Introduction
There is accumulating evidence that intercellular
communication is essential in the spread of signals between cells.
It is involved in a number of physiological (rhythmic electrical
activity) and pathological (schizophrenic disorders and
inflammatory responses) processes. Gap junctions are the only
junctional structures that are conserved in all multicellular
organisms, and not only connect neighboring cells but also permit
the exchange of molecules between the cytoplasm and the
extracellular space (1). Pannexins
(Panxs), which were described as a new member of the family of gap
junctions in 2000, are permeable to relatively large molecules,
including adenosine triphosphate (ATP) (2–5). ATP
in turn binds to metabotropic P2Y receptors, leading to
Ca2+ release from intracellular stores. Ca2+
then activates Panx-1 hemichannels again inducing the release of
ATP. This is termed ATP-induced ATP release. By facilitating
ATP-induced ATP release and Ca2+-wave propagation,
Panx-1 has an important function in a number of cellular processes
(3,6,7). For
instance, Panx-1 mediates neuronal death, affects keratinocyte
differentiation and regulates the proliferation of human
subcutaneous fibroblasts, and neural stem and progenitor cells
(8–11).
Astrocytes are the most abundant non-neuronal cells
in the central nervous system and are crucial to a number of
physiological and pathophysiological processes. Glioma is the most
common form of malignant brain tumor, and is associated with a poor
prognosis. A study has shown that Panx-1 acts as a tumor-suppressor
protein in the development of C6 gliomas (12). However, the association between
Panx-1 and astrocyte proliferation remains poorly understood. The
current study examined the effect of Panx-1 on the proliferation of
U87-MG malignant glioma cells as well as examining the effects of
ATP on cell proliferation and apoptosis in U87-MG cells that were
or were not expressing Panx-1. This was achieved through the use of
immunohistochemistry, short interfering RNA (siRNA) transfection
and Cell Counting kit-8 (CCK-8) assays. Proliferating cell nuclear
antigen (PCNA) was used to identify proliferating cells.
Materials and methods
Cell lines and cell culture
The U87 human malignant glioma cell line (U87-MG)
were obtained from the Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in Dulbecco’s modified Eagle’s
medium (HyClone, Logan, UT, USA) containing a high concentration of
glucose, supplemented with 10% fetal bovine serum (FBS; Gibco Life
Technologies, Grand Island, NY, USA) and a mixture of
antibiotics-antimycotics (HyClone) in an atmosphere of 5%
CO2/95% air at 37°C. The cells were passaged every 4–5
days to maintain exponential growth and were not used beyond the
twentieth passage.
Immunocytochemistry
U87-MG cells grown on coverslips were fixed with 4%
paraformaldehyde for 30 min, washed several times with 0.1 M
phosphate-buffered saline (pH 7.4) and then incubated for 20 min in
0.3% H2O2, which had been diluted in methanol
to quench the endogenous peroxidase activity. Coverslips were
blocked with 10% normal goat serum for 30 min. A rabbit polyclonal
anti-Panx-1 antibody (Abcam, Cambridge, UK) was diluted to 1:1,000.
The secondary antibody was goat anti-rabbit immunoglonulin G
conjugated to horseradish peroxidase (HRP) for detection
(Changdao-Bio, China). Subsequently, 3,3′-diaminobenzidine
(Maixin-Bio, Fuzhou, China) was used to develop the color reaction.
Finally, sections were counterstained with hematoxylin, dehydrated
and coverslipped. Immunostaining was also performed in samples
prepared without the primary antibody as a negative control.
siRNA transfection
Cell transfection was conducted with Lipofectamine
2000TM reagent (Invitrogen Life Technologies, Carslbad,
CA, USA), according to the manufacturer’s instructions. Briefly,
U87-MG cells were seeded in 6-well plates. Once the cells were
50–80% confluent, the appropriate treatments were applied. For
siRNA experiments, media lacking antibiotics was used to improve
transfection efficiency. U87-MG cells were transfected with 75 μM
Panx-1-specific siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) using the Lipofectmine 2000TM reagent.
Scrambled control siRNA (Santa Cruz Biotechnology, Inc.) with no
homology to any mammalian sequence was used as a negative control.
Cells were harvested 48 h after transfection for analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted with TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. The quality of the RNA was confirmed using
formaldehyde-agarose gel electrophoresis. RNA (500 ng) was used to
obtained template cDNA using a PrimeScriptTM RT master
mix (Takara Bio, Inc., Shiga, Japan) for qPCR using SYBR Premix Ex
TaqII on a ABI 7300 system (Takara Bio, Inc.). The following
specific PCR primers designed by Bio-TNT (Shanghai, China) were
used: Forward: 5′-AAT CTG TGA CTT CTG CGA CAT-3′ and reverse:
5′-CCA TTT CCA TTA GGG ACT CAA-3′ for Panx-1; forward: 5′-TTA GCT
CCA GCG GTG TAA AC-3′ and reverse: 5′-CAG CGG TAG GTG TCG AA-3′ for
PCNA; and forward: 5′-AAGGTGACAGCAGTCGGTT-3′ and reverse:
5′-TGTGTGGACTTGGGAGAGG-3′ for β-actin (the reference gene). Samples
were run in triplicate and the relative levels of mRNA expression
were analyzed relative to β-actin levels using the comparative
cycle (Ct) threshold method. Following PCR amplification, all the
samples were verified by 2% agarose gel electrophoresis.
Western blot analysis
Total cellular proteins were extracted with sodium
dodecyl sulfate (SDS) lysis buffer, heated for 5 min at 99°C and
then centrifuged for at 16,000 × g for 5 min. Protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Beyotime, Shanghai, China). Samples (20 μg/well) were
resolved on a 10% SDS-polyacrylamide gel electrophoresis gel and
then electrophoretically transferred to Immobilon®-P
membranes (Millipore, Billerica, MA, USA). Pre-stained molecular
markers (Fermentas, Pittsburgh, PA, USA) were used as a reference
for the molecular weight of the proteins. Membranes were blocked
with 5% non-fat milk in 1× Tris-buffered saline with Tween-20 for 1
h and subsequently incubated with either rabbit polyclonal
anti-Panx-1 antibody (1:1,000) or mouse polyclonal anti-PCNA
(1:400; Cruz Biotechnology, Inc.) antibodies overnight at 4°C,
prior to incubation with the appropriate HRP-conjugated goat
monoclonal anti-rabbit (1:1000; Millipore, Billerica, MA, USA) or
goat monoclonal anti-mouse (1:1,000; Millipore, Billerica, MA, USA)
secondary antibodies for 1 h at room temperature. Immunoreactivity
was detected by enhanced chemiluminescence detection using the
chemiluminescent HRP substrate kit (Millipore). The band densities
were quantified with the image-analysis software (Tinon Software,
Zhongshan, China). All data were normalized to GAPDH.
Cell proliferation assay
Cells transfected with Panx-1-specific siRNA or
scrambled siRNA for 24 h were seeded on 96-well plates at a density
of 1,000 cells/100 μl culture medium containing 10% FBS per well.
Cell proliferation was estimated using CCK-8 (Dojindo, Kumamoto,
Japan) according to the manufacturer’s instructions at 1, 6, 12, 24
and 48 h after cells were seeded. Briefly 10 μl reagent was mixed
with 100 μl culture medium and incubated for 1–3 h in a cell
incubator (Lishen, Shanghai, China). Absorbance at 450 nm was
measured using an enzyme-linked analyzer (Biotek Instruments Inc.,
Winooski, VT, USA). Experiments were repeated five times.
ATP treatment
U87-MG cells were seeded in 96-well plates in five
parallel wells and treated with 0.1, 1 and 5 μmol/ml ATP
(Sigma-Aldrich, St. Louis, MO, USA). At the predetermined time
points, cell proliferation was estimated using a CCK-8 assay.
Protein levels of Panx-1 and PCNA were estimated by western blot
analysis, as described above.
Statistical analysis
Calculations were performed with GraphPad InStat,
Version 5.0 (GraphPad Prism Software, San Diego, CA, USA).
Student’s two-tailed t-test was utilized for all data analysis and
values are expressed as the mean ± standard error of the mean
acquired from at least two independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
Panx-1 expression is increased in mitotic
U87-MG cells
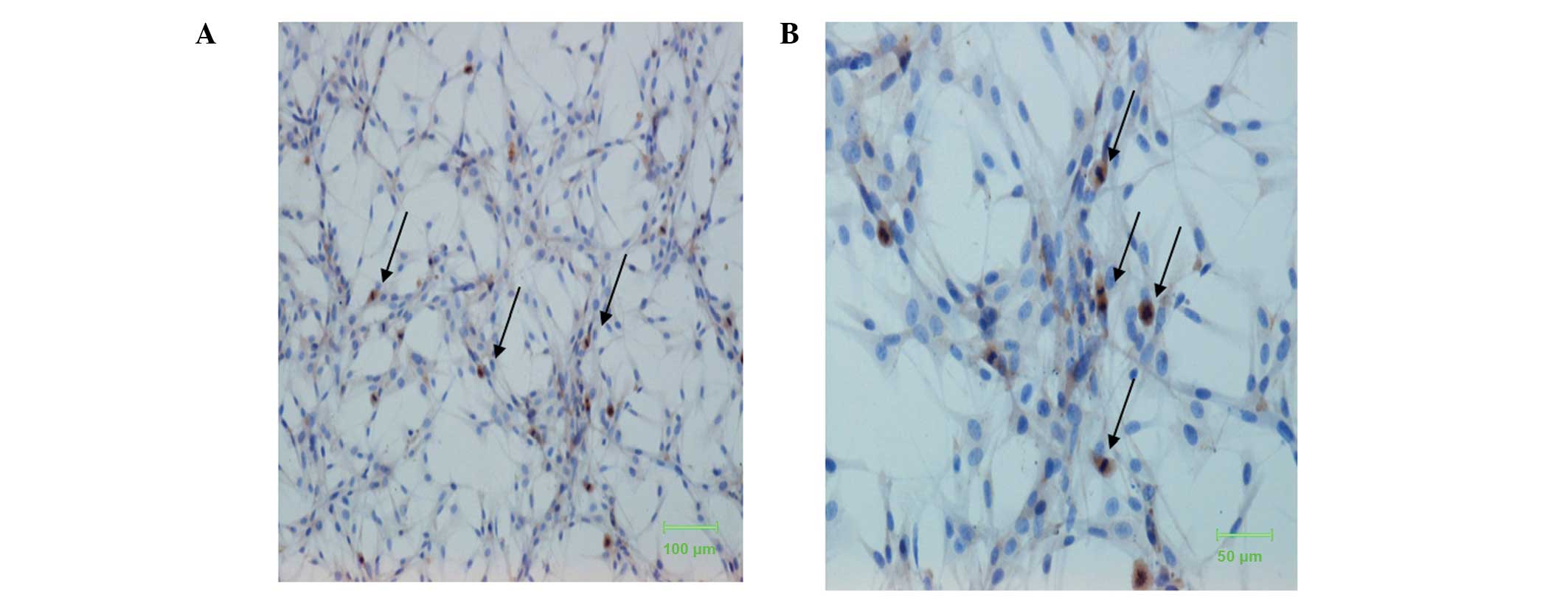
The results from the immunocytochemistry experiments
showed that Panx-1 protein was located in the cytoplasm. The degree
of staining varied between cells. As shown in Fig. 1, Panx-1 staining was predominantly
observed in cells in the process of mitosis. Cells at the mitotic
phase, with chromosomes arranging in a flower-like ring and
arranging at the equator of the spindle, and cells just finishing
mitosis were positively immunostained. This suggests that Panx-1
expression may be associated with the proliferation of U87-MG
cells.
Panx-1 and PCNA mRNA and protein
expression following cell transfection
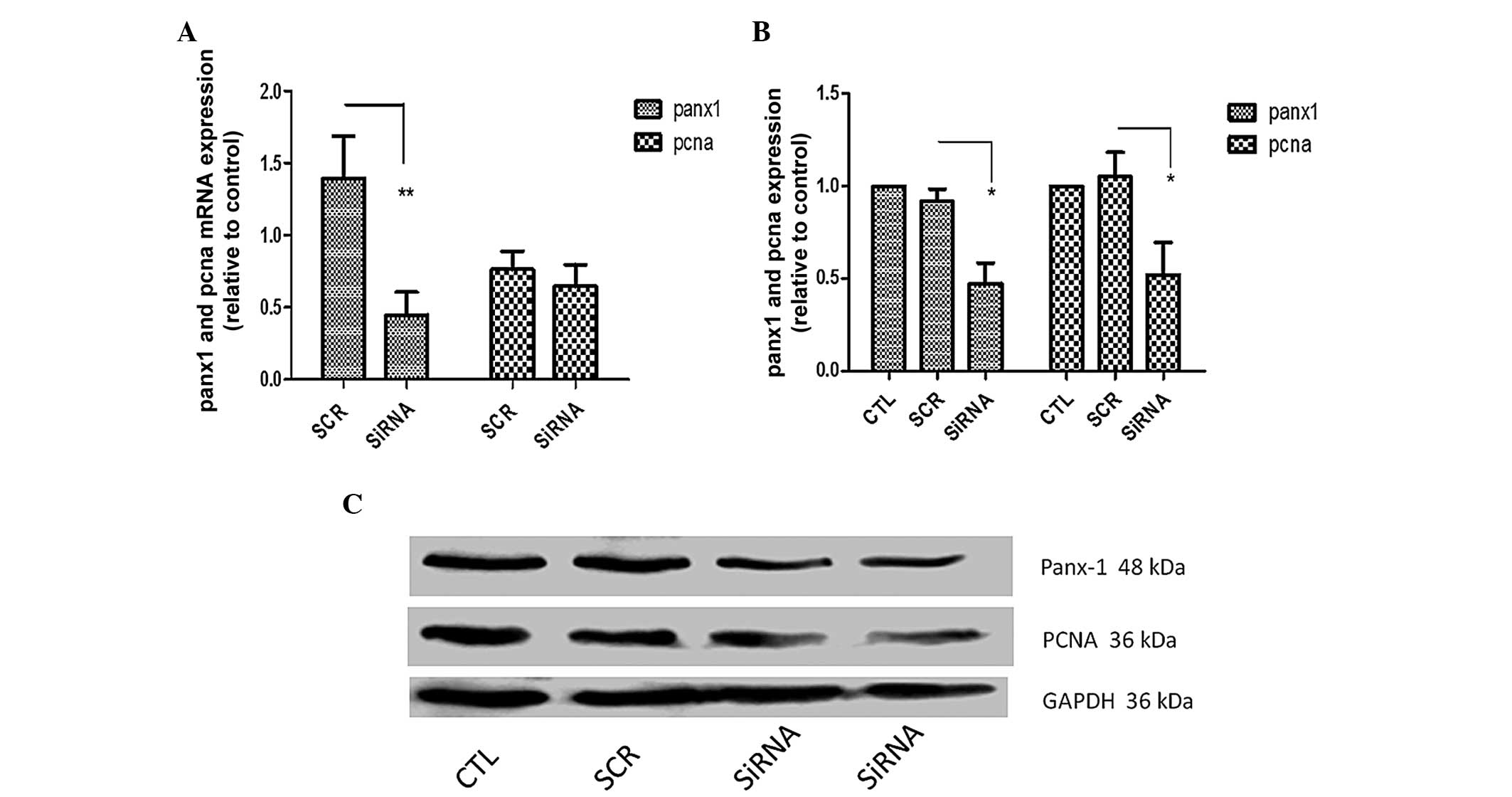
Based on the immunohistochemical results, cell
transfection experiments were conducted. Panx-1-specific siRNA was
effective in knocking down endogenous expression of Panx-1 at mRNA
(Fig. 2A, 66%, P<0.01) and
protein levels (Fig. 2B, 52.5%,
P<0.05) in U87-MG cells. Knockdown of Panx-1 did not alter PCNA
mRNA levels, but western blot analysis revealed that PCNA protein
expression in the Panx-1-specific siRNA transfection group was
reduced by 47.8% (P<0.05).
Effects of Panx-1 silencing on the
proliferation of U87-MG cells
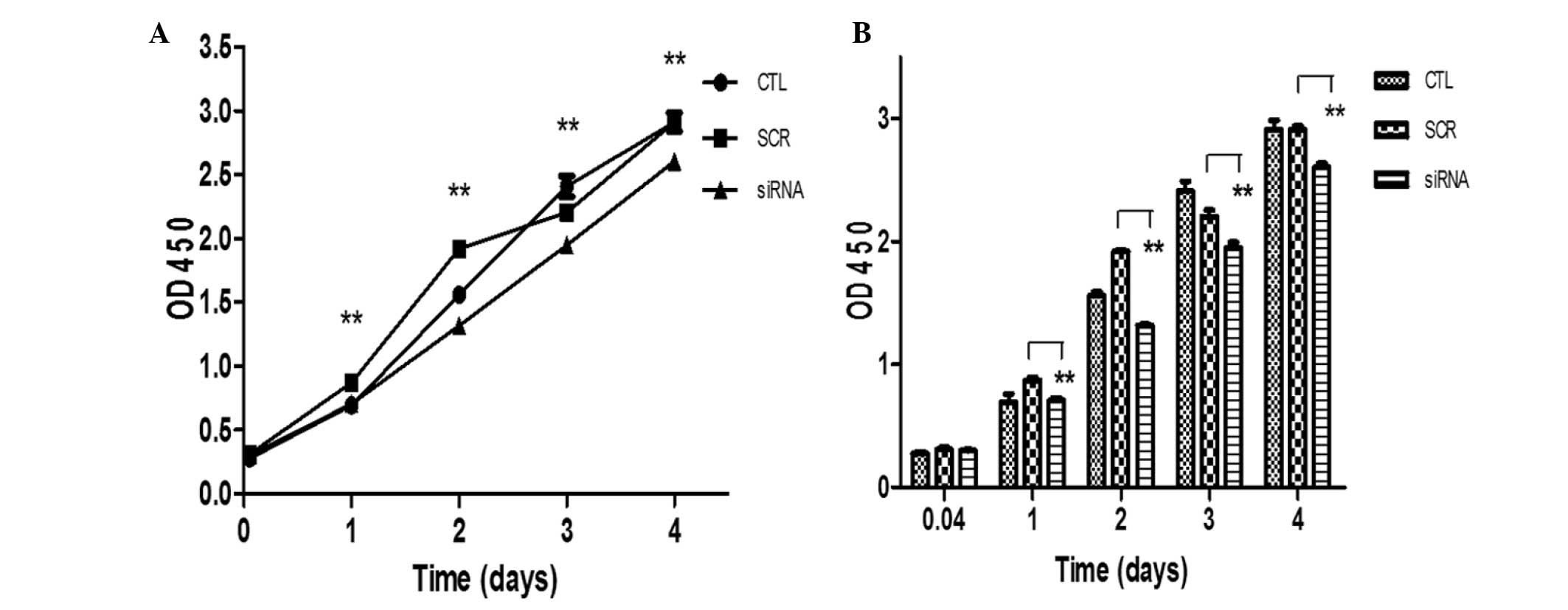
To investigate the role of Panx-1 in the regulation
of cell proliferation, the effects of Panx-1-specific siRNA on the
proliferation of U87-MG cells were investigated. Cells were
transfected either with Panx-1-specific siRNA or a scrambled
control siRNA using Lipofectamine 2000 and the proliferation of
U87-MG cells was determined using a CCK-8 assay at various
timepoints (1 h, and 1, 2, 3 and 4 days) after cells had been
seeded. The results from the CCK-8 assay showed that the optical
density (OD) at 450 of the Panx-1-specific siRNA group was
decreased significantly compared with the scrambled siRNA control
at 1–4 days (Fig. 3). This
indicates that Panx-1 silencing significantly inhibits the
proliferation of U87-MG cells.
ATP-induced decrease in cell
proliferation is enhanced by Panx-1 silencing
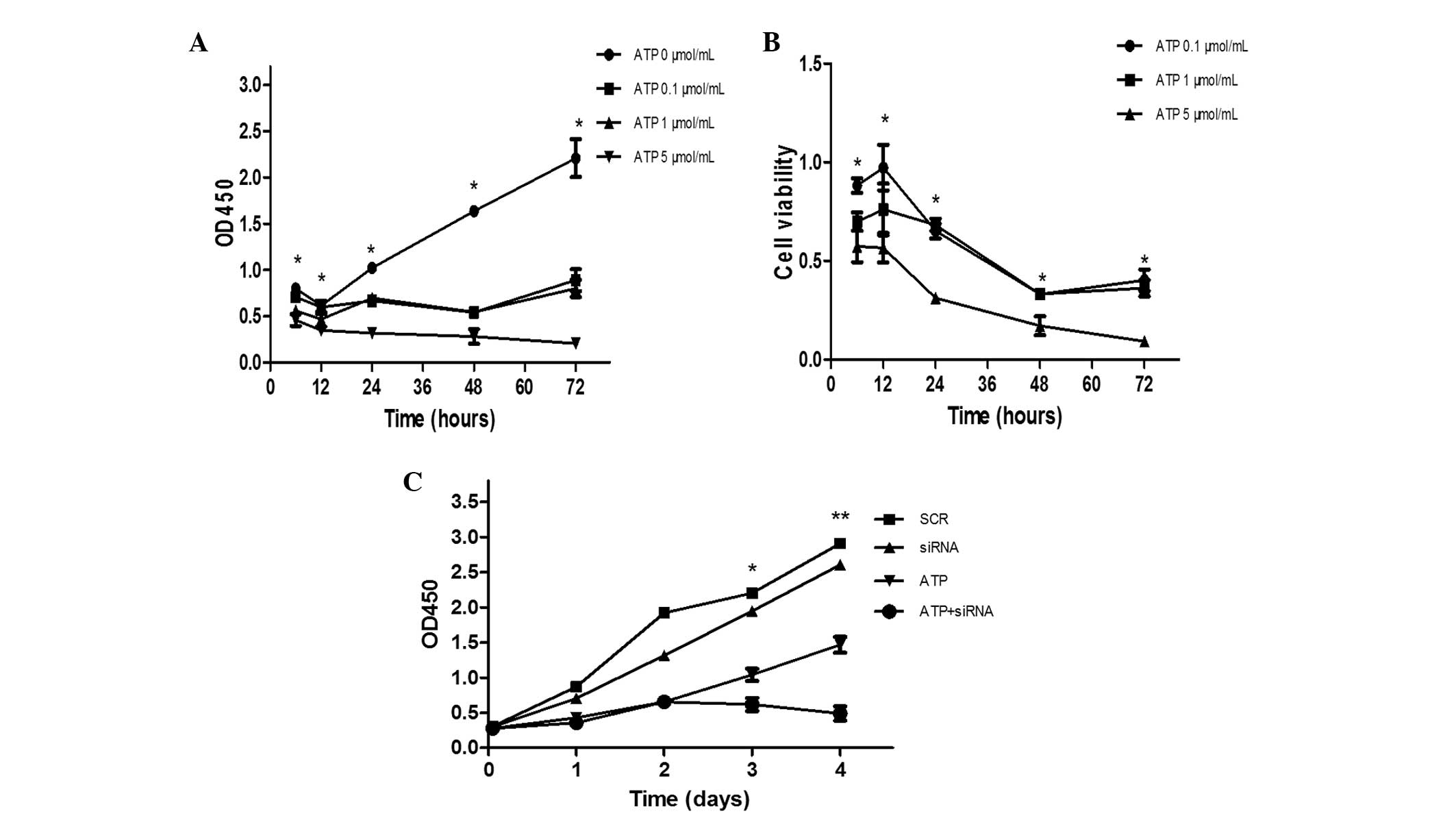
In order to investigate the correlation between
Panx-1 and cell proliferation, the effect of varying levels of ATP
in Panx-1 knockdown cells was observed. The effects of different
concentrations of ATP on cell proliferation and cell viability were
examined. As shown in Fig. 4A,
each of the three concentrations led to a reduction in U87-MG cell
proliferation, and the effect of the 5 μmol/ml dose was
statistically significant. It can also be seen from Fig. 4B that 5 μmol/ml ATP led to a
significant decrease in cytotoxicity. However, the moderate
concentration (1.0 μmol/ml) of ATP was the most effective at
inhibitong cell proliferation and cell viability at the 6 h time
point. For this reason, 1.0 μmol/ml ATP was selected for the
remainder of the cell viability assays. Fig. 4C shows that combining Panx-1
knockdown with the administration of ATP markedly inhibited the
proliferation of U87-MG cells compared with either of these factors
alone.
Discussion
Panxs are a novel group of gap junction proteins and
have a structure similar to that of connexins (Conxs). Panxs and
Conxs are four-pass transmembrane proteins, with intracellular
amino-(NH2) and carboxy- (COOH) termini. The Panx family comprises
three members: Panx-1, Panx-2 and Panx-3 (1,2).
Panx-1 is highly expressed in the central nervous system, while
Panx-2 is largely restricted to the brain and Panx-3 is found in
the skin, the male reproductive tract of the adult rat, osteoblasts
and mature growth plate chondrocytes (13–15).
Panx-1 is able to form homomeric and heteromeric channels in
combination with Panx-2. It is expressed by several organisms and
has been shown to be critical in mediating cell growth (7–12,16,17).
However, little is known about the effect of Panx-1 on glioma cell
development. The present study reports that Panx-1 expression and
U87-MG cell proliferation are closely correlated.
Using immunocytochemistry, it was shown that Panx-1
was expressed in the cytoplasm of U87-MG cells. This was consistent
with a prior study that used reverse transcription PCR, but which
did not demonstrate the expression of Panx-1 in the U87-MG cells
using immunocytochemistry or immunofluorescence (12). However, the results of the current
study were different from those of previous studies, which
demonstrated that Panx-1 is predominantly located on the cellular
membrane in primary cultured astrocytes (18,19).
To the best of our knowledge, this is the first study demonstrating
the location of Panx-1 in U87-MG cells. In addition, Panx-1
staining was predominantly observed in mitotic cells, which
provides support for the hypothesis that Panx-1 may participate in
the growth of glioma cells. A knockdown model of Panx-1 was
therefore produced in order to test this hypothesis. PCNA, an
ancestral nuclear protein involved in DNA replication, has a strong
association with cancer transformation (20–22).
It was used in the present study as a marker to track cell
proliferation during the cell transfection process. It was shown
that expression of Panx-1 was silenced by specific siRNAs at the
mRNA and protein level. In addition, as shown in Fig. 2, the change in protein levels of
Panx-1 was correlated with that observed in PCNA protein levels.
However, it is noteworthy that PCNA mRNA levels did not change in
the same manner. This may be attributed to different regulatory
mechanisms acting on the synthesis and degradation of mRNA and
proteins, which affect their quantity (23). This result provided strong evidence
that Panx-1 may be involved in the regulation of U87-MG cell
proliferation.
Prior studies have shown Panx-1 may be gated by
membrane depolarization, mechanical stimulation, extracellular
K+, intracellular Ca2+ release and ATP.
Furthermore, ATP has been implicated in the regulation of skeletal
muscle proliferation, differentiation and regeneration (24,25).
It was hypothesized that, as Panx-1 acts as an ATP-releasing
channel, Panx-1 knockdown may induce a downregulation of
extracellular ATP concentration. The present study demonstrated
that ATP markedly inhibited the proliferation of U87-MG cells,
therefore, Panx knockdown may increase cell proliferation. A study
conducted in C6 glioma cells provided evidence in support of this
theory (12). It revealed that
Panx was not expressed in these cells, but that transfection with
Panx-1 resulted in suppression of glioma cell growth. The current
study used a CCK-8 assay to analyze whether there was a synergistic
effect in inhibiting cell proliferation between ATP treatment and
Panx-1 silencing. Contrary to the hypothesis that Panx-1 knockdown
ultimately leads to an increase in cell proliferation via reducing
extracellular ATP, treatment with ATP led to a greater
downregulation of cell proliferation in the Panx-1-specific
siRNA-transfected U87-MG cells compared with control
siRNA-transfected cells, knockdown cells or ATP treated cells
alone. This suggests that Panx-1 located in the intracellular space
is not able to form an ATP-releasing channel, meaning that Panx-1
silencing had no effect on the extra- and intracellular ATP
concentration, and therefore was unable activate the purinergic
signaling pathway. However, further research is required to test
this hypothesis, including the use of BrdU or EdU incorporation
assays or flow cytometry to define progress through the cell
cycle.
Glioma cell proliferation and migration are
associated with the metastasis of cancer cells. The modulation of
cell proliferation is therefore important in cancer biology
research. Probenecid, which has been used as an effective clinical
drug for the treatment of arthritis, has been shown to be an
effective Panx-1 channel blocker (26–28).
This may prove to be a novel method of treatment for glioma.
However, its nonspecificity not only limits its use as a Panx-1
channel blocker, as it is also used as a multidrug resistance
transporter-1 (MRP1) blocker (29–31).
As a novel clinical treatment, gene therapy is currently accepted
as one of the most promising strategies for cancer therapy. siRNAs
have been shown to have effective biomedical genetic-therapy
applications for a number of diseases. siRNAs induce
sequence-specific gene silencing of target mRNAs and thus alter the
expression of molecules involved in tumor development. However, the
majority of these studies have been conducted at the cellular level
and further studies are required to explore the effects of siRNA
interference in vivo (32–36).
Acknowledgements
This study was supported by grants from the Shanghai
Municipal Health Bureau (grant no. 2012-234) to Mr. Yinghui
Chen.
References
|
1
|
Barbe MT, Monyer H and Bruzzone R:
Cell-cell communication beyond connexins: the pannexin channels.
Physiology (Bethesda). 21:103–114. 2006. View Article : Google Scholar
|
|
2
|
Panchin Y, Kelmanson I, Matz M, et al: A
ubiquitous family of putative gap junction molecules. Curr Biol.
10:R473–R474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suadicani SO, Iglesias R, Wang J, et al:
ATP signaling is deficient in cultured Pannexin1-null mouse
astrocytes. Glia. 60:1106–1116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dahl G and Keane RW: Pannexin: from
discovery to bedside in 11±4 years? Brain Res. 3:150–159. 2012.
View Article : Google Scholar
|
|
5
|
Penuela S, Gehi R and Laird DW: The
biochemistry and function of pannexin channels. Biochim Biophys
Acta. 1828:15–22. 2013. View Article : Google Scholar
|
|
6
|
D’hondt C, Ponsaerts R, De Smedt H, et al:
Pannexin channels in ATP release and beyond: An unexpected
rendezvous at the endoplasmic reticulum. Cell Signal. 23:305–316.
2011. View Article : Google Scholar
|
|
7
|
Gulbransen BD, Bashashati M, Hirota SA, et
al: Activation of neuronal P2×7 receptor-pannexin-1 mediates death
of enteric neurons during colitis. Nat Med. 18:600–604. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Celetti SJ, Cowan KN, Penuela S, et al:
Implications of pannexin 1 and pannexin 3 for keratinocyte
differentiation. J Cell Sci. 123:1363–1372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orellana JA, Froger N, Ezan P, et al: ATP
and glutamate released via astroglial connexin 43 hemichannels
mediate neuronal death through activation of pannexin 1
hemichannels. J Neurochem. 118:826–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pinheiro AR, Paramos-de-Carvalho D, Certal
M, et al: Histamine induces ATP release from human subcutaneous
fibroblasts, via pannexin-1 hemichannels, leading to
Ca2+ mobilization and cell proliferation. J Biol Chem.
288:27571–27583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wicki-Stordeur LE, Dzugalo AD, Swansburg
RM, et al: Pannexin 1 regulates postnatal neural stem and
progenitor cell proliferation. Neural Devel. 7:112012. View Article : Google Scholar
|
|
12
|
Lai CP, Bechberger JF, Thompson RJ, et al:
Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer
Res. 67:1545–1554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwamoto T, Nakamura T, Doyle A, et al:
Pannexin 3 regulates intracellular ATP/cAMP levels and promotes
chondrocyte differentiation. J Biol Chem. 285:18948–18958. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bond SR, Lau A, Penuela S, et al: Pannexin
3 is a novel target for Runx2, expressed by osteoblasts and mature
growth plate chondrocytes. J Bone Miner Res. 26:2911–2922. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turmel P, Dufresne J, Hermo L, et al:
Characterization of pannexin1 and pannexin3 and their regulation by
androgens in the male reproductive tract of the adult rat. Mol
Reprod Dev. 78:124–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chekeni FB, Elliott MR, Sandilos JK, et
al: Pannexin 1 channels mediate ‘find-me’ signal release and
membrane permeability during apoptosis. Nature. 467:863–867. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan C, Voss U, Svensson S, et al: High
glucose and free fatty acids induce beta cell apoptosis via
autocrine effects of ADP acting on the P2Y(13) receptor. Purinergic
Signal. 9:67–79. 2013. View Article : Google Scholar :
|
|
18
|
Santiago MF, Veliskova J, Patel NK, et al:
Targeting pannexin1 improves seizure outcome. PLoS One.
6:e251782011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karpuk N, Burkovetskaya M, Fritz T, et al:
Neuroinflammation leads to region-dependent alterations in
astrocyte gap junction communication and hemichannel activity. J
Neurosci. 31:14–425. 2011. View Article : Google Scholar
|
|
20
|
Stoimenov I and Helleday T: PCNA on the
crossroad of cancer. Biochem Soc Trans. 37:605–613. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiara AD, Pederzoli-Ribeil M, Burgel PR,
et al: Targeting cytosolic proliferating cell nuclear antigen in
neutrophil-dominated inflammation. Front Immunol. 3:3112012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): a key factor in DNA
replication and cell cycle regulation. Ann Bot. 107:1127–1140.
2011. View Article : Google Scholar :
|
|
23
|
de Sousa Abreu R, Penalva LO, Marcotte EM,
et al: Global signatures of protein and mRNA expression levels. Mol
Biosyst. 5:1512–1526. 2009.PubMed/NCBI
|
|
24
|
Ryten M, Dunn PM, Neary JT and Burnstock
G: ATP regulates the differentiation of mammalian skeletal muscle
by activation of a P2×5 receptor on satellite cells. J Cell Biol.
158:345–355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ryten M, Yang SY, Dunn PM, et al:
Purinoceptor expression in regenerating skeletal muscle in the mdx
mouse model of muscular dystrophy and in satellite cell cultures.
FASEB J. 18:1404–1406. 2004.PubMed/NCBI
|
|
26
|
Bao BA, Lai CP, Naus CC and Morgan JR:
Pannexin1 drives multicellular aggregate compaction via a signaling
cascade that remodels the actin cytoskeleton. J Biol Chem.
287:8407–8416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKuen MJ, Dahl G and Fields KA: Assessing
a potential role of host Pannexin 1 during Chlamydia trachomatis
infection. PLoS One. 8:e637322013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Zhu R, Huang Z, et al:
Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer
cells promotes cell survival and proliferation in hepatocellular
carcinoma. Dig Dis Sci. 58:2223–2236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YH, Wang CC, Xiao X, et al: Multidrug
resistance-associated protein 1 decreases the concentrations of
antiepileptic drugs in cortical extracellular fluid in amygdale
kindling rats. Acta Pharmacol Sin. 34:473–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tietje K, Rivera-Ingraham G, Petters C, et
al: Reporter dyes demonstrate functional expression of multidrug
resistance proteins in the marine flatworm Macrostomum lignano: the
sponge-derived dye Ageladine A is not a substrate of these
transporters. Mar Drugs. 11:3951–3969. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Furugen A, Yamaguchi H, Tanaka N, et al:
Contribution of multidrug resistance-associated proteins (MRPs) to
the release of prostanoids from A549 cells. Prostaglandins Other
Lipid Mediat. 106:37–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu J, Huang W and He Z: Dendrimers as
carriers for siRNA delivery and gene silencing: a review.
ScientificWorldJournal. 29:6306542013.
|
|
33
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu XW, Ding BW, Zhu CR, et al:
PU.1-silenced dendritic cells prolong allograft survival in rats
receiving intestinal transplantation. World J Gastroenterol.
19(43): 7766–7771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang ZH, Wang CY, Zhang WL, et al: Histone
deacetylase HDAC4 promotes gastric cancer SGC-7901 cells
progression via p21 repression. PLoS One. 9:e988942014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou M, Zhou L, Zheng L, et al: miR-365
Promotes Cutaneous Squamous Cell Carcinoma (CSCC) through Targeting
Nuclear Factor I/B (NFIB). PLoS One. 9:e1006202014. View Article : Google Scholar : PubMed/NCBI
|