Introduction
Lipid accumulation in the kidney contributes to the
progression of chronic kidney disease and independently induces
renal dysfunction (1,2). Hyperlipidemia induces a significant
increase in the expression of monocyte chemoattractant protein-1
(MCP-1) in apolipoprotein E (ApoE)−/− mice fed a high-fat diet
(3,4).
During the process of kidney injury, the expression
of MCP-1 is significantly increased via numerous cells expressing
MCP-1 in kidney inflammation, including those in glomerular
(5–7) and tubular areas (8–10)
and primary endothelial cells (11). Tashiro et al suggested that
urinary MCP-1 may be useful for evaluating the degree of renal
injuries in patients with diabetic nephropathy (12). By contrast inhibition of the
expression of MCP-1 protects renal injury by reducing macrophage
infiltration (13,14).
The increased expression of MCP-1 and subsequent
macrophage infiltration is a substantial source of pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α and interleukin
(IL)-6, which induce further damage in kidney dysfunction.
Increased expression levels of IL-6 are often observed in patients
with progressive kidney dysfunction and can affect the outcome of
long-term kidney diseases (15),
suggesting that MCP-1 and IL-6 are important in
hyperlipidemia-induced kidney dysfunction.
Baicalin is one of several pharmacologically-active
flavones and is widely used in traditional Chinese herbal medicine
(16). It has a number of
pharmacological effects, including inhibitory effects against
several viruses (17–19) and anti-inflammatory, anti-allergic
and antibacterial effects in a variety of inflammatory diseases
(20,21). However, the effects of baicalin on
MCP-1 and IL-6 in the kidneys of ApoE-knockout (KO) mice have not
been investigated.
Due to its anti-inflammatory effect, baicalin may
have a protective effect against kidney dysfunction via regulating
the expression of MCP-1 and IL-6.
Materials and methods
Drug administration
Baicalin (Sigma-Aldrich, St. Louis, MO, USA; cat.
no. 21967-41-9; purity, 98%; molecular formula,
C21H18O11; molecular weight,
446.36) was dissolved in normal saline and the pH was adjusted to
pH 7.4 using NaOH.
Experimental animal models and tissues
preparation
Male C57BL/6J mice, and ApoE−/− mice (8-week-old)
which were of a C57BL/6J background, were purchased from The
Jackson Laboratory (Bar Harbor, ME, USA). The mice were bred and
maintained at the Animal Center of Beijing University (Beijing,
China), following the guidelines provided in the Guide for the Care
and Use of Laboratory Animals, published by the US National
Institutes of Health (NIH Publication No. 85-23, revised 1996). All
animal procedures were reviewed and approved by the ethics
committee of Huazhong University os Science and Technology (Wuhan,
China). The mice were acclimated to their environment, which
consisted of controlled lighting (12 h light/dark cycle) and
temperature (25°C), for a period of 1 week.
Following adaptation, the ApoE−/− mice were randomly
assigned to one of two groups. Group 1 (hypercholesterol control
group; n=6) were freely fed a high-cholesterol diet containing
1.25% cholesterol and 10% coconut oil (22) and received normal saline by gavage
feeding once daily for 12 weeks. Group 2 (baicalin administration
group; n=6) were fed a high-cholesterol diet (100 mg/kg/day) and
received baicalin treatment (200 μl/kg/day) by gavage feeding once
daily for 12 weeks. A group of wild-type male C57BL/6J mice (normal
control group, n=6) were fed a standard diet (free feeding; mouse
food, Oriental Yeast, Co, Ltd., Tokyo, Japan) and received normal
saline by gavage feeding once daily for 12 weeks. Water was
available ad libitum to all mice groups.
At 21 weeks of age, the mice were anesthetized with
2% pentobarbital solution (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) by intraperitoneal injection, and then
sacrificed by bleeding through the aorta. The heart and aorta were
immediately removed following perfusion with phosphate-buffered
saline (PBS; Wuhan Boster Biological Technology, Ltd., Wuhan,
China). The kidneys of the mice were then rapidly frozen in liquid
nitrogen (Huazhong University of Science and Technology, Wuhan,
China) and stored until subsequent protein and RNA extraction.
Plasma TC profile
The mice were anesthetized at 21 weeks of age and
blood was obtained from the angular veins using a capillary siphon.
The plasma levels of TC were detected using a Hitachi 917
auto-analyzer (Hitachi, Ltd., Tokyo, Japan).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis of the mRNA
expression levels of MCP-1, IL-6 and vascular cell adhesion
molecule 1 (VCAM-1)
Total RNA was extracted from the mouse renal tissues
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. mRNA was then
converted into cDNA with the ReverTra Ace® qPCR RT Kit
(Toyobo, Osaka, Japan). Following amplification by RT, the mRNA
expression levels of MCP-1, IL-6 and VCAM-1 were detected by
RT-qPCR, performed using a SYBR PrimeScript RT-PCR kit (Takara Bio,
Inc., Otsu, Japan) and the ABI PRISM 7900HT sequence detection
system (Applied Biosystems Life Technologies, Foster City, CA,
USA). The relative gene expression levels of the target genes,
normalized to the endogenous control gene β-actin, were calculated
using the comparative Ct method formula 2−ΔΔCT. The
following PCR primers (GenScript Inc, Piscataway, NJ, USA) were
used: MCP-1, forward 5′-TGTCCCAAAGAAGCTGTAGT-3′ and reverse
5′-ACAGAAGTGCTTGAGGTGGT-3′; IL-6, forward
5′-GTTGCCTTCTTGGGACTGATG-3′ and reverse
5′-GTATAGACAGGTCTGTTGGGAG-3′; VCAM-1, forward
5′-TGGCTCCAGACATTTACCCAGTTT-3′ and reverse
5′-GTTCTTTGACAGTCTCCCTTTCTTT-3′ and β-actin, forward
5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse
5′-TAAAGACCTCTATGCCAACACAGT-3′. The PCR cycling conditions were as
follows: 1 cycle of 50°C for 2 min, 95°C for 10 min, followed by 40
cycles of 95°C for 30 sec and 60°C for 30 sec.
Western blot analysis of the protein
levels of MCP-1, IL-6 and VCAM-1
The expression levels of proteins in the kidneys
were determined using western blot analysis. The tissues, which
were frozen in liquid nitrogen following isolation and stored at
−80°C, were homogenized in 25 mM Tris-HCl (pH 7.4), 1 mM
dithiolthreitol and 1 mM sodium orthovanadate (Invitrogen Life
Technologies), a protease inhibitor cocktail tablet (complete
protease; Roche NimbleGen, Inc., Madison, WI, USA), phosphatase
inhibitor cocktail (PhosSTOP; Roche NimbleGen, Inc.) and 1% Triton
X-100 (Invitrogen Life Technologies) using a glass homogenizer
(Wuhan Boster Biological Technology, Ltd.). The total protein
samples were prepared from the homogenates of pooled arteries and
protein concentrations were determined using a bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
Equal quantities of the protein extracts were separated on 7.5, 10
and 15% SDS-PAGE gels and immobilized onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were then sequentially blocked using Tris-buffered saline
containing 5% non-fat milk and incubated overnight at 4°C with the
following primary antibodies: Rabbit polyclonal anti-MCP-1 (1:200;
ab9669; Abcam, Cambridge, MA, USA); rabbit polyclonal
immunoglobulin G (IgG) anti-IL-6 (1:800; sc-1265-R; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and rabbit polyclonal
anti-VCAM-1 (1:600; BS6005; Bioworld Technology, Inc., St. Louis
Park, MN, USA). Following incubation, the membranes were washed
with Tris-buffered saline (Wuhan Boster Biological Technology,
Ltd.) and further incubated with horseradish peroxidase-conjugated
goat anti-rabbit polyclonal IgG secondary antibody (1:10,000; Wuhan
Boster Biological Technology, Ltd.) at 37°C for 1 h. Following
several additional washes, immune-positive bands were detected by
electrochemiluminescence (Beijing Liuyi Instrument Plant, Beijing,
China). Immunoreactive signals were visualized using SuperSignal
West Dura Extended Duration Substrate (Thermo Fisher Scientific),
and detected using a Kodak XRS system (Eastman Kodak, Fair Lawn,
NJ, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Comparison of the means was performed by Student’s
t-test for two groups and one-way analysis of variance for multiple
groups using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
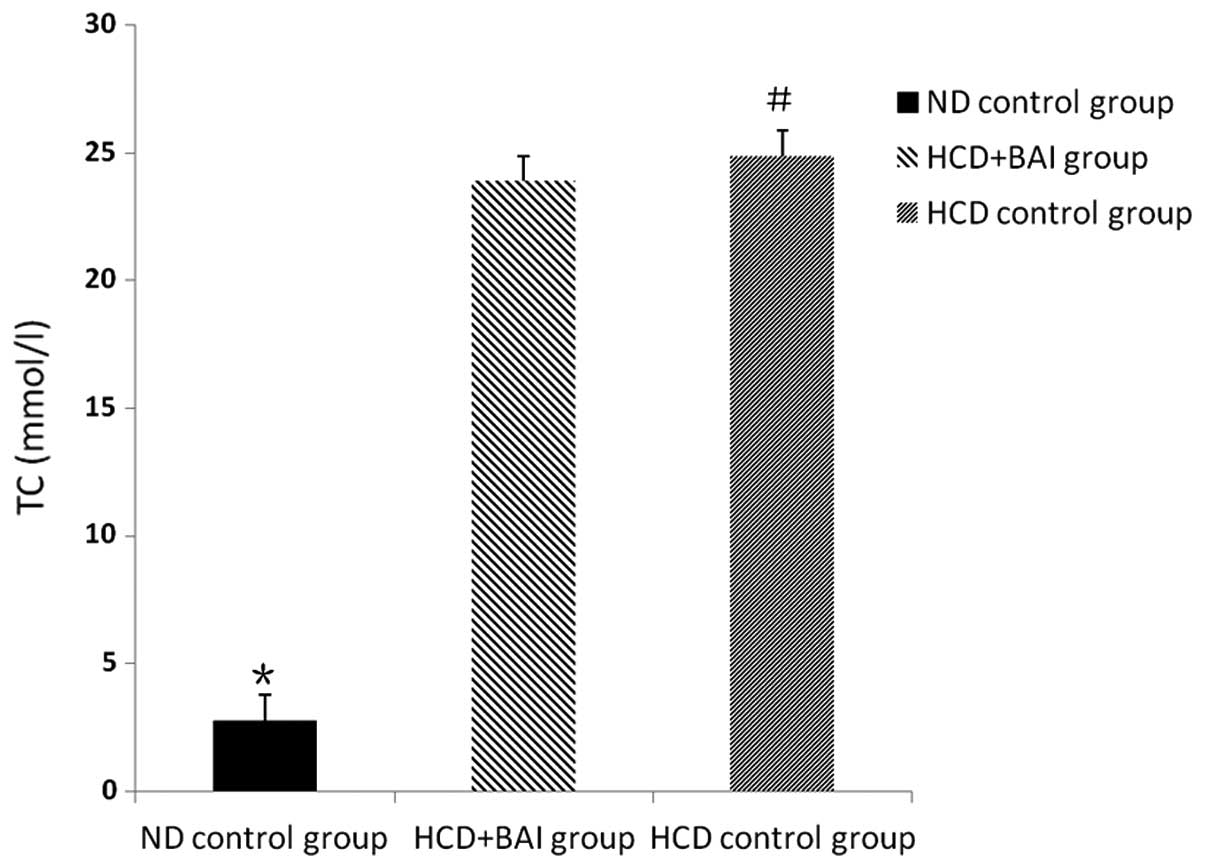
Impact of baicalin on plasma TC levels in
mice
Following the administration of baicalin for 12
weeks, the levels of TC in the male wild-type C57BL/6J mice were
lower compared with the ApoE−/− mice fed a high cholesterol diet
(P<0.05). No significant difference in the levels of TC were
observed between the baicalin administration group and
hypercholesterol control group (P>0.05). Baicalin caused no
decrease in the level plasma TC in the ApoE−/− mice fed a high
cholesterol diet (Fig. 1).
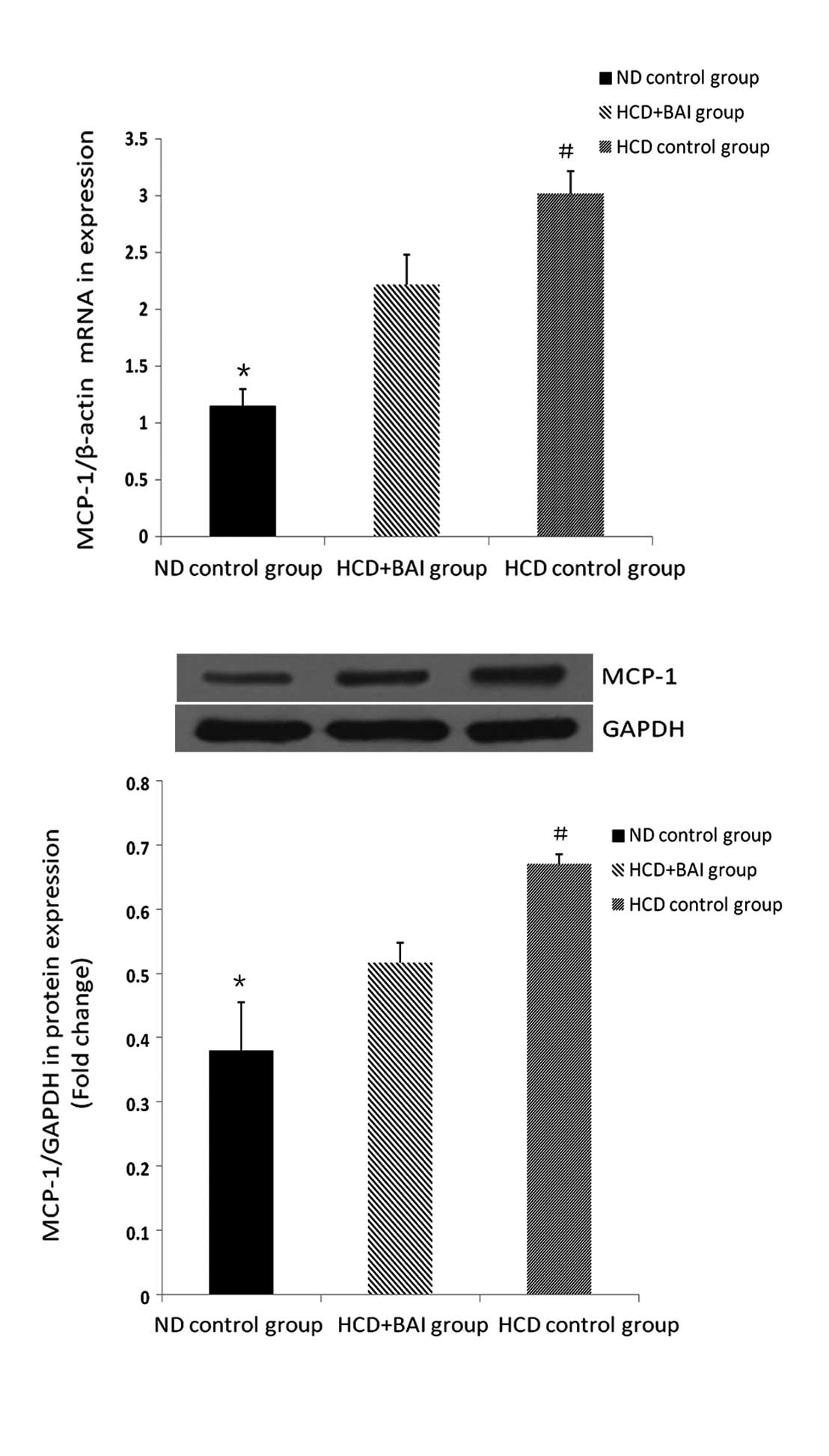
RT-qPCR and western blot analysis of the
effect of baicalin on the mRNA and protein expression of MCP-1 in
the mouse kidneys
To investigate the effect of baicalin on the
expression of MCP-1, the mRNA and protein expression levels of
MCP-1 were examined in the mouse kidneys. The expression of MCP-1
in the wild-type male C57BL/6J mice was lower compared with all the
groups of ApoE−/− mice fed a high cholesterol diet (P<0.05) and
the expression of MCP-1 was highest in the hypercholesterol control
group (P<0.05). Following administration of baicalin, the
expression of MCP-1 was reduced compared with that in the
hypercholesterol control group (P<0.05). Baicalin reduced the
expression of renal MCP-1 in the ApoE−/− mice fed a high
cholesterol diet (Fig. 2).
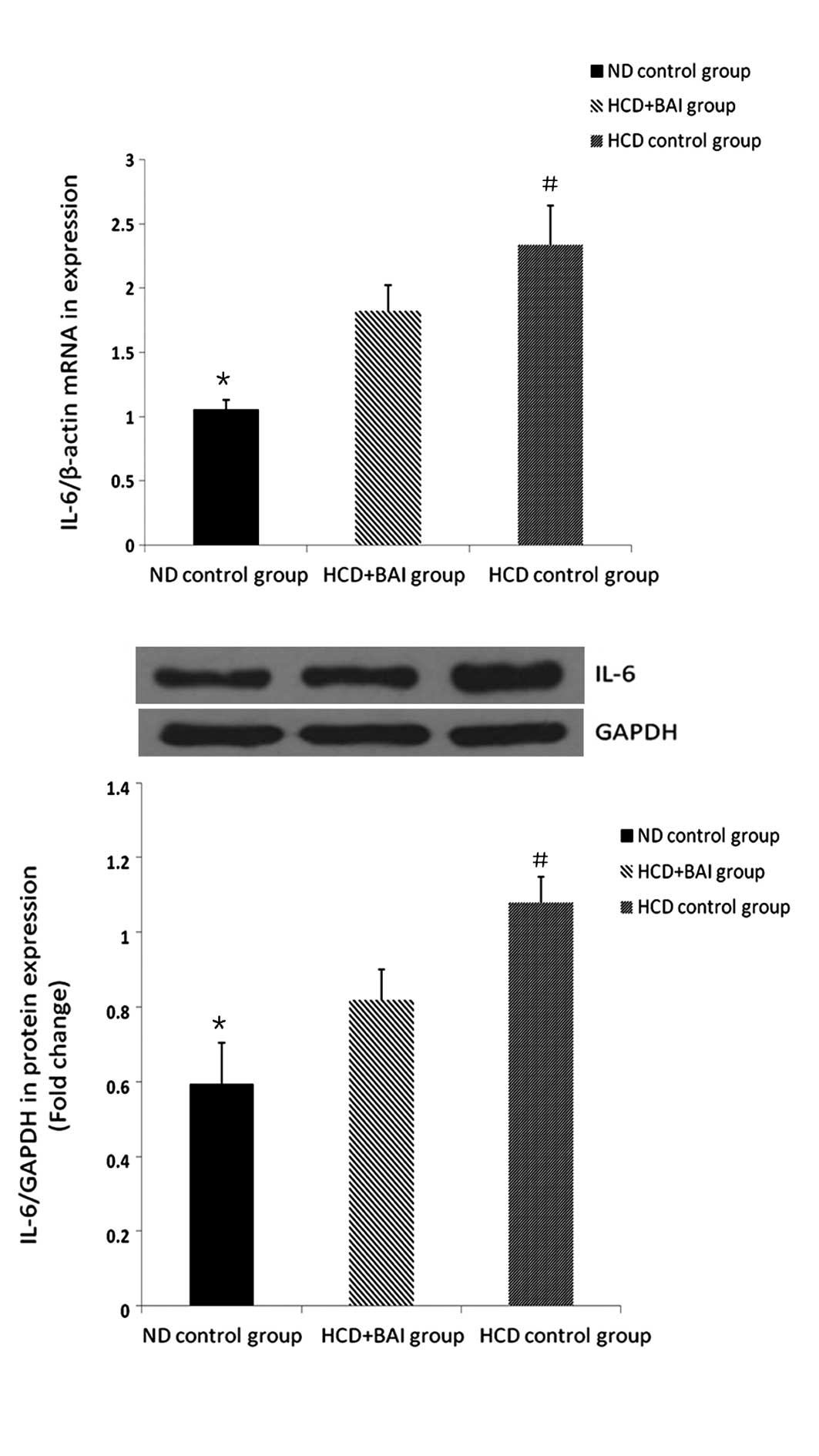
RT-qPCR and western blot analysis of the
effect of baicalin on the mRNA and protein expression of IL-6 in
the mouse kidneys
To investigate the effect of baicalin on the
expression of IL-6, the mRNA and protein expression levels of IL-6
were examined in the mouse kidneys. The expression of IL-6 in the
wild-type male C57BL/6J mice was lower compared with all the groups
of ApoE−/− mice fed a high cholesterol diet (P<0.05) and the
expression of IL-6 was highest in the hypercholesterol control
group (P<0.05). Following administration of baicalin, the
expression of IL-6 was reduced compared with that in the
hypercholesterol control group (P<0.05). Baicalin also reduced
the expression of renal IL-6 in the ApoE−/− mice fed a high
cholesterol diet (Fig. 3).
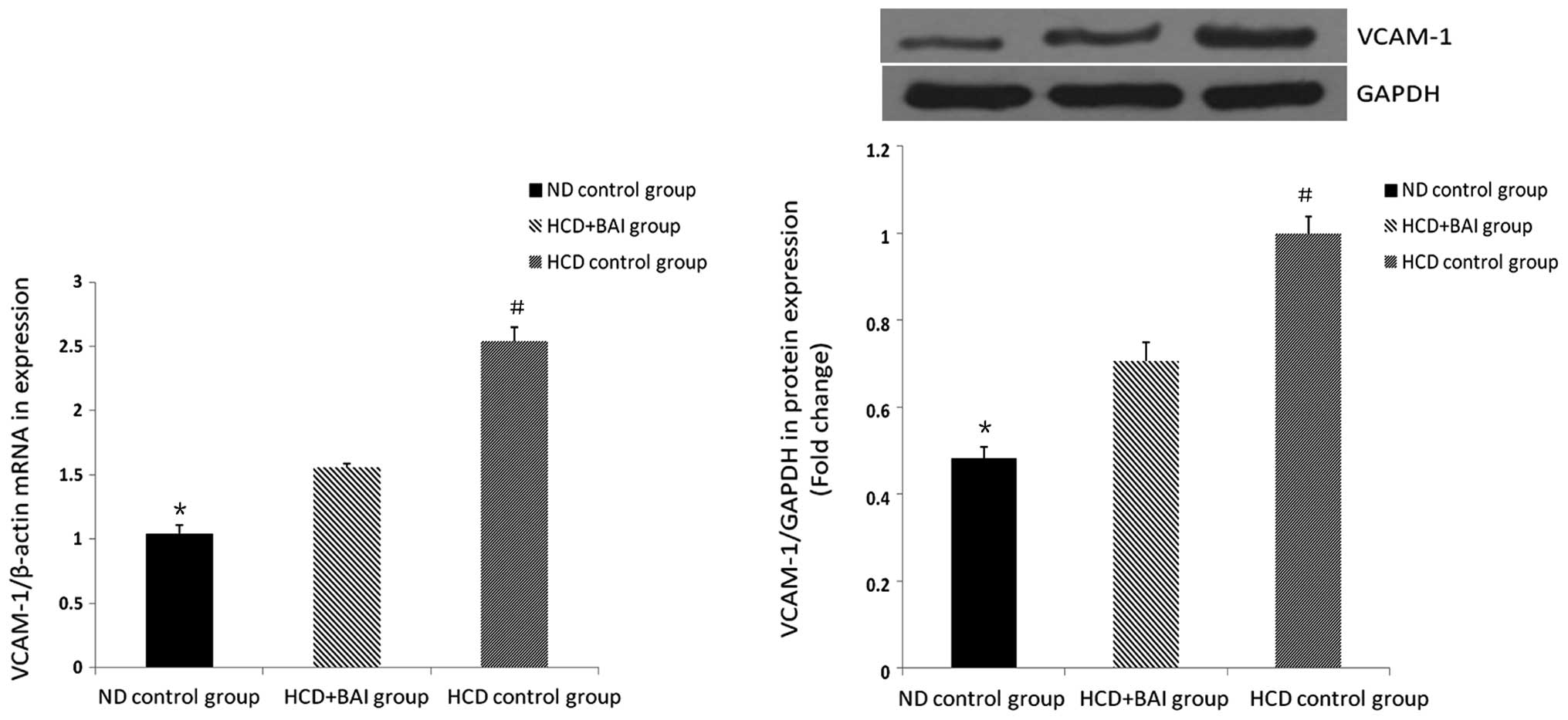
RT-qPCR and western blot analysis of the
effect of baicalin on the mRNA and protein expression of VCAM-1 in
the mouse kidneys
The mRNA and protein expression of VCAM-1 was also
assessed in the mouse kidneys administered with baicalin. The
expression of VCAM-1 in wild-type male C57BL/6J mice was lower
compared with all the groups of ApoE−/− mice fed a high cholesterol
diet (P<0.05) and the expression of VCAM-1 was highest in the
hypercholesterol control group (P<0.05). Following baicalin
administration, the expression of VCAM-1 was reduced compared with
that in the hypercholesterol control group (P<0.05). Baicalin
reduced the expression of renal VCAM-1 in the ApoE−/− mice fed a
high cholesterol diet (Fig.
4).
Discussion
The present study demonstrated that baicalin
attenuated the expression levels of MCP-1 and IL-6 in the renal
tissues, resulting in suppression of the secretion of VCAM-1 in the
ApoE−/− mice fed a high cholesterol diet. However, no decrease in
the plasma TC was observed.
Baicalin inhibits the activation of nuclear factor
(NF)-κB via the NF-κB-inducing kinase/inhibitor of NF-κB kinase,
extracellular-signal-regulated kinases and p38-mitogen activated
protein kinase pathways in the kidney tissues of rats (23). It also significantly suppresses
iron overload-induced kidney injury (24) and protects the kidneys of rats with
severe acute pancreatitis by inhibiting inflammatory mediators and
inducing apoptosis (25). The
present study revealed that baicalin attenuated the expression of
MCP-1, IL-6 and VCAM-1 in the kidney tissues from the ApoE−/− mice,
consistent with previous studies on the inhibition of inflammatory
mediators.
As an activator of macrophages and monocytes, MCP-1
recruits macrophages and monocytes, inducing the infiltration of
inflammatory cells into the kidney tissues in several types of
kidney disease, including diabetic nephropathy (26), glomerulonephritis (27) and chronic renal injury in obesity
(28). In the present study, the
ApoE−/− mice group fed a high cholesterol diet, exhibited increased
expression levels of MCP-1 and IL-6 and this may promote the
progression of kidney dysfunction. The results demonstrated that
baicalin reduced the expression levels of MCP-1 and IL-6 in the
kidney tissues of the AopE−/− mice fed a high cholesterol diet.
Although no decrease in the level of TC was observed in the mice
treated with baicalin, it may inhibit hypercholesterol-associated
pro-inflammatory mediators in kidney tissues, reduce leucocyte
recruitment into the kidney and alleviate inflammation.
Previous studies have demonstrated that the
expression of VCAM-1 increases within the kidneys in several types
of kidney diseases, including lupus nephritis (29), chronic renal failure (30,31)
and immunoglobulin (Ig)A nephropathy (32). Furthermore, in IgA nephropathy,
levels of plasma VCAM-1 can be used in patients as a marker, not
only for severe clinical statuses, but also for severe histological
lesions (32,33). Severe hyperlipidaemia in ApoE null
mice fed with a 0.15% cholesterol Western diet leads to glomerular
injury, glomerular endothelial cell activation and increased
expression levels of major histocompatability complex class II
(I-Ab) and VCAM-1, which recruit leucocytes to the kidney and
induces renal inflammatory diseases (34). Following treatment with baicalin in
the present study, the expression of VCAM-1 in the kidney tissues
decreased significantly, accompanied by a decreased production of
MCP-1 and IL-6. Therefore, the inhibitory effect of baicalin may be
due to its ability to attenuate pro-inflammatory producers under
hyperlipidemia.
The present study had certain limitations. Baicalin
was not compared with other drugs and only a single concentration
of baicalin, in accordance with previous studies, was used.
Therefore, no comparison was made on the effects of different
concentrations of baicalin.
In conclusion, the present study demonstrated that
the administration of baicalin induced a substantial decrease in
the expression levels of MCP-1, IL-6 and VCAM-1 in the kidney
tissue from ApoE−/− mice fed a high cholesterol diet and revealed
that baicalin may prevent the process of inflammatory
mediator-induced kidney dysfunction in several types of renal
disease.
References
|
1
|
Moorhead JF, Chan MK, El-Nahas M and
Varghese Z: Lipid nephrotoxicity in chronic progressive glomerular
and tubulo-interstitial disease. Lancet. 2:1309–1311. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
uan XZ, Varghese Z and Moorhead JF: An
update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol.
5:713–721. 2009. View Article : Google Scholar
|
|
3
|
Kim E, Tolhurst AT, Qin LY, Chen XY,
Febbraio M and Cho S: CD36/fatty acid translocase, an inflammatory
mediator, is involved in hyperlipidemia-induced exacerbation in
ischemic brain injury. J Neurosci. 28:4661–4670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sultan A, Strodthoff D, Robertson AK, et
al: T cell-mediated inflammation in adipose tissue does not cause
insulin resistance in hyperlipidemic mice. Circ Res. 104:961–968.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perez de Lema G, Maier H, Nieto E, et al:
Chemokine expression precedes inflammatory cell infiltration and
chemokine receptor and cytokine expression during the initiation of
murine lupus nephritis. J Am Soc Nephrol. 12:1369–1382.
2001.PubMed/NCBI
|
|
6
|
Anders HJ, Belemezova E, Eis V, et al:
Late onset of treatment with a chemokine receptor CCR1 antagonist
prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am
Soc Nephrol. 15:1504–1513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rovin BH, Rumancik M, Tan L and Dickerson
J: Glomerular expression of monocyte chemoattractant protein-1 in
experimental and human glomerulonephritis. Lab Invest. 71:536–542.
1994.PubMed/NCBI
|
|
8
|
Zoja C, Liu XH, Donadelli R, et al: Renal
expression of monocyte chemoattractant protein-1 in lupus
autoimmune mice. J Am Soc Nephrol. 8:720–729. 1997.PubMed/NCBI
|
|
9
|
Tesch GH, Schwarting A, Kinoshita K, Lan
HY, Rollins BJ and Kelley VR: Monocyte chemoattractant protein-1
promotes macrophage-mediated tubular injury, but not glomerular
injury, in nephrotoxic serum nephritis. J Clin Invest. 103:73–80.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chow F, Ozols E, Nikolic-Paterson DJ,
Atkins RC and Tesch GH: Macrophages in mouse type 2 diabetic
nephropathy: correlation with diabetic state and progressive renal
injury. Kidney Int. 65:116–128. 2004. View Article : Google Scholar
|
|
11
|
He X, Schoeb TR, Panoskaltsis-Mortari A,
et al: Deficiency of P-selectin or P-selectin glycoprotein ligand-1
leads to accelerated development of glomerulonephritis and
increased expression of CC chemokine ligand 2 in lupus-prone mice.
J Immunol. 177:8748–8756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tashiro K, Koyanagi I, Saitoh A, et al:
Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and
interleukin-8 (IL-8), and renal injuries in patients with type 2
diabetic nephropathy. J Clin Lab Anal. 16:1–4. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanamori H, Matsubara T, Mima A, et al:
Inhibition of MCP-1/CCR2 pathway ameliorates the development of
diabetic nephropathy. Biochem Biophys Res Commun. 360:772–777.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chow FY, Nikolic-Paterson DJ, Ozols E,
Atkins RC, Rollin BJ and Tesch GH: Monocyte chemoattractant
protein-1 promotes the development of diabetic renal injury in
streptozotocin-treated mice. Kidney Int. 69:73–80. 2006. View Article : Google Scholar
|
|
15
|
Garibotto G, Sofia A, Balbi M, et al:
Kidney and splanchnic handling of interleukin-6 in humans.
Cytokine. 37:51–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Lin G and Zuo Z: Pharmacological
effects and pharmacokinetics properties of Radix scutellariae and
its bioactive flavones. Biopharm Drug Dispos. 32:427–445. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baylor NW, Fu T, Yan YD and Ruscetti FW:
Inhibition of human T cell leukemia virus by the plant flavonoid
baicalin (7-glucuronic acid, 5,6-dihydroxyflavone). J Infect Dis.
165:433–437. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li BQ, Fu T, Dongyan Y, Mikovits JA,
Ruscetti FW and Wang JM: Flavonoid baicalin inhibits HIV-1
infection at the level of viral entry. Biochem Biophys Res Commun.
276:534–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng Y, Song C, Ding X, Ji X, Yi L and Zhu
K: Baicalin reduces the severity of experimental autoimmune
encephalomyelitis. Braz J Med Biol Res. 40:1003–1010. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XP, Tian H, Wu DJ, et al:
Pathological changes in multiple organs of rats with severe acute
pancreatitis treated by baicalin and octreotide. Hepatobiliary
Pancreat Dis Int. 8:85–92. 2009.PubMed/NCBI
|
|
21
|
Zhang X, Tian H, Wu C, et al: Effect of
baicalin on inflammatory mediator levels and microcirculation
disturbance in rats with severe acute pancreatitis. Pancreas.
38:732–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zwai M, Chen R, Li Z, et al: Deletion of
angiotensin II type 2 receptor exaggerated atherosclerosis in
apolipoprotein E-null mice. Circulation. 112:1636–1643. 2005.
View Article : Google Scholar
|
|
23
|
Kim DH, Kim HK, Park S, et al: Short-term
feeding of baicalin inhibits age-associated NF-kappaB activation.
Mech Ageing Dev. 127:719–725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Gao Z, Liu J and Xu Z: Protective
effects of baicalin and quercetin on an iron-overloaded mouse:
comparison of liver, kidney and heart tissues. Nat Prod Res.
25:1150–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang XP, Tian H, Lai YH, et al:
Protective effects and mechanisms of Baicalin and octreotide on
renal injury of rats with severe acute pancreatitis. World J
Gastroenterol. 13:5079–5089. 2007.PubMed/NCBI
|
|
26
|
Lv SS, Liu G, Wang JP, et al: Mesenchymal
stem cells transplantation ameliorates glomerular injury in
streptozotocin-induced diabetic nephropathy in rats via inhibiting
macrophage infiltration. Int Immunopharmacol. 17:275–282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tesch GH, Maifert S, Schwarting A, Rollins
BJ and Kelley VR: Monocyte chemoattractant protein 1-dependent
leukocytic infiltrates are responsible for autoimmune disease in
MRL-Fas(lpr) mice. J Exp Med. 190:1813–1824. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu CP, Lee IT, Sheu WH, et al: The levels
of circulating and urinary monocyte chemoattractant protein-1 are
associated with chronic renal injury in obese men. Clin Chim Acta.
413:1647–1651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu T, Xie C, Wang HW, et al: Elevated
urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC
chemokine ligand 16 in multiple murine lupus strains and human
lupus nephritis. J Immunol. 179:7166–7175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bolton CH, Downs LG, Victory JG, et al:
Endothelial dysfunction in chronic renal failure: roles of
lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial
Transplant. 16:1189–1197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cottone S, Mule G, Amato F, et al:
Amplified biochemical activation of endothelial function in
hypertension associated with moderate to severe renal failure. J
Nephrol. 15:643–648. 2002.PubMed/NCBI
|
|
32
|
Zhu L, Shi S, Liu L, Lv J and Zhang H:
Increased plasma sVCAM-1 is associated with severity in IgA
nephropathy. BMC Nephrol. 14:212013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nelson CL, Karschimkus CS, Dragicevic G,
et al: Systemic and vascular inflammation is elevated in early IgA
and type 1 diabetic nephropathies and relates to vascular disease
risk factors and renal function. Nephrol Dial Transplant.
20:2420–2426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bruneval P, Bariety J, Belair MF, Mandet
C, Heudes D and Nicoletti A: Mesangial expansion associated with
glomerular endothelial cell activation and macrophage recruitment
is developing in hyperlipidaemic apoE null mice. Nephrol Dial
Transplant. 17:2099–2107. 2002. View Article : Google Scholar : PubMed/NCBI
|