Introduction
Heat shock factor 1 (HSF1) is a member of heat shock
transcription factors that are able to differentially regulate the
expression of heat shock proteins in response to a variety of
stresses (1,2). These heat shock proteins function as
molecular chaperones, regulating cellular homeostasis via
modulating protein folding, assortment, stability and
protein-protein interactions. This adaptive process is known as the
heat shock response (1). HSF1 is
the predominant heat shock response transcription factor. In
addition to mediating the heat shock response, it is associated
with numerous other cellular processes, including tissue
development (e.g. brain, testis and placenta) (3), inflammation and tumorigenesis
(4). In humans, HSF1 and its
associated heat shock proteins are upregulated in the majority of
tumor tissue types (e.g. lymphoma, lung, breast and prostate
cancer) (5–7), and are involved in regulating tumor
cell hyperproliferation, metabolism, metastasis and chemotherapy
resistance (8–11). Certain heat shock proteins (e.g.
HSP90 and HSP70) have been targeted for cancer therapy and the
creation of tumor prognostic biomarkers (12–14).
In animal models, HSF1 is important for p53 mutation-induced
lymphoma (15), diethylnitrosamine
(DEN)-induced hepatocellular carcinoma (16), dimethylbenz(a)anthracene
(DMBA)-tetradecanoylphorbol acetate (TPA)-induced skin cancer
(4) and human epidermal growth
factor receptor 2 (Her2)-induced breast cancer (5,9). In
these tumor models, HSF1 is involved in regulating tumor
initiation, development and metastasis, and is considered a novel
non-oncogenic oncogene. These data suggested that HSF1 may act as a
novel candidate for the development of new cancer prognostic
biomarkers.
The biomarkers that represent HSF1 transcription
activation in tumor tissues are currently being investigated.
Protein post-translational modifications, e.g. phosphorylation,
have important roles during HSF1 activation. Under physiological
conditions, HSF1 forms monomers or heterodimers with an HSP90-HSP70
chaperone complex without transcriptional activity (17,18)
and is constitutively phosphorylated at serine (S)303 and S307
(19,20). Upon heat shock, HSF1 dissociates
from the HSF1-HSP90-HSP70 chaperone complex and becomes activated
upon hyperphosphorylation at S230 and S326 (21–23).
Mutations of HSF1/S326 disrupt its transcriptional activity under
heat shock conditions, referring the phosphorylation of S326 as a
biomarker of HSF1 activation (23). Recently, Mendillo et al
(7) reported that
hyperphosphorylation of HSF1/S326, which is upregulated in breast
cancer compared with the normal counterparts, was used as a
biomarker to indicate HSF1 activation in breast cancer. The
constitutive activation of HSF1 in breast cancer contributes to the
expression of a group of malignant program genes in addition to the
heat shock proteins and this HSF1-regulated malignant program was
also active in colon and lung cancer (7).
Hepatocellular carcinoma is the fifth most common,
with the third highest mortality rate of all cancer types
worldwide. It dominantly occurs in Asian countries, including
China, Japan and Southeast Asian countries (24). HCC is closely correlated with the
infection of hepatitis B virus (HBV), HCV, aspergillus flavus
infections, as well as cirrhosis and obesity (25). A number of proteins have been
identified as biomarkers for HCC diagnosis and prognosis, including
alpha-fetoprotein (AFP), AFPLens culinaris agglutinin-reactive AFP,
des-gamma-carboxy prothrombin, glypican-3, osteopontin, and others,
including squamous cell carcinoma antigen-immunoglobulin M
complexes, alpha-1-fucosidase, chromogranin A, human hepatocyte
growth factor and insulin-like growth factor (26). However, none of these biomarkers
are efficacious for the early diagnosis of HCC, and therefore,
further studies are required to identify novel specific biomarkers
of HCC to improve the prognosis. The accumulative evidence
indicates that HSF1 and its downstream HSPs are upregulated in HCC
tissues. Knockdown HSP70 and HSF1 triggered apoptosis of an HCC
cell line in vitro (27)
and the inhibition of DEN-induced HCC in vivo (16). HSP27, which is upregulated in HCC
tissues, is also elevated in HCC patient serum and is correlated
with HCC prognosis (28). It was
reported that HSF1 is upregulated in prostate cancer and HCC
(27). However, the possible role
of HSF1 as a prognostic marker of HCC has not been well
studied.
The present study investigated HSF1 protein
expression and its phosphorylaton at S326 in HCC tumor tissues and
HCC cell lines. Knockdown of HSF1 in the HCC cell line plc/pfr5 was
achieved with small hairpin (sh)RNA, and its effects on protein
expression, cell growth and colony formation were assessed. It was
explored whether of HSF1 and phospho-S326 may be used as biomarkers
of HCC progression and as potential candidate targets for HCC
therapeutics.
Materials and methods
Cell culture and plasmids
The cell lines SMMC7042 (Chinese Academy of
Sciences, Shanghai, China), HepG2 (ATCC, Manassas, VA, USA),
plc/prf5 (ATCC), SM7721 (Chinese Academy of Sciences) and Chang
liver cells (China Military Medical Science Academy, Beijing,
China) were routinely grown in Dulbecco’s modified Eagle’s medium
(DMEM) containing 10% FBS and 100 μg/ml ampicillin-streptomycin
mixture in a 37°C incubator with 5% CO2. The cells were
passaged every two days. The pLTHR-shRNA-HSF1 plasmid was used as a
retroviral vector expressing the shRNA targeting the human HSF1
sequence CAG GAG CAG CTC CTT GAG A (29). The pLTHR-shRNA-enhanced green
fluorescence protein (EGFP) was used as the scrambled shRNA.
Recombinant retrovirus expresses
shRNA-HSF1
The pLTHR-shRNA-HSF1 and pLTHR-scramble constructs
were transiently transfected into 293 amph cells for retrovirus
packaging. The cell supernatants, which were collected and mixed
with 2 μg/ml polybrane, were used to infect the plc/prf5 cells for
12 h. Following selection with 2 μg/ml of puromycin for three days,
the live cells were pooled and used for the experiments (e.g.
immunoblotting, cell growth and colony formation assay and cell
cycle analysis).
Immunohistochemical staining
Primary HCC tissues were kindly provided by Dr. Song
Zhenshun (Department of Surgery, Shanghai Tenth Hospital Affiliated
to Tongji University, Shanghai, China) were imbedded in paraffin
and selected for immunohistochemical staining using the standard
method. Briefly, following deparaffinization, rehydration and
antigen retrieval, the slides were blotted in 3% bovine serum
albumin/phosphate-buffered saline buffer for 1 h and incubated with
primary rabbit polyclonal antibody against Hsf1 for 1–2 h.
Following washing out unbound primary antibody, the slides were
then incubated with secondary antibody conjugated with alkaline
phosphatase (AP). The slides were developed in DAB buffer and
counterstained with hematoxylin. The slides were photographed with
the ZEISS 540 microscope under 40x-index (Carl Zeiss, Jena,
Germany). The study was approved by the Ethics Committee of
Shanghai Tenth Hospital Affiliated to Tongji University.
Immunoblotting, immunoprecipitation and
glutathione S-transferase (GST)-pull down
The cells were lysed in modified
radioimmunoprecipitation assay buffer [50 mM Tris-Cl, pH 7.4, 150
mM NaCl, 0.25% deoxycholate, 0.5% NP-40, 1× protein inhibitor
cocktail and 1× phosphatase inhibitor cocktail (Sigma, St. Louis,
MO, USA)]. The procedures for immunoblotting, immunoprecipitation
and in vivo GST-pull down were performed as described
previously (Zhang et al (30), 2010). The rabbit polyclonal
antibodies against HSF1 and HSP70 were purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). The rabbit polyclonal
anti-phospho-Hsf1/S326 antibody was purchased from Enzo Life
Sciences, Inc., Famingdale, NY, USA). The antibodies against pRB
and p53 were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA).
MTT assay, colony formation and cell
cycle analysis
For the MTT assay, 2×103 plc/prf5 cells
that stably expressed shRNA-HSF1 and plc/prf5-scramble were
seeded into 96-well plates and grown for up to seven days. Every
day, MTT reagent was added to the media 4 h prior to cell
collection. The MTT-labeled cells were homogenized in lysis buffer
containing 0.1% NP-40/isopropanol for 10 min. The optical density
(OD) value was calculated at an absorbance wavelength of 599 nm.
For colony formation, 500 cells were seeded into 60-mm plates and
grown for seven days. The cells were stained with 0.1% crystal
violet for 30 min. The data expressed represent three independent
experiments. For cell cycle analysis, equal numbers of the cells
expressing shRNA-HSF1 or plc/prf5-scramble were cultured for
24 h. The cells were then collected and fixed in 70% ethanol.
Following propidium iodide (PI) staining, the cell cycles were
analyzed by flow cytometry (BD FACSCalibur, San Jose, CA, USA).
Statistical analysis
The χ2-test and Spearman’s rho analysis
using SPSS software (SPSS, Inc., Chicago, IL, USA), and Student’s
t-test using Quantity One software were applied for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
HSF1 protein and phospho-S326/HSF1 are
upregulated in HCC cell lines and tissues
To further elucidate the activity of HSF1 in human
HCC, the expression levels of HSF1 and phospho-S326/HSF1 in the
four HCC cell lines and in the immortalized hepatocyte Chang liver
cells were assessed by immunoblotting. As indicated in Fig. 1A and B, HSF1 is upregulated in the
four HCC cell lines when compared with the immortalized Chang liver
cells, but the expression levels of HSF1 vary between the four HCC
cell lines (lanes 1, 2, 4 and 5). The expression levels of HSF1 in
SM7721 and M7024 was higher than those in HepG2 and plc/prf5 cells.
HepG2 and plc/prf5 are two highly differentiated HCC cell lines
(31). Consistent with HSF1, the
phosphorylation of HSF1/S326 is also upregulated in the HCC cell
lines compared with the Chang liver cells (Fig. 1A, upper panel). The phosphorylation
ratio of S326 was increased six-fold in SM7024, 18-fold in SM7721,
1.3-fold in HepG2 and 2.5-fold in the plc/prf5 cells (Fig. 1B) compared with that in Chang liver
cells. To determine whether levels of HSF1 expression and
phospho-S326/HSF1 were upregulated in HCC tissues, nine primary
human HCC tissues and their corresponding adjacent normal tissues
were assessed by immunoblotting. HSF1 and phospho-S326/HSF1 were
upregulated in the HCC tissues compared with their parental
adjacent normal tissues (Fig. 1C and
D). Consistently, immunohistochemical staining indicated that
the HSF1 protein and its phopho-S326 derivative were upregulated in
the HCC tissues compared with their adjacent normal tissues
(Fig. 1E). These results
demonstrated that HSF1 protein expression and transcriptional
activity were upregulated in HCC tissues.
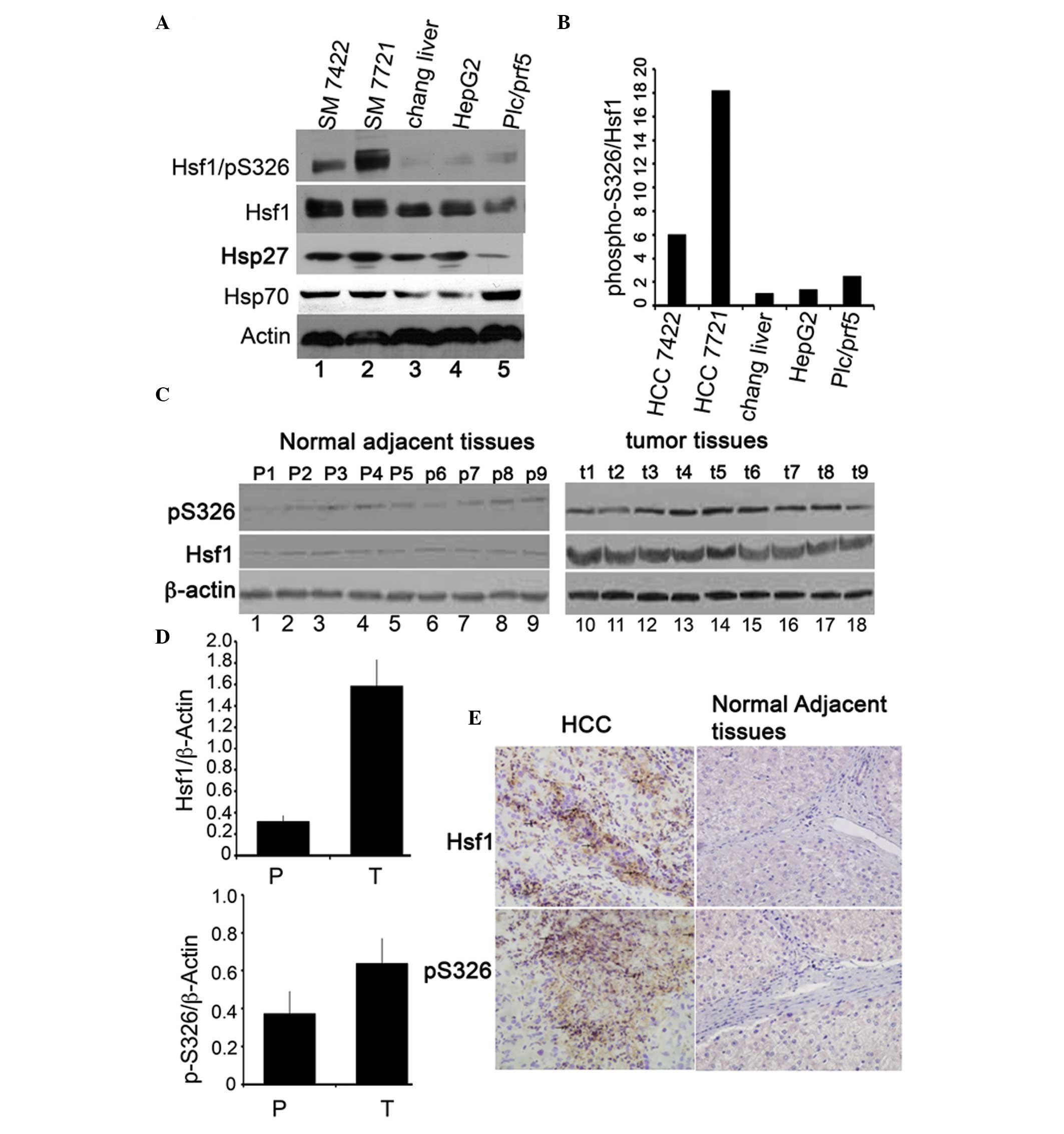 | Figure 1Protein expression of HSF1 and
phosphorylation of HSF1/S326 in HCC cell lines and tissues. (A)
Immunoblotting of HSF1, phospho-S326/HSF1 and β-actin in four HCC
cell lines: Lane 1, SM7024; lane 2, SM7721; lane 3, immortalized
Chang liver cell line; lane 4, HepG2; lane 5, Plc/prf/5. (B) The
percentage of phospho-S326/HSF1 was determined by normalization of
the density of phosphorylation of HSF1/S326 to the density of the
HSF1 protein. (C) Immunoblotting of HSF1 and phospho-S326/HSF1 in
HCC tissues and the normal counterparts. N1–N9 denote nine cases of
normal counterpart tissues (left panel) and T1–T9 represent nine
cases of HCC tissues (right panel). (D) Quantity of the Hsf1
proteins (upper panel) and phospho-S326 (lower panel). (E)
Immunohistochemical staining of the expression of HSF1 and
phospho-S326/HSF1 in HCC tissue (left panels) and in the normal
counterparts. Magnification, ×40. *P<0.05, the
expression levels of Hsf1 and phospho-Hsf1/S326 in normal adjacent
tissues compared with that in HCC tissues. HSF1, heat shock factor
1; HCC, hepatocellular carcinoma. |
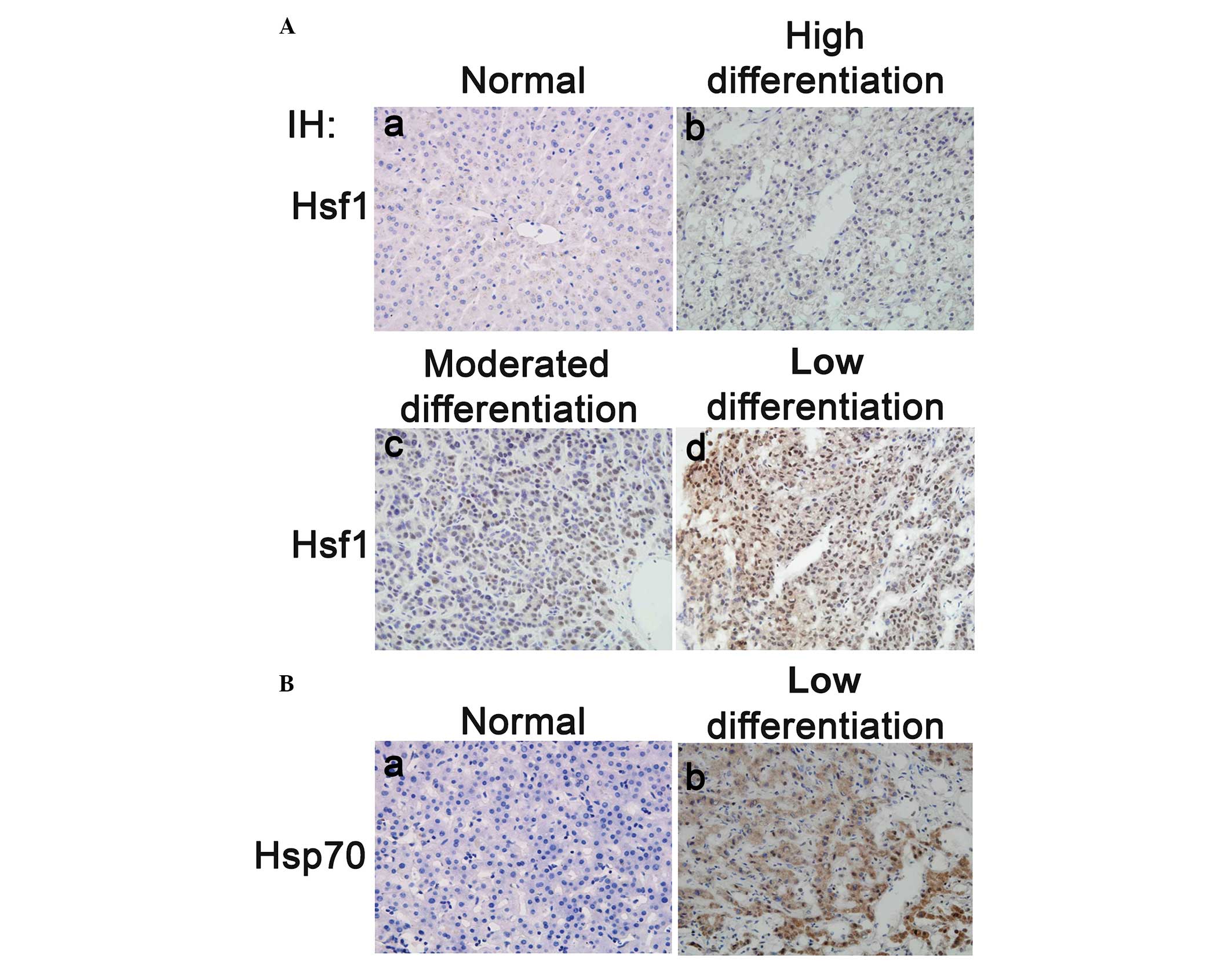
HSF1 expression is correlated with HCC
progression
The immunoblotting results demonstrated that both
HSF1 protein expression and phosphorylation of S326 were
significantly upregulated in the HCC tissues compared with their
adjacent normal counterparts. It was therefore hypothesized that
HSF1 may act as an effective prognostic marker of HCC. To prove
this hypothesis, 67 HCC tissues from HCC patients (who had not
received any prior chemotherapy and/or radiotherapy) and 21 normal
liver tissues were used to examine the expression of HSF1 by
immunohistochemical staining. The results indicated that the
expression levels of HSF1 in the moderately and poorly
differentiated HCC tissues were notably higher than those in the
highly differentiated HCC and normal liver tissues (Fig. 2A). Statistical analysis of cohort
studies indicated that 68.7% (n=46/67) of the HCC patients were
HSF1-positive compared with 28.6% (n=6/21) of the normal liver
biopsies (χ2=10.628, P=0.001; Table I), and HSF1 protein levels were
significantly increased in HCC tissues. Furthermore, the
correlation between the HSF1 expression and HCC malignancies
(including HCC metastasis, cancer cell differentiation, early phase
HCC and late phase HCC, aging, gender and HBV infection) was
studied. Of the 67 HCC patients, 14 out of 27 HCC patients who had
intact tumor membranes were HSF1-positive (51.9%). By contrast, 32
out of 40 (80.0%) HCC patients with broken tumor membranes were
HSF1-positive and HSF1 expression levels were notably higher in
membrane-broken HCC than those in membrane-intact tumors. The
χ2 analysis results indicated that the expression of
HSF1 was correlated with HCC invasion and metastasis
(χ2=9.76; P=0.015). According to the tumor
differentiation characteristics, there were 26, 31 and 10 patients
out of the total 67 patients who were diagnosed as poorly,
moderately and well-differentiated HCC, respectively. The
immunohistochemistry results demonstrated that 96.2% (n=25/26) of
the poorly differentiated HCC, 61.3% (n=19/31) of the moderately
differentiated HCC and 20% (n=2/10) of the well-differentiated HCC
patients were HSF1-positive. Statistical analysis indicated that
HSF1 expression was significantly different between the poorly and
moderately differentiated HCC, and between the moderately and
well-differentiated HCC samples (χ2=5.159; P<0.05).
This suggested that HSF1 expression was closely associated with
malignant HCC progression. Based on the clinical diagnosis [using
the tumor, nodes and metastasis grading system], 44.0% of phase
I-II HCC tissues (n=11/25) demonstrated low levels of HSF1 protein
expression, which was significantly different to the 83.3% of phase
III and IV HCC tissues that exhibited high HSF1 expression.
However, the expression of HSF1 was not correlated to HCC patient
age, gender, HBV infection status, AFP expression levels, Ceacam1
expression levels and portal vein thrombosis (Table I).
 | Table ICohort study of HSF1 protein
expression in HCC tissues. |
Table I
Cohort study of HSF1 protein
expression in HCC tissues.
| Clinical
factors | Cases (n) | HSF1
expression | Positive ratio
(%) | χ2 | P-value |
|---|
|
|---|
| Positive | Negative |
|---|
| Age, years |
| <55 | 36 | 23 | 13 | 63.9 | 0.822 | 0.365a |
| ≥55 | 31 | 23 | 8 | 74.2 | | |
| Gender |
| Male | 55 | 37 | 18 | 67.3 | 0.273 | 0.740b |
| Female | 12 | 9 | 3 | 75.0 | | |
| HBV |
| HBsAg (+) | 53 | 39 | 14 | 73.6 | 2.863 | 0.112b |
| HBsAg (−) | 14 | 7 | 7 | 50.0 | | |
| AFP |
| Positive | 39 | 26 | 13 | 66.7 | 0.172 | 0.679a |
| Negative | 28 | 20 | 8 | 71.4 | | |
| CEA |
| Positive | 56 | 41 | 15 | 73.2 | 3.292 | 0.086b |
| Negative | 11 | 5 | 6 | 45.5 | | |
| Wrap membrane |
| Intact | 27 | 14 | 13 | 51.9 | 5.935 | 0.015a |
| Broken | 40 | 32 | 8 | 80.0 | | |
| Portal V
thrombosis |
| Yes | 31 | 24 | 7 | 77.4 | 2.059 | 0.151a |
| No | 36 | 22 | 14 | 61.1 | | |
| Differention |
| Low | 26 | 25 | 1 | 96.2 | 9.762 | 0.002a |
| Middle | 31 | 19 | 12 | 61.3 | 5.159 | 0.032b |
| High | 10 | 2 | 8 | 20.0 | | |
| TNM phase |
| Phase I+II | 25 | 11 | 14 | 44.0 | 11.267 | 0.001a |
| Phase III+IV | 42 | 35 | 7 | 83.3 | | |
To determine whether the expression of HSP70 is
correlated with HSF1 in HCC, 40 poorly differentiated HCC tissues
were immunohistochemically stained with an HSP70 antibody. Similar
to HSF1, HSP70 protein expression was significantly upregulated in
the poorly differentiated HCC tissues compared with the
non-cancerous tissues (Fig. 2B).
The correlation between HSF1 and HSP70 in HCC was studied in a
cohort of 40 HCC tissues. A total of 22 HCC samples were both HSF1-
and HSP70-positive, 10 samples were both negative for HSF1 and
HSP70, 3 HCC samples were HSF1-postive but HSP70-negative, and 5
samples were HSF1-negative but HSP70-positive. The Spearman test
results indicated that HSF1 expression was significantly correlated
with HSP70 expression (Table II).
Taken together, these results strongly supported that the
expression of HSF1 is closely correlated with HCC progression and
HSP70 is one of the downstream targets of HSF1 in HCC tissues.
 | Table IICorrelation between HSF1 and
Hsp70. |
Table II
Correlation between HSF1 and
Hsp70.
| Hsp70 | |
|---|
|
| |
|---|
| HSF1 | + | − | Total |
|---|
| + | 22a | 3 | 25 |
| − | 5 | 10 | 15 |
| Total | 27 | 13 | |
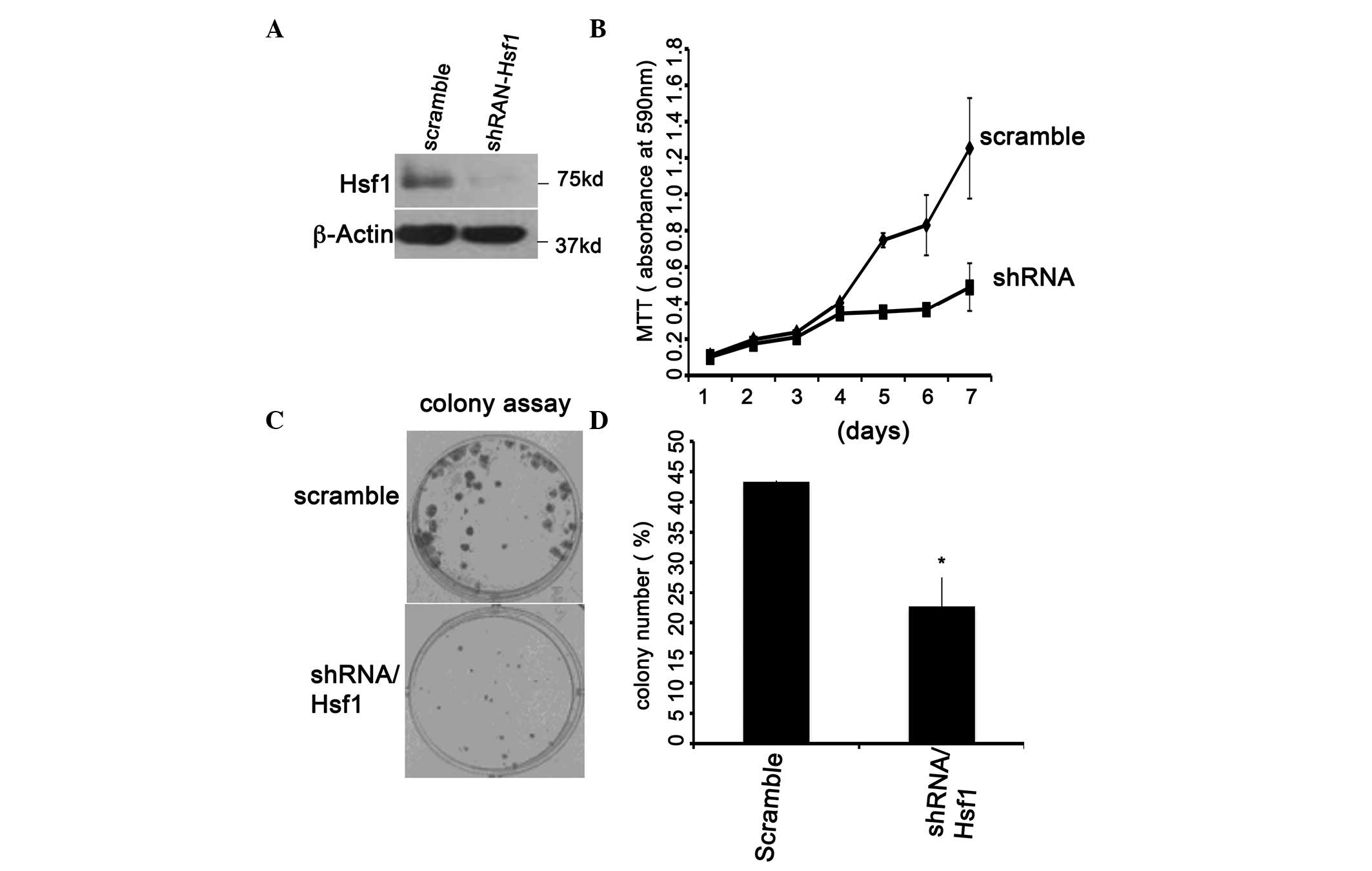
HSF1 knockdown inhibits plc/prf5 cell
proliferation
The close correlation between HSF1 and HCC
progression in Table I suggested
that HSF1 may be a novel therapeutic target of HCC. To determine
its therapeutic roles, the plc/prf5 cells were selected for further
study as manipulating them for transient transfection in
vitro is a simple process. The scrambled shRNA and shRNA
against HSF1 were transiently transfected into plc/prf5 cells. HSF1
was significantly downregulated by shRNA-HSF1 but not by the
scrambled shRNA (Fig. 3A, lanes 1
and 2). Knockdown of HSF1 expression may significantly inhibit
prc/prf5 cell proliferation and colony formation (Fig. 3B and C). These data were consistent
with observations reported for other cell types (9) and demonstrated that HSF1 may be a
target for HCC therapy.
HSF1 knockdown induces pRB protein
expression
It has been previously reported that a depletion of
HSF1 expression may induce the expression of p53 in
E1A-immortalized mouse embryonic fibroblast (MEF) cells, resulting
in cell growth inhibition (32).
To investigate whether the induction of p53 was also involved in
the slow growth of HSF1-knockdown prc/plf5 cells, the cells
expressing shRNA-HSF1 or scrambled shRNA were subjected to
immunoblot analysis. The results shown in Fig. 4A showed no difference in p53, HSP90
and HSP27 expression levels between the cells expressing
shRNA-HSF1 and those expressing scrambled shRNA. However,
pRB, another tumor suppressor, was evidently overexpressed in the
shRNA-HSF1-expressing cells compared with the cells
expressing scrambled shRNA (Fig.
4A, lanes 2 and 1). Cell cycle analysis indicated that 42% of
the shRNA-transfected cells were in G1 phase, as compared with 35%
of scrambled cells in G1 phase. There was no statistically
significant difference in the number of cells in S and G2 phase
between the two cell lines. These results indicated that knockdown
of HSF1 may arrest the cell cycle in G1 phase (Fig. 4B) by upregulating pRB protein
expression.
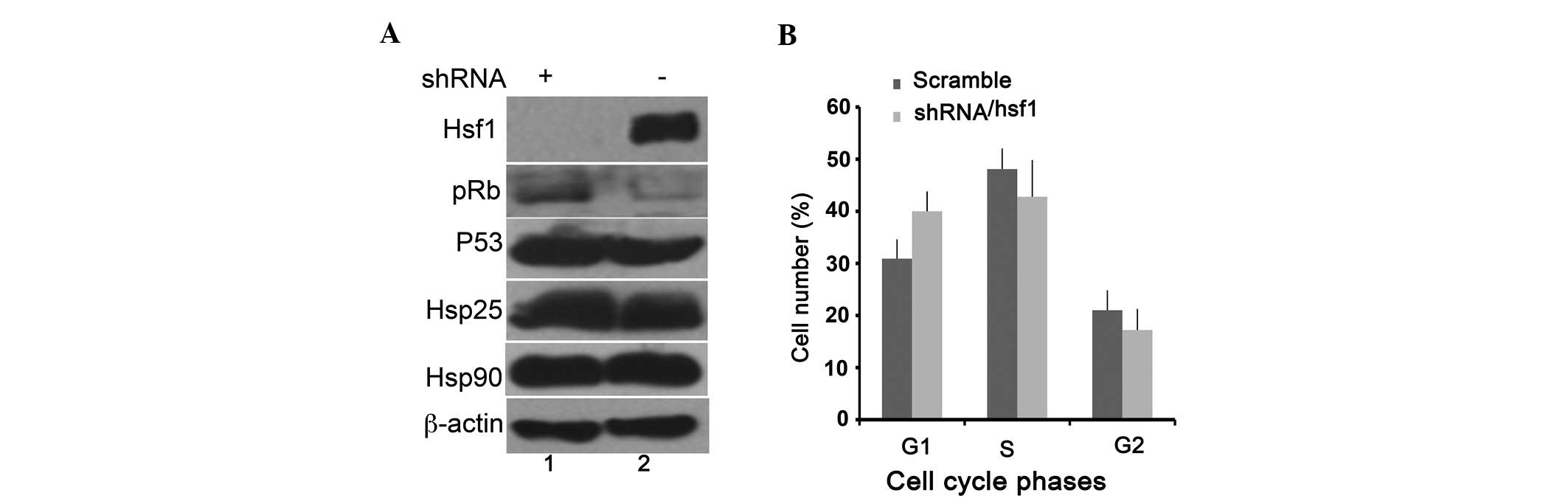 | Figure 4Knockdown of Hsf1 arrests the cells
at G1 phase by upregulating the expression of pRB. (A)
Immunobotting of the expression of HSF1, pRB, p53, HSP27, HSP90 and
β-actin in the plc/prf5 cells containing shRNA-HSF1 (lane 1) and
scrambled shRNA (lane 2). (B) Cell cycle analysis of plc/prf5 cells
containing shRNA-HSF1 (black bar) or scrambled shRNA (gray bar).
*P<0.05, scramble shRNA vs. shRNA-Hsf1. HSF1, heat
shock factor 1; HSP, heat shock protein; HCC, hepatocellular
carcinoma; pRB, retinoblastoma protein; shRNA, small hairpin
RNA. |
Discussion
Identification of the proteins that are specifically
expressed in tumor tissues has been used for targeted tumor therapy
and prognosis (33). The present
study provided evidence to support the hypothesis that HSF1 may be
used as a prognostic biomarker and therapeutic target for HCC. The
data demonstrated that HSF1 protein expression and its
phospho-S326/HSF1 were significantly upregulated in HCC tissues
compared with the adjacent normal tissues. Statistical analysis
demonstrated that HSF1 expression and transcriptional activities
were closely correlated with HCC development and invasion.
Knockdown of HSF1 inhibited prc/plf5 cell growth and colony
formation, induced the expression of pRB protein and arrested the
cell cycle at G1 phase. The results demonstrated that HSF1 may be a
novel prognostic marker and therapeutic target for HCC.
An association of HSF1 with cancer initiation and
development has been found in animal models and human cancer
tissues (4,9). Knockdown of HSF1 may inhibit mutant
p53-induced lymphoma; Her2 induced breast cancer and DEN induced
hepatocellular carcinoma in mouse models (4,16).
Defective HSF1 slowed down SV40-Tag- (unpublished data) and
E1A-induced MEF cell transformation in vitro (32). These data strongly suggested that
HSF1 activation is closely associated with cell transformation. In
addition, knockdown of HSF1 with siRNA may induce human tumor cell
apoptosis in vitro (4),
which supports the evidence that HSF1 has important roles in
maintaining tumor development. HSF1 has been found to be associated
with several oncogenic pathways. For example, both HSF1 and HSF2
were identified to regulate p53 protein stability by controlling
the expression of the proteasome subunits Psmb5 and gankyrin
(34). p53 protein is upregulated
in E1A-immortalized HSF1−/− MEF cells compared with its
parental tissues (32). HSF1 is
able to associate with cell cycle regulators cdc20 and polo-like
protein kinase 1, participating in the regulation of tumor cell
chromosomal stability (35).
Furthermore, it is able to modulate the metabolism of glucose and
lipids by indirectly regulating insulin receptor protein
expression, which has been demonstrated to be important for tumor
initiation and development (16).
HSF1 was reported to regulate interleukin (IL)-6 expression by
binding to and triggering the demethylation of the IL-6 promoter,
the latter of which was found to be the key inflammatory factor
involved in chronicle inflammation-induced cell transformation
(36,37). In breast cancer, HSF1 knockdown
inhibited Erb2-induced breast tissue tumorigenesis and tumor
metastasis in mouse models (5,9).
Deletion of HSF1 was identified to interfere with the HSP90-Her2
complex association, induce p21 protein accumulation and reduce
survivin expression (5). By
immunohistochemistry and immunoblotting it was identified in the
present study that the expression of HSF1 was highly correlated
with HCC development and prognosis. HSF1 expression was
significantly upregulated in the poorly differentiated,
membrane-broken HCC, rather than the normal and highly
differentiated HCC tissues (Fig. 2
and Table II). These data
provided further supporting evidence that HSF1 is closely
associated with HCC development and prognosis, which is consistent
with the results of previous studies (27). To determine the underlying
signaling pathways involved, HSF1 expression was silenced with
shRNA in vitro in the HCC cell line plc/prf5. HSF1 knockdown
induced significant upregulation of pRB expression, suggesting that
in plc/prf5 cells, HSF1 is associated with tumor suppressor pRB
instead of p53, which may explain why deletion of HSF1 causes cell
growth arrest (Fig. 4B). HSF1 has
been reported to modulate p53 protein stability by regulating the
expression of 26S proteasome subunits Psmb5 and gankyrin (34). It also directly binds to the
promoter, initiating the transcription of target genes, including
IL-6 and HSPs. However, it remains elusive how HSF1 negatively
regulates pRB expression.
Furthermore, it remains elusive how HSF1 is
activated in tumorigenesis. HSF1 is activated by heat shock and
other stresses, and the activation of HSF1 is a complex process,
involving HSF1 homotrimerization, hyperphosphorylation,
acetylation, sumoylation and nuclear translocation. It has been
reported that phosphorylation of S230 and/or S326 is an important
process for HSF1 activation (22,23).
Mutation of S326 and S230 impairs HSF1′s transcriptional activity
under heat shock conditions. Ca2+/calmodulin-dependent
protein kinase II (CAMKII), c-Jun N-terminal kinase (JNK) 1/2 and
mammalian target of rapamycin (mTOR) were found to be able to
phosphorylate these two sites in response to different stresses
(22,23,35).
CAMKII is activated by heat shock and glutamine and mediates the
phosphorylation of HSF1/S230 (38). JNK1-mediated phosphorylation of
HSF1/S320 participates in the regulation of IL-6 expression during
the inflammation-induced cell transformation and malignant
suspension (36). mTOR is able to
sensitize the protoxic signals or nutrient signals and participates
in the phosphorylation of S326. Knockdown of mTOR blocks S326
phosphorylation and inhibits the activation of HSF1 in heat shock
conditions. These results demonstrate that the phosphorylation of
S326 serves as a hallmark for HSF1 activation under heat shock
stress. Mendillo et al (7)
reported that phosphorylation of S326 was detected in breast cancer
tissues but not in immortalized breast epithelial cells and normal
breast tissues, suggesting that phosphorylation of S326 may be a
marker of active HSF1 in tumor tissues (7). According to this evidence, in the
present study, the expression of HSF1 and phospho-S326/HSF1 was
compared between HCC tissues and adjacent normal tissues. The
results demonstrated that the expression of HSF1 protein and
phosphorylation of S326 were significantly elevated in HCC tissues
compared with the adjacent normal tissues (Fig. 1A and C). The results indicated that
HSF1 was activated in HCC tissues. However, the biological effects
of HSF1 in HCC remain elusive. Generally, it is noted that the
dominant role of HSF1 is to regulate the HSPs chaperone expression,
which is important for tumorigenesis. However, Mendillo et
al’s recent study demonstrated that heat shock and
tumorigenesis-activated HSF1 are involved in different signaling
pathways (7). Heat shock-activated
HSF1 is mainly responsive to the expression of heat shock proteins,
while activation of HSF1 in tumor tissues is predominantly
responsible for controlling the expression of non-heat shock
proteins, e.g. CKS2, LY6K, RBM23, CCT6A, CKS1B, ST13 and EIF4A2. In
transformed breast epithelial cells, active HSF1 prefers to bind to
the promoters of non-heat shock proteins. By contrast, HSF1 is
facilitated to recognize HSPs (HSP70 and HSP90) under heat shock
conditions. The distinctive promoter-binding preferences of HSF1
are hypothesized to be regulated by post-translational
modifications, including phosphorylation, acetylation, sumoylation
and glycosylation.
Clinically, well-established cancer biomarkers are
used as diagnostic and prognostic indicators. For example,
prostate-specific antigen (PSA) is used for prostate cancer, Erb2
and estrogen for breast cancer and a p53 mutant for lung cancer. In
HCC, a number of biomarkers have been proposed to be associated
with HCC prognosis. The most commonly used biomarker is the
alpha-fetoprotein (AFP) protein. In addition, the transcription
factor forkhead box C1 and cyclin G1, which are highly expressed in
the majority of HCC tissues, are correlated with HCC metastasis by
associating with snail expression and the AKT-signaling pathway
(39). SAL4 protein, which is
upregulated in HCC stem cells, has been used as an HCC stem marker
and HCC prognostic marker (39,40).
However, these biomarkers are not sufficient for interpreting the
intricate prognosis of HCC clinically. These data indicate that
HSF1 is upregulated in the most developed (late stage) HCC tissues.
Its expression and transcriptional activity is closely correlated
with HCC malignancy and invasion, which implies that HSF1 may be
used as a novel biomarker in HCC prognosis.
In conclusion, the present study demonstrated that
HSF1 is upregulated in HCC tissues and cell lines. Its expression
levels and transcriptional activity are correlated with HCC
development. Cancer stage progression is likely to be correlated
with the expression of HSF1, which indicates that HSF1 may be a
useful biomarker for HCC prognosis and the development of novel
therapeutic strategies for its treatment.
Acknowledgements
The authors are grateful to Muhan Hu, Department of
Medicine of University of Alabama, for language editing. The
present study was supported by National Natural Science Foundation
of China (NSFC; grant nos. 30971508 and 81270985).
References
|
1
|
Sorger PK: Heat shock factor and the heat
shock response. Cell. 65:363–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qian SB, McDonough H, Boellmann F, et al:
CHIP-mediated stress recovery by sequential ubiquitination of
substrates and HSP70. Nature. 440:551–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christians E: HSF1 knock-out. J Biol Chem.
286:le26–le27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai C, Whitesell L, Rogers AB and
Lindquist S: Heat shock factor 1 is a powerful multifaceted
modifier of carcinogenesis. Cell. 130:1005–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng L, Gabai VL and Sherman MY:
Heat-shock transcription factor HSF1 has a critical role in human
epidermal growth factor receptor-2-induced cellular transformation
and tumorigenesis. Oncogene. 29:5204–5213. 2010. View Article : Google Scholar
|
|
6
|
Wang Y, Theriault JR, He H, et al:
Expression of a dominant negative heat shock factor-1 construct
inhibits aneuploidy in prostate carcinoma cells. J Biol Chem.
279:32651–32659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendillo ML, Santagata S, Koeva M, et al:
HSF1 drives a transcriptional program distinct from heat shock to
support highly malignant human cancers. Cell. 150:549–562. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santón A, García-Cosío M, Cristóbal E, et
al: Expression of heat shock proteins in classical Hodgkin
lymphoma: correlation with apoptotic pathways and prognostic
significance. Histopathology. 58:1072–1080. 2011.PubMed/NCBI
|
|
9
|
Xi C, Hu Y, Buckhaults P, et al: Heat
shock factor HSF1 cooperates with ErbB2 (Her2/Neu) protein to
promote mammary tumorigenesis and metastasis. J Biol Chem.
287:35646–35657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng Q, Chang JT, Geradts J, et al:
Amplification and high-level expression of heat shock protein 90
marks aggressive phenotypes of human epidermal growth factor
receptor 2 negative breast cancer. Breast Cancer Res. 14:R622012.
View Article : Google Scholar
|
|
11
|
Calderwood SK and Gong J: Molecular
chaperones in mammary cancer growth and breast tumor therapy. J
Cell Biochem. 113:1096–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamal A, Thao L, Sensintaffar J, et al: A
high-affinity conformation of Hsp90 confers tumour selectivity on
Hsp90 inhibitors. Nature. 425:407–410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang F, Lazorchak AS, Liu D, et al:
Inhibition of the mTORC2 and chaperone pathways to treat leukemia.
Blood. 119:6080–6088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Modi S, Stopeck A, Linden H, et al: HSP90
inhibition is effective in breast cancer: a phase II trial of
tanespimycin (17-AAG) plus trastuzumab in patients with
HER2-positive metastatic breast cancer progressing on trastuzumab.
Clin Cancer Res. 17:5132–5139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Min JN, Huang L, Zimonjic DB, et al:
Selective suppression of lymphomas by functional loss of HSF1 in a
p53-deficient mouse model for spontaneous tumors. Oncogene.
26:5086–5097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin X, Moskophidis D and Mivechi NF: Heat
shock transcription factor 1 is a key determinant of HCC
development by regulating hepatic steatosis and metabolic syndrome.
Cell Metab. 14:91–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou J, Guo Y, Guettouche T, et al:
Repression of heat shock transcription factor HSF1 activation by
HSP90 (HSP90 complex) that forms a stress-sensitive complex with
HSF1. Cell. 94:471–480. 1998. View Article : Google Scholar
|
|
18
|
Shi Y, Mosser DD and Morimoto RI:
Molecular chaperones as HSF1-specific transcriptional repressors.
Genes Dev. 12:654–666. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bulman AL, Hubl ST and Nelson HC: The
DNA-binding domain of yeast heat shock transcription factor
independently regulates both the N- and C-terminal activation
domains. J Biol Chem. 276:40254–40262. 2001. View Article : Google Scholar
|
|
20
|
Hietakangas V, Ahlskog JK, Jakobsson AM,
et al: Phosphorylation of serine 303 is a prerequisite for the
stress-inducible SUMO modification of heat shock factor 1. Mol Cell
Biol. 23:2953–2968. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng W, Zhang Y, Zheng M, et al:
Cardioprotection by CaMKII-deltaB is mediated by phosphorylation of
heat shock factor 1 and subsequent expression of inducible heat
shock protein 70. Circ Res. 106:102–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holmberg CI, Hietakangas V, Mikhailov A,
et al: Phosphorylation of serine 230 promotes inducible
transcriptional activity of heat shock factor 1. EMBO J.
20:3800–3810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou SD, Prince T, Gong J and Calderwood
SK: mTOR is essential for the proteotoxic stress response, HSF1
activation and heat shock protein synthesis. PLoS One.
7:e396792012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han KH, Kudo M, Ye SL, et al: Asian
consensus workshop report: expert consensus guideline for the
management of intermediate and advanced hepatocellular carcinoma in
Asia. Oncology. 81:158–64. 2011. View Article : Google Scholar
|
|
25
|
Yoshimoto S, Loo TM, Atarashi K, et al:
Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature. 499:97–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bertino G, Ardiri A, Malaguarnera M, et
al: Hepatocellular carcinoma serum markers. Semin Oncol.
39:410–433. 2012. View Article : Google Scholar
|
|
27
|
Fang F, Chang R and Yang L: Heat shock
factor 1 promotes invasion and metastasis of hepatocellular
carcinoma in vitro and in vivo. Cancer. 118:1782–1794. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gruden G, Carucci P, Lolli V, et al: Serum
heat shock protein 27 levels in patients with hepatocellular
carcinoma. Cell Stress Chaperones. 18:235–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rossi A, Ciafrè S, Balsamo M, et al:
Targeting the heat shock factor 1 by RNA interference: a potent
tool to enhance hyperthermochemotherapy efficacy in cervical
cancer. Cancer Res. 66:7678–7685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Hu YZ, Xueli L, et al: The
inhibition of CMV promoter by heat shock factor 4b is regulated by
Daxx. Int J Biochem Cell Biol. 42:1698–1707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raney AK, Milich DR, Easton AJ and
McLachlan A: Differentiation-specific transcriptional regulation of
the hepatitis B virus large surface antigen gene in human hepatoma
cell lines. J Virol. 64:2360–2368. 1990.
|
|
32
|
Jin X, Moskophidis D, Hu Y, et al: Heat
shock factor 1 deficiency via its downstream target gene
alphaB-crystallin (Hspb5) impairs p53 degradation. J Cell Biochem.
107:504–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huynh H: Molecularly targeted therapy in
hepatocellular carcinoma. Biochem Pharmacol. 80:550–560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lecomte S, Desmots F, Le Masson F, et al:
Roles of heat shock factor 1 and 2 in response to proteasome
inhibition: consequence on p53 stability. Oncogene. 29:4216–4224.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim EH, Lee YJ, Bae S, et al: Heat shock
factor 1-mediated aneuploidy requires a defective function of p53.
Cancer Res. 69:9404–9412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rokavec M, Wu W and Luo JL: IL6-mediated
suppression of miR-200c directs constitutive activation of
inflammatory signaling circuit driving transformation and
tumorigenesis. Mol Cell. 45:777–789. 2012. View Article : Google Scholar
|
|
37
|
Chew V, Tow C, Teo M, et al: Inflammatory
tumour microenvironment is associated with superior survival in
hepatocellular carcinoma patients. J Hepatol. 52:370–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue H, Slavov D and Wischmeyer PE:
Glutamine-mediated dual regulation of heat shock transcription
factor-1 activation and expression. J Biol Chem. 287:40400–40413.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen W, Ding J, Sun W, et al: Cyclin
G1-mediated epithelial-mesenchymal transition via phosphoinositide
3-kinase/Akt signaling facilitates liver cancer progression.
Hepatology. 55:1787–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia L, Huang W, Tian D, et al:
Overexpression of forkhead box C1 promotes tumor metastasis and
indicates poor prognosis in hepatocellular carcinoma. Hepatology.
57:610–624. 2013. View Article : Google Scholar : PubMed/NCBI
|