of carbohydrate in the
intestine, including α-glucosidase inhibitors and acarbose; or iv)
enhance the action of insulin, including thiazolidinediones,
pioglitazone and rosiglitazone. However, none of these agents is
completely effective (2).
Numerous pharmaceutical studies have focused on the
development of novel drugs to reduce the symptoms associated with
the long-term complications of diabetes (3–5).
Glucagon-like peptide-1 (GLP-1) is an incretin hormone of the
enteroinsular axis. In healthy individuals, GLP-1 is secreted
subsequent to eating, and lowers glucose concentrations by
increasing insulin secretion and suppressing glucagon release.
Furthermore, GLP-1 impairs gastric emptying, suppresses appetite
and has been suggested to inhibit β-cell apoptosis (6). However, native GLP-1 is degraded
within 2–3 min in the circulation (6). Therefore, a number of GLP-1 receptor
agonists have been developed to prolong the in vivo effect
of GLP-1. Exenatide, a GLP-1 receptor agonist that exhibits
sustained activity, has potential for the treatment of T2DM due to
its ability to enhance insulin secretion and increase β-cell mass
(7–9). Although the preparation and
antidiabetic activity of exenatide have been widely reported
(10,11), the molecular mechanism by which
exenatide improves β-cell mass is yet to be elucidated.
Experimental evidence from animals and healthy
subjects indicates that GLP-1 may be involved in the control of
appetite and the intake of energy in patients with T2DM (12,13).
Furthermore, GLP-1 has been suggested as a potential treatment for
T2DM due to its effects on glycemic control, insulin sensitivity
and β-cell function (14). β cells
function to promote insulin production and maintain glucose
homeostasis (15), which is
critical for the prevention and treatment of T2DM. Adiponectin is a
fat-derived hormone that is known to exhibit antidiabetic and
anti-atherogenic effects. Adiponectin acts to stimulate the uptake
of glucose into skeletal muscle cells via the activation of insulin
receptor substrate-1-mediated phosphatidylinositol-3 kinase
function, suppress the production of glucose by the liver and
enhance β-oxidation in the muscle via the activation of 5′
adenosine monophosphate-kinase (16). C-reactive protein (CRP) has been
shown to be an independent predictor of risk for the development of
diabetes (17). However, it has
been reported that, in patients with T2DM, exenatide treatment has
durable and persistent beneficial effects on CRP (18). In addition, exenatide increases
adiponectin levels in patients with T2DM (19). Thus, we hypothesized that exenatide
could enhance β-cell proliferation through an adiponectin-induced
reduction in β-cell apoptosis (20) and a reduction in the levels of CRP,
a sign of inflammation and potential risk in patients with T2DM
(17).
Materials and methods
Plasmid constructs, antibodies and
reagents
Exenatide is a single, non-glycosylated peptide
containing 39 amino acids (HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAP
PPS) and has a molecular mass of 4,186.6 Da. Full-length genes of
exenatide and adiponectin were constructed by overlap extension
polymerase chain reaction (PCR), followed by the subcloning of the
constructs into various vectors. Histidine (His)-tag exenatide was
expressed in BL21 (DE3) E. coli and purified using
Ni-nitrilotriacetic acid resin. Anti-Myc antibody (1 mg/ml;
Clontech Laboratories Inc., Mountain View, CA, USA) was used for
the assay of the results of the co-immunoprecipitation (Co-IP)
test. Anti-Flag monoclonal antibody (1 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) and anti-green fluorescent protein (GFP) antibody
(2 mg/ml; Cell Signaling Technology, Inc., Danvers, MA, USA) were
used for fluorescence staining. Anti-exenatide and -adiponectin
polyclonal antibodies (1 mg/ml; Abcam, Cambridge, UK) were used for
western blot analysis. Anti-glutathione S-transferase (GST; 0.5
mg/ml) and -His (0.1 mg/ml) antibodies were used for the assay of
the results of the GST-pull-down analysis, and were purchased from
Tiangen Biotech Co. Ltd. (Beijing, China). GAPDH (1 mg/ml) and
secondary antibodies (1 mg/ml) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Various vectors were
amplified in E. coli, isolated using a QIAprep®
Miniprep kit (Qiagen, Inc., Chatsworth, CA, USA) and verified by
automated DNA sequencing. Anti-CRP antibody (1 mg/ml; Shengshi
Zhongfang BioSci & Tech) was used for western blot analysis.
β-actin antibody was purchased from Abcam. The present study was
approved by the ethics committee of the People’s Hospital of Hainan
Province (Haikou, China).
Rat INS-1 cell culture and
incubation
INS-1 cells were purchased from the Shanghai Cell
Bank (Shanghai, China). INS-1 cells were grown in monolayer
cultures in RPMI-1640 medium at 37°C in a humidified atmosphere,
with 5% CO2 and 95% air. INS-1 cells were harvested and
divided into three groups. The cells in the three groups were
exposed to normal concentrations of glucose (5 mM), high
concentrations of glucose (30 mM) or high concentrations of glucose
(30 mM) plus exenatide (100 nM), for 24 h each. The levels of
adiponectin and CRP and INS-1 cell proliferation were determined
after three days of culturing. INS-1 cell proliferation was
determined by direct cell counting. For direct cell counting,
5×104 cells were seeded and harvested after three days
of culture, then counted using a hemacytometer (Hausser Scientific,
Horsham, PA, USA).
Quantitative (q)PCR
Total RNA was isolated from cells using QIAshredder
and RNeasy® Mini kits (Qiagen, Inc.). cDNA was
synthesized using 500 ng RNA extracts in a volume of 20 μl using
Avian Myeloblastosis Virus reverse transcriptase XL (Takara Bio
Inc., Dalian, China) priming with random 9-mers at 42°C for 10 min.
The cDNA was stored at 20°C until use. qPCR was performed using
SYBR®-Green I Master Mix in the Light-Cycler®
480 System (Roche, Mannheim, Germany). RNA was isolated from
non-transfected and transfected INS-1 cells, followed by cDNA
synthesis and data analysis as described previously (9). The primers for qPCR were as follows:
Adiponectin, 5′-GTCCTAAGGGAGACATCG GTG-3′ (forward) and
5′-CCATACACCTGGAGCCAGAC-3′ (reverse); CRP,
5′-CTGTCCTCGACCCGTGGGTAC-3′ (forward) and 5′-CTGGTGACAGCACAAAGTC-3′
(reverse) and GAPDH, 5′-CCCTTCATTGACCTCAACTAC-3′ (forward) and
5′-CCACCTTCTTGATGTCATCAT-3′ (reverse). GAPDH was used as an
internal control. The AmpliTaq Gold® enzyme was
activated by heating for 10 min at 95°C, and all genes were
amplified by 50 cycles of heating for 15 sec at 95°C, followed by 1
min at 60°C.
For the construction of standard curves of positive
controls, the total RNA of primary neuronal cells was
reverse-transcribed into cDNA and serially diluted in water in five
or six log steps to generate four-fold serial dilutions of cDNA
between 100 pg and 100 ng. These cDNA serial dilutions were stored
at 20°C. The coefficient of linear regression was calculated for
each standard curve, and using the cycle threshold value for each
sample, the relative concentration of adiponectin, CRP and GAPDH
were calculated. To normalize for differences in the quantity of
total RNA in each starting reaction, GAPDH expression was used as
an endogenous control. The data represent the average expression of
target genes, relative to GAPDH, from three independent
cultures.
Transfection of INS-1 cells and western
blot analysis
The INS-1 cells (2×105/p-35 plate) were
transfected with various vectors. Transfection was performed in
50–60% confluent cells in plates using 9 μl Lipofectamine 2000™
(Applied Biosystems, Foster City, CA, USA). Forty-eight hours
subsequent to transfection, the cells were split and clones were
selected for antibiotic resistance. Resistant colonies were either
pooled or cloned by ring isolation.
All transfected and non-transfected cells were
homogenized in radioimmunoprecipitation assay buffer [150 mM NaCl,
1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.1% SDS and 50
mM Tris-HCl (pH 8.0)] with Complete Mini Protease Inhibitor
(Roche). Following debris removal, the supernatants were boiled and
mixed with an equal volume of 20% glycerol containing 0.02%
bromophenol blue. Proteins were separated by SDS-PAGE and
transferred to a polyvinylidene difluoride membrane (Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skimmed
milk in 10 mM Tris (pH 7.5), 100 mM NaCl and 0.1% Tween-20 (TBST)
and incubated with primary antibodies in TBST with 0.5% skimmed
milk overnight at 4°C. The membrane was treated with primary
antibodies and horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G secondary antibodies, diluted 1:3,000 (Amersham
Biosciences, Amersham, UK). Immunoreactive bands were visualized by
enhanced chemiluminescence (GE Healthcare, Amersham, UK) and
quantified by densitometry using ImageJ 1.45 software (National
Institutes of Health, Bethesda, MD, USA) according to the
manufacturer’s instructions.
Fluorescence microscopy
At 24 h post-transfection, the cells were fixed with
2% paraformaldehyde for 10 min, prior to washing with
phosphate-buffered saline (PBS). The cells were subsequently
permeabilized using 1% Triton X-100 for 10 min, washed with PBS and
incubated with monoclonal antibodies for 1 h, followed by
incubation with secondary antibodies for 1 h. The cell nuclei were
stained with 0.1 g/ml DAPI and the cells were observed using a
fluorescence microscope.
Immunoprecipitation
The cells were harvested and lysed in 20 mM HEPES
(pH 7.2), 50 mM NaCl, 0.5% Triton X-100, 1 mM NaF and 1 mM
dithiothreitol (HEPES lysis buffer). The lysate was incubated with
the indicated antibodies for 3 h at 4°C, prior to the addition of
protein A/G-plus agarose. Immunoprecipitates were washed three
times in lysis buffer, and analyzed using western blotting.
GST pull-down assay
Bacteria-expressed GST or GST-adiponectin proteins
were immobilized on glutathione-Sepharose 4B beads (Amersham) and
washed. The beads were then incubated with exenatide and washed
with GST binding buffer (100 mM NaCl, 50 mM NaF, 2 mM EDTA and 1%
NP-40). The proteins were subsequently eluted, prior to use in
western blot analysis.
RNA interference (RNAi)
Small interfering (si)RNA directed against
adiponectin (5′-GTTGCTGGGAGCTGTTCTACT-3′), CRP (5′-GAG
TCGGATACTTCCTATGTA-3′) and non-target control siRNA
(5′-UUCUCCGAACGUGUCACGU-3′) were synthesized by Shanghai GenePharm
Co., Ltd. (Shanghai, China).
Statistical analysis
All results are presented as the mean ± standard
deviation. Student’s unpaired t-tests were performed for the
comparison of individual data and two-way analysis of variance with
Fisher’s protected least significant difference post hoc tests was
performed for repeated measures over time. χ2 tests were
performed to compare differences in distribution. A value of
P<0.05 was considered to indicate statistical significance.
Results
Relative adiponectin and CRP mRNA levels
in INS-1 cells
qPCR analysis revealed that the mRNA levels of
adiponectin were significantly reduced and those of CRP
significantly increased in the INS-1 cells in the 30 mM group,
compared with those in the 5 mM group. However, upon the addition
of exenatide, mRNA levels of adiponectin were found to increase and
those of CRP decrease in the cells in the 30 mM group (Fig. 1). These results suggest that
exenatide can increase the mRNA levels of adiponectin and reduce
those of CRP in INS-1 cells.
Adiponectin and CRP protein levels in
INS-1 cells
In accordance with the qPCR results, western blot
analysis revealed that the protein levels of adiponectin were
reduced and those of CRP were increased in INS-1 cells in the 30 mM
group compared with those in the 5 mM group. However, upon the
addition of exenatide, the protein levels of adiponectin were
increased by 20% (P<0.05) and those of CRP were reduced by 50%
(P<0.01) in the cells in the 30 mM group (Fig. 2). These findings suggest that
exenatide can increase the protein levels of adiponectin and reduce
those of CRP in INS-1 cells.
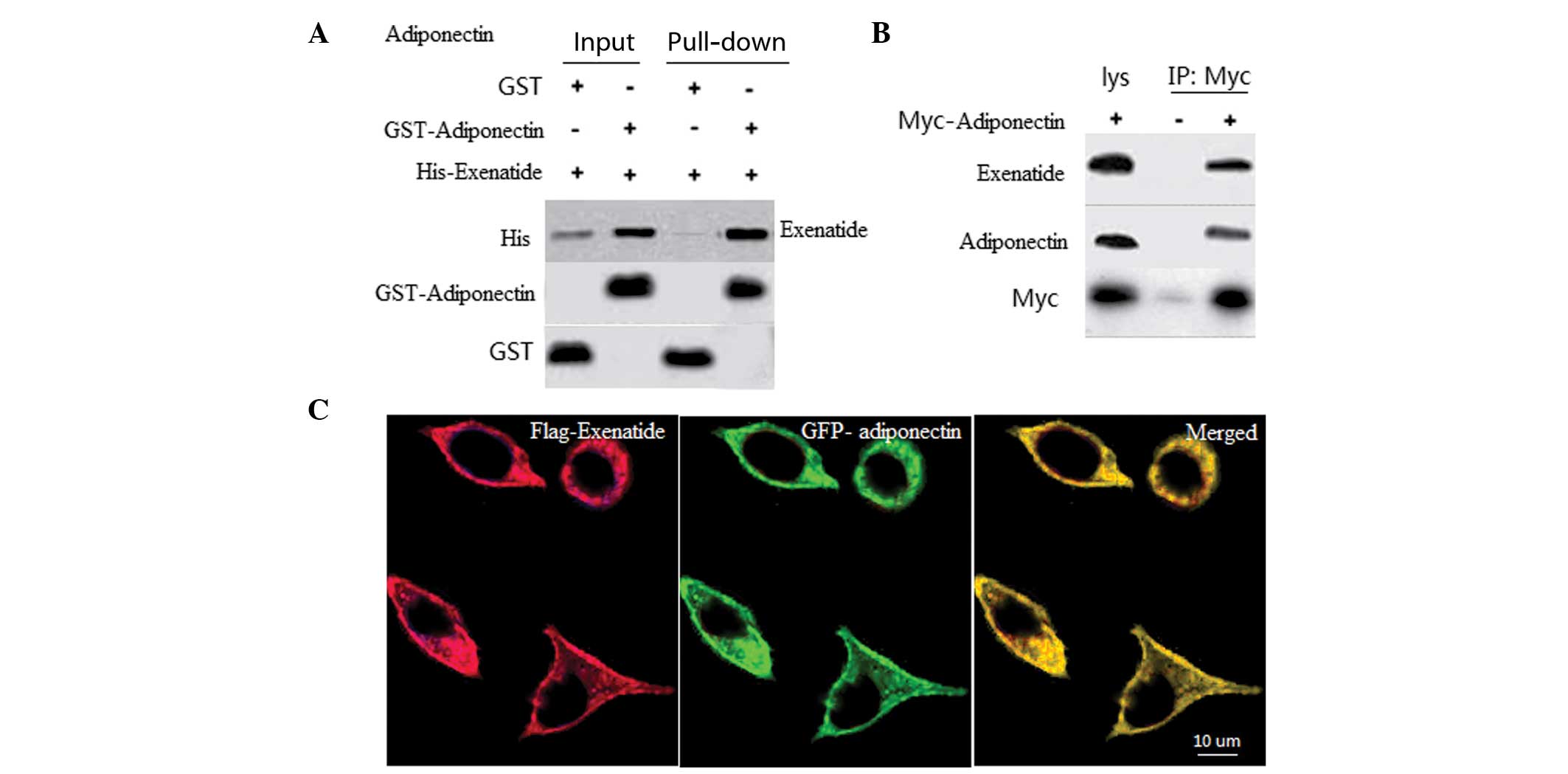
Exenatide interacts with adiponectin
To determine the interaction between exenatide and
adiponectin, in vitro GST pull-down assays using recombinant
GST-adiponectin and His-exenatide were performed. A specific
interaction was observed between exenatide and adiponectin, but not
with GST alone (Fig. 3A). To
assess whether exenatide interacts with adiponectin, a Co-IP assay
was performed in INS-1 cells. The Co-IP results further revealed an
association between exenatide and adiponectin (Fig. 3B), which suggests that these two
proteins may co-localize. To assess the subcellular localization of
exenatide and adiponectin, INS-1 cells were transfected with
GFP-adiponectin and Flag-exenatide. When coexpressed, exenatide and
adiponectin were observed to be co-localized in the membrane of the
INS-1 cells (Fig. 3C).
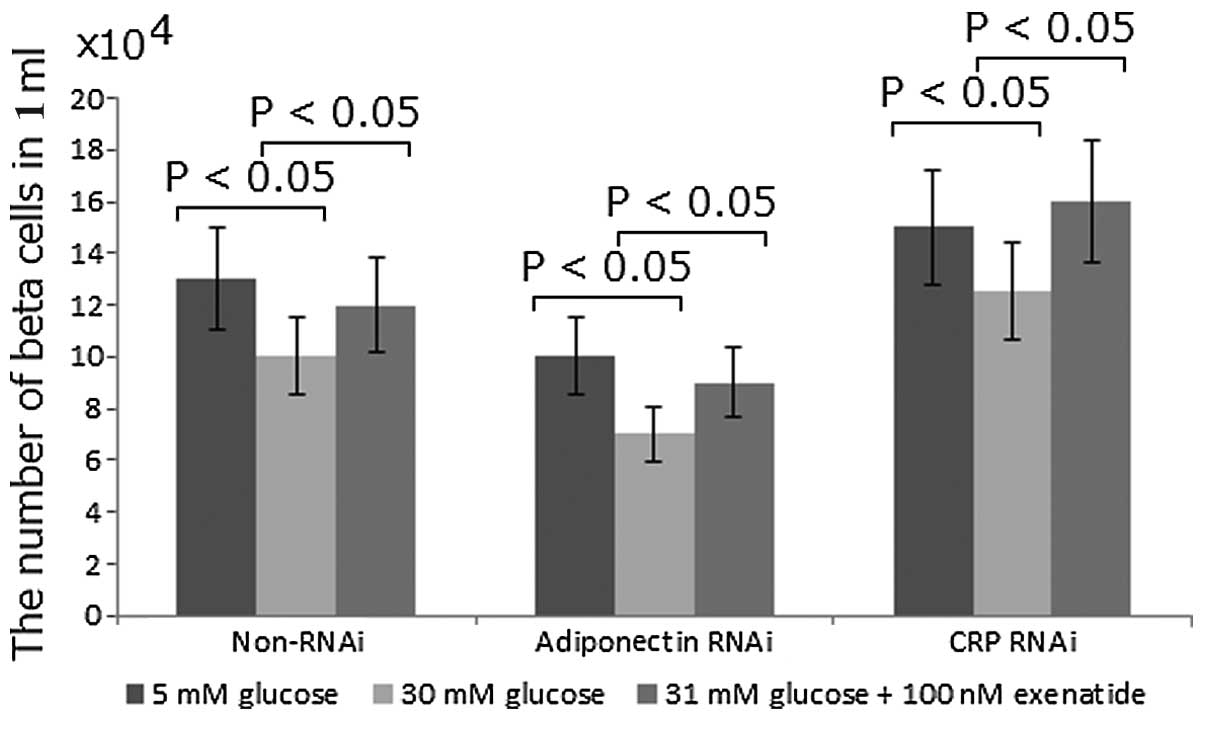
Exenatide enhances the INS-1 rat
pancreatic β-cell mass by increasing the protein levels of
adiponectin and reducing the levels of CRP
It has previously been reported that exenatide
treatment may activate adiponectin protein expression, which may
have a protective effect against β-cell apoptosis (20). In the present study, to determine
whether adiponectin could increase β-cell mass, the function of
adiponectin was inhibited using RNAi. Compared with the non-RNAi
group, the number of β cells was reduced by 20% with RNAi treatment
(Fig. 4). By contrast, compared
with the non-RNAi group, the inhibition of CRP using RNAi was
observed to increase the number of β cells by 15% (Fig. 4). In all the cases, high
concentrations of glucose were found to reduce the number of β
cells while exenatide was capable of correcting the change by
increasing the number of β cells (Fig.
4). Exenatide treatment was observed to increase the number of
β cells by 20%. These results suggest that exenatide may improve
the INS-1 rat pancreatic β-cell mass by increasing the protein
levels of adiponectin and reducing the level of CRP.
Discussion
In the present study, compared with the control
group, the levels of CRP were found to be significantly increased
in the high-dose glucose group while those of adiponectin were
reduced. Reduced levels of adiponectin and high levels of CRP are
frequently followed by a decrease in the number of β cells.
Adiponectin acts against cytokine- and fatty acid-induced β-cell
apoptosis and completely restores the function of insulin-producing
cells that have been disrupted by autoimmunity and lipotoxicity
(21). In addition, CRP is closely
associated with obesity in T2DM (22), while free fatty acids in obesity
may cause β-cell dysfunction and depletion (23).
It has previously been reported that high glucose
levels can induce apoptosis in cultured human pancreatic islets
(24). High concentrations of
glucose may elevate the level of oxidative stress in INS-1 cells,
leading to an increase in apoptosis and a decrease in
proliferation. It has been suggested that exenatide may have a
protective effect on INS-1 cells exposed to high-glucose
environments. In the present study, exenatide was observed to be
capable of reversing the increase in CRP and the decrease in
adiponectin levels in the high-dose glucose group; therefore,
exenatide may enhance INS-1 rat pancreatic β-cell mass by
increasing the protein levels of adiponectin and reducing the
levels of CRP.
Exenatide monotherapy has been of clinical interest
in patients with T2DM due to reported improvements in glycemic
control and weight with the use of exenatide in combination with
oral antidiabetic agents (25,26).
However, its limitations include dose-dependent glucoregulatory
activity and dose-limiting nausea and vomiting. A gradual
escalation in the dose of exenatide can reduce the incidence of
dose-limiting nausea and vomiting, without a concurrent loss of
glucoregulatory activity; therefore, gradual dose-escalation may be
beneficial for attenuating the gastrointestinal side-effects of
exenatide (27). However,
exenatide therapy is also limited by the relatively short half-life
of the drug (28). It has been
reported that modified exenatide, a synthetic form of exendin-4,
which is a glucagon-like peptide hormone that regulates insulin
secretion, has a native half-life of 2.4 h and shows a projected
half-life of 139 h (29).
Therefore, expressing novel modified exenatide in various
eukaryotic systems will be the focus of our future
investigations.
In conclusion, the present study demonstrated that
Exenatide can increase adiponectin protein levels and reduce the
level of CRP in INS-1 cells, resulting in an increase in INS-1 rat
pancreatic β-cell mass. These findings suggest that Exenatide may
ameliorate T2DM by increasing adiponectin protein levels and
reducing the level of CRP.
References
|
1
|
Anvari M: Use of metabolic surgery for the
treatment of type 2 diabetes. Can J Diabetes. 35:99–108. 2011.
View Article : Google Scholar
|
|
2
|
Srinivasan K and Ramarao P: Animal models
in type 2 diabetes research: an overview. Indian J Med Res.
125:451–472. 2007.PubMed/NCBI
|
|
3
|
Bennett WL, Maruthur NM, Singh S, et al:
Comparative effectiveness and safety of medications for type 2
diabetes: an update including new drugs and 2-drug combinations.
Ann Intern Med. 154:602–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pathania S, Randhawa V and Bagler G:
Prospecting for novel plant-derived molecules of Rauvolfia
serpentina as inhibitors of Aldose Reductase, a potent drug
target for diabetes and its complications. PloS one.
8:e613272013.PubMed/NCBI
|
|
5
|
Sugimoto T and Kashiwagi A: The
cutting-edge of medicine; novel therapeutic agents for the
treatment of diabetes sodium-glucose co-transporter (SGLT) 2
inhibitors. Nihon Naika Gakkai Zasshi. 102:1474–1483. 2013.(In
Japanese).
|
|
6
|
Meier JJ: GLP-1 receptor agonists for
individualized treatment of type 2 diabetes mellitus. Nat Rev
Endocrinol. 8:728–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gedulin BR, Nikoulina SE, Smith PA, et al:
Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell
mass in insulin-resistant obese fa/fa Zucker rats independent of
glycemia and body weight. Endocrinology. 146:2069–2076. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bunck MC, Diamant M, Cornér A, et al:
One-year treatment with exenatide improves beta-cell function,
compared with insulin glargine, in metformin-treated type 2
diabetic patients: a randomized, controlled trial. Diabetes Care.
32:762–768. 2009.
|
|
9
|
Fehse F, Trautmann M, Holst JJ, et al:
Exenatide augments first- and second-phase insulin secretion in
response to intravenous glucose in subjects with type 2 diabetes. J
Clin Endocrinol Metab. 90:5991–5997. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tripathy NR, Basha S, Jain R, Shetty S and
Ramachandran A: Exenatide and acute pancreatitis. J Assoc
Physicians India. 56:987–988. 2008.PubMed/NCBI
|
|
11
|
Kendall DM, Riddle MC, Rosenstock J, et
al: Effects of exenatide (exendin-4) on glycemic control over 30
weeks in patients with type 2 diabetes treated with metformin and a
sulfonylurea. Diabetes Care. 28:1083–1091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gutzwiller JP, Drewe J, Göke B, et al:
Glucagon-like peptide-1 promotes satiety and reduces food intake in
patients with diabetes mellitus type 2. Am J Physiol.
276:R1541–R1544. 1999.PubMed/NCBI
|
|
13
|
Zander M, Madsbad S, Madsen JL and Holst
JJ: Effect of 6-week course of glucagon-like peptide 1 on glycaemic
control, insulin sensitivity, and beta-cell function in type 2
diabetes: a parallel-group study. Lancet. 359:824–830. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Navarro M, Rodriquez de Fonseca F, Alvarez
E, et al: Colocalization of glucagon-like peptide-1 (GLP-1)
receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat
hypothalamic cells: evidence for a role of GLP-1 receptor agonists
as an inhibitory signal for food and water intake. J Neurochem.
67:1982–1991. 1996. View Article : Google Scholar
|
|
15
|
Nir T, Melton DA and Dor Y: Recovery from
diabetes in mice by beta cell regeneration. J Clin Invest.
117:2553–2561. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwaki M, Matsuda M, Maeda N, et al:
Induction of adiponectin, a fat-derived antidiabetic and
antiatherogenic factor, by nuclear receptors. Diabetes.
52:1655–1663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freeman DJ, Norrie J, Caslake MJ, et al:
C-reactive protein is an independent predictor of risk for the
development of diabetes in the West of Scotland Coronary Prevention
Study. Diabetes. 51:1596–1600. 2002. View Article : Google Scholar
|
|
18
|
Bergenstal RM, Wysham C, Macconell L, et
al: DURATION-2 Study Group: Efficacy and safety of exenatide once
weekly versus sitagliptin or pioglitazone as an adjunct to
metformin for treatment of type 2 diabetes (DURATION-2): a
randomised trial. Lancet. 376:431–439. 2010. View Article : Google Scholar
|
|
19
|
Derosa G, Putignano P, Bossi AC, et al:
Exenatide or glimepiride added to metformin on metabolic control
and on insulin resistance in type 2 diabetic patients. Eur J
Pharmacol. 666:251–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wijesekara N, Krishnamurthy M,
Bhattacharjee A, Suhail A, Sweeney G and Wheeler MB:
Adiponectin-induced ERK and Akt phosphorylation protects against
pancreatic beta cell apoptosis and increases insulin gene
expression and secretion. J Biol Chem. 285:33623–33631. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rakatzi I, Mueller H, Ritzeler O,
Tennagels N and Eckel J: Adiponectin counteracts cytokine-and fatty
acid-induced apoptosis in the pancreatic beta-cell line INS-1.
Diabetologia. 47:249–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kahn SE, Zinman B, Haffner SM, et al:
ADOPT Study Group: Obesity is a major determinant of the
association of C-reactive protein levels and the metabolic syndrome
in type 2 diabetes. Diabetes. 55:2357–2364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boden G and Shulman GI: Free fatty acids
in obesity and type 2 diabetes: defining their role in the
development of insulin resistance and beta-cell dysfunction. Eur J
Clin Invest. 32(Suppl 3): 14–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Federici M, Hribal M, Perego L, et al:
High glucose causes apoptosis in cultured human pancreatic islets
of Langerhans: a potential role for regulation of specific Bcl
family genes toward an apoptotic cell death program. Diabetes.
50:1290–1301. 2001. View Article : Google Scholar
|
|
25
|
Moretto TJ, Milton DR, Ridge TD, et al:
Efficacy and tolerability of exenatide monotherapy over 24 weeks in
antidiabetic drug-naive patients with type 2 diabetes: a
randomized, double-blind, placebo-controlled, parallel-group study.
Clin Ther. 30:1448–1460. 2008. View Article : Google Scholar
|
|
26
|
Gentilella R, Bianchi C, Rossi A and
Rotella CM: Exenatide: a review from pharmacology to clinical
practice. Diabetes Obes Metab. 11:544–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fineman MS, Shen LZ, Taylor K, Kim DD and
Baron AD: Effectiveness of progressive dose-escalation of exenatide
(exendin-4) in reducing dose-limiting side effects in subjects with
type 2 diabetes. Diabetes Metab Res Rev. 20:411–417. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brubaker PL: Incretin-based therapies:
mimetics versus protease inhibitors. Trends Endocrinol Metab.
18:240–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Constantinou A and Deonarain M:
Modulating antibody pharmacokinetics using hydrophilic polymers.
Expert Opin Drug Deliv. 8:1221–1236. 2011. View Article : Google Scholar : PubMed/NCBI
|