Introduction
Cervical cancer is a common malignancy in females,
which is associated with a severe risk of cancer-associated
mortality. The etiology and pathogenesis of cervical cancer are not
yet fully understood (1). However,
increased risk is considered to be associated with marriage at a
young age, multiparity, cervical laceration, poor local hygiene and
numerous other factors. In the past two decades, the viral etiology
of cervical cancer has received increasing attention (2). Human papilloma virus (HPV) infection
is considered to be an important pathogenic risk factor for
cervical cancer (3). Currently,
surgery remains the most effective treatment option for cervical
cancer. However, upon development of infiltrating lesions, the
disease progresses very rapidly and often results in mortality
within 2–5 years if untreated (4,5).
Therefore, the occurrence of tumor metastasis often causes failure
of cervical cancer treatment (6).
With recent advances in oncological research,
microRNA (miRNA/miR) has attracted interest among researchers as a
potential biotherapy. miRNAs are a class of endogenous,
small-molecule, non-coding, single-stranded RNAs, which are ~19–24
nucleotides (nt) long (7). miRNAs
have been shown to be highly conserved during evolution. The
specific complementary pairing of miRNAs with the bases of target
gene mRNA, may result in the degradation or inhibition of the
target gene mRNA translation, leading to widespread negative
regulation of target gene expression (8). Currently, >400 miRNAs have been
identified, which have the ability to regulate the expression
levels of one third of the genes in the human body (9). Mutations of miRNA can activate the
expression of associated oncogenes or inhibit the expression of
tumor suppressor genes, leading to carcinogenesis (10).
To date, the role of miRNAs in cancer progression
remains unclear. A previous study demonstrated that miR-146b-5p is
able to inhibit the growth and metastasis of various types of
cancer (11). The present study
investigated the inhibitory effects and mechanisms of miR-146b-5p
on the proliferation and metastatic potential of Caski human
cervical cancer cells. The aim was to determine a novel direction
for cancer biotherapy and provide a potential target for the
prevention and determination of cancer metastasis.
Materials and methods
Cells and cell culture
The Caski human cervical cancer cell line (American
Type Culture Collection, Manassas, VA, USA) was routinely
subcultured in Dulbecco’s modified Eagle’s medium (Corning Life
Sciences, Manassas, VA, USA) supplemented with 10% fetal bovine
serum (FBS; Corning Life Sciences), 100 U/ml penicillin and 100
U/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C, in a
humidified atmosphere containing 5% CO2. Upon reaching
80% confluence, the cells were digested with 0.25% trypsin,
followed by repetitive pipetting to disperse the cells into a
single-cell suspension for routine subculture. The present study
was approved by the ethics committee of Qilu Hospital of Shandong
University (Jinan, China).
The expression plasmid, pGPU6/Neo-miR-146b-5p, and
the nonsense scrambled sequence, pGPU6/Neo-NC, were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). The cells
(3×105) were seeded into six-well plates and cultured
for 24 h at 37°C with 5% CO2, followed by transfection
with the pGPU6/Neo-miR-146b-5p or pGPU6/Neo-NC plasmids, using
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer’s instructions.
To establish the stable sub-cell lines, the cells were screened
with 200 mg/l G418 (Sigma-Aldrich). Untreated Caski cells were
considered as the control group, Caski cells transfected with
pGPU6/Neo-NC were the negative group and Caski cells transfected
with pGPU6/Neo-miR-146b-5p were the miR-146b-5p group. The
expression of miR-146b-5p was quantified using stem-loop
quantitative polymerase chain reaction (qPCR), with U6 as the
internal control (12).
MTS cell proliferation assay
A total of 2×103 cells were seeded into
96-well plates and cultured at 37°C for 24 h in an atmosphere
containing 5% CO2. Fresh culture medium and 20 μl MTS
solution (Promega Corp., Madison, WI, USA) were then added to each
well and cultured for 2 h. The absorbance was detected at 490 nm
using a microplate reader (iMark; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A cell growth curve was then generated with
time as the abscissa and the absorbance value as the ordinate.
Transwell invasion assay
The bottom of transwell chambers was coated with 60
μl Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). A total of
5×105 cells in 200 μl serum-free culture medium were
added to the upper chamber and 600 μl complete culture medium,
containing 10% FBS, was added to the lower chamber that was
pre-coated with 10 μg/ml fibronectin (Sigma-Aldrich). Next, the
samples were incubated for 24 h at 37°C and 5% CO2. The
chamber membranes were then stained with crystal violet
(Sigma-Aldrich) at room temperature for 25 min and washed three
times with phosphate-buffered saline (PBS). Subsequently, the cells
of four randomly selected central and peripheral visual fields were
counted (BX53; Olympus Corp., Tokyo, Japan).
Cell adhesion assay
A total of 5×104 cells were seeded into a
96-well plate that was pre-coated with Matrigel and incubated at
37°C in a 5% CO2 incubator for 3 h. The cells were then
stained with 0.1% crystal violet for 25 min and washed five times
with PBS. Subsequently, 100 μl 10% acetic acid was added to each
well and the cells were lysed for 10 min. The absorbance values of
the samples were detected at 570 nm using a microplate reader.
Cell cycle determination
To investigate the cell cycle, 3×105
cells were seeded into 6-well plates and incubated at 37°C in a 5%
CO2 incubator for 24 h. Following digestion with 0.25%
Trypsin (Sigma-Aldrich) and centrifugation at 500 × g for 5 min,
70% pre-cooled ethanol was added to the cells, which were fixed at
4°C overnight. Next, the samples were centrifuged and the ethanol
solution was discarded, followed by staining with propidium iodide
(Sigma-Aldrich) containing DNaseA (Beyotime, Shanghai, China) for
30 min in the dark. The distribution of cells in the cell cycle was
then determined using a flow cytometer (FACSAria; BD
Biosciences).
qPCR
A total of 3×105 cells were seeded into
6-well plates and incubated at 37°C in an atmosphere containing 5%
CO2 for 24 h. Total RNA (200 ng/μl) was extracted from
the cells using TRIzol® reagent (Invitrogen Life
Technologies). RNA was then reverse transcribed into cDNA,
according to the manufacturer’s instructions of the RNA reverse
transcription kit (Invitrogen Life Technologies). cDNA was mixed
with ABI SYBR® Green Master Mix (Applied Biosystems Life
Technologies, Foster City, NJ, USA) and specific gene primers,
followed by amplification using an ABI 7500 Real-time PCR system
(Applied Biosystems Life Technologies). The primer sequences
(Invitrogen Life Technologies) used were as follows: C-X-C
chemokine receptor type 4 (CXCR4) forward,
5′-ATCTTCCTGCCCACCATCTACTCCATCATC-3′, and reverse,
5′-ATCCAGACGCCAACATAGACCACCTTTTCA-3′; matrix metalloproteinase
(MMP)-2 forward, 5′-TGATGGTGTCTGCTGGAAAG-3′, and reverse,
5′-GACACGTGAAAAGTGCCTTG-3′; MMP-9 forward, 5′-GCCTTTGGACACGCACG-3′,
and reverse, 5′-AGCGGTCCTGGCAGAAATAG-3′; c-Myc forward,
5′-TACCCTCTCAACGACAGCAG-3′, and reverse,
5′-TCTTGACATTCTCCTCGGTG-3′; cyclin D1 forward,
5′-TCTAAGATGAAGGAGACCATC-3′, and reverse,
5′-GCGGTAGTAGGACAGGAAGTTGTT-3′; human papilloma virus (HPV)16
forward, 5′-GTCAAAAGCCACTGTGTCCT-3′, and reverse,
5′-CCATCCATTACATCCCGAC-3′; p27 forward, 5′-CAAGTACGAGTGGCAAGAGG-3′,
and reverse, 5′-GTAGAAGAATCGTCGGTTGC-3′; and p53 forward,
5′-AACCTACCAGGGCAGCTACG-3′, and reverse 5′-TTCCTCTGTGCGCCGGTCTC-3′.
The results were normalized to GAPDH forward,
5′-GCAGGGGGGAGCCAAAAGGG-3′, and reverse,
5′-TGCCAGCCCCAGCGTCAAAG-3′. Amplification conditions were as
follows: Pre-denaturation at 94°C for 5 min, followed by 40 cycles
of 95°C for 5 min and 65°C for 40 sec. Results were quantified
using MJ Opticon Monitor Software 3.1 (Bio-Rad Laboratories,
Inc.).
Western blot analysis
To perform western blot analysis, 3×105
cells were seeded into 6-well plates and incubated at 37°C in an
atmosphere containing 5% CO2 for 24 h. Pre-cooled lysis
buffer (Millipore, Billerica, MA, USA) was then added to isolate
the protein from the cells and the samples were centrifuged at
10,000 × g at 4°C for 20 min. The protein concentrations were
determined using the Bradford method (13). Total protein (80 μg) was separated
on a 12% polyacrylamide gel (Pierce Biotechnology, Inc., Rockford,
IL, USA) and transferred onto polyvinylidene fluoride membranes
(Millipore). Next, the membranes were blocked in 5% skim milk at
room temperature for 1 h, and incubated with the following primary
mouse anti-human monoclonal antibodies: CXCR4, MMP-2, MMP-9, c-Myc,
cyclin D1, p27, p53, phospho (p)-protein kinase B (Akt) and p-c-Jun
N-terminal protein kinase (JNK), which were all purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) at a dilution of
1:500, as well as β-actin (1:5,000; Sigma-Aldrich) at 4°C
overnight. The membranes were washed three times with Tris-buffered
saline/Tween 20 (TBST) and then incubated with horseradish
peroxidase-conjugated secondary immunoglobulin G antibodies
(1:5,000) at room temperature for 30 min. Subsequently, the
membranes were washed a further three times with TBST and treated
with an enhanced chemiluminescence reagent (Millipore) for 1–2 min,
prior to exposure to an X-ray film (SDT Film Co., Ltd., Suzhou,
China). The results were normalized to β-actin.
Telomerase activity assay
For determination of the telomerase activity,
3×105 cells were seeded into 6-well plates and incubated
at 37°C in a 5% CO2 incubator for 24 h. Pre-cooled lysis
buffer was then added and the cells were centrifuged at 10,000 × g
at 4°C for 20 min. Next, the protein concentrations were determined
using the Bradford method. Total protein (2 μg) was used to perform
a telomerase activity assay using the TeloTAGGG Telomerase PCR
ELISA (Roche Diagnostics, Basel, Switzerland), according to the
manufacturer’s instructions. The PCR reaction conditions were set
at: 25°C for 20 min; 94°C for 5 min; 30 cycles at 94°C for 30 sec,
50°C for 30 sec and 72°C for 90 sec; and 72°C for 10 min. PCR ELISA
was performed using the PCR products (5 μl) to detect the
telomerase activity at 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
ELISA
In total, 3×105 cells were seeded into
6-well plates and incubated at 37°C in a 5% CO2
incubator for 24 h. Following the 24-h culture, the culture medium
was replaced with serum-free culture medium and the cells were
cultured for a further 24 h. The supernatants were collected
through centrifugation at 300 × g for 10 min and the concentrations
of the transforming growth factor (TGF)-β (human TGF-β 1 Quantikine
ELISA kit), monocyte chemoattractant protein (MCP)-1 (human MCP-1
Quantikine ELISA kit) and tumor necrosis factor (TNF)-α (human
TNF-α Quantikine ELISA kit) were measured using ELISA kits (R&D
Systems, Inc., Minneapolis, MN, USA), according to the
manufacturer’s instructions.
Dual luciferase report assay
The cells were transfected with 2 μg nuclear factor
(NF)-κB, signal transducer and activator of transcription (STAT)3
and STAT5 fluorescent reporter plasmid or 0.02 μg control plasmid
(Beyotime) were transfected using Lipofectamine 2000 (Invitrogen
Life Technologies) for 24 h. Subsequently, the luciferase activity
of the cells was detected using a Dual Luciferase®
Reporter assay, according to the manufacturer’s instructions.
Statistical analysis
SPSS version 13.0 software (SPSS Inc., Chicago, IL,
USA) was used for statistical analyses. The experimental data are
presented as the mean ± standard deviation and the statistical
significance of differences between the groups was determined using
a one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of miR-146b-5p on the biological
characteristics of Caski cells
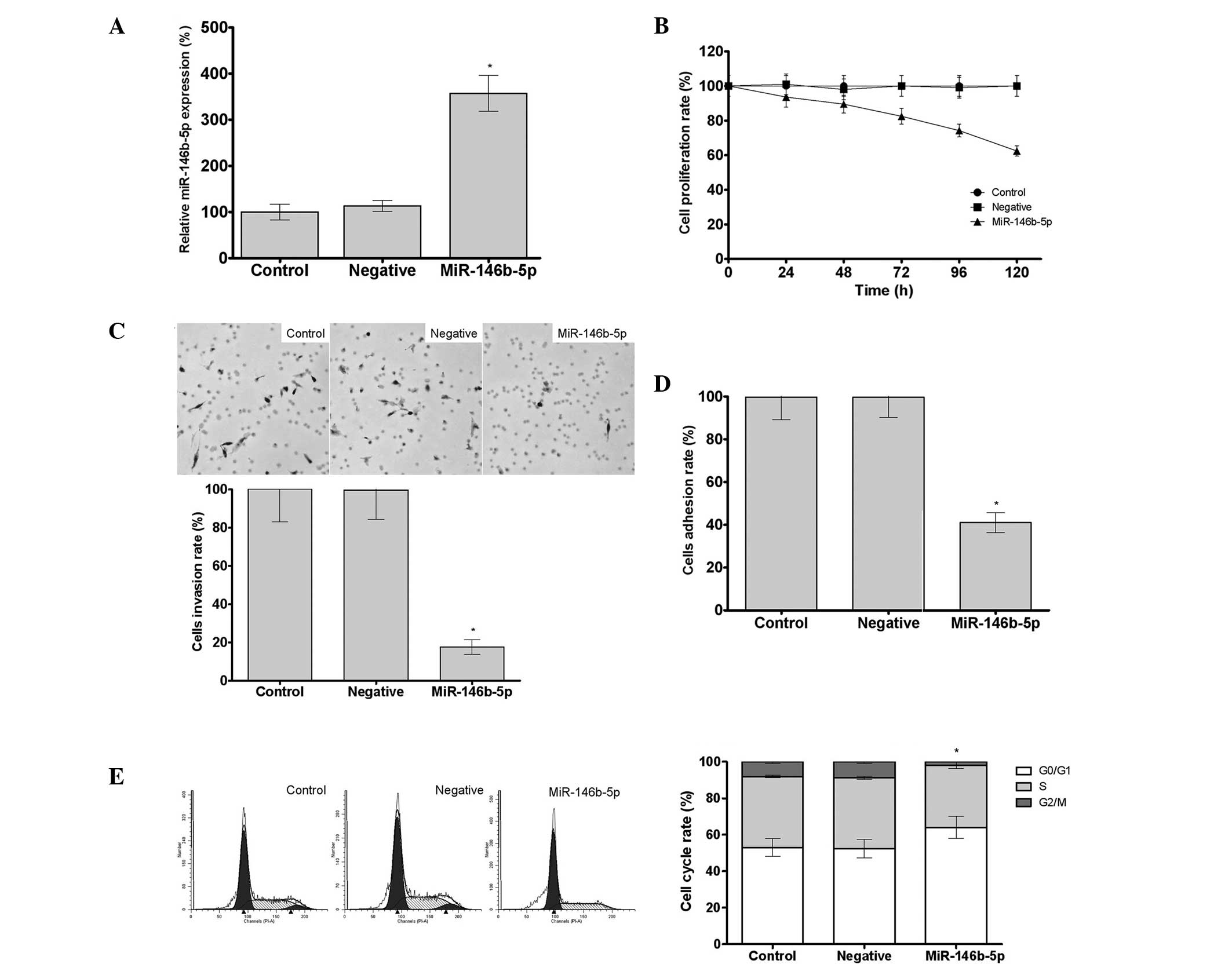
Increased expression levels of miR-146b-5p were
observed in the miR-146b-5p group, when compared with the control
and negative groups, as determined by qPCR (Fig. 1A). Based on the results of the MTS
assay, the 120-h relative proliferation rate in the miR-146b-5p
group was found to be 62.4% of the control group rate (P<0.05).
Therefore, transfection with miR-146b-5p was able to significantly
inhibit the proliferation of Caski cells in vitro (Fig. 1B). In addition, the results of the
transwell assay demonstrated that transfection with miR-146b-5p was
found to significantly inhibit the invasion of Caski cells in
vitro, since the cell invasion rate in the miR-146b-5p group
was 23.5% that of the control group (P<0.05) (Fig. 1C). Furthermore, transfection with
miR-146b-5p significantly inhibited the adhesion of Caski cells
in vitro, since the cell adhesion rate in the miR-146b-5p
group was 43.5% that of the control group (P<0.05) (Fig. 1D). In addition, the percentage of
cells in the G0/G1 phase of the cell cycle
was found to be significantly increased in the miR-146b-5p group,
indicating that miR-146b-5p was capable of arresting the Caski
cells in the cell cycle (Fig.
1E).
Effect of miR-146b-5p on cytokine
secretion and telomerase activity of Caski cells
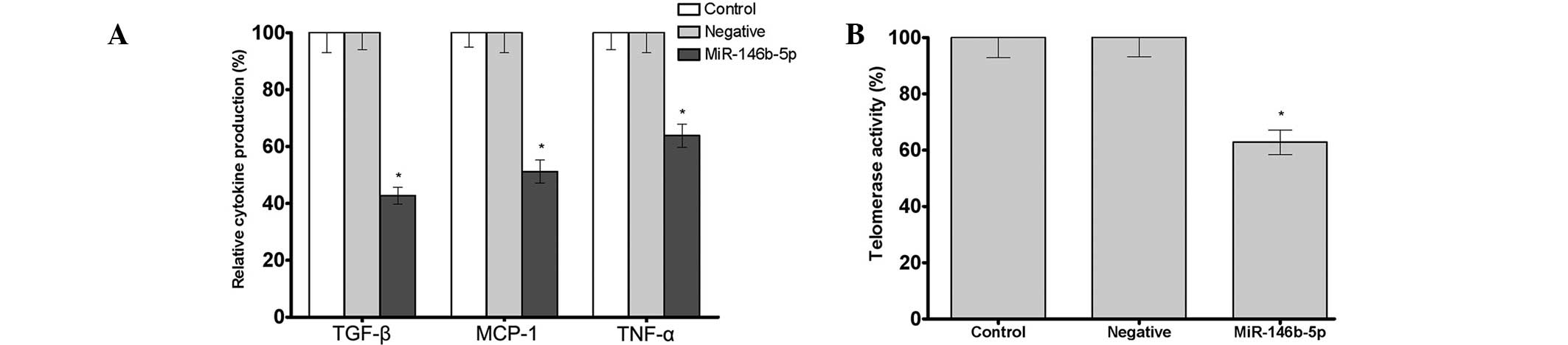
As determined by ELISA, transfection with
miR-146b-5p was able to significantly inhibit the secretion levels
of TGF-β, MCP-1 and TNF-α in Caski cells. The secretion levels of
TGF-β, MCP-1 and TNF-α in the miR-146b-5p group was 42.7, 51.2 and
63.8% that of the control group, respectively (P<0.05) (Fig. 2A). In addition, transfection with
miR-146b-5p was found to significantly inhibit the telomerase
activity of Caski cells, as determined by qPCR analysis. The
telomerase activity in the miR-146b-5p group was 62.8% that of the
control group (P<0.05) (Fig.
2B).
Effect of miR-146b-5p on Caski cell
proliferation and metastasis-associated gene expression
As demonstrated by western blot analysis,
transfection with miR-146b-5p significantly inhibited the protein
expression levels of CXCR4, MMP-2, MMP-9, c-Myc, cyclin D1 and
HPV16, while it increased the protein expression levels of p27 and
p53 in the Caski cells (Fig. 3A).
Furthermore, the mRNA expression levels of CXCR4, MMP-2, MMP-9,
c-Myc, cyclin D1, HPV16, p27 and p53 in the miR-146b-5p group were
found to be 38.4, 27.2, 21.1, 22.7, 18.6, 43.6, 267.3 and 255.0%
that of the control group (P<0.05), respectively, as determined
by qPCR (Fig. 3B). These results
indicated that miR-146b-5p was capable of modifying the biological
characteristics of tumor cells by regulating tumor cell
proliferation and the expression levels of metastasis-associated
genes.
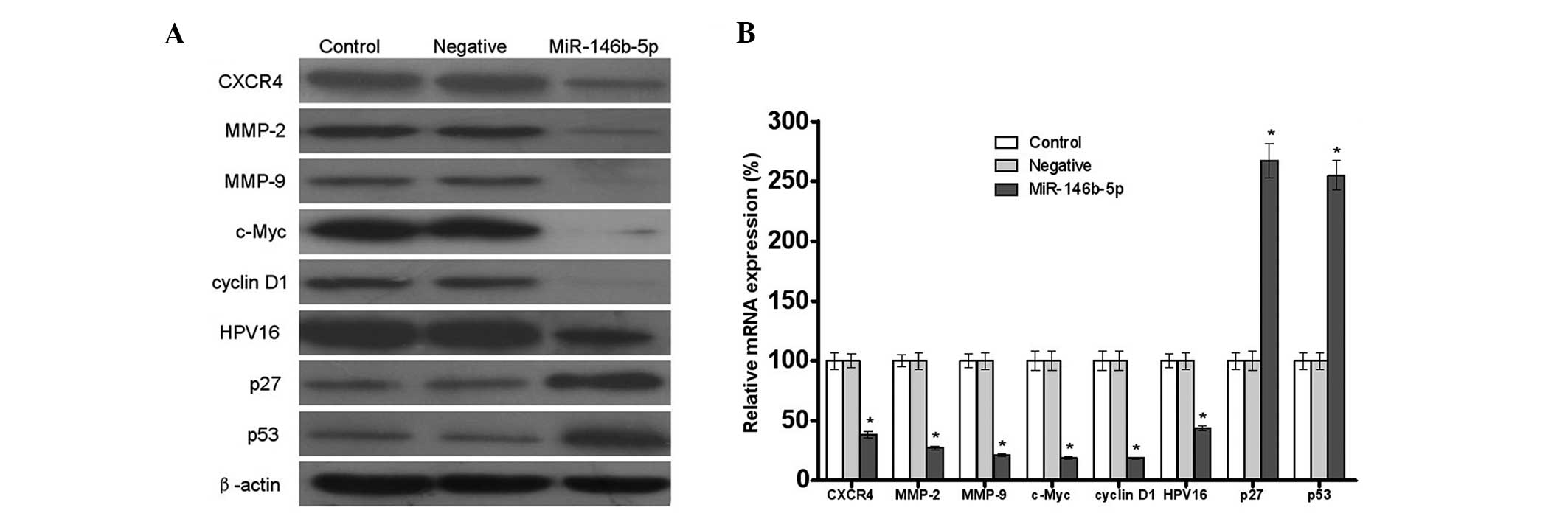 | Figure 3Effects of miR-146b-5p on
proliferation and metastasis-associated gene expression of Caski
human cervical cancer cells. (A) Protein expression levels,
determined by western blot analysis (using β-actin as an internal
loading control), and (B) mRNA expression levels, determined by
quantitative polymerase chain reaction (using GAPDH as an internal
loading control). The protein and mRNA expression levels of CXCR4,
MMP-2 and 9, c-Myc, cyclin D1 and HPV16 were found to be decreased,
whereas the protein and mRNA expression levels of p27 and p53 were
found to be increased, in the miR-146b-5p group when compared with
the control and negative groups. All the data are presented as the
mean ± standard deviation from at least three independent
experiments. *P<0.05, vs. control group. miR,
microRNA; CXCR4, C-X-C chemokine receptor type 4; MMP, matrix
metalloproteinase; HPV, human papilloma virus. |
Effect of miR-146b-5p on the signal
transduction pathway of Caski cells
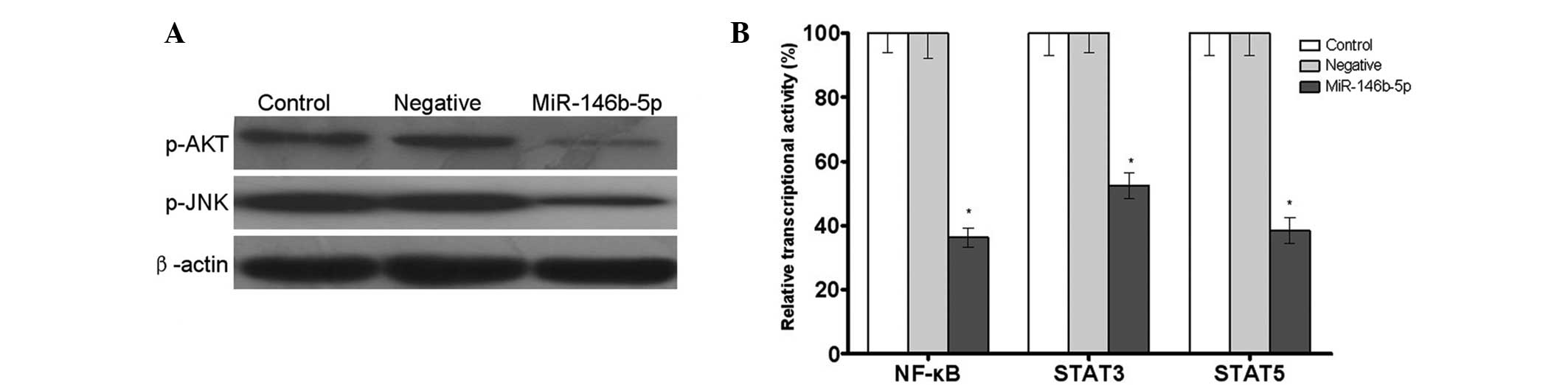
Western blot analysis demonstrated that transfection
with miR-146b-5p significantly inhibited the phosphorylation of Akt
and JNK in Caski cells (Fig. 4A).
Furthermore, a dual luciferase reporter assay indicated that the
transcriptional activities of NF-κB, STAT3 and STAT5 in the
miR-146b-5p group were 36.3, 52.4 and 38.5% that of the control
group, respectively (P<0.05) (Fig.
4B). These results indicated that miR-146b-5p was capable of
modifying the biological characteristics of tumor cells by
regulating the activity of tumor cell signal transduction molecules
(Fig. 4).
Discussion
Cervical cancer is one of the most common cancers in
females. Although surgery is the primary treatment strategy for
cervical cancer, tumor metastasis often leads to poor therapeutic
efficacy. Therefore, research on the identification of cervical
cancer metastasis inhibitors has received increasing attention.
Tumor cell metastasis is a complex process that involves numerous
factors, and the underlying mechanisms include enhanced heterotypic
cell-cell adhesion, upregulated MMP expression and increased
secretion of pro-metastatic cytokines (14).
Tumor-associated genes, including CXCR4, matrix
metalloproteinase-2/9, c-Myc, cyclin D1, p27 and p53 have important
roles in proliferation and metastasis (15). Tumor invasion and metastasis are
often associated with the upregulation of CXCR4 expression, which
is an important chemotactic factor. CXCR4 can improve the
chemotactic activity of tumor cells, enabling their invasion and
migration to surrounding tissues during the process of tumor
metastasis. In addition, the upregulation of CXCR4 expression has
been shown to be directly correlated with tumor invasion and
metastasis (16). MMPs are
involved in the degradation of the extracellular matrix (ECM) and
basilar membrane, as well as in numerous physiological and
pathological processes. In addition, MMPs are important regulatory
molecules involved in tumor infiltration, metastasis and
angiogenesis, and therefore play a key role in tumor progression
(17). MMP-2 is a zinc-dependent
protease, with substrates including types IV, VII and X collagen
and gelatin, which are the main components of the ECM and vascular
basilar membrane. Overexpression of MMP-2 accelerates the
degradation of the ECM and vascular basilar membrane, enabling the
tumor cells to migrate from tumor nests and across blood vessels,
therefore facilitating tumor invasion and metastasis.
Overexpression of MMP-2 is associated with the enhanced invasive
and metastatic capacity of numerous tumors (18). MMP-9 is one of the most important
proteases capable of degrading the basilar membrane within the MMP
enzyme family. MMP-9 is initially secreted as a zymogen by the
cells, which can induce enzymolysis following activation by various
factors. Enzymolysis of the ECM enhances tumor growth into the
surrounding area. Numerous studies have observed fairly high
expression levels of MMPs in various tumor tissues, including
nasopharyngeal carcinoma, and these expression levels have been
found to be associated with tumor invasiveness (19).
Previous studies have demonstrated that p27 and p53
genes control cell death and proliferation and also limit the
metastatic capability of cancer cells, by regulating the expression
levels of oncogenes (20).
Therefore, the loss of p27 and p53 greatly enhances tumor
progression and metastasis, while the lack of p27 and p53
expression has been shown to be directly correlated with tumor
invasion and metastasis (21,22).
In tumor cells, the proteins that control progression of the cell
cycle, such as cyclins, are often overexpressed; by contrast, the
proteins that suppress cell division, such as cyclin-dependent
kinase (CDK) inhibitors, are often inactivated. Among the numerous
cell cycle regulatory factors, cyclin D1 is significant. Cyclin D1
forms a complex with CDK4/6 in G1 phase, thereby driving
cells from G1 to S phase and causing cell division or
conversion. A previous study has reported that overexpression of
cyclin D1 was detected in lung, breast, thyroid and prostate cancer
tissues, as well as in other tumors, thus indicating a certain
association between cyclin D1 and tumorigenesis (23).
Telomerase activity has been previously demonstrated
to be associated with tumor metastatic potential, due to shortened
telomeres (24). The present study
aimed to determine the role of telomerase activity on the ability
of miR-146b-5p to inhibit metastasis of Caski cells. The results
indicated that telomerase activity was decreased in Caski cells
overexpressing miR-146b-5p. In addition, the present study provided
evidence regarding a potential mechanism for miR-146b-5p-inhibited
metastasis of Caski cells. Human papilloma virus (HPV) is also an
important factor in cervical carcinogenesis and cancer progression,
while HPV16 is considered to be the most common high-risk type of
HPV. HPV16 E7 participates in the tumorigenesis of the majority of
cervical cancers. Extensive clinical and pathological studies have
confirmed that HPV16 E7 expression is closely correlated with poor
tumor differentiation, susceptibility to lymph node metastasis and
poor prognosis in oral, breast and gastric cancer, as well as in
various other human epithelial malignancies (25,26).
Previous studies have also observed an upregulation of TGF-β, MCP-1
and TNF-α expression in HPV16-infected malignant tumor tissues,
which was considered to be associated with tumor metastasis, signal
transduction pathways and the upregulation of the expression of
proliferation-associated regulatory factors (27).
Akt is a downstream effector molecule in the
phosphoinositide 3-kinase signaling pathway, the most important
target enzyme of which is JNK. JNK is frequently detected during
carcinogenesis and the progression of malignant tumors. The
phosphorylation of Akt and JNK, as well as the activity of NF-κB,
STAT3 and STAT5, play an important role in the signaling pathways
of tumor invasion and metastasis, through a series of substrate
phosphorylation (28,29). These pathways can regulate the
expression levels of CXCR4, MMP-2, MMP-9 and other oncogenes and
may also participate in tumor cell invasion and migration.
In conclusion, the present study demonstrated that
miR-146b-5p was able to inhibit the in vitro proliferative,
adhesive and invasive ability of tumor cells, and arrest the cell
cycle in G0/G1 phase in Caski cells. In
addition, transfection with miR-146b-5b was hypothesized to be
associated with the downregulation of CXCR4, MMP-2, MMP-9, c-Myc,
cyclin D1 and telomerase activity, the upregulation of p27 and p53,
the inhibition of TGF-β, MCP-1, TNF-α and other cytokine
secretions, and the inhibition of Akt phosphorylation and
transcriptional activity of NF-κB, STAT3 and STAT5. Furthermore,
miR-146b-5p was able to downregulate HPV16 E7 expression in the
Caski cervical cancer cells, thereby laying the experimental basis
for further studies of cervical cancer gene therapy.
References
|
1
|
Sriplung H, Singkham P, Iamsirithaworn S,
Jiraphongsa C and Bilheem S: Success of a cervical cancer screening
program: trends in incidence in songkhla, southern Thailand,
1989–2010, and prediction of future incidences to 2030. Asian Pac J
Cancer Prev. 15:10003–10008. 2014. View Article : Google Scholar
|
|
2
|
Üreyen I, Aksoy Ü, Dündar B, Tapisiz ÖL,
Karalök MA, Turan AT, Boran N and Tulunay HG: Does lymph node
involvement affect the patterns of recurrence in stage IB cervical
cancer? Turk J Med Sci. 44:844–852. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chawla N, Breen N, Liu B, Lee R and
Kagawa-Singer M: Asian American Women in California: a pooled
analysis of predictors for breast and cervical cancer screening. Am
J Public Health. 18:e1–e12. 2014.
|
|
4
|
Li C, Ma C, Zhang W and Wang J: The immune
function differences and high-risk human papillomavirus infection
in the progress of cervical cancer. Eur J Gynaecol Oncol.
5:557–561. 2014.
|
|
5
|
Bai LX, Wang JT, Ding L, Jiang SW, Kang
HJ, Gao CF, Chen X, Chen C and Zhou Q: Folate deficiency and FHIT
hypermethylation and HPV 16 infection promote cervical
cancerization. Asian Pac J Cancer Prev. 21:9313–9317. 2014.
View Article : Google Scholar
|
|
6
|
García-Zepeda SP, García-Villa E,
Díaz-Chávez J, Hernández-Pando R and Gariglio P: Resveratrol
induces cell death in cervical cancer cells through apoptosis and
autophagy. Eur J Cancer Prev. 22:577–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gocze K, Gombos K, Kovacs K, Juhasz K,
Gocze P and Kiss I: MicroRNA expressions in HPV-induced cervical
dysplasia and cancer. Anticancer Res. 1:523–530. 2015.
|
|
8
|
Shishodia G, Verma G, Srivastava Y,
Mehrotra R, Das BC and Bharti AC: Deregulation of microRNAs Let-7a
and miR-21 mediate aberrant STAT3 signaling during human
papillomavirus-induced cervical carcinogenesis: role of E6
oncoprotein. BMC Cancer. 14:9962014.PubMed/NCBI
|
|
9
|
Ribeiro J and Sousa H: MicroRNAs as
biomarkers of cervical cancer development: a literature review on
miR-125b and miR-34a. Mol Biol Rep. 3:1525–1531. 2014. View Article : Google Scholar
|
|
10
|
Wang X, Wang HK, Li Y, Hafner M, Banerjee
NS, Tang S, Briskin D, Meyers C, Chow LT, et al: MicroRNAs are
biomarkers of oncogenic human papillomavirus infections. Proc Natl
Acad Sci USA. 11:4262–4267. 2014. View Article : Google Scholar
|
|
11
|
Kutty RK, Nagineni CN, Samuel W,
Vijayasarathy C, Jaworski C, Duncan T, Cameron JE, Flemington EK,
Hooks JJ and Redmond TM: Differential regulation of microRNA-146a
and microRNA-146b-5p in human retinal pigment epithelial cells by
interleukin-1β, tumor necrosis factor-α, and interferon-γ. Mol Vis.
19:737–750. 2013.
|
|
12
|
Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S,
Wang Q, Yan Y, Kang C, Jin S, An T, Shi C, Xu J, Wei C, Liu J, Sun
J, Wen Y, Zhao S and Kong Y: miR-146b-5p inhibits glioma migration
and invasion by targeting MMP16. Cancer Lett. 339:260–269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bergot AS, Ford N, Leggatt GR, Wells JW,
Frazer IH and Grimbaldeston MA: HPV16-E7 expression in squamous
epithelium creates a local immune suppressive environment via CCL2-
and CCL5- mediated recruitment of mast cells. PLoS Pathog.
10:e10044662014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Zhang Y and Zhang S: MicroRNA-92
regulates cervical tumorigenesis and its expression is upregulated
by human papilloma virus-16 E6 in cervical cancer cells. Oncol
Lett. 6:468–474. 2013.PubMed/NCBI
|
|
15
|
Zhu Y, Zhang L, Zhang GD, Wang HO, Liu MY,
Jiang Y, Qi LS, Li Q and Yang P: Potential mechanisms of benzyl
isothiocyanate suppression of invasion and angiogenesis by the
U87MG human glioma cell line. Asian Pac J Cancer Prev.
19:8225–8228. 2014. View Article : Google Scholar
|
|
16
|
Li J, Jiang K, Qiu X, Li M, Hao Q, Wei L,
Zhang W, Chen B and Xin X: Overexpression of CXCR4 is significantly
associated with cisplatin-based chemotherapy resistance and can be
a prognostic factor in epithelial ovarian cancer. BMB Rep.
47:33–38. 2014. View Article : Google Scholar :
|
|
17
|
Nowak E, Galilejczyk A, Sypniewski D and
Bednarek I: MMP-9 directed shRNAs as relevant inhibitors of matrix
metalloproteinase 9 activity and signaling. Postepy Hig Med Dosw
(Online). 67:742–749. 2013. View Article : Google Scholar
|
|
18
|
Gutschalk CM, Yanamandra AK, Linde N,
Meides A, Depner S and Mueller MM: GM-CSF enhances tumor invasion
by elevated MMP-2, -9, and -26 expression. Cancer Med. 2:117–129.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan X, Wu W, Shi H and Han J: RNA
interference targeting CD147 inhibits the invasion of human
cervical squamous carcinoma cells by downregulating MMP-9. Cell
Biol Int. 37:737–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Y, Zhu L, Lu L, Zhang L, Zhang G, Wang
Q and Yang P: Role and mechanism of the alkylglycerone phosphate
synthase in suppressing the invasion potential of human glioma and
hepatic carcinoma cells in vitro. Oncol Rep. 1:431–436. 2014.
|
|
21
|
Kuroda H, Jomen W, Miura S, Arihara Y,
Yamada M, Hirako T, Abe T, Sakurai T, Fujii S, Maeda M, et al:
Bendamustine-rituximab therapy is effective for transformed
follicular lymphoma with significant expression of p53. Gan To
Kagaku Ryoho. 40:1055–1058. 2013.(In Japanese). PubMed/NCBI
|
|
22
|
Zhang M, Li J, Wang L, Tian Z, Zhang P, Xu
Q, Zhang C, Wei F and Chen W: Prognostic significance of p21, p27
and survivin protein expression in patients with oral squamous cell
carcinoma. Oncol Lett. 6:381–386. 2013.PubMed/NCBI
|
|
23
|
Portari EA, Russomano FB, de Camargo MJ,
Machado Gayer CR, da Rocha Guillobel HC, Santos-Rebouças CB and
Brito Macedo JM: Immunohistochemical expression of cyclin D1,
p16Ink4a, p21WAF1, and Ki-67 correlates with the severity of
cervical neoplasia. Int J Gynecol Pathol. 32:501–508. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sui X, Kong N, Wang Z and Pan H:
Epigenetic regulation of the human telomerase reverse transciptase
gene: A potential therapeutic target for the treatment of leukemia
(Review). Oncol Lett. 6:317–322. 2013.PubMed/NCBI
|
|
25
|
Kim MS, Bak Y, Park YS, Lee DH, Kim JH,
Kang JW, Song HH, Oh SR and Yoon do Y: Wogonin induces apoptosis by
suppressing E6 and E7 expressions and activating intrinsic
signaling pathways in HPV-16 cervical cancer cells. Cell Biol
Toxicol. 29:259–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Almajhdi FN, Senger T, Amer HM, Gissmann L
and Ohlschläger P: Design of a highly effective therapeutic HPV16
E6/E7-Specific DNA vaccine: Optimization by different ways of
sequence rearrangements (shuffling). PLoS One. 9:e1134612014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masuda S, Kumano K, Shimizu K, Imai Y,
Kurokawa M, Ogawa S, Miyagishi M, Taira K, Hirai H and Chiba S:
Notch1 oncoprotein antagonizes TGF-beta/Smad-mediated cell growth
suppression via sequestration of coactivator p300. Cancer Sci.
96:274–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geraldo MV, Yamashita AS and Kimura ET:
MicroRNA miR-146b-5p regulates signal transduction of TGF-β by
repressing SMAD4 in thyroid cancer. Oncogene. 31:1910–1922. 2012.
View Article : Google Scholar
|