Introduction
Bone is a dynamic tissue that constantly undergoes
remodeling. Bone remodeling is a coupled process of bone formation
mediated by osteoblasts and resorption regulated by osteoclasts,
which continues throughout life (1). An imbalance between bone formation
and resorption may result in excessive bone loss, which is a
feature of chronic inflammatory diseases, including rheumatoid
arthritis, osteomyelitis, bacterial arthritis and infection of
orthopedic implants (2).
Lipopolysaccharide (LPS), a component of the outer
membranes of all gram-negative bacteria, has been demonstrated to
be capable of inducing bone resorption in vivo and in vitro
(3–8). Furthermore, LPS is able to inhibit
osteoblast differentiation and function in cell culture (9–12).
However, effective therapies against bacteria-induced bone
destruction are limited to antibiotic regimens and surgical
strategies in chronic inflammatory diseases. Therefore, the
investigation and development of potential drugs that restore
osteoblast function remains a major goal in the prevention of bone
destruction in infective bone diseases.
Quercetin, a dietary flavonoid, has been highlighted
as a bioactive substance, due to its biological, pharmacological
and medicinal activities. Evidence suggests that quercetin inhibits
bone loss by affecting osteoclastogenesis and regulating a variety
of systemic and local factors, including hormones and inflammatory
cytokines (13–15). By contrast, the effect of quercetin
on osteoblastogenesis remains a matter of controversy (16–17).
Mitogen-ctivated protein kinases (MAPK) pathways,
including c-Jun N-terminal kinases (JNK), are involved in the
progression of inflammatory responses and cell apoptosis in
osteoblasts (18). Quercetin has
been reported to demonstrate an inhibitory effect on MAPK
activation and cyclooxygenase-2 (COX-2) expression induced by LPS
(19). In our previous study it
was identified that quercetin triggered apoptosis and inhibited
bone absorption of LPS induced-osteoclasts through activation of
MAPK p38 and JNK pathways (20).
Therefore, the aim of the present study was to examine the effect
of quercetin on osteoblast differentiation in MC3T3-E1 osteoblasts
stimulated with LPS. Furthermore, the mechanism underlying the
effect of quercetin on MAPK phosphorylation in MC3T3-E1 osteoblasts
stimulated with LPS was also examined.
Materials and methods
Reagents
Escherichia coli LPS (serotype 055:B5), quercetin,
β-glycerophosphate (β-GP),
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and
ascorbic acid were purchased from Sigma Chemical Co. (St. Louis,
MO, USA). The protease inhibitor cocktail, and the selective MAPK
inhibitors, PD98059 and SP600125, were purchased from Calbiochem
(San Diego, CA, USA). Dulbecco’s modified Eagle’s medium (DMEM),
fetal bovine serum (FBS) and penicillin/streptomycin were purchased
from Gibco (Gibco, Rockville, MD, USA). Antibodies against JNK,
phosphorylated JNK, extracellular signal-regulated kinase (ERK1/2)
and phosphorylated ERK 1/2 were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Antibodies against Osterix
(Osx) and β-actin were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). MC3T3-E1 cells, an osteoblast-like cell
line, was obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Other chemicals and reagents used in the
present study were of analytical grade.
Cell culture
MC3T3-E1 cells were grown in DMEM supplemented with
10% (v/v) FBS, 1% (v/v) penicillin-streptomycin solution, 10 mM
HEPES solution and incubated at 37°C in 5% CO2 humidified air. To
examine the effect of quercetin on osteoblast differentiation
stimulated with LPS, MC3T3-E1 cells at 5×104 cells/cm2 were
cultured in osteogenic differentiation medium (DMEM with 10% FBS,
10 mM HEPES, 50 μg/ml L-ascorbic acid and 5 mM β-GP) for two days.
On differentiation day three, the cells were treated with LPS at
100 ng/ml or without LPS in osteogenic differentiation medium for
one day. The cells were then incubated with various concentrations
of quercetin (5, 10, 15, 25 or 50 μM) or without quercetin in the
presence of LPS (100 ng/ml) for the indicated times.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from MC3T3-E1 cells treated
with 10, 50, 100, 200 or 1,000 ng/ml LPS for 24 h, or treated with
5, 10, 15, 25 or 50 μM quercetin in the presence of 100 ng/ml LPS
for 24 h. Total RNA was isolated using TRIzol reagent (Invitrogen,
Grand Island, NY, USA) and quantified by spectrophotometry
(Eppendorf BioSpectrometer; Eppendorf AG, Hamburg, Germany).
Following isolation, 3 μg total RNA from each sample was reverse
transcribed utilizing the HiFi-MMLV cDNA kit (Beijing CoWin Biotech
Co. Ltd., Beijing, China) according to the manufacturer’s
instructions. Primers for alkaline phosphatase (ALP), runt-related
transcription factor 2 (Runx2), Osx, bone sialoprotein (BSP),
osteocalcin (OCN), type I collagen α1 (Col1α1) and β-actin (Sangon
Biotech Co. Ltd, Shanghai, China) and the annealing temperatures
used in the present study are listed Table I. In each reaction, 1 μl cDNA, 12.5
μl 2X Taq MasterMix (Beijing CoWin Biotech Co. Ltd., Beijing,
China) and 0.4 μM forward and reverse primer in a total volume of
25 μl were used. The initial denaturation was performed at 94°C for
3 min. Then, the products were subjected to denaturation at 94°C
for 30 sec, annealing temperature for 30 sec, extension at 72°C for
30 sec for 32 cycles and a final elongation at 72°C for 5 min. The
PCR products were separated by 1.5% agarose gel electrophoresis,
photographed using Gel-Doc (Bio-Rad, Hercules, CA, USA) and
quantified by density determination using Quantity One image
analysis software (Bio-Rad). β-actin was used as the internal
control.
 | Table IPrimer sequences and cycle conditions
for polymerase chain reaction amplification. |
Table I
Primer sequences and cycle conditions
for polymerase chain reaction amplification.
| Gene | Primer sequences
(5′-3′) | Accession number | Annealing temperature
(°C) | Product size
(bp) |
|---|
| ALP | F:
GAGCGTCATCCCAGTGGAG
R: TAGCGGTTACTGTAGACACCC | NM_007433 | 62 | 158 |
| Runx2 | F:
TTCAACGATCTGAGATTTGTGGG
R: GGATGAGGAATGCGCCCTA | NM_001146038 | 62 | 221 |
| Osx | F:
ATGGCGTCCTCTCTGCTTG
R: TGAAAGGTCAGCGTATGGCTT | NM_130458 | 62 | 156 |
| BSP | F:
CAGGGAGGCAGTGACTCTTC
R: AGTGTGGAAAGTGTGGCGTT | NM_008318 | 58 | 158 |
| OCN | F:
GAGGGCAATAAGGTAGTGAA
R: CATAGATGCGTTTGTAGGC | NM_001037939 | 62 | 160 |
| Col1α1 | F:
CCCTGCCTGCTTCGTGTA
R: TTGAGTTTGGGTTGTTCGTC | BC003198 | 63 | 101 |
| JNK1 | F:
CTCCAGCACCCATACATC
R: CATTGACAGACGGCGAAG | BC053027 | 62 | 247 |
| ERK1 | F:
GAGCGGCTGAAGGAGTTG
R: GGGATTGGAGTGGGAGAA | NM_011952 | 62 | 260 |
| β-actin | F:
GGCTGTATTCCCCTCCATCG
R: CCAGTTGGTAACAATGCCATGT | NM_007393 | 60 | 154 |
Quantitative (q)PCR
Total RNA was isolated using TRIzol reagent
(Invitrogen) and quantified by spectrophotometry. Following
isolation, 3 μg total RNA from each sample was reverse transcribed
utilizing the HiFi-MMLV cDNA kit (Beijing CoWin Biotech) according
to the manufacturer’s instructions. The primer sequences of JNK1
and ERK1 (Sangon Biotech Co. Ltd, Shanghai, China) and annealing
temperatures used in this study are also listed in Table I. qPCR was performed with a
RealSYBR mixture (Beijing CoWin Biotech Co. Ltd.) according to the
manufacturer’s instructions. All qPCR reactions were performed
using the ABI PRISM 7700 sequence detection system (Applied
Biosystems, Grand Island, NY, USA). In each reaction, 1 μl cDNA, 10
μl 2X RealSYBR mixture, and 0.25 μM forward and reverse primer in a
total volume of 20 μl were used. The reaction condition was as
follows: One cycle of 95°C for 5 min followed by 40 cycles of 95°C
for 15 sec, annealing temperature for 30 sec and extension at 72°C
for 30 sec. qPCR for each sample was run in triplicate. β-actin was
used as the internal control and all of the results were analyzed
using the standard 2-ΔΔCT method as described previously (21).
Western blot analysis
At the end of treatment, the cell culture medium was
aspirated and the cells were detached in phosphate-buffered saline
by scrapping. The detached cells were centrifuged at 15,000 × g at
4°C for 15 min. The cell pellets were then lysed in 300 μl lysis
buffers (Cytobuster protein extraction reagent; Novagen, Darmstadt,
Germany) with 25 mM NaF, 1 mM Na3VO4 and 1X protease inhibitor
cocktail. The protein concentrations were quantified by
spectrophotometry. For western blotting, equal quantities of
protein from each sample were loaded on SDS-PAGE and
electrotransferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). These membranes were then blocked
with 5% (w/v) bovine serum albumin in TBST [10 mM Tris, 150 mM NaCl
and 0.1% (v/v) Tween-20; pH 7.5] for 1 h at room temperature and
incubated overnight at 4°C with the following primary antibodies:
Rabbit polyclonal anti-JNK, phosphorylated JNK and β-actin, goat
polyclonal anti-Osx (all 1:500; Santa Cruz Biotechnology, Inc.) and
rabbit polyclonal anti-ERK1/2 and phosphorylated ERK1/2 (1:1,000;
Cell Signaling Technology, Inc.). The secondary antibodies used for
detected were horseradish peroxidase (HRP)-conjugated goat
anti-rabbit immunoglobulin (Ig)G (Santa Cruz Biotechnology, Inc.)
and HRP-conjugated rabbit anti-goat IgG (Santa Cruz Biotechnology,
Inc). Incubation was conducted at room temperature for 2 h (Santa
Cruz Biotechnology, Inc.). Enhanced chemiluminescence (Beyotime,
Shanghai, China) was used to detect the immunoreactive protein
signals. The protein signals were visualized on films, then scanned
and quantified using the Image J software (National Institutes of
Health Image, Bethesda, MA, USA). For re-probing, PVDF membranes
were stripped with 0.2 M NaOH for 10 min prior to blocking with
another primary antibody. The expression of the molecules of
interest was determined relative to that of β-actin.
Statistical analysis
The data are expressed as the mean ± standard
deviation for three or more independent experiments. The
significant differences were determined using factorial analysis of
variance. Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of quercetin on the mRNA
expression levels of osteoblast-specific genes in MC3T3-E1 cells
stimulated with LPS
Effect of quercetin on the mRNA expression levels of
osteoblast-specific genes, including ALP, Osx, Runx2, Col1α1, OCN
and BSP in the MC3T3-E1 cells stimulated with LPS was determined by
RT-PCR. The mRNA expression levels of osteoblast-specific genes in
MC3T3-E1 cells were significantly inhibited by LPS, whereas
quercetin significantly restored the LPS-suppressed mRNA expression
of osteoblast-related genes in a dose-dependent manner in MC3T3-E1
cells (Fig. 1). Quercetin at 50 μM
was demonstrated to completely restore mRNA expression levels of
osteoblast-specific genes compared with the non-quercetin-treated
cultures in MC3T3-E1 cells stimulated with LPS.
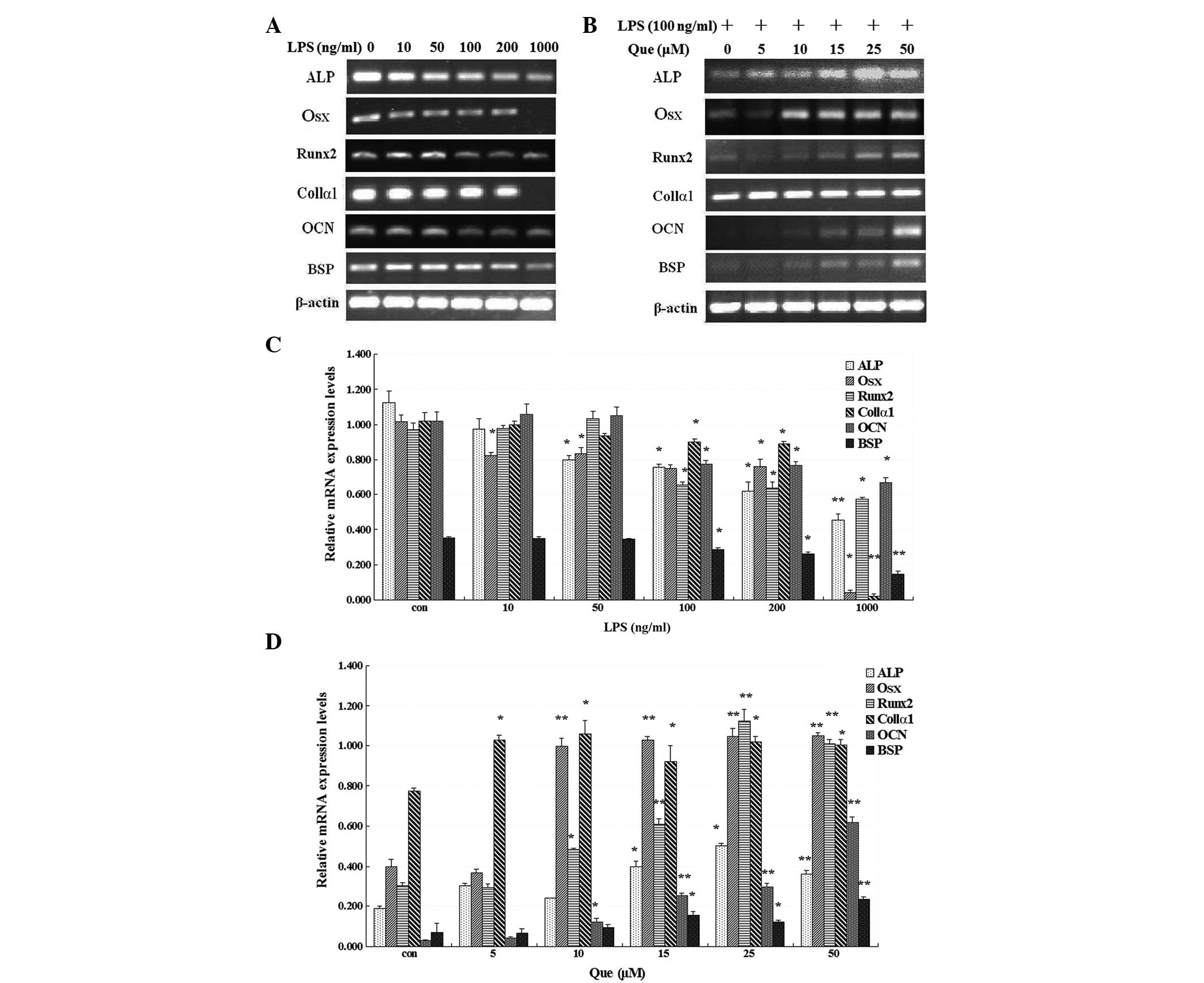 | Figure 1mRNA expression of ALP,
OSX, Runx2, Col1α1, OCN and BSP
(A) following treatment with LPS at concentrations of 10, 50, 100,
200 and 1,000 ng/ml, or without LPS, for 24 h or (B) with Que
treatment (0, 5, 10, 15, 25, or 50 μM) for one day in MC3T3-E1
cells stimulated with LPS (100 ng/ml). (C) MC3T3-E1 cells were
treated with LPS (0, 10, 50, 200 and 1,000 ng/ml) for 24 h and (D)
with Que at concentrations of 0, 5, 10, 15, 25 and 50 μM.
*P<0.05, and **P<0.01, compared with
the control and the other groups. Data represent the mean ±
standard deviation from three independent experiments. LPS,
lipopolysaccharide; Que, quercetin; ALP, alkaline
phosphatase; Osx, osterix; Runx2, runt-related
transcription factor 2; Col1α1, type I collagen α1;
OSN, osteocalcin; BSP, bone sialoprotein. |
Effect of quercetin on the protein level
of Osx in MC3T3-E1 cells stimulated with LPS
The protein level of Osx in MC3T3-E1 cells was
significantly inhibited by LPS after cell exposure to LPS >16 h
(Fig. 2A). To examine the effect
of quercetin on the protein level of Osx in MC3T3-E1 cells
stimulated with LPS, the cells were treated with LPS at 100 ng/ml
for one day. Then, the cells were incubated with various
concentrations of quercetin (5, 10, 15, 25 or 50 μM), or without
quercetin, in the presence of LPS (100 ng/ml) for one day.
Quercetin significantly upregulated the protein expression of Osx
in a dose-dependent manner in MC3T3-E1 cells stimulated with LPS at
one day compared with the non-quercetin-treated cultures (Fig. 2B).
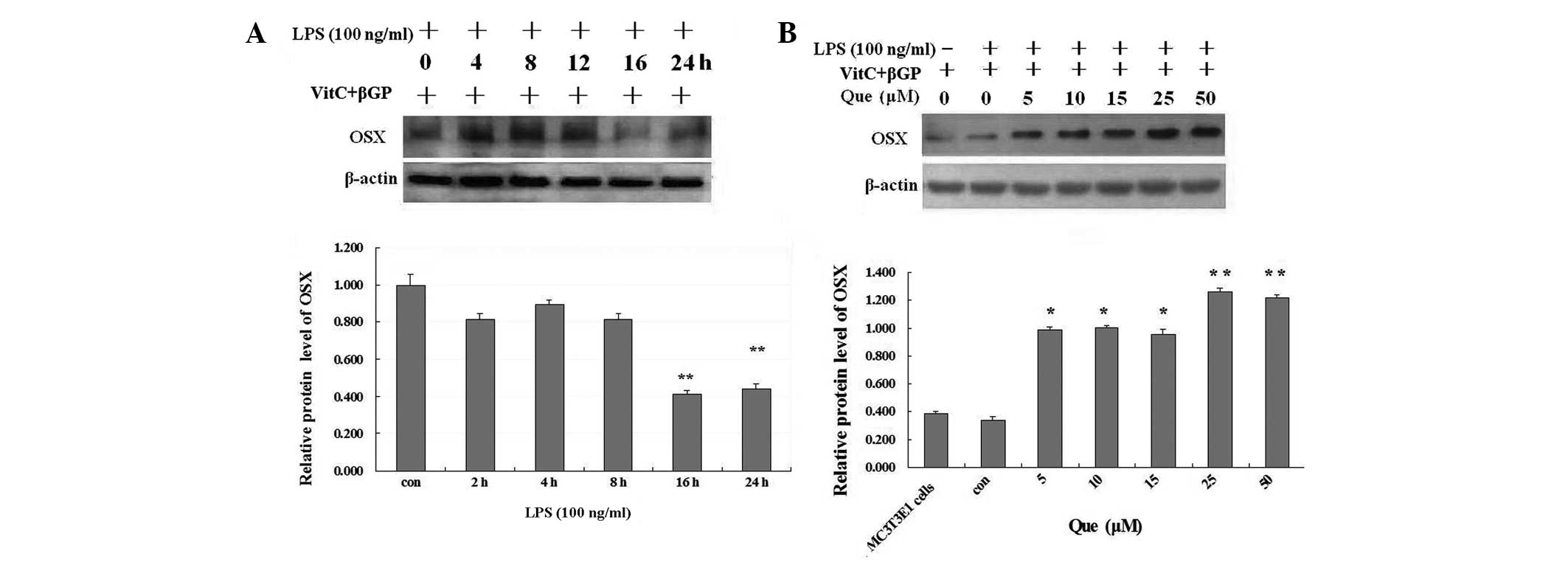 | Figure 2(A) Effect of LPS on the protein
expression of Osx in MC3T3-E1 cells. MC3T3-E1 cells were treated
with LPS at 100 ng/ml or without LPS for 24 h. (B) Effect of Que on
the protein expression of Osx in MC3T3-E1 cells stimulated with
LPS. MC3T3-E1 cells were treated with Que at concentrations of 5,
10, 15, 25 and 50 μM, or without Que, for one day in the presence
of LPS (100 ng/ml). *P<0.05 and
**P<0.01, compared with the control and the other
groups. LPS, lipopolysaccharide; Que, quercetin; Osx, osterix;
β-GP, β-glycerophosphate. |
Effect of quercetin on the protein levels
of MAPKs in MC3T3-E1 cells stimulated with LPS
MC3T3-E1 cells at 5×104 cells/cm2 were cultured in
osteogenic differentiation medium for two days. On differentiation
day three, the cells were treated with LPS at 100 ng/ml in
osteogenic differentiation medium for one day. Then, the cells were
incubated with various concentrations of quercetin (5, 10, 15, 25
or 50 μM), or without quercetin, in the presence of LPS (100 ng/ml)
for 2 h or 30 min. Quercetin significantly enhanced the protein
levels of phosphorylated ERK1/2 and decreased the protein levels of
phosphorylated JNK (Fig. 3).
Quercetin at 50 μM was demonstrated to inhibit the protein
expression of phosphorylated JNK compared with the other
quercetin-treated cultures.
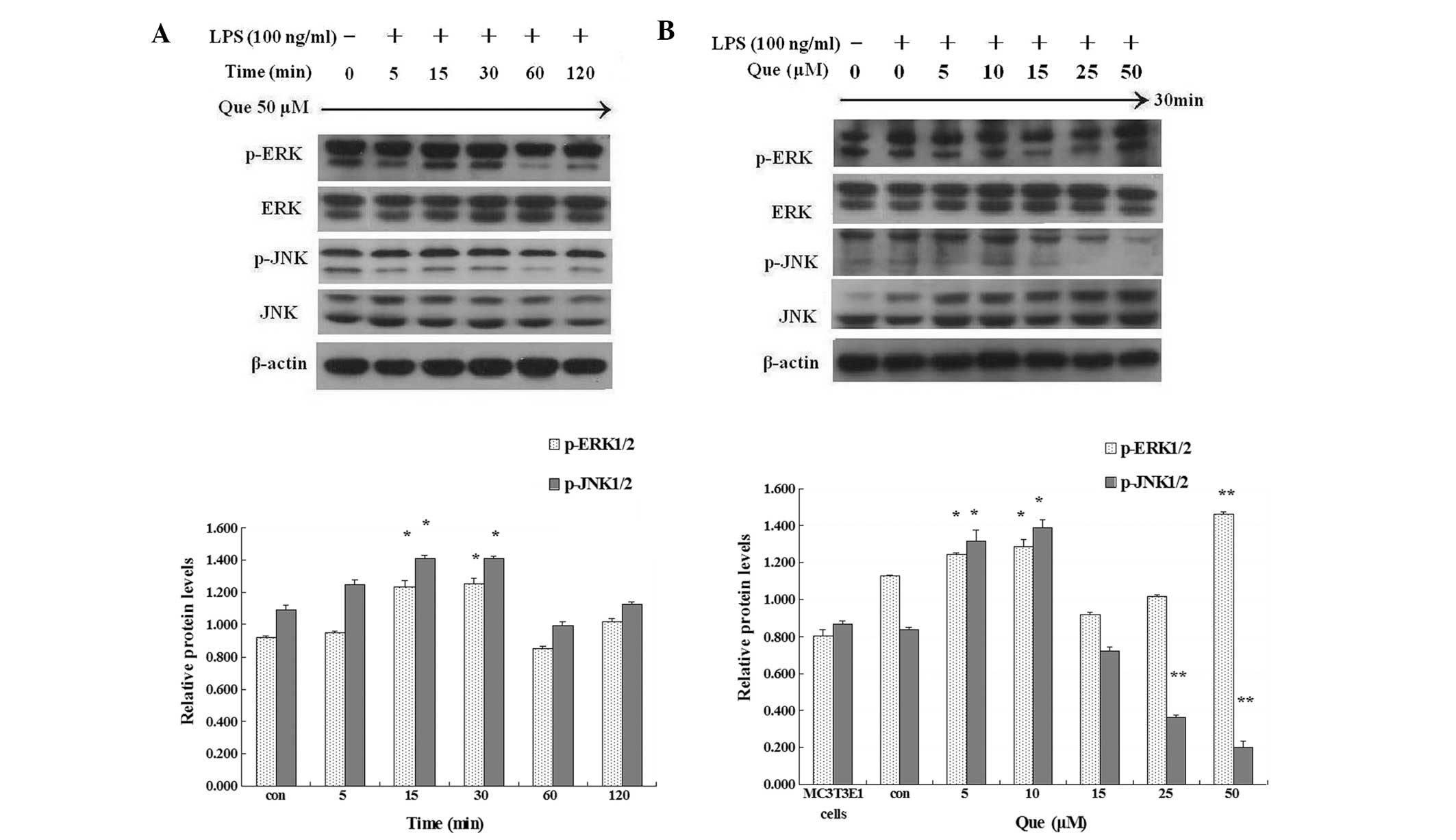 | Figure 3(A) Effect of Que at 50 μM on the
protein expression of MAPKs in MC3T3-E1 cells in the presence of
LPS (100 ng/ml) for 2 h. (B) Effect of Que at 5, 10, 15, 25 or 50
μM, or without Que, on the protein expression of MAPKs in MC3T3-E1
cells in the presence of LPS (100 ng/ml) for 30 min.
*P<0.05 and **P<0.01. Data represent
the mean ± standard deviation from three independent experiments.
MAPKs, mitogen-activated protein kinases; LPS, lipopolysaccharide;
Que, quercetin; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p, phosphorylated. |
MAPK inhibitors, PD98059 and SP600125, were applied
for 2 h prior to quercetin treatment and the protein samples were
prepared 30 min following quercetin treatment. The results
demonstrated that MAPK inhibitors selectively decreased the
quercetin-enhanced protein level of phosphorylated ERK1/2 (by
PD98059), while restored quercetin-induced downregulation of the
protein expression of JNK (by SP600125; Fig. 4).
Effect of quercetin on the mRNA
expression levels of MAPK in MC3T3-E1 cells stimulated with
LPS
MC3T3-E1 cells were cultured in osteogenic
differentiation medium with 100 ng/ml LPS for one day. Then, the
cells were incubated with various concentrations of quercetin (5,
10, 15, 25 or 50 μM), or without quercetin, in the presence of LPS
(100 ng/ml) for one day. The results demonstrated that quercetin
enhanced the mRNA expression of ERK1, which was downregulated by
LPS, and decreased the mRNA expression of JNK1, which was
upregulated by LPS in a dose-dependent manner in MC3T3-E1 cells
(Fig. 5).
 | Figure 5Effect of Que on the mRNA expression
of MAPKs in MC3T3-E1 cells stimulated with LPS. (A) The mRNA
expression of ERK1 and JNK1 following LPS treatment
(10, 50, 100, 200 and 1,000 ng/ml), or without LPS, for one day in
MC3T3-E1 cells. (B) The mRNA expression of ERK1 and
JNK1 following Que treatment (5, 10, 15, 25 or 50 μM), or
without Que, for one day in the presence of LPS (100 ng/ml) in
MC3T3-E1 cells. *P<0.05 and **P<0.01,
compared with the control and the other groups. Data represent the
mean ± standard deviation from three independent experiments. Que,
quercetin; MAPKs, mitogen-activated protein kinases; LPS,
lipopolysaccharide; ERK, extracellular signal-regulated kinase;
JNK, c-Jun N-terminal kinase. |
Discussion
Excessive bone resorption in chronic inflammatory
diseases, including septic arthritis, osteomyelitis and infected
orthopedic implant failure, is at least partially caused by
bacteria-induced activation of inflammatory responses (2). LPS, a pro-inflammatory glycolipid
component of the gram-negative bacteria cell wall, is well
documented in mediation with gram-negative bacterial bone
destruction. LPS stimulates osteoclastic bone resorption in vivo
(3,4), and promotes osteoclast
differentiation in whole bone marrow cell culture (5) or in preosteoclasts (6,7).
Furthermore, LPS also inhibits osteoblast differentiation and
function in vitro (9–12). Therefore, the investigation of
potential drugs that restore osteoblast function remains a major
goal in the prevention of bone destruction in infective bone
diseases. In our previous study it was identified that quercetin
triggered the apoptosis and inhibited bone absorption of LPS
induced-osteoclasts through activation of the MAPK p38 and JNK
pathways (20). In the present
study, the effect of quercetin on osteoblast differentiation in
MC3T3-E1 osteoblasts stimulated with LPS was examined. The results
demonstrated that quercetin reversed the inhibition of osteoblast
differentiation induced by LPS through MAPK signaling.
The reverse effect of quercetin on LPS-induced
inhibition of osteoblast differentiation was confirmed by examining
the effect of quercetin treatment on mRNA expression levels of
osteoblast-related genes. ALP has been suggested to be involved in
the early-stage molecular events of osteoblast differentiation,
whereas OCN and BSP are involved in the late-stage molecular events
(18,22). Col1, the most abundant and widely
distributed type of collagen, is required and sufficient for
extracellular matrix mineralization to occur in bone. Col1 is
composed of two chains, α1 and α2, encoded by two distinct genes,
Col1α1 and Col1α2, which are highly expressed in osteoblasts
(22–24). In the present study, the mRNA
expression levels of ALP, Osx, Runx2, Col1α1, OCN and BSP in
MC3T3-E1 cells were significantly downregulated by LPS. By
contrast, quercetin significantly restored the LPS-suppressed mRNA
expression of osteoblast-related genes in a dose-dependent manner.
The data indicated that the reverse effect of quercetin on
LPS-induced inhibition of osteoblast differentiation may be due to
the restored expression of osteoblast-related genes.
Bone formation is a highly regulated process
involving the differentiation of mesenchymal stem cells to
osteoblasts. Runx2 (Cbfa1) is required for mesenchymal cell
differentiation into preosteoblasts (25). Osx, downstream of Runx2, is an
osteoblast-specific transcription factor essential for osteoblast
differentiation and bone formation (26). Forced expression of Osx in vitro
induces the expression of several osteoblastic genes, including
Col1α1 and osteocalcin (27,28).
The present study demonstrated that quercetin restored the
expression of Osx and Runx2, which were suppressed by LPS in
MC3T3-E1 cells. Based on these results, it is suggested that
restoration of osteoblast differentiation induced by quercetin also
resulted from enhancing the expression of Osx and Runx2.
MAP kinases are activated by various stresses,
including LPS and affect apoptosis either positively or negatively
(29). In numerous cell types, JNK
and p38 MAPK contribute to the induction of apoptosis, whereas ERK
inhibits apoptotic processes (30–32).
In the present study, treatment with quercetin enhanced the protein
levels of phosphorylated ERK1/2, whereas it reduced the protein
levels of phosphorylated JNK. Quercetin also enhanced the mRNA
expression of ERK1, while decreased mRNA expression of JNK1 in a
dose-dependent manner in MC3T3-E1 cells stimulated with LPS. The
quercetin-enhanced phosphorylation of ERK1/2 was attenuated by
PD98059; while SP600125 restored quercetin-induced downregulation
of phosphorylated JNK. The present study confirms that quercetin
was able to restore the inhibition of osteoblast differentiation
induced by LPS through MAPK signaling (18).
In conclusion, these data suggest that quercetin may
reverse LPS-induced inhibition of osteoblast differentiation
through MAPK signaling. These results indicate that quercetin may
be an effective drug for the treatment of abnormal human bone loss
induced by LPS in chronic inflammatory diseases.
Acknowledgements
The present study was supported by a research grant
from the National Natural Science Foundation of China (no.
81371981) and The School Fund of Luohe Medical College (no
2012-DF001).
References
|
1
|
Roodman GD: Advances in bone biology: the
osteoclast. Endocr Rev. 17:308–332. 1996.PubMed/NCBI
|
|
2
|
Nair SP, Meghji S, Wilson M, et al:
Bacterially induced bone destruction: Mechanisms and
misconceptions. Infect Immun. 64:2371–2380. 1996.PubMed/NCBI
|
|
3
|
Orcel P, Feuga M, Bielakoff J and De
Vernejoul MC: Local bone injections of LPS and M-CSF increase bone
resorption by different pathways in vivo in rats. Am J Physiol.
264:E391–E397. 1993.PubMed/NCBI
|
|
4
|
Chiang CY, Kyritsis G, Graves DT and Amar
S: Interleukin-1 and tumor necrosis factor activities partially
account for calvarial bone resorption induced by local injection of
lipopolysaccharide. Infect Immun. 67:4231–4236. 1999.
|
|
5
|
Itoh K, Udagawa N, Kobayashi K, et al:
Lipopolysaccharide promotes the survival of osteoclasts via
Toll-like receptor 4, but cytokine production of osteoclasts in
response to lipopolysaccharide is different from that of
macrophages. J Immunol. 170:3688–3695. 2003. View Article : Google Scholar
|
|
6
|
Islam S, Hassan F, Tumurkhuu G, et al:
Bacterial lipopolysaccharide induces osteoclast formation in RAW
264.7 macrophage cells. Biochem Biophys Res Commun. 360:346–351.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mörmann M, Thederan M, Nackchbandi I, et
al: Lipopolysaccharides (LPS) induce the differentiation of human
monocytes to osteoclasts in a tumour necrosis factor (TNF)
alpha-dependent manner: a link between infection and pathological
bone resorption. Mol Immunol. 45:3330–3337. 2008.PubMed/NCBI
|
|
8
|
Zou W and Bar-Shavit Z: Dual modulation of
osteoclast differentiation by lipopolysaccharide. J Bone Miner Res.
17:1211–1218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadono H, Kido J, Kataoka M, et al:
Inhibition of osteoblastic cell differentiation by
lipopolysaccharide extract from Porphyromonas gingivalis. Infect
Immun. 67:2841–2846. 1999.PubMed/NCBI
|
|
10
|
Xing Q, Ye Q, Fan M, et al: Porphyromonas
gingivalis lipopolysaccharide inhibits the osteoblastic
differentiation of preosteoblasts by activating Notch 1 signaling.
J Cell Physiol. 225:106–114. 2010. View Article : Google Scholar
|
|
11
|
Bandow K, Maeda A, Kakimoto K, et al:
Molecular mechanisms of the inhibitory effect of lipopolysaccharide
(LPS) on osteoblast differentiation. Biochem Biophys Res Commun.
402:755–761. 2010. View Article : Google Scholar
|
|
12
|
Ochi H, Hara Y, Tagawa M, et al: The roles
of TNFR1 in lipopolysaccharide-induced bone loss: dual effects of
TNFR1 on bone metabolism via osteoclastogenesis and osteoblast
survival. J Orthop Res. 28:657–663. 2010.PubMed/NCBI
|
|
13
|
Wattel A, Kamel S, Mentaverri R, et al:
Potent inhibitory effect of naturally occurring flavonoids
quercetin and kaempferol on in vitro osteoclastic bone resorption.
Biochem Pharmacol. 65:35–42. 2003. View Article : Google Scholar
|
|
14
|
Woo JT, Nakagawa H, Notoya M, et al:
Quercetin suppresses bone resorption by inhibiting the
differentiation and activation of osteoclasts. Biol Pharm Bull.
27:504–509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqui JA, Swarnkar G, Sharan K, et al:
A naturally occurring rare analog of quercetin promotes peak bone
mass achievement and exerts anabolic effect on osteoporotic bone.
Osteoporos Int. 22:3013–3027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prouillet C, Mazière JC, Mazière C, et al:
Stimulatory effect of naturally occurring flavonols quercetin and
kaempferol on alkaline phosphatase activity in MG-63 human
osteoblasts through ERK and estrogen receptor pathway. Biochem
Pharmacol. 67:1307–1313. 2004. View Article : Google Scholar
|
|
17
|
Notoya M, Tsukamoto Y, Nishimura H, et al:
Quercetin, a flavonoid, inhibits the proliferation,
differentiation, and mineralization of osteoblasts in vitro. Eur J
Pharmacol. 485:89–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuguchi T, Chiba N, Bandow K, et al:
JNK activity is essential for Atf4 expression and late-stage
osteoblast differentiation. J Bone Miner Res. 24:398–410. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gutiérrez-Venegas G, Jiménez-Estrada M and
Maldonado S: The effect of flavonoids on transduction mechanisms in
lipopolysaccharide-treated human gingival fibroblasts. Int
Immunopharmacol. 7:1199–1210. 2007.PubMed/NCBI
|
|
20
|
Guo C, Hou GQ, Li XD, et al: Quercetin
triggers apoptosis of lipopolysaccharide (LPS)-induced osteoclasts
and inhibits bone resorption in RAW264.7 cells. Cell Physiol
Biochem. 30:123–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
22
|
Kim HK, Cho SG, Kim JH, et al: Mevinolin
enhances osteogenic genes (ALP, type I collagen and osteocalcin),
CD44, CD47 and CD51 expression during osteogenic differentiation.
Life Sci. 84:290–295. 2009. View Article : Google Scholar
|
|
23
|
Thunyakitpisal P, Alvarez M, Tokunaga K,
et al: Cloning and functional analysis of a family of nuclear
matrix transcription factors (NP/NMP4) that regulate type I
collagen expression in osteoblasts. J Bone Miner Res. 16:10–23.
2001. View Article : Google Scholar
|
|
24
|
Kern B, Shen J, Starbuck M and Karsenty G:
Cbfa1 contributes to the osteoblast-specific expression of type I
collagen genes. J Biol Chem. 276:7101–7107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Komori T, Yagi H, Nomura S, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar
|
|
26
|
Nakashima K, Zhou X, Kunkel G, et al: The
novel zinc finger-containing transcription factor osterix is
required for osteoblast differentiation and bone formation. Cell.
108:17–29. 2002. View Article : Google Scholar
|
|
27
|
Fu H, Doll B, McNelis T and Hollinger JO:
Osteoblast differentiation in vitro and in vivo promoted by
Osterix. J Biomed Mater Res A. 83:770–778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kurata H, Guillot PV, Chan J and Fisk NM:
Osterix induces osteogenic gene expression but not differentiation
in primary human fetal mesenchymal stem cells. Tissue Eng.
13:1513–1523. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pearson G, Robinson F, Beers Gibson T, et
al: Mitogen-activated protein (MAP) kinase pathways: regulation and
physiological functions. Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
30
|
Xia Z, Dickens M, Raingeaud J, et al:
Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis.
Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fister S, Günthert AR, Aicher B, et al:
GnRH-II antagonists induce apoptosis in human endometrial, ovarian,
and breast cancer cells via activation of stress-induced MAPKs p38
and JNK and proapoptotic protein Bax. Cancer Res. 69:6473–6481.
2009. View Article : Google Scholar
|
|
32
|
Park GB, Kim YS, Lee HK, et al:
Endoplasmic reticulum stress-mediated apoptosis of EBV-transformed
B cells by cross-linking of CD70 is dependent upon generation of
reactive oxygen species and activation of p38 MAPK and JNK pathway.
J Immunol. 185:7274–7284. 2010. View Article : Google Scholar
|



















