Introduction
Kidney stones, also termed renal calculi, are
crystal aggregations formed in the kidneys from dietary minerals in
the urine. Urolithiasis is the term used for the condition where
urinary stones are formed or located in the urinary system. In
humans, calcium oxalate is a key component of the majority of
urinary stones. Compared with normal subjects, patients with
calcium stones excrete significantly more calcium and oxalate.
Kidney stones are more prevalent in males than females with ~80% of
individuals with kidney stones being male (1). Urinary stone disease is a common
disease, which affects 10–12% of the population in industrialized
countries (2). In males, the
highest prevalence of the disease occurs between the age of 20 and
40 years, while in females the highest incidence of the disease
occurs later (3). Numerous
treatment modalities are used to treat kidney stones. However, the
high recurrence rate is a serious concern in urinary stone disease
and in the majority of cases the rate of recurrence may be >50%
after 10 years (4). At present,
numerous treatment regimens are available to treat kidney stone
recurrence, including extracorporeal shock wave lithotripsy (SWL)
and endourological procedures, including ureteroscopy, as well as
percutaneous extraction procedures. However, these treatments are
associated with severe side effects. The side effects associated
with SWL include traumatic effects caused by the shock waves,
severe hematuria, pancreatitis, infection and continual residual
fragments, which may serve as nidi for the formation of further
calculi. Moreover, side effects associated with endourological and
percutaneous procedures include extravasation of irrigating fluid,
urosepsis and ureteral damage (5–10).
Various orally administered drugs are used to treat the formation
of renal calculi; however, their long-term use is limited by severe
side effects and their lack of universal tolerance by patients.
Thus, alternative treatment options have arisen, including herbal
medicines, which have been used to treat urinary stone disease for
hundreds of years without evident harmful side effects. Certain
herbal extracts exert their antilithogenic effects through changing
the ionic composition of urine, such as calcium ions and magnesium
ions. Furthermore, a number of the herbal extracts are rich in
saponins, which disarrange mucoprotein suspensions and promote
crystallization (11).
Urtica dioica, commonly termed ‘Stinging
Nettle,’ is a herbaceous perennial flowering plant and the best
known member of the nettle genus Urtica. Nettle leaf has a long
history of traditional medicinal use for arthritis in Germany
(12). Urtica dioica has
been used in traditional Austrian medicine internally (as tea or
fresh leaves), as well as for the treatment of kidney, urinary
tract, gastrointestinal tract and locomotor disorders. Furthermore,
nettle root extracts have been studied in human clinical trials as
a treatment for benign prostatic hyperplasia. In the present study,
the antiurolithiatic activity of the methanol extract of Urtica
dioica leaves was investigated in male rats. Phytochemical
analysis of the extract was also performed using liquid
chromatography-electrospray ionization tandem mass spectrometry
(LC-ESI-MS-MS) and high-performance liquid chromatography with
photodiode array detection (HPLC-DAD), which to the best of our
knowledge is the first study to do so (13–15).
Materials and methods
Plant material and preparation of the
extract
Leaves of U. dioica were collected between
May and June 2013 from Jiuzhaigou, China. The plant material was
confirmed by a taxonomist. The leaves of U. dioica were
washed with water, shade-dried and homogenized. Methanol (95%) was
used for hot extraction, which was performed for 3 h using a
Soxhlet extraction apparatus (Benang Instrument Co., Ltd.,
Shanghai, China). The extract was then concentrated under reduced
pressure in a rotary evaporator (Yarong Biochemistry Instrument
Factory, Shanghai, China) at 40°C and was stored at 4°C prior to
use.
LC-ESI-MS-MS-HPLC analysis
An Agilent 6410B Triple quadrupole LC/MS system
equipped with a 1260 Infinity chromatographic system coupled with
an Agilent triple quadrupole mass spectrometer fitted with an ESI
source (Agilent Technologies, Santa Clara, CA, USA) was used for
the LC-ESI-MS-MS-HPLC analysis. The MS conditions were as follows:
MS range, 100–1,200 Da; nebulizer gas, 45 psi; gas temperature,
325°C; and capillary voltage, 4,000 V. MSn spectra were obtained
using positive and negative modes. HPLC analysis was performed
using an Agilent 1260 Infinity series HPLC system (Agilent
Technologies). A Chromolith® RP-18e column (internal
diameter, 4.6 mm ID; length, 50 mm; Merck & Co., Inc.,
Readington, NJ, USA) was also used. The mobile phase consisted of
aqueous formic acid (A; 0.1%) and methanol (B). The gradient
conditions were: 0–8 min, linear gradient from 12 to 25% of B; 8–12
min, isocratic conditions at 25% of B; 12–16 min, linear gradient
between 25 and 40% of B; 16–40 min, linear gradient between 40 and
50% of B; and 40–50 min, linear gradient between 50 and 100% of B.
The flow rate was 1 ml/min.
Experimental animals
Male Sprague-Dawley rats (Experimental Animal Centre
of Sichuan University, Chengdu, China) weighing between 100 and 170
g were used in the present study. Rats were maintained under
hygienic conditions in cages in a temperature- and
humidity-controlled room (30±2°C and 50%, respectively), with a
12-h light/dark cycle. Food and water were available ad
libitum. Animals were treated in accordance with the Guide for
the Care and Use of Laboratory Animals (National Institutes of
Health; 1996) and all procedures were approved by the Ethics
Committee of the General Hospital of Chengdu Military Region
(Chengdu, China).
Experimental induction of urinary stones
in rats
To induce calcium oxalate urinary calculi in rats,
the rats were allowed free access to drinking water containing
0.55% (v/v) ethylene glycol and 1% (w/v) ammonium chloride for 10
days, as described previously (16). Rats were then divided into four
groups containing 10 rats per group, prior to treatment.
Experimental design, acute toxicity study
and dose selection
For the Urtica dioica extract dose selection,
rats were divided into five groups containing 10 rats per group and
were fasted overnight with free access to drinking water. Rats in
group I received only distilled water (10 ml/kg body weight
orally). Rats in groups II, III, IV and V were administered a
single oral dose of the Urtica dioica extract at 10, 50, 600
or 2,000 mg/kg body weight, respectively, through gastric
intubation using a soft catheter. Rats were observed continuously
for 4 h after the administration of the extract and then observed
at one hour intervals for 48 h and the everyday for 15 days to
monitor mortalities. The extract was determined to be non-toxic as
no animal mortalities were observed with doses ≤2000 mg/kg body
weight. The doses selected for the experimental investigations were
0.7 and 1.4 g/kg body weight. The final experimental groups were as
follows: Group I, rats without the experimental induction of
urinary stones (normal); group II, urinary stone-induced rats
treated with 10 ml/kg body weight distilled water (control); group
III, urinary stone-induced rats treated with 0.7 g/kg body weight
Urtica dioica extract; and group IV, urinary stone-induced
rats treated with 1.4 g/kg body weight Urtica dioica
extract.
Determination of the antiurolithiatic
activity of the extract
Following hydration with 10 ml distilled water
administered orally, rats were put in separate metabolic cages and
urine samples were collected after 48 h from the overnight-fasted
rats on day 30. Urine samples were centrifuged at 3,000 rpm at
25±2°C for 10 min. The supernatants of the urine samples were used
to estimate the pH and to quantitatively measure the levels of
oxalate, calcium and creatinine, as described previously (17–19).
Rats were sacrificed using cervical dissection on day 31. The
kidneys were then removed and washed using ice-cold saline solution
and were sectioned. Ice-cold 0.10 M KCl solution was used to wash
one kidney from each rat, which was then weighed. The kidney was
sectioned into two equal halves. One half of the kidney was then
homogenized with 5% HCl solution and the homogenate was centrifuged
at 3,000 × g at 25±2°C for 10 min. The supernatant was used to
determine the renal deposition of oxalate and calcium as described
previously (15,16).
Histopathological studies of the
kidneys
The remaining half of the kidney sample was rapidly
fixed using 10% neutralized formalin (pH 7.4). Paraffin-embedded
kidney sections were prepared and stained with hematoxylin and
eosin, prior to being histopathologically assessed.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One way analysis of variance was used to measure
inter-group variation. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Toxicological and histopathological
results
The methanol extract was determined to be non-toxic
as no animal mortalities were reported upon treatment with doses
≤2,000 mg/kg body weight. In the control rats (group II), following
ethylene glycol and ammonium chloride ingestion for 30 days, levels
of urinary oxalate, calcium and creatinine were observed to
significantly increase compared with the rats in group I (Fig. 1). Furthermore, compared with the
normal rats, an increase in the renal deposition of calcium oxalate
was found in the rats in the control group, as revealed by phase
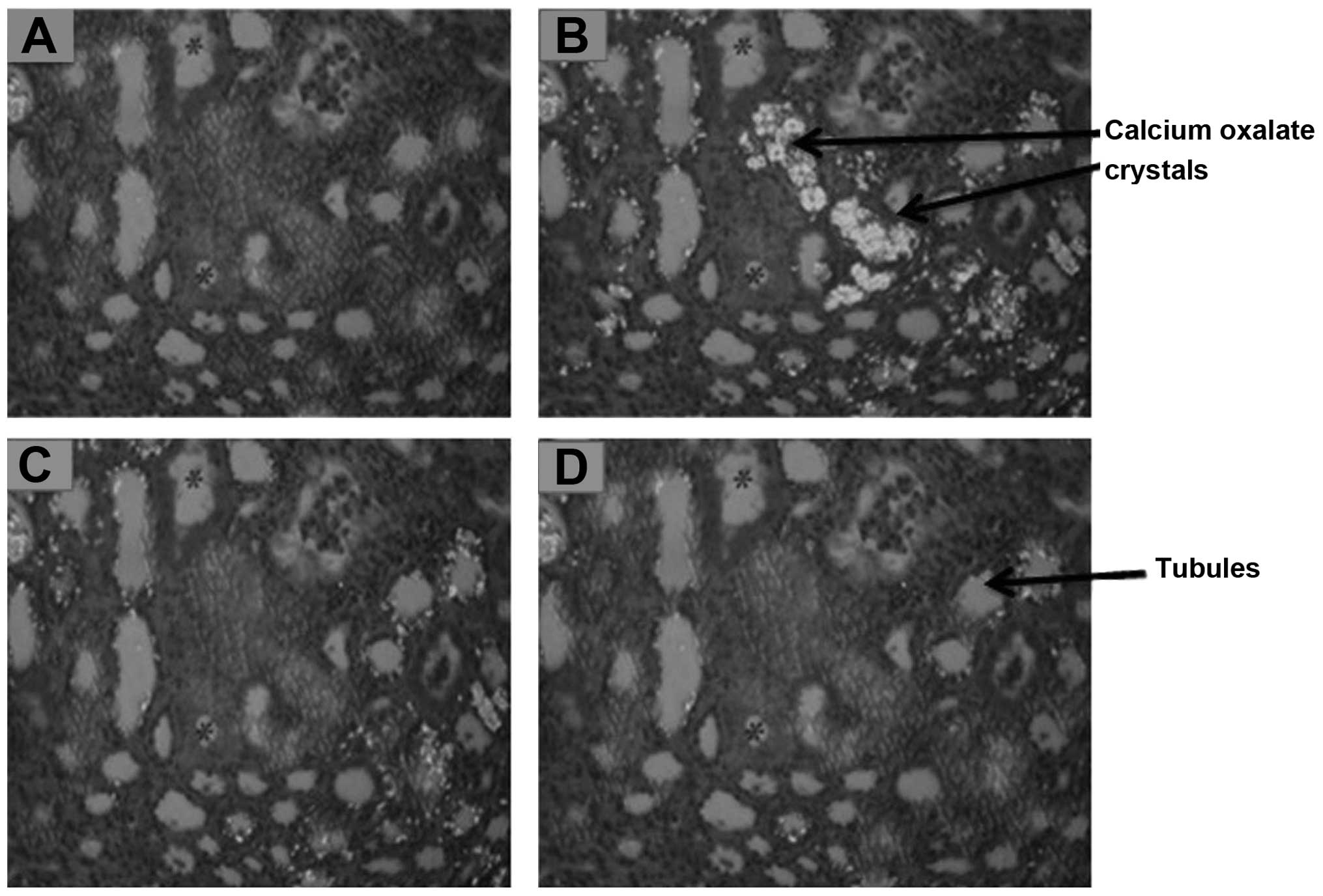
contrast microscopy (Fig. 2). A
significant decrease in urine pH (from 7.0–7.5 to 5.0–5.7, P≤0.01;
data not shown) and an increase in kidney weight (Fig. 1) were also observed in the control
rats compared with the normal rats. In addition, histopathological
studies using phase contrast microscopy revealed inflammation of
tubules and atrophy of glomeruli, as well as intra-tubular and
interstitial calcium oxalate crystal deposition in the kidneys of
the rats in the control group compared with those in the normal
group.
Antiurolithiatic effects of the U. dioica
extract
Oral administration of the U. dioica methanol
extract to rats in Group III (0.7 g/kg body weight) and Group IV
(1.4 g/kg body weight from day 10–30 was found to induce a
significant reduction in urinary excretion (Fig. 1), as well as a reduction in the
renal deposition of oxalate and calcium (Fig. 2). Furthermore, the U. dioica
methanol extract was found to decrease kidney weight and urinary
creatinine levels (Fig. 1), and
restore urinary pH levels (data not shown). Histopathological
analysis using phase contrast microscopy revealed that treatment
with the U. dioica extract also reduced calcium oxalate
crystal deposition and renal damage, and promoted the regeneration
of the renal tubules and glomeruli compared with the control group
(Fig. 2). The reduction in calcium
oxalate crystal deposition and renal injury in the renal tubules
and glomeruli suggests that treatment with the U. dioica
extract promoted the dissolution of preformed calcium oxalate
crystals. The decrease in kidney weight observed in the U.
dioica extract-treated rats supports this hypothesis.
Furthermore, the more marked decrease in urinary and renal
parameters observed in the high-dose group IV rats compared with
the low-dose group III rats, suggests that the U. dioica
extract had a dose-dependent effect on calcium oxalate stone
formation. In the group IV rats, which received 1.4 g/kg body
weight U. dioica extract, the decrease in the renal
deposition of calcium and oxalate and the decrease in urinary
excretion were also more pronounced, compared with the group III
rats. Moreover, in the group IV rats, the reduction in kidney
weight and levels of urinary creatinine were more pronounced,
further indicating a dose-dependent effect of the U. dioica
extract.
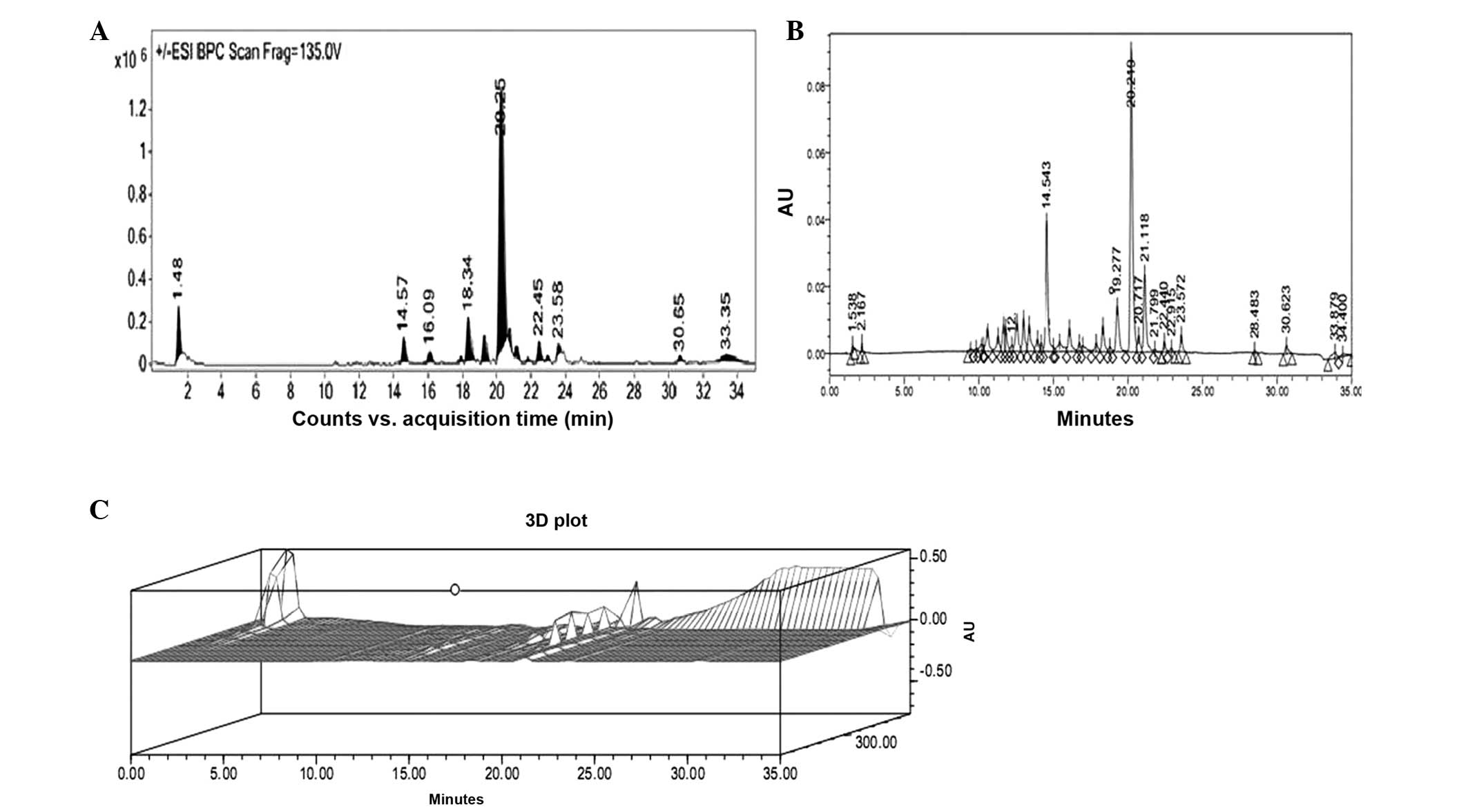
LC-ESI-MS-MS-HPLC analysis
Phytochemical analysis of the U. dioica
methanol extract was performed using LC-ESI-MS-MS and HPLC-DAD. The
extract was run under positive and negative ESI-MS conditions and
was observed to be composed of several major and minor ionic
species. The total ion MS chromatogram, HPLC profile and HPLC 3D
plot are shown in Fig. 3.
Fragmentation of the major peaks was used for the identification of
the compounds. The identification of the chemical compounds was
also performed by comparing the molecular ion peaks and the MS
fragmentation pattern with those reported in the literature. The
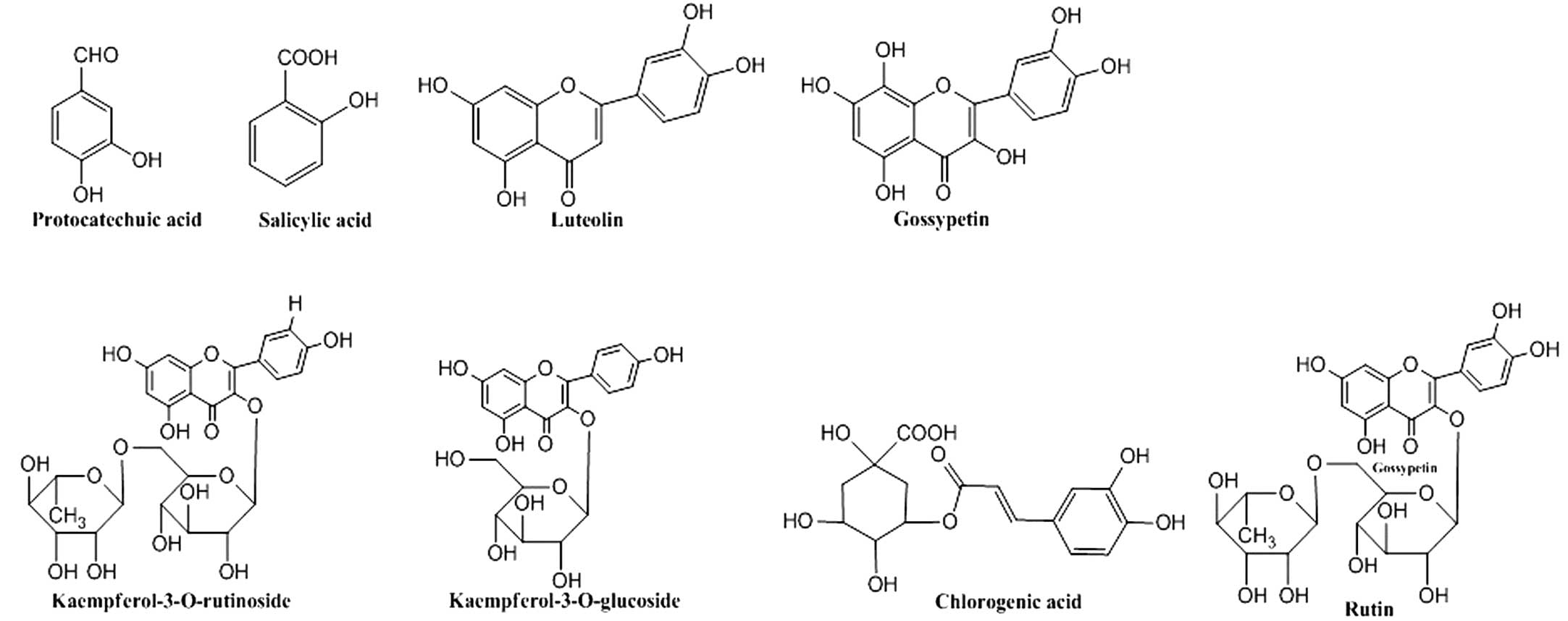
eight chemical constituents identified were protocatechuic acid,
salicylic acid, luteolin, gossypetin, rutin,
kaempferol-3-O-rutinoside, kaempferol-3-O-glucoside and chlorogenic
acid (Fig. 4). The phytochemicals
present in the aerial parts of the Urtica species have been found
to be primarily phenolic compounds, including caffeic acid,
chlorogenic acid, 2-O-caffeoylmalic acid and flavonoids, such as
quercetin and isorhamnetin glycosides. Moreover, the biological
activities of nettle leaves are often assigned to the flavonoid
fraction (20–23).
Discussion
In recent years, there have been marked advances in
phytotherapy for urolithiasis, with the United States investing
>1.5 billion dollars annually (3). Although phytotherapeutic extracts are
popular in certain cultures, their specific mechanism of action in
urolithiasis remains unclear. Elucidating the mechanism of action
of these herbal extracts in urolithiasis has diagnostic value with
regard to the nature of this disease, as well as potential
therapeutic implications in this future field of research. Based on
the traditional medicinal claims of Urtica dioica for the
treatment of various urinary disorders, the present study aimed to
investigate the antiurolithiatic effect of a methanol extract of
the leaves of Urtica dioica in male rats and define its
chemical composition using LC-ESI-MS-MS and HPLC-DAD, which to the
best of our knowledge is the first study to do so.
Numerous studies have reported the presence of
flavonoids, saponins and anthocyanins in U. dioica, thus the
decrease in the renal deposition of calcium and oxalate in the
U. dioica extract-treated rats observed in the present
study, may be induced by these phytochemicals (24–26).
Saponins and flavonoids prevent calcium and oxalate deposition
through disintegrating mucoproteins, which have a high affinity for
calcium oxalate crystal surfaces and thus promote the growth and
deposition of crystals (27).
In the present study, a significant increase in
urinary creatinine was observed after 48 h in the control rats,
suggesting the occurrence of hyperoxaluria-induced renal damage,
which may cause decreased urine out-put and subsequent
supersaturation of lithogenic promoting agents. Furthermore,
hyperoxaluria-induced renal damage and stone formation was found to
be associated with calcium oxalate crystal deposition and damage to
the kidney. Urinary pH has been reported to affect crystaluria,
with alterations to urinary pH found to induce urinary stone
dissolution. A urinary pH between 5.0 and 6.3 promotes calcium
oxalate stone formation (28). In
the present study, the decrease in the urinary pH from 7.0–7.3 to
5.0–5.4 supports the formation of calcium oxalate calculi.
Furthermore, restoration of the urinary pH (5.4–7.3) was found to
support the dissolution of preformed calcium oxalate crystals.
In conclusion, the present study revealed that the
methanol extract of U. dioica efficiently dissolves calcium
oxalate renal stones in male Sprague-Dawley rats. The extract
showed a dose-dependent curative effect on urinary and renal
parameters, including calcium oxalate renal stone formation. These
findings support previous reports that the extract can be used for
the treatment of kidney and urinary tract disorders.
Acknowledgements
This study was supported by grants from the Shandong
Province Natural Science Foundation of China (grant no.
ZR2009CL027), the Science and Technology Star Plans Foundation of
Jinan Technology Bureau of China (grant no. 20100118) and the
Medical Foundation of Shandong Academy of Medical Science (grant
no. 201023).
References
|
1
|
Robertson WG, Peacock M, Heyburn PJ,
Marshall DH and Clark PB: Risk factors in calcium stone disease of
the urinary tract. Br J Urol. 50:449–454. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buttterwick V and Khan SR: Herbal
medicines in the management of urolithiasis: alternative or
complimentary? Planta Med. 75:1095–1103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pak CY: Citrate and renal calculi. Miner
Electrolyte Metab. 13:257–266. 1987.PubMed/NCBI
|
|
4
|
Gürocak S and Küpeli B: Consumption of
historical and current phytotherapeutic agents for urolithiasis: a
critical review. J Urol. 176:450–455. 2006.PubMed/NCBI
|
|
5
|
Wasserstein AG: Nephrolithiasis: acute
management and prevention. Dis Mon. 44:196–213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaussy C, Schmeidt E, Jochman D, et al:
First clinical experience with extracorporeally induced destruction
of kidney stones by shocks waves. J Urol. 127:417–420.
1982.PubMed/NCBI
|
|
7
|
Cass AS: Comparison of first generation
(Dornier HM3) and second generation (Medstone STS) lithotriptors:
treatment results with 13, 863 renal and ureteral calculi. J Urol.
153:588–592. 1995. View Article : Google Scholar
|
|
8
|
Gronau E, Pannek J, Böhme M and Senge T:
Results of extracorporeal shock wave lithotripsy with a new
electrohydraulic shock wave generator. Urol Int. 71:355–360. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lingeman JE, Siegel YI, Steele B, et al:
Management of lower pole nephrolithiasis: a critical analysis. J
Urol. 151:663–667. 1994.PubMed/NCBI
|
|
10
|
Kerbl K, Clayman RV, Chandhoke PS, et al:
Percutaneous stone removal with the patient in a flank position. J
Urol. 151:686–688. 1994.PubMed/NCBI
|
|
11
|
Grases F and Costa-Bauzá A: Study of
factors affecting calcium oxalate crystalline aggregation. Br J
Urol. 66:240–244. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hughes RE, Ellery P, Harry T, Jenkins V
and Jones E: The dietary potential of the common nettle. J Sci Food
Agric. 31:1279–1286. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vogl S, Picker P, Milhaly-Bison J,
Fakhrudin N, Atanasov AG, Heiss EH, Wawrosch C, Reznicek G, Dirsch
VM, Saukel J and Kopp B: Ethanopharmacological in vitro studies on
Austria’s folk medicine - an unexplored lore in vitro
anti-inflammatory activities of 71 Austrian traditional herbal
drugs. J Ethanopharmacol. 149:750–771. 2013.PubMed/NCBI
|
|
14
|
Lopatkin N, Sivikov A, Walther C, Schläfke
S, Medvedev A, Avdeichuk J, Golubev G, Melnik K, Elenberger N and
Engelman U: Long-term efficiency and safety of a combination of
sabal and urtica extract for lower urinary tract symptoms - a
placebo-controlled, double-blind, multicenter trial. World J Urol.
23:139–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Safarinejad MR: Urtica dioica for
the treatment of benign prostatic hyperplasia: a prospective,
randomized, double-blind, placebo-controlled, crossover study. J
Herb Pharmacother. 5:1–11. 2005. View Article : Google Scholar
|
|
16
|
Khan SR and Glenton PA: Deposition of
calcium phosphate and calcium oxalate crystals in the kidneys. J
Urol. 153:811–817. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lorentz K: Improved determination of serum
calcium with 2-cresolphthalein complexone. Clin Chim Acta.
126:327–334. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hodgkinson A: Calcium-containing stones:
their causation and treatment. Postgrad Med J. 53(Suppl 2): 25–34.
1977.PubMed/NCBI
|
|
19
|
Bowers LD and Wong ET: Kinetic serum
creatinine assays. II A critical evaluation and review. Clin Chem.
26:555–561. 1980.PubMed/NCBI
|
|
20
|
Akbay P, Basaran AA, Undeger U and Basaran
N: In vitro immunomodulatory activity of flavonoid glycosides from
Urtica dioica L. Phytother Res. 17:34–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Budzianowski J: Caffeic acid esters from
Urtica dioica and U. urens. Planta Med. 57:5071991.
View Article : Google Scholar
|
|
22
|
Grevsen K, Frette XC and Christensen LP:
Concentration and composition of flavonol glycosides and phenolics
acids in aerial parts of stinging nettle (Urtica dioica L.)
are affected by nitrogen fertilization and by harvest times. Eur J
Hortic Sci. 73:20–27. 2008.
|
|
23
|
Kavtaradze NS, Alaniya MD and Aneli JN:
Chemical components of Urtica dioica growing in Georgia.
Chem Nat Compd. 37:2872001. View Article : Google Scholar
|
|
24
|
Basaran AA, Akbay P, Undeger U and Basaran
N: In vitro immunomodulatory and mutagenic activity of the
flavonoid glycosides from Urtica dioica L. Toxicology. 164:171–172.
2001.
|
|
25
|
Chaurasia N and Wichtl M: Flavonol
glycosides from Urtica dioica. Planta Med. 53:432–434.
1987.
|
|
26
|
Fu HY, Chen SJ, Chen RF, Ding WH,
Kuo-Huang LL and Huang RN: Identification of oxalic acid and
tartaric acid as major persistent pain-inducing toxins in the
stinging hairs of nettles, Urtica thunbergiana. Ann Bot.
98:57–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leal JJ and Finlayson B: Adsorption of
naturally occurring polymers onto calcium oxalate crystal surfaces.
Invest Urol. 14:278–283. 1977.PubMed/NCBI
|
|
28
|
King JS Jr: Etiologic factors involved in
urolithiasis: a review of recent research. J Urol. 97:583–591.
1967.PubMed/NCBI
|