Introduction
Breast adenocarcinoma is the second leading cause of
cancer-associated mortality after lung cancer and is the most
common type of malignancy diagnosed in women in China (1,2).
Breast cancer is the most common type of invasive cancer in women
with an incidence ranging between 0.193% in eastern Africa to
0.897% in western Europe (3).
JWA, also termed ADP-ribosylation-like factor 6
interacting protein 5, was initially cloned from human tracheal
bronchial epithelial cells (4). As
a tumor suppressor gene, it is widely expressed in the majority of
organ tissues and cultured cells, including in melanoma, gastric
cancer, hepatocellular carcinoma, esophageal squamous cell
carcinoma and ovarian cancer (5–9).
Furthermore, certain studies have revealed that the expression of
JWA in malignant tumor tissues is lower compared with the matched
non-tumor tissues (8,10). However, the expression of JWA in
breast cancer remains to be fully elucidated.
The mitogen-activated protein kinase (MAPK)
signaling pathway, which has been observed in several studies, is
aberrantly activated in a number of tumor cells (11,12).
Three distinct MAPK pathways have been defined, including
extracellular signal-regulated kinase (ERK) 1/2, also termed
p44/p42; c-Jun N-terminal kinase (JNK), also termed
stress-activated protein kinases (SAPK) and CSBP/RK/Mpk2 kinase
(p38) (13). The development of
breast cancer is closely associated with the activation of the MAPK
pathway. The activation of p38 MAPK, but not JNK or ERK1/2, has
been observed to increase by arctigenin (ATG), a natural lignan
product of Arctium lappa in human breast cancer MDA-MB-231
cells (14).
Previous studies have reported that the expression
level of JWA, a structurally novel microtubule-associated protein,
is associated with the MAPK pathway in the regulation of tumor
proliferation, invasion and apoptosis in vitro and in
vivo (9,15). In addition, the overexpression of
JWA induces apoptosis and inhibits migration and invasion in the
human esophageal squamous cell carcinoma (ESCC) cell lines
(15). Activation of the p38 MAPK
signaling pathway has been found to contribute to JWA-induced
tubulin polymerization, which is involved in
As2O3-induced apoptosis (9).
In present study, the protein levels of JWA in
breast cancer tissues and normal tissues were assessed to confirm
whether the expression was reduced in the tumor tissues and whether
the JWA gene was associated with the MAPK pathway in inducing the
progression of breast cancer. Cell proliferation, migration,
invasion and apoptosis were assessed in the JWA-knockdown
MDA-MB-231 cell lines. The expression levels of three major MAPK
pathways following JWA-knockdown in the MDA-MB-231 cells were also
assessed. The aims were to examined the association between the
MAPK pathway, the JWA gene and in the development of breast cancer
cells to elucidate the possible mechanism underlying the malignant
process of breast cancer.
Materials and methods
Breast cancer specimens
The tumor specimens and paired normal breast tissue
specimens were obtained from consenting patients (15 female
patients, aged from 26–53, who underwent breast surgery) and
approved by the Medical Ethics Committee of Yixing People’s
Hospital (Yixing, China). None of the patients had received
radiotherapy or chemotherapy prior to surgery.
Cell culture
The MDA-MB-231 breast cancer cells were purchased
from American Type Culture Collection (Manassas, VA, USA) and were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone
Laboratories, South Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS; Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Zhejiang, China), 100 U/ml penicillin and 100
mg/l streptomycin (Beyotime Institute of Biotechnology, Shanghai,
China). The cells were cultured in a humidified incubator
containing 5% CO at 37°C.
JWA small interfering (si)RNA
transfection
The JWA siRNA was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA; Cat. no, sc-60820) and
siRNA transfection was performed using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). The
concentration of siRNA was 150 nm after 6 h. The cells were
collected after 48 h and nonsense siRNA was used as a negative
control and a blank control.
MTT assay
Cell proliferation was measured using an MTT assay.
The cells were collected 6 h after transfection and seeded at
2×104cells/well in DMEM containing 10% FBS in
96-well-plates. A total of five duplicate wells were used for each
group and the assay was repeated three times. After 48 h, 20 μl 5
mg/ml MTT solution in PBS was added to each well for 4 h. The
absorbance of each well was determined using an Infinite F50
Microplate Reader (Tecan Group, Ltd., Männedorf, Switzerland) at a
wavelength of 570 nm. According to the optical densities,
proliferation curves were plotted and the proliferation of the two
groups were compared prior to and following transfection.
Transwell assay
Cell migration and invasion were determined using a
Costar® transwell (Corning Costar, Cambridge, MA, USA)
with a pore size of 0.8 μm. Matrigel (100 μl; BD Biosciences,
Franklin Lakes, NJ, USA) was added to a 24-well-Transwell chamber,
the normal chamber was used for cell migration assays and the
Matrigel-coated chamber was used for cell invasion assays. Cell
suspension (100 μl) at a concentration of 2×105/ml was
added to the upper chamber and DMEM containing 10% FBS was added to
the lower chamber. Following incubating for 24 h at 37°C, the cells
in the upper chamber were carefully removed using a cotton swab and
the cells that had traversed to the reverse side of the membrane
were fixed with methanol, stained with Giemsa (Sangon, Shanghai,
China) and the penetrating cells were counted under a light
microscope at ×200 magnification (Olympus BX41, Olympus
Corporation, Tokyo, Japan).
Western blot analysis
The proteins were extracted from the MDA-MB-231
cells using radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) 72 h after transfection. The
proteins (40 μg) were separated by SDS-PAGE and then blotted onto a
hybond enhanced chemilluminescence (ECL) nitrocellulose membrane
(GE Healthcare Life Sciences, Shanghai, China). The membrane was
subsequently blocked using 5% nonfat milk at room temperature
(15–25°C) for 1.5 h and incubated at 4°C overnight (15–17 h) with a
rabbit polyclonal antibody to B-cell lymphoma 2 (Bcl-2; Abcam,
Cambridge, UK), a rabbit polyclonal antibody to Bcl2-associated X
protein (BAX), a rabbit monoclonal antibody to mitogen-activated
protein kinase (MEK), a rabbit monoclonal antibody to extracellular
signal-regulated kinase (ERK) 1/2, a rabbit monoclonal antibody to
p38, a rabbit monoclonal antibody to c-Jun N-terminal kinases
(JNK), a rabbit monoclonal antibody to phosphorylated (p-)MEK, a
rabbit monoclonal antibody to p-ERK1/2, p-p38, a rabbit monoclonal
antibody to p-JNK (all from Cell Signaling Technology, Inc.,
Danvers, MA, USA, at a dilution of 1:1,000) and a mouse anti-human
GAPDH monoclonal antibody (Beyotime Institute of Biotechnology;
1:1,000).
After 15–17 h, the membrane was washed with
Tris-buffered saline prior to incubation for 2 h at room
temperature with the secondary antibody of immunoglobulin G (Merck
KGaA, Darmstadt, Germany), labelled with alkaline phosphatase and
colored by ECL, at room temperature. The membrane was then scanned
using a Hewlett-Packard Development Company, 5590 (Hewlett-Packard,
Palo Alto, CA, USA) to determine the relative value of protein
expression.
Statistical analysis
All data were analyzsed using SPSS 14.0 software
(SPSS, Inc., Chicago, IL, USA). The results of the quantitative
experiments are expressed as the mean ± standard deviation. The
samples were compared using Student’s t-test or one-way analysis of
variance (ANOVA). P<0.05 was considered to indicate a
statistically significant difference.
Results
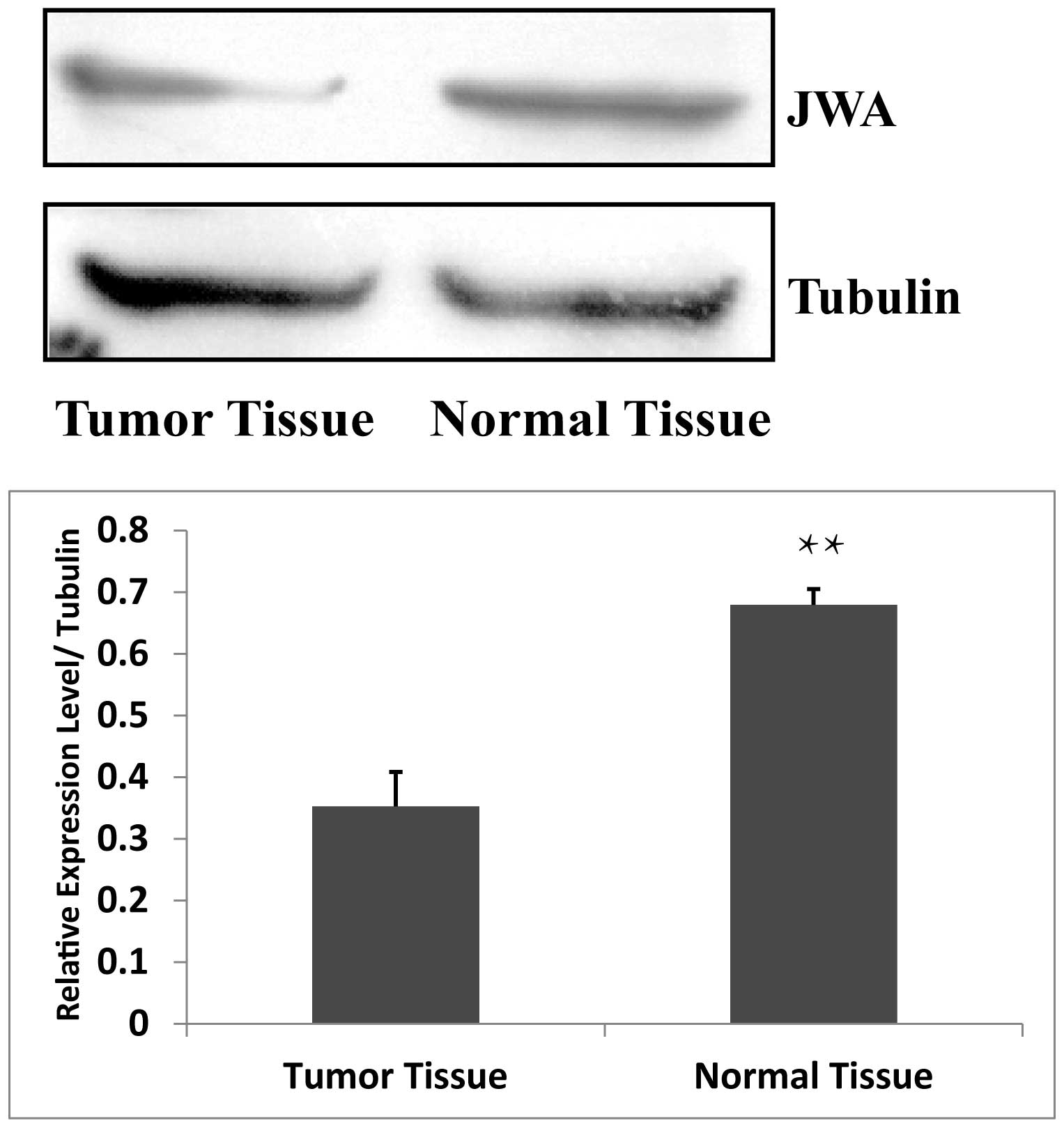
Expression level of JWA in breast cancer
tissues and normal breast tissues
JWA is widely expressed in a number of organ tissues
and cultured cells (5–9). The present study hypothesized that
JWA was also present in breast tissue. Therefore, the expression
levels of JWA in breast tumor tissue and in normal breast tissue
were assessed using Western blot analysis. The expression of JWA
was significantly higher in the normal breast tissue compared with
the breast tumor tissue (Fig. 1),
indicating that the expression of JWA is low in breast cancer
tissues.
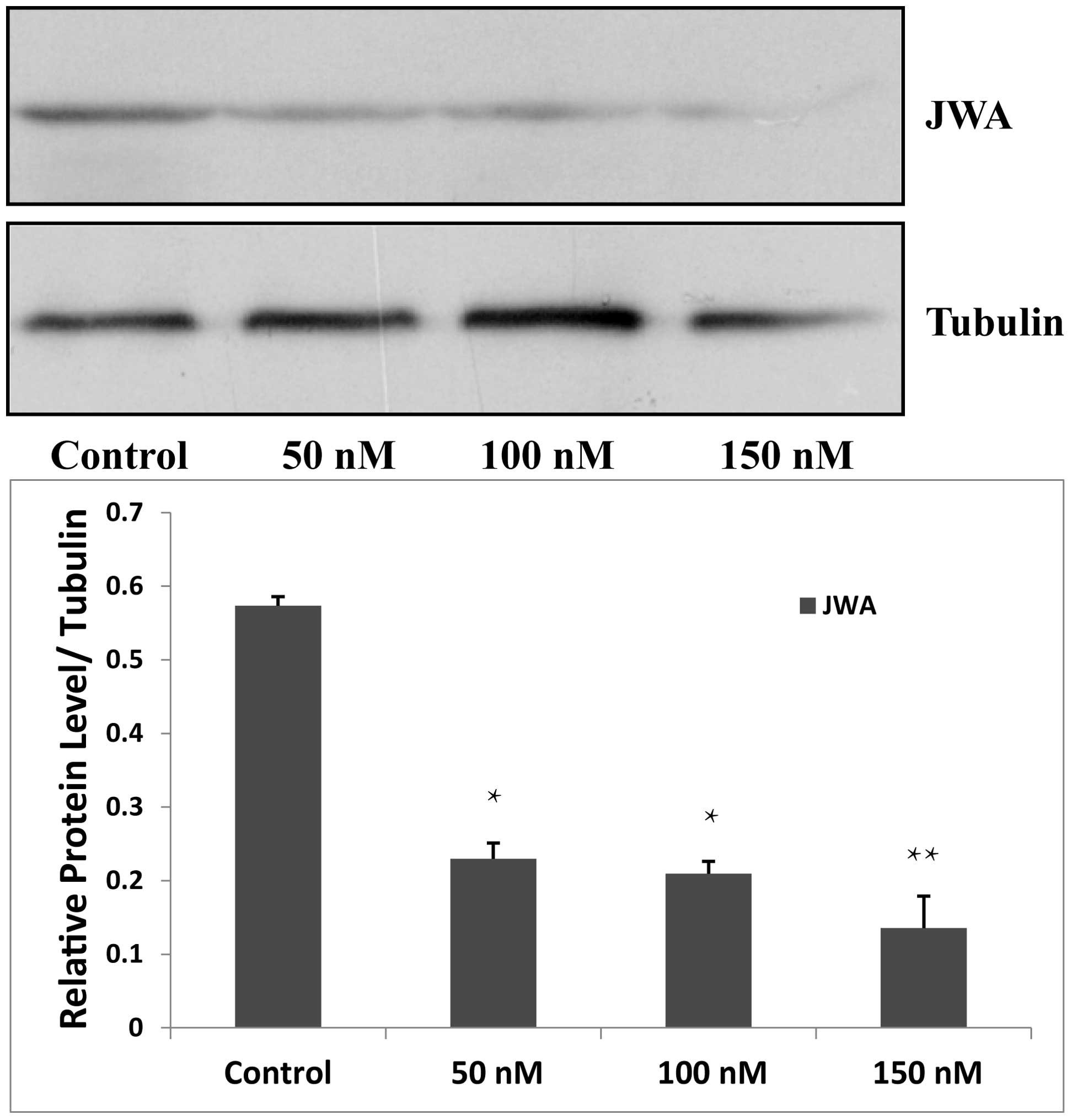
siRNA downregulates the expression of JWA
in MDA-MB-231 cells
In the present study, siRNA was used to knock down
JWA in the MDA-MB-231 cells. siRNA targeting JWA was used at
concentrations of 50, 100 and 150 nM for the siRNA group and
nonsense siRNA was used for the negative control group. Untreated
wild type cells were used as a blank control group. Following
transfection of the MDA-MB-231 cells by siRNA, 150 nM JWA siRNA was
the most effective concentration and was used for subsequent
Western blot analysis (Fig.
2).
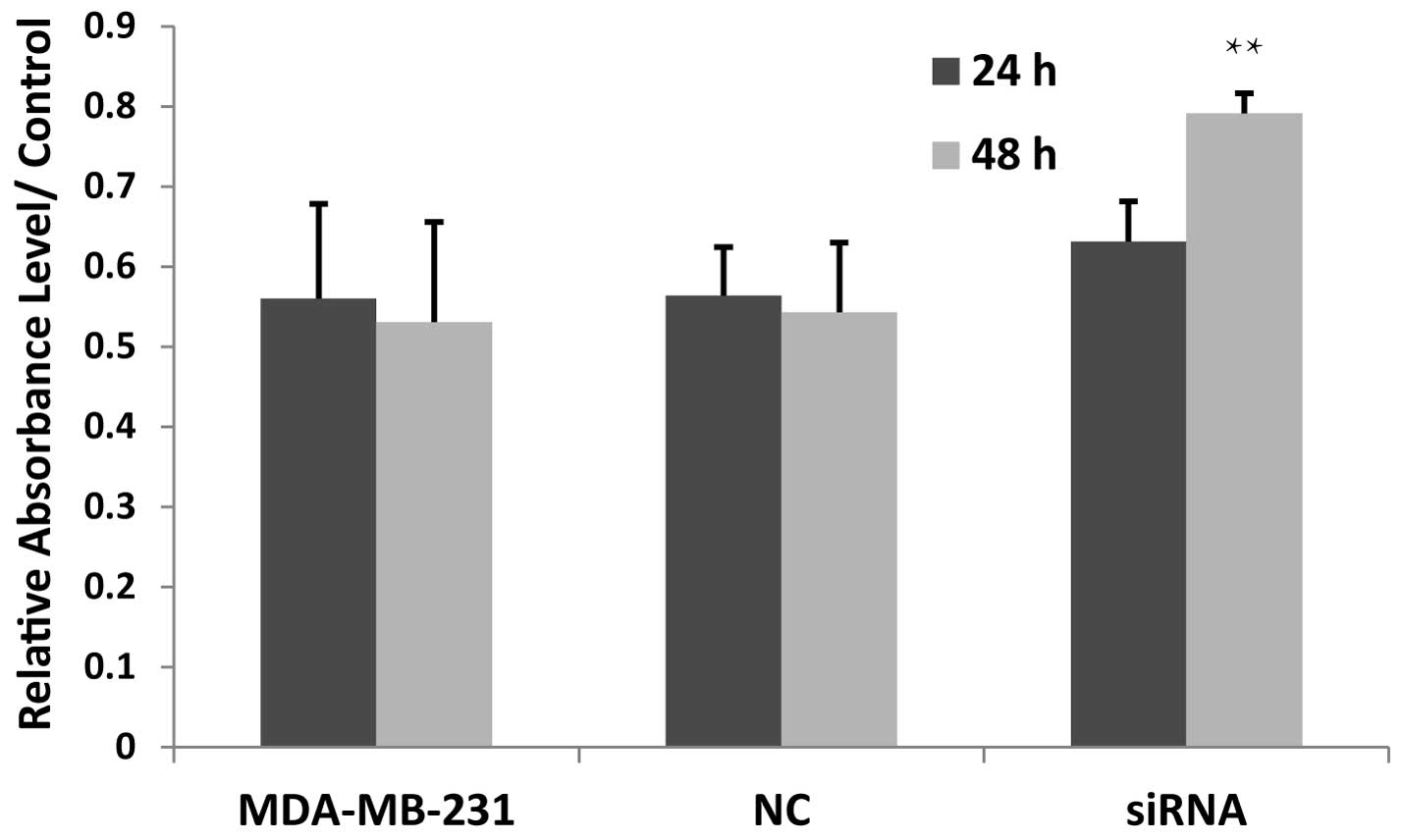
Knocking down JWA increases the
proliferation rate of tumor cells
To determine the effect of JWA in breast cancer
cells, the effects of siRNA on the expression of JWA were assessed
by MTT assay in the MDA-MB-231 cells. The proliferation rate
markedly increased compared with the negative control and blank
control groups after 24 h, although these results were not
statistically significant. After 48 h, the proliferation of the
MDA-MB-231 cells transfected with JWA siRNA was significantly
increased (Fig. 3), indicating
that the JWA-knockdown upregulated the proliferation rate of breast
cancer cells and that siRNA transfection had a time-dependent
effect on proliferation in the MDA-MB-231 cells.
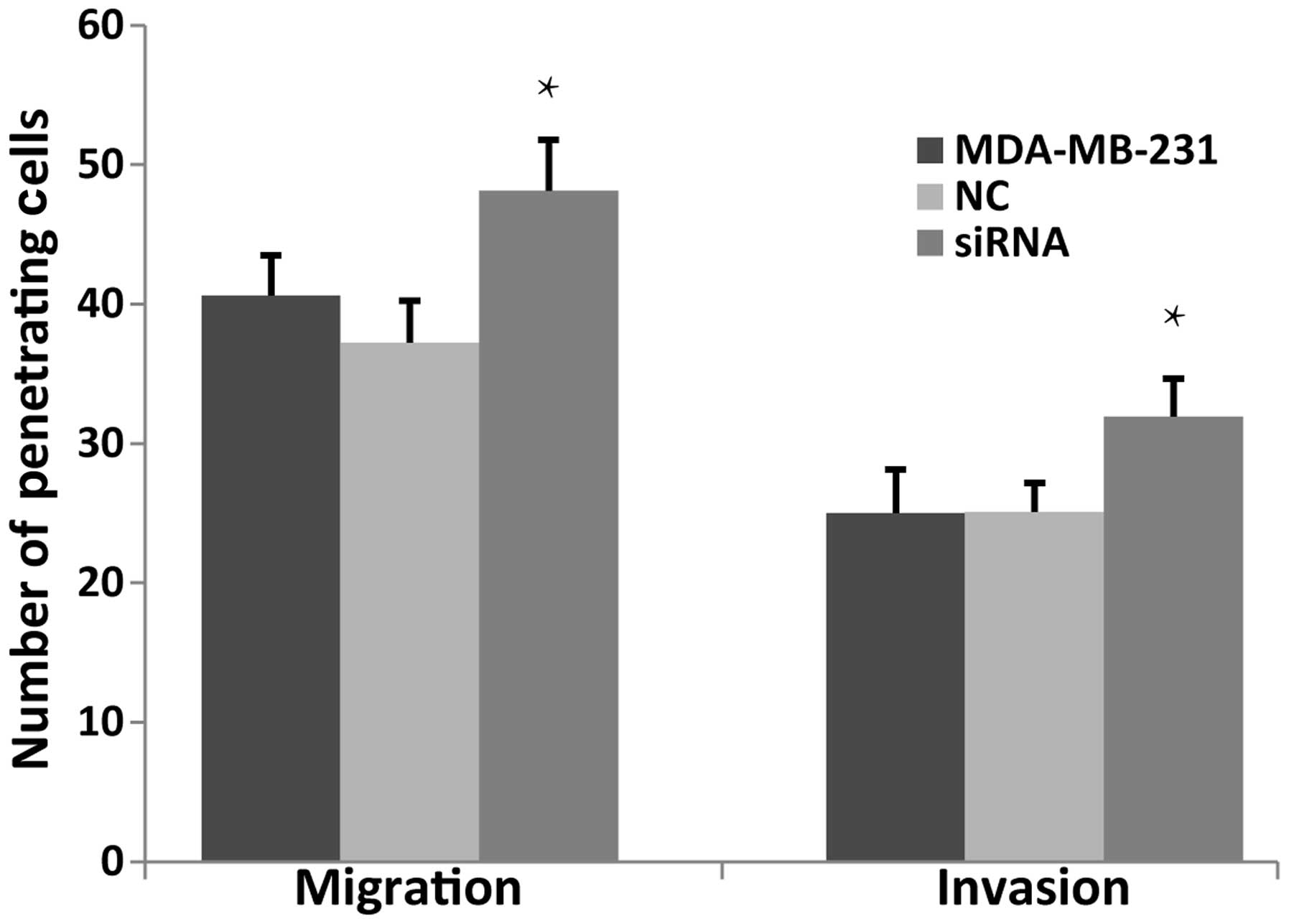
JWA siRNA enhances the migration and
invasion of MDA-MB-231 cells
Migration and invasion are basic biological
characteristic of tumor cells. JWA is a tumor suppressor gene and
knocking down JWA enhances the migration of several types of tumor
cell, whereas overexpression of JWA inhibits cell migration
(16). The present study aimed to
measure the capability of migration and invasion of the MDA-MB-231
cells using a Transwell assay. The number of penetrating cells in
the JWA siRNA group increased in the non-basement membrane chamber
and the Matrigel-coated chamber (Fig.
4). These results suggested that knock down of JWA markedly
enhanced the migration and invasion of the MDA-MB-231 cells.
Knock down of JWA reduces the levels of
apoptosis in tumor cells
Previous studies have indicated that JWA is
important in the As2O3- and C/EBPα-induced
apoptotic processes (17,18) and that overexpression of JWA
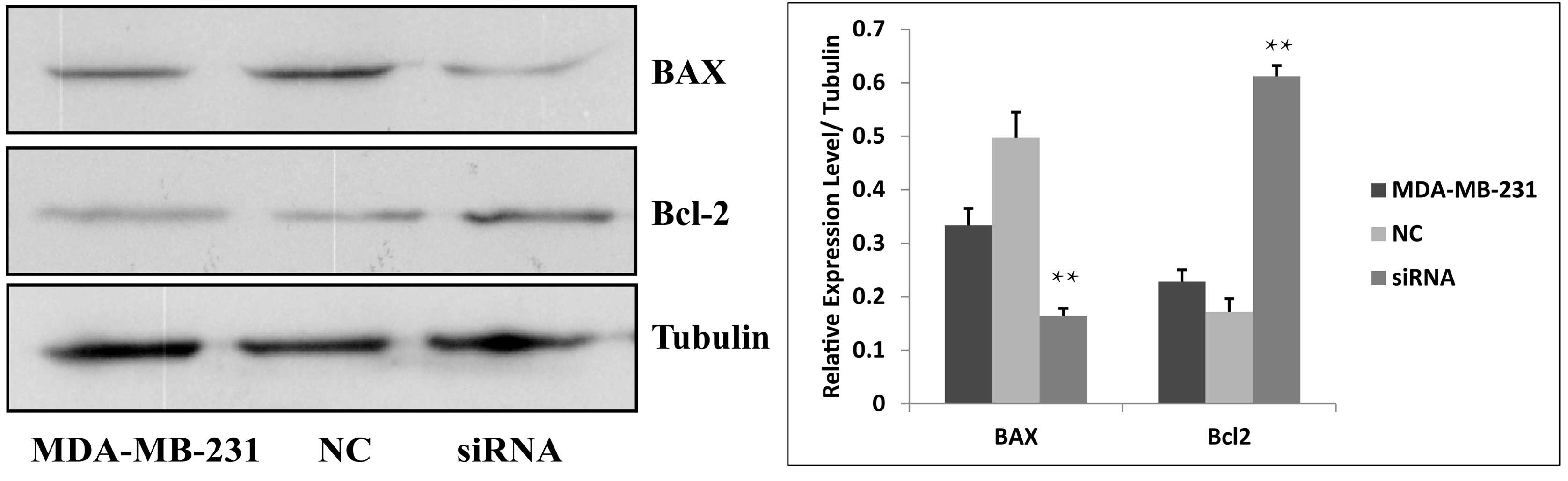
increases the apoptosis of esophageal cancer cells (15). In this process, Bcl-2 and BAX
independently regulate apoptosis by inhibiting cell death or
promoting apoptosis, respectively (19). The present study demonstrated the
effect of downregulating the expression of JWA on apoptosis in the
MDA-MB231 cells. The protein expression of BAX was significantly
decreased and the protein expression of Bcl-2 was significantly
increased in the JWA siRNA group compared with the control groups
(Fig. 5). These results indicated
that downregulating the expression of JWA increases the apoptosis
of MDA-MB-231 breast cancer cells.
p38 pathway activation following
JWA-knockdown
It has been previously revealed that all
trans-retinoic acid (ATRA) inhibits proliferation and induces
apoptosis in Hela cells by inducing ERK phosphorylation, whereas
JWA downregulation inhibits ATRA-induced ERK phosphorylation
(20). To investigate the
association between JWA and the MAPK pathways in breast cancer
cells, the levels of phosphorylated and non-phosphorylated MAPK
proteins were determined by Western blot analysis using MDA-MB-231
cells transfected with JWA siRNA. Among the three MAPK pathways,
only the expression of p-p38 decreased following siRNA
transfection. No changes were detected in the levels of ERK1/2,
p-ERK1/2, JNK, p-JNK or p38 in response to siRNA transfection.
These data revealed that JWA regulated a certain biological
function in the progression of breast cancer cells via the p38
pathway (Fig. 6).
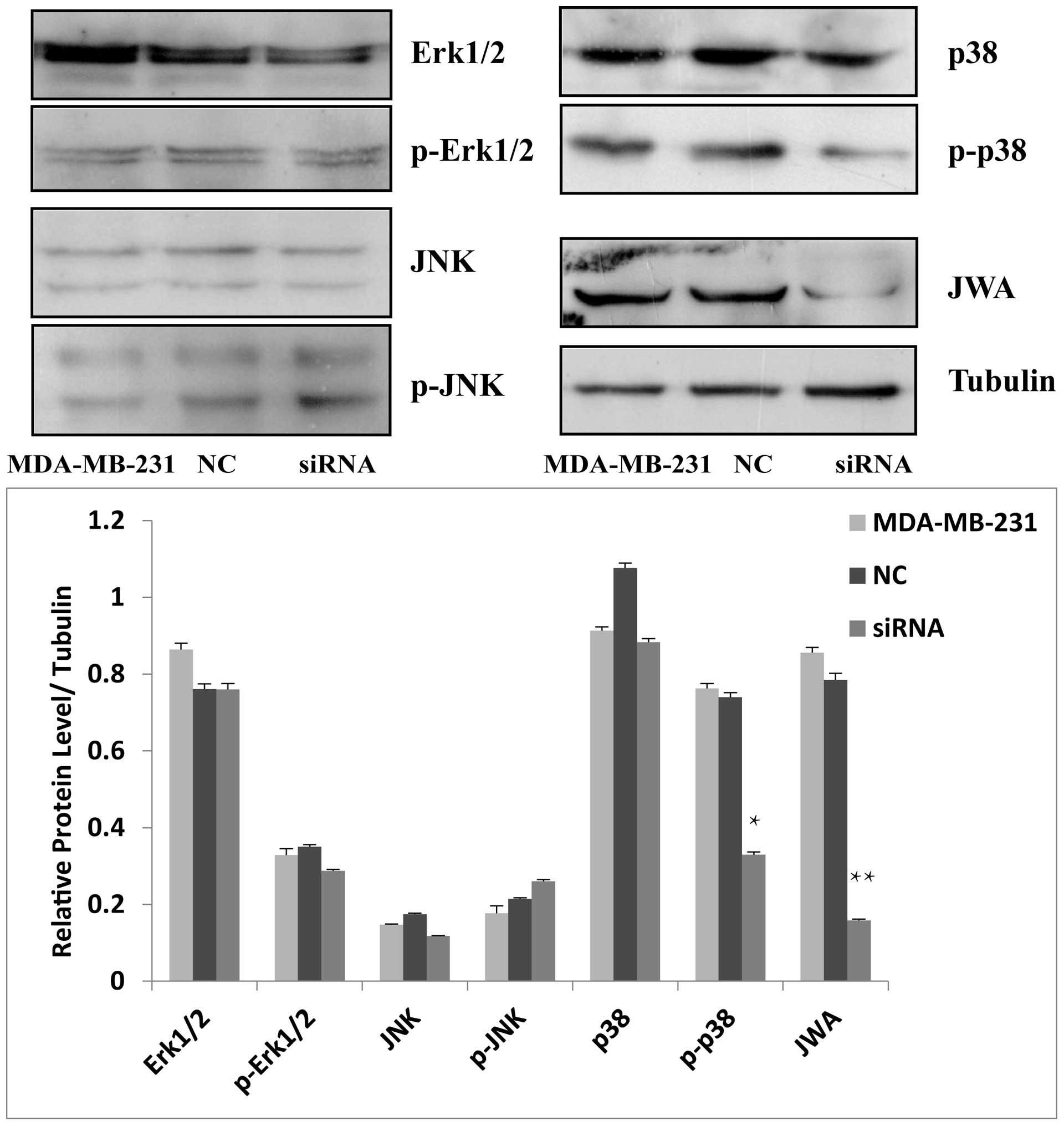 | Figure 6Protein expression levels of the MAPK
pathway following JWA siRNA treatment. In the MDA-MB-231 cells, the
levels of p-p38 were significantly reduced in the siRNA group
compared with the NC and untreated cell groups
(**P<0.05). No significant change in p-Erk1/2 or JNK
was detected (P>0.05). NC, normal control, Erk1/2, extracellular
signal-regulated kinases 1/2; p-Erk1/2, JNK, p-JNK, p38 or p-p38.
siRNA, small interfering ribonucleic acid, p-, phosphorylated-;
Erk, extracellular signal regulated kinase; JNK, c-Jun N-terminus
kinase; p38, CSBP/RK/Mpk2 kinase. |
Discussion
Breast cancer is a common type of malignancy
occurring worldwide and its development involves multiple factors,
stages and numerous oncogenes and tumor suppressor genes
alternating at the molecular level. Early systemic dissemination
and local tumor progression are usually the major hallmarks of
breast cancer (2). Therefore, it
is vital to understand the metastais by determining the mechanisms
underlying tumor progression.
Although the role of JWA has been investigated in
gastric cancer, human bronchial epithelial cells, bladder cancer,
human esophageal squamous cells and several other tumor cell lines
(8,10,15,21,22),
few studies have investigated the role of JWA in breast cancer
cells. The present study investigated whether the expression levels
of JWA affected the proliferation, invasion, migration and
apoptosis of breast cancer cells and whether JWA regulated the
biological behavior of breast cancer by activating the MAPK
pathway.
Cancer cells, including breast cancer cells, are
characterized by rapid proliferation rate. The results of the
present study demonstrated that following the knock down of the
tumor suppressor gene JWA, the proliferation rate of MDA-MB-231
cell lines increased. BAX and Bcl-2 independently regulate
apoptosis, where Bcl-2 inhibits cell death and BAX promotes
apoptosis (23). In addition, the
expression of Bcl-2 has a synergistic effect with a lack of BAX on
apoptosis (24). Shi et al
observed that overexpression of JWA induces apoptosis in ESCC cells
(15). The present study
identified that the expression of BAX decreased and the expression
of Bcl-2 increased in the cells transfected with JWA siRNA. These
data demonstrated that a low expression level of JWA inhibited
apoptosis in breast cancer cells.
A previous study reported that JWA is associated
with cytoskeletal proteins and the cell cycle (16). In ESCC cells, overexpression of JWA
leads to inhibition of cell migration and invasion (15). In melanoma cells, Bai et al
identified that invasive ability was inhibited following siRNA JWA
transfection (25). This was also
detected in HeLa, B16 and HCCLM3 cancer cells (16). Overexpression of JWA inhibits cell
migration and the opposite effect is observed when JWA is knocked
down (16). The results of the
present study revealed that the migration and invasion of the cells
in the siRNA JWA group were increased significantly compared with
the negative and blank control groups. These results may be
associated with the characteristics of JWA, associated with
cytoskeletal proteins and the cell cycle (16). These findings suggested that JWA
inhibited the migration and invasion of human breast cancer
cells.
The MAPK signaling pathway is important in a number
of the metabolic processes of tumor progression (26). Previous studies have demonstrated
that a low level of JWA expression inhibits the MEK/ERK signaling
pathway in MCF-7 and HeLa cells (16,18,23).
The present study detected the expression levels of proteins
involved in the three major pathways of the MAPK signaling pathway
following JWA knockdown in the MDA-MB-231 human breast
adenocarcinoma cell line. Notably, the p38 signaling pathway was
inhibited following siRNA treatment, however no significant changes
were observed in the MEK/ERK or JNK/SAPK signaling pathways. These
findings differ from these of Ye et al, which revealed that
a low level of JWA expression inhibited the c-Raf/MEK/ERK signaling
pathway in the MCF-7 cells (23).
This may be associated with the selection of different human breast
adenocarcinoma cell lines. The results of the present study
suggested that JWA is an important regulatory protein in the p38
signaling pathway in MDA-MB-231 cells.
In conclusion, the results of the present study led
to the hypothesis that JWA regulates cell proliferation, migration,
invasion and apoptosis through the p38 pathways in the MDA-MB-231
cells and that JWA has the potential to control the behavior and
development of breast cancer.
Acknowledgements
This study was supported, in part, by the Natural
Science Foundation of Jiangsu Province (no. BK2012563), the
Development Fund of Clinical Science and Technology, Jiangsu
University (no. JLY20120062), the Foundation of Yixing (no.
2013-21) and of Wuxi (no. MD201202).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
4
|
Gong Z, Shi Y, Zhu Z, et al: JWA
deficiency suppresses dimethylbenz (a) anthracene-phorbol ester
induced skin papillomas via inactivation of MAPK pathway in mice.
PLoS One. 7:e341542012. View Article : Google Scholar
|
|
5
|
Lu J, Tang Y, Cheng Y, et al: ING4
regulates JWA in angiogenesis and their prognostic value in
melanoma patients. Br J Cancer. 109:2842–2852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Huang Y, Huang Y, et al: JWA
suppresses tumor angiogenesis via Sp1-activated matrix
metalloproteinase-2 and its prognostic significance in human
gastric cancer. Carcinogenesis. 35:442–451. 2014. View Article : Google Scholar
|
|
7
|
Wu X, Chen H, Gao Q, et al: Downregulation
of JWA promotes tumor invasion and predicts poor prognosis in human
hepatocellular carcinoma. Mol Carcinog. 53:325–336. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Ge Z, Tan Y, Jiang G, Feng J, Wang
H and Shi G: Downregulation of JWA expression in human esophageal
squamous cell carcinoma and its clinical significance. Oncol Res.
20:157–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen L, Xu W, Li A, Ye J and Zhou J: JWA
enhances As2O3-induced tubulin polymerization
and apoptosis via p38 in HeLa and MCF-7 cells. Apoptosis.
16:1177–1193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Wu X, Chen Y, et al: Prognostic
and predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aguirre-Ghiso JA, Estrada Y, Liu D and
Ossowski L: ERK(MAPK) activity as a determinant of tumor growth and
dormancy; regulation by p38(SAPK). Cancer Res. 63:1684–1695.
2013.
|
|
12
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peti W and Page R: Molecular basis of MAP
kinase regulation. Protein Sci. 22:1698–1670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsieh CJ, Kuo PL, Hsu YC, Huang YF, Tsai
EM and Hsu YL: Arctigenin, a dietary phytoestrogen, induces
apoptosis of estrogen receptor-negative breast cancer cells through
the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol
Med. 67:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi GZ, Yuan Y, Jiang GJ, et al: PRAF3
induces apoptosis and inhibits migration and invasion in human
esophageal squamous cell carcinoma. BMC Cancer. 12:972012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen H, Bai J, Ye J, et al: JWA as a
functional molecule to regulate cancer cells migration via MAPK
cascades and F-actin cytoskeleton. Cell Signal. 19:1315–1327. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang GL, Shi X, Salisbury E and Timchenko
NA: Regulation of apoptotic and growth inhibitory activities of
C/EBPalpha in different cell lines. Exp Cell Res. 314:1626–1639.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Ye J, Zhao X, Li A and Zhou J: JWA
is required for arsenic trioxide induced apoptosis in HeLa and
MCF-7 cells via reactive oxygen species and mitochondria linked
signal pathway. Toxicol Appl Pharmacol. 230:33–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mao WG, Liu ZL, Chen R, Li AP and Zhou JW:
JWA is required for the antiproliferative and pro-apoptotic effects
of all-trans retinoic acid in Hela cells. Clin Exp Pharmacol
Physiol. 33:816–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu YQ, Li AP, Chen R and Zhou JW: The role
of JWA in N-methyl-N′-nitro-N-nitrosoguanidine induced human
bronchial epithelial cell apoptosis. Zhonghua Lao Dong Wei Sheng
Zhi Ye Bing Za Zhi. 24:205–208. 2006.(In Chinese). PubMed/NCBI
|
|
22
|
Li CP, Zhu YJ, Chen R, et al: Functional
polymorphisms of JWA gene are associated with risk of bladder
cancer. J Toxicol Environ Health A. 70:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye J, Li A, Liu Q, Wang X and Zhou J:
Inhibition of mitogen-activated protein kinase kinase enhances
apoptosis induced by arsenic trioxide in human breast cancer MCF-7
cells. Clin Exp Pharmacol Physiol. 32:1042–1048. 2005. View Article : Google Scholar
|
|
24
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai J, Zhang J, Wu J, et al: JWA regulates
melanoma metastasis by integrin alphaVbeta3 signaling. Oncogene.
29:1227–1237. 2010. View Article : Google Scholar
|
|
26
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|