Introduction
Elimination of excess ammonia is the primary method
of prevention and treatment of hepatic encephalopathy (HE), a
progressive condition in which neurological function is impaired as
the liver fails to remove toxic metabolites, particularly ammonia,
from the bloodstream. The majority of intestinal ammonia is
produced in the colon; however, no clinically viable oral treatment
exists for the elimination of this excess ammonia, resulting in
continuous low clearance levels due to poor ammonia elimination in
the body.
HE is a syndrome caused by serious hepatic diseases
as a result of metabolic disturbance and dysfunction of the central
nervous system. Its clinical manifestation includes deposition
changes, ethological abnormalities and mental retardation. In
severe cases, it may develop into a coma or even be
life-threatening. A number of researchers have divided HE into
three categories: Type A is associated with acute liver failure;
type B is caused by severe portosystemic shunting without liver
disease; and type C is associated with chronic liver disease and
hepatic cirrhosis (1). Acute liver
failure models are well recognized as a representative model of
type A HE. These models may be used to examine the pathological
response of HE to various treatment methods in animal models
(2).
HE may occur for a variety of reasons, and it is
widely associated with numerous other pathological conditions. The
classic theory holds that ammonia intoxication is the root cause of
the symptoms, and a number of studies have demonstrated that the
function of the cranial nerve is improved by the removal of
intestinal ammonia (3–5). Ethological manifestations,
electroencephalography (EEG), blood ammonia, intestinal ammonia,
levels of alanine aminotransferase (ALT) and total bilirubin (TBIL)
and liver pathology have each been used as previous indicators of
HE in rat models and humans (6–8).
Effective treatment options for HE include reducing
the bacterial production of ammonia and enhancing its elimination,
inhibiting the growth of intestinal bacteria, regulating the amino
acid metabolism and protecting brain function. Commonly used
methods to counteract HE may be divided into several types
according to treatment access: Administration via the oral, rectal
or intravenous routes; artificial liver transplantation; and full
liver transplantation (9–13). Oral drugs mainly act by reducing
the production and absorption of ammonia. Drugs including
lactulose, lactitol and kactitok operate by this mechanism
(14,15). These drugs pass through the small
intestine without being absorbed or hydrolyzed and thus reduce the
intestinal burden of ammonia by interacting with colonic bacteria
to acidify the intestinal tract. This acidification increases the
number of protein-synthesizing bacteria and reduces the number of
protein-degrading bacteria (16,17).
These treatments, however, also have unpleasant side effects that
include diarrhea and flatulence (18). Alternative medications, fradiomycin
and rifaximin, decrease ammonia production by inhibiting bacterial
RNA synthesis, thereby reducing intestinal ammonia as well as
nervous and mental symptoms (19,20).
However, these treatments also have relatively serious renal and
hepatic side effects.
Ammonia, considered to be the primary cause of HE
symptoms, is principally produced in the colon by intestinal
bacterial action from undigested protein and amino acids. A small
amount of ammonia is generated by the diffusion of urea from the
blood to the intestine, where it is hydrolyzed by bacterial urease,
and an intestinal alkaline environment is conducive to the
absorption of ammonia.
Microemulsions for the removal of colonic ammonia
(ME-RCA) were prepared using a formulation containing Tween-80
(surfactant), ethylene glycol (cosurfactant), dimethyl silicone oil
(external oil phase) and an ammonia absorbent with a solution of
30% acetic acid and 0.9% sodium chloride (internal phase). The
principle was that acetic acid was used as the water phase for
removing ammonia (an alkaline substance in accordance with the
theory of acid-base balance). The surfactant and cosurfactant play
a significant role in maintaining stability. Microemulsion has been
widely used as a drug carrier from as early as the 1980s. Based on
this observation, ammonia adsorbent acetic acid solution could be
designed to be embedded in a microemulsion as an inner aqueous
phase to establish ME-RCA (21).
Previous studies have demonstrated that ME-RCAs are effective in
the removal of ammonia in artificial colonic fluids; however, these
compounds are not stable in the mimetic gastrointestinal (GI)
environment, indicating the likelihood of poor effectiveness in
vivo (21–24). These ME-RCAs could be further
pH-intelligentialized using macromolecular materials, including
sodium alginate, to obtain pH-resistant MBG-RCAs suitable for oral
administration.
MBG-RCA as a compound includes Tween-80, ethylene
glycol, dimethyl silicone oil, acetic acid and sodium alginate. The
latter controls the release of drugs according to different pH
values along the GI tract (25–27).
In the acidic environment of the stomach, the sodium alginate gel
contracts, without release of the drug, whereas it swells in
certain pH environments (pH range 7.4–10.4) (28,29)
in the colon (30,31). MBG-RCA reaches the colon following
administration and swells to release ME-RCA. It absorbs intestinal
ammonia through acid-base neutralization, and is then carried out
of the body. Since the slightly acidic conditions of the colon,
generally ranging from pH 5.5–7.0, are responsible for this
instability, a pH-sensitive microemulsion-based gel has been
proposed for the removal of colonic ammonia (MBG-RCA).
Macromolecular materials, including sodium alginate, enclose and
protect the original ME-RCA, making it particularly resilient to
the varied pH levels encountered upon oral administration.
Furthermore, these molecules have been previously used as carriers
to release bioactive molecules to targeted areas of the body, and
this delivery system has been used to release ME-RCA directly in
the colon (21). By adding a
protective layer to existing microemulsion molecules, an increased
resistance to pH may be achieved that increases the pharmacological
usefulness of these materials in the treatment of HE.
Over the course of the present study, rat models of
HE were induced and offered a high-protein diet, likely to
stimulate ammonia in the colon. These models were simultaneously
administered varying levels of MBG-RCA via the intragastric route
through oral administration in order to observe the effect on
ammonia levels in the blood and intestine. A decrease in these
levels could indicate the potential for a future clinically
relevant treatment capable of preventing and treating HE in rats
and, in the future, even human subjects. The development of a
novel, clinically applicable agent for the oral elimination of
intestinal ammonia may revolutionize the treatment of HE.
Materials and methods
Animal care
The experimental protocols were approved by the
Animal Care and Use Committee of Dalian Medical University (Dalian,
Liaoning, China) in accordance with guidelines established by the
Canadian Council on Animal Care.
A total of 80 male Sprague Dawley rats (250–300 g,
10 weeks of age) were purchased from the Animal Care and Use
Committee of Dalian Medical University. Rats were well developed
and exhibited no health-related abnormalities or previous
pathological conditions. Rats were subsequently divided into 20
A-type stainless steel cages with a sink and a manger. A maximum of
five rats were housed in each cage and marked accordingly. Rats
were fed common granular fodder containing protein (23%), fat
(4.7%), sodium (0.24%) and tap water. The temperature was
maintained at 17–24°C, and rats were subjected to a day-night light
cycle reflecting a normal circadian rhythm.
All animal testing was conducted in the laboratory
of the College of Pharmacy of Dalian Medical University. The
experimental protocols were approved by the Animal Care and Use
Committee of Dalian Medical University in accordance with
guidelines established by the Canadian Council on Animal Care.
Reagents
MBG-RCA was prepared using a formulation containing
Tween-80 (surfactant), ethylene glycol (cosurfactant), dimethyl
silicone oil (external oil phase), an ammonia absorbent with a
solution of 30% acetic acid and 0.9% sodium chloride (internal
phase) and sodium alginate by the Membrane Science and Technology
Center at the Dalian University of Technology (21). Lactulose was produced by the
Dandong Rehabilitation Pharmaceutical Co. Ltd. (production lot
H10890057; Dandong, Liaoning, China) at a concentration of 5%.
Thioacetamide (TAA) was produced by Shanghai Chemical Reagent
Company (production lot 920419; Shanghai, China). ALT and TBIL
reagent kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, Jiangsu, China). All materials used were of the
highest reagent grade, AR.
Animal model grouping
The eighty rats were divided at random into eight
groups of ten. The result was eight distinct groups, consisting of
four control groups (normal, blank, lactulose and acetic acid), an
HE group, and three MBG-RCA therapeutic groups by dosage (high,
medium and low doses of 15, 10 and 5 ml/kg MBG-RCA, respectively).
The normal control and blank control groups were fed a normal diet.
Conversely, the HE group, acetic acid and lactulose control groups,
and the therapeutic MBG-RCA groups were fed a high-protein diet
(with 20% casein added to the normal diet). The rats in the HE
group, acetic acid and lactulose control groups, and the MBG-RCA
therapeutic groups were prepared as HE models according to the
method proposed by Li et al (32). In this method, HE rat models are
induced by intraperitoneal injection of TAA (350 mg/kg·day) for
three consecutive days.
Treatment administrations were conducted once a day
over the course of four days. Two hours prior to model
establishment, therapeutic MBG-RCA groups were administered 15
ml/kg (high), 10 ml/kg (medium) and 5 ml/kg (low) doses of MBG-RCA.
The blank control group was administered equivalent blank
microemulsion-based gels. The lactulose control group was
administered an equal volume of lactulose (10 ml/kg). The normal
control and HE groups were administered equal volumes of normal
(0.9%) saline. Finally, the acetic acid control group was
administered acetic acid solution (1.15%). All administrations were
conducted using similar tools under similar conditions at a single
laboratory setting.
Sample collection
After four days (four doses) rats were consecutively
weighed and subsequently placed in an ether anesthesia induction
box for ~3 min in order to induce anesthesia. Rats were removed
after full anesthesia had been induced, and 1 ml of blood was drawn
from the angular vein of each subject. Simultaneously, abdominal
skin was disinfected and the abdominal cavity was opened to expose
the inferior vena cava (IVC). Blood (1.5 ml) was drawn from the IVC
using a 5-ml syringe. The syringe needle was sealed after drawing
blood to prevent ammonia leakage. The sample was immediately placed
in a vacuum vessel containing ethylenediamine tetra-acetic acid and
centrifuged at 999 × g for 5 min at 10–15°C. The mesentery was
separated and the cecum was resected following ligation of both
ends, and the excised tissue was placed in a vessel filled with
normal (0.9%) saline. The colonic wall was cut to release the
contents and capped synchronously. The sample was stored at 4°C
overnight. Supernatant fluid was obtained following centrifugation
at a time, temperature and speed consistent with those used for the
blood sample.
Subsequently, rats were sacrificed and their livers
were truncated for embedding in formalin (10%) and fixation with
neutral balsam.
Liver function analyses
Blood samples were used to evaluate liver function
using ALT and TBIL reagent kits (Nanjing Jiancheng Bioengineering
Institute). The principle of detection was the enzyme-linked
immunosorbent assay and the diazo method, and testing was conducted
according to the instructions provided by the manufacturer.
Pathological sections of livers were observed with
hematoxylin and eosin (H&E) staining. Resultant observations
included the structure of hepatic cords and sinuses; shapes of
liver cells; number, appearance and location of the cell nuclei;
and infiltration of inflammatory cells, edema, necrosis or other
abnormalities, if present.
Blood ammonia level analyses
Blood samples taken from the IVC were analyzed for
ammonia content. Blood ammonia was detected using an Automatic
Dry-type Biochemical Analyzer (Johnson & Johnson, New
Brunswick, NJ, USA). The entire process was completed within 30 min
of collection from the specimens and blood was stored in sealed
vials to prevent ammonia leakage prior to testing.
Additionally, intestinal ammonia in the supernatant
fluid collected from the excised cecum tissue was detected using
the Automatic Dry-type Biochemical Analyzer. The data were
corrected according to the weight of the excised tissue.
Measurement of ethological
indicators
The general condition of rats, including mental
state, sleeping time (lethargic time) and mortality was observed
using the Infrared Night Vision Surveillance System (Jinan
dimensional Century Technology Co., Ltd., Jinan, Shandong, China),
consisting of community monitoring by video capture cards embedded
in cage-mounted digital devices (Guangzhou Ao Qiman Electronic
Technology Co., Ltd., Guangzhou, Guangdong, China). Food intake and
sleep duration were determined using electronic scales and
observational methods, respectively, and recorded once daily.
Initial and final rat body weights were noted on the first and
fourth day using electronic scales. The grading score of HE was
analyzed according to the standards by Zimmerman et al
(33). In brief, the gradings were
as follows: Level 0, normal; Level I, drowsiness, slow responses,
reduced locomotor activity and normal reflexes; Level II, ataxia
but with normal reflexes; Level III, gradual disappearance of
reflexes; and Level IV, lack of corneal reflex/unconsciousness.
Electroencephalographic analyses
On the fourth day, prior to sample collection, rat
subjects were anesthetized using pelltobarbitalum natricum (50
mg/kg) and fixed on a stereotaxic instrument ~30 min afterwards.
The rats were awake for all subsequent tests. EEG was applied to
all rats using the Biological Function of Experimental System
(model BL-420F; Chengdu Taimeng Technology Co., Ltd., Chengdu,
China). EEG was traced for 10 min with a time constant of 0.3 and a
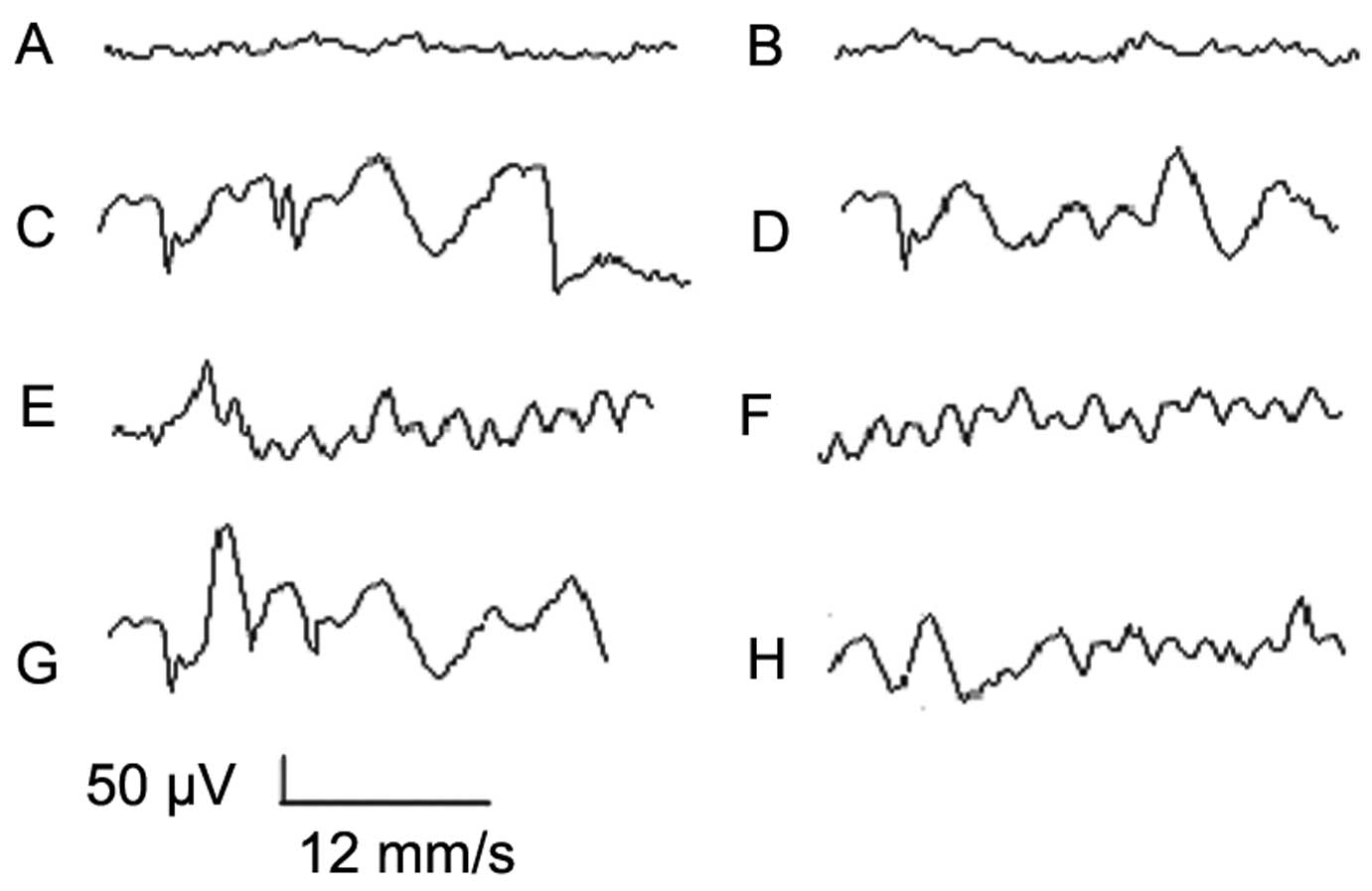
high-frequency filter setting of 100 Hz. Frequency and amplitude
were indicated in the EEG, and α waves were primarily on top of the
brains of normal rats (frequency, 8–13 times/sec; voltage, 50–100
μV). Mild EEG abnormalities were displayed as low-rhythm α waves
mingled with θ waves (frequency primarily at 8–11 times/sec,
voltage at 150–250 μV). Severe EEG abnormalities were revealed as
extensive wide and deep δ waves (frequency, 5–7 times/sec; voltage
160–280 μV). All rats were awake for ~4–6 h.
Statistical analyses
Data analyses were conducted using SPSS 11.5
software (Chicago, IL, USA). Measurement data are presented as the
mean ± standard deviation, matching normal distribution. One-factor
analysis of variance was used for group comparison whereas least
square difference testing was applied for pairwise comparisons. The
rank-sum test was used to assess ranked data. Rank correlation was
used for correlation analyses. The χ2 test was used for
comparison of enumeration data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Qualitative condition of subjects
Compared with the normal control group, no
abnormality was observed in the blank control group. Rats in the HE
group, however, exhibited visual signs of depressed activity
levels, a hunched or irregular stature, and jittery or inconsistent
movement patterns. Additionally, these subjects exhibited a
visually apparent decreased desire for foraging, and two subjects
developed hemorrhagia nasalis. Therapeutic groups, as well as the
acetic acid and lactulose control groups, exhibited reduced or
milder manifestations of the above symptoms, generally concurrent
with the increasing dosage of MBG-RCA in the therapeutic
groups.
Control group results
No significant differences between the normal
control group and blank control group were observed in terms of
food intake, sleep duration, weight change, HE grading score, ALT
and TBIL levels, blood ammonia levels or intestinal ammonia levels
(P>0.05). Furthermore, there were no mortalities in the normal
or blank control groups (100% survival). The EEG of the normal and
blank control groups showed no abnormality and presented mainly as
α waves (frequency, 8–13 times/sec; voltage, 50–100 μV). H&E
staining in the excised hepatic tissue of the normal and blank
control groups revealed no obvious abnormalities.
Food intake
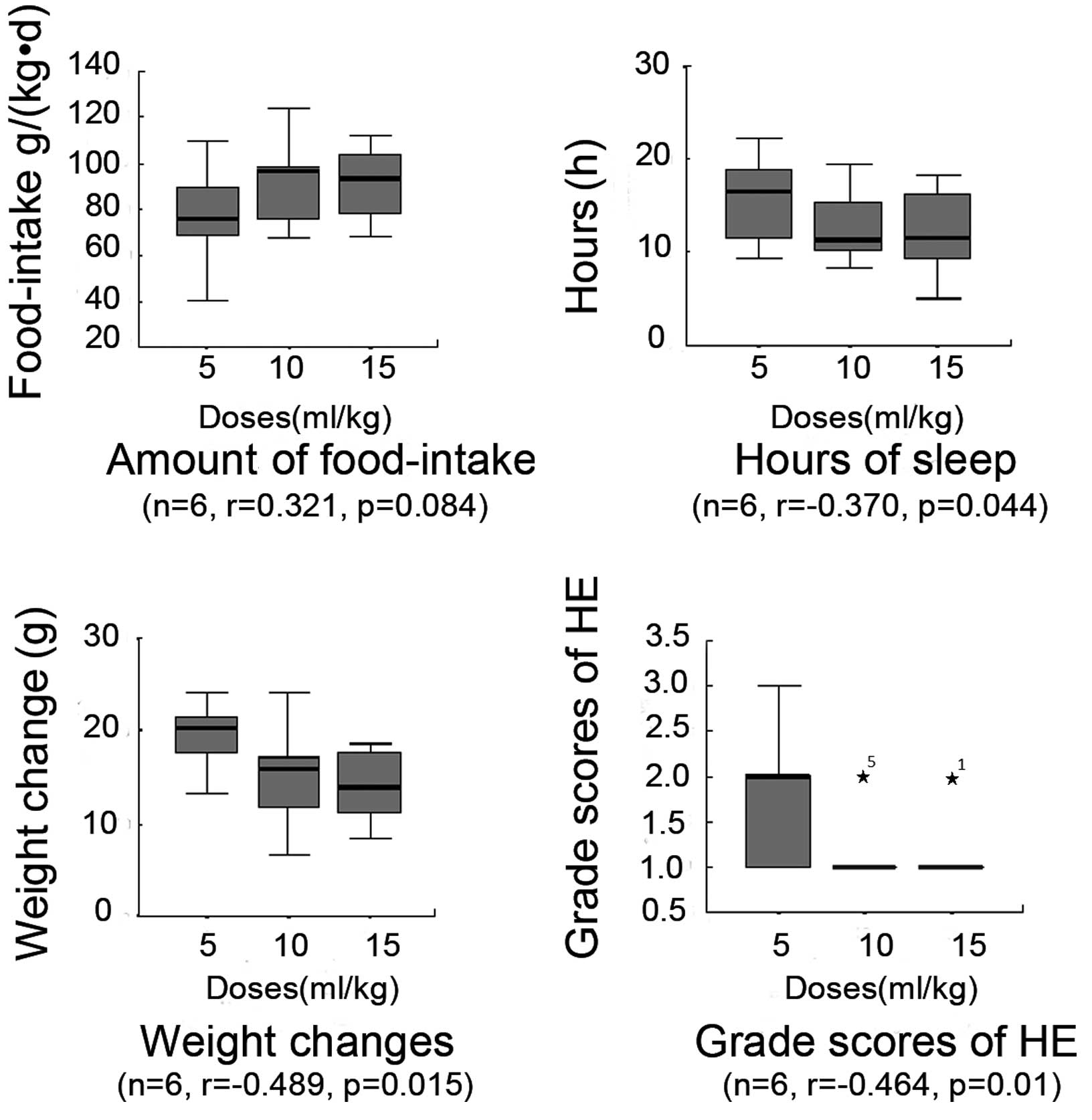
The food intake amount in each therapeutic group and
the lactulose and acetic acid control groups exhibited recovery
compared with the HE group. The HE group food intake was observed
to be 51±18 g/kg·day. The high-, medium- and low-dose MBG-RCA
therapeutic groups exhibited a greatly increased observed food
intake of 92±15 (P=0.000), 92±16 (P=0.000) and 77±20 g/kg·day
(P=0.018), respectively. The lactulose and acetic acid control
groups demonstrated a moderate food intake of 75±23 (P=0.024) and
72±20 g/kg·day (P=0.022), respectively. Food intake amounts in the
high-dose MBG-RCA group were significantly higher than those in the
lactulose and acetic acid control groups by 122 and 129%,
respectively. Compared with the medium-dose MBG-RCA group, food
intake in the acetic acid and lactulose control groups was
significantly lower, by 78 and 82%, respectively. No significant
differences, however, were noted among the acetic acid and
lactulose control groups and low-dose MBG-RCA groups (Table I). Statistically, no clear
correlation was observed between the food intake and dosage of
MBG-RCA; however, the level of food intake was similarly elevated
in the medium- and high-dosage therapeutic groups (Fig. 1).
 | Table IComparisons of associated indicators
among rat groups (mean ± standard deviation). |
Table I
Comparisons of associated indicators
among rat groups (mean ± standard deviation).
| Indicators | C | D | E | F | G | H |
|---|
| Food intake
(g/kg·day) | 54.0±18.0a | 77.0±20.0 | 92.0±16.0d | 92.0±15.0d | 72.0±20.0 | 75.0±23.0 |
| Sleep duration
(h/24 h) | 20.0±2.0a,d | 16.0±4.0 | 13.0±3.0d | 12.0±4.0a,d | 17.0±5.0 | 16.0±5.0 |
| Weight changes
(g) | 37.0±8.0c,d | 37.0±8.0c,d | 15.0±5.0b,d | 14.0±4.0c,d | 21.0±2.0 | 22.0±4.0 |
| HE grading
score | 2.7±0.9a | 1.8±0.8 | 1.2±0.4a | 1.1±0.3b,d | 2.0±0.8 | 1.9±0.7 |
| Blood ammonia
(μmol/l) | 286.0±27.0c,d | 243.0±37.0 | 209.0±28.0c,d | 199.0±42.0a,d | 252.0±28.0 | 246.0±33.0 |
| Intestinal ammonia
(μmol/l) | 410.0±56.0a,d | 317.0±47.0d | 270.0±88.0 | 251.0±69.0a | 246.0±70.0 | 336.0±72.0 |
| ALT (U/l) | 252.0±22.0d | 219.0±23.0 | 202.0±22.0a | 196.0±24.0a,d | 226.0±18.0 | 223.0±20.0 |
| TBIL (μmol/l) | 55.0±12.0a | 45.0±10.0 | 38.0±5.0b,d | 36.0±6.0b,d | 48.0±10.0 | 46.0±5.0 |
Sleep duration
In comparison with the HE sleep duration of 20±2
h/24 h, the therapeutic groups were observed to have decreased
sleep durations of 12±4.0 (P=0.001), 13±3 (P=0.001) and 16±4 h/24 h
(P=0.006) in the high, medium and low MBG-RCA dosage groups,
respectively. The lactulose and acetic acid control groups
exhibited sleep durations of 16±5 (P=0.030) and 17±5 h/24 h
(P=0.037), respectively. The sleep duration in the high-dose
MBG-RCA group was significantly shorter than that observed in the
lactulose and acetic acid control groups, by 74 and 73%,
respectively. Conversely, when compared with the medium-dose
MBG-RCA group, the lactulose and acetic acid control group
demonstrated increased sleep durations, by 130 and 132%,
respectively. No significant differences, however, were noted among
the acetic acid and lactulose control groups and the low-dose
MBG-RCA group. The observed sleep duration in high- and medium-dose
MBG-RCA groups was shorter than that observed in the low-dose
MBG-RCA group by 75 and 78%, respectively. These results indicate a
negative correlation between sleep duration and increasing dosages
of MBG-RCA (Table I and Fig. 1).
Weight changes
Rats in the HE group exhibited a weight change of
37±8 g. The high-, medium- and low-dosage MBG-RCA groups exhibited
weight changes of 14±4 (P=0.000), 15±5 (P=0.000) and 37±8 g
(P=0.001), respectively. The lactulose and acetic acid control
groups exhibited weight changes of 22±4 (P=0.001) and 21±2 g
(P=0.001), respectively. The weight decrease in the high-dose
MBG-RCA group was significantly lower than that observed in the
lactulose and acetic acid control groups, by 65 and 67%,
respectively. Compared with the medium-dose MBG-RCA group, the
lactulose and acetic acid control groups demonstrated decreased
weight changes of 70 and 71%, respectively. No significant
differences among the acetic acid and lactulose control groups and
the low-dose MBG-RCA group were observed. Weight decreases in the
high- and medium-dose MBG-RCA groups were significantly lower than
that observed in the low-dose MBG-RCA group, by 38 and 41%,
respectively. Accordingly, the results indicate a negative
correlation between the decreasing change in weight and the
increasing dosage of MBG-RCA (Table
I and Fig. 1).
HE grading score
Rats in each therapeutic group demonstrated higher
HE grading scores compared with the HE group score of 2.7±0.9,
demonstrating high, medium and low HE grading scores of 1.1±0.3
(P=0.001), 1.2±0.4 (P=0.004) and 1.8±0.8 (P=0.188), respectively.
The lactulose and acetic acid control groups exhibited HE grading
scores of 1.9±0.7 (P=0.000) and 2.0±0.8 (P=0.225), respectively.
The HE grading score in the high-dose MBG-RCA group was
significantly higher than those observed in the lactulose and
acetic acid control groups by 58 and 55%, respectively. Compared
with the medium-dose MBG-RCA group, the lactulose and acetic acid
control groups demonstrated increased HE scores by 70 and 74%,
respectively. No significant differences among the acetic acid and
lactulose control groups and the low-dose MBG-RCA group were noted.
Furthermore, the HE grading scores in the high- and medium-dose
MBG-RCA groups, as well as in the medium- and low-dose MBG-RCA
groups, revealed no significant differences. HE grading scores of
the high-dose MBG-RCA group were lower than those of the low-dose
MBG-RCA group by 61%. Thus, the results suggest a negative
correlation between weight decrease and dosage of MBG-RCA (Table I and Fig. 1).
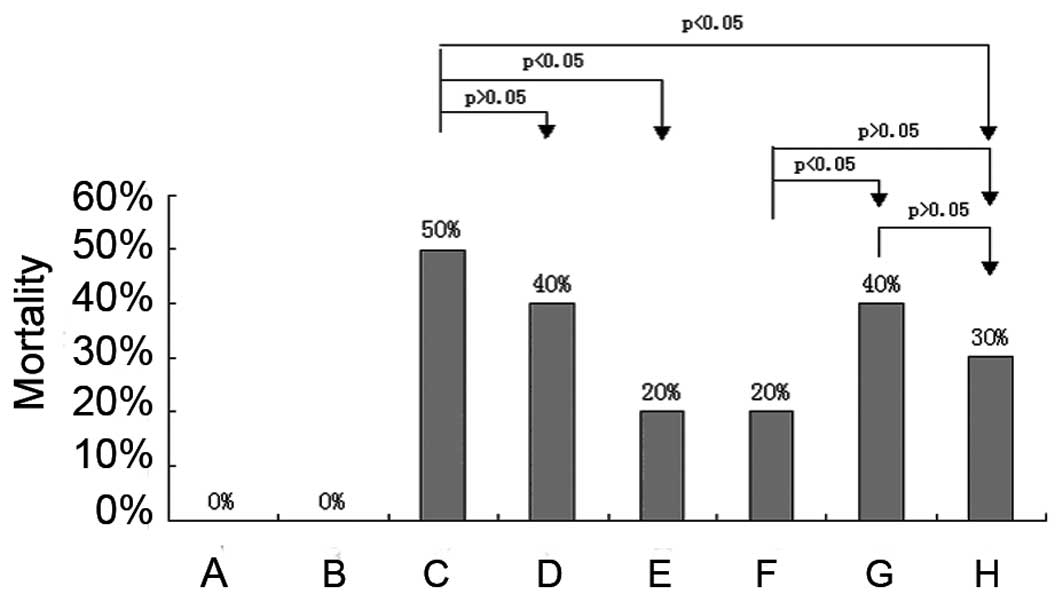
Mortality
The prevalence of mortality in the HE group and in
the rats receiving therapeutic high, medium and low doses of
MBG-RCA was 50, 20, 20 and 40%, respectively. The lactulose and
acetic acid control group treatments exhibited mortality rates of
30 and 40%, respectively. Rats in the high- and medium-dose MBG-RCA
groups as well as those in the lactulose control group revealed a
statistically significant lower mortality than those in the HE
group, although the low-dose MBG-RCA and acetic acid control groups
exhibited no significant difference from the HE group. The
mortality of each group is shown in Fig. 2.
EEG
EEGs of the HE group were notably abnormal,
revealing extensive wide and deep δ waves (frequency, 5–7
times/sec; voltage, 160–280 μV). Medium- and high-dose MBG-RCA
groups revealed low-to-moderate EEG abnormalities characterized by
a mixture of low-rhythm α waves and θ waves (frequency mainly at
8–11 times/sec; voltage, 150–250 μV). In the acetic acid and
lactulose control groups, as well as the low-dose MBG-RCA group,
EEGs were observed to be moderately abnormal, presenting abnormal θ
waves (frequency, 7–9 times/sec; voltage, 130–200 μV) as shown in
Fig. 3.
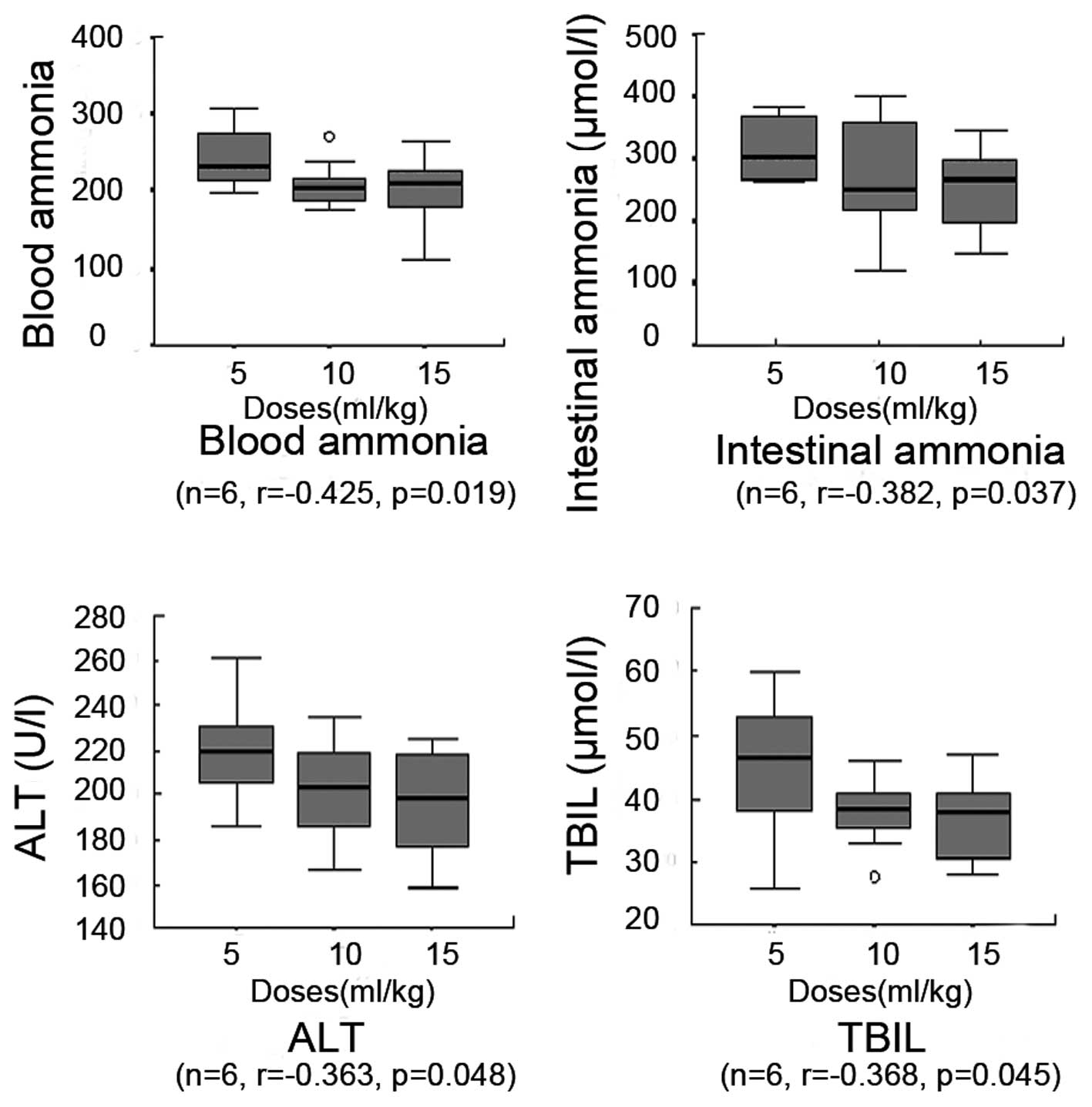
Blood ammonia level
Rats receiving therapeutic treatments exhibited
reduced blood ammonia levels compared with the HE group, which had
a blood ammonia level of 286±27 μmol/l. The high-, medium- and
low-dose MBG-RCA groups exhibited blood ammonia levels of 199±42
(P=0.000), 209±28 (P=0.001) and 243±37 μmol/l (P=0.054),
respectively. The lactulose and acetic acid control groups
exhibited blood ammonia levels of 246±33 (P=0.027) and 252±28
μmol/l (P=0.155), respectively. Blood ammonia in the high-dose
MBG-RCA group was significantly lower than that observed in the
lactulose and acetic acid control groups, by 81 and 80%,
respectively. Compared with the medium-dose MBG-RCA group, the
lactulose and acetic acid control groups exhibited increasing
levels of blood ammonia by 118 and 121%, respectively. No
significant differences among the acetic acid and lactulose control
groups and the low-dose MBG-RCA group were observed. Table I shows the blood ammonia levels in
each group. The blood ammonia levels observed in the high- and
medium-dose MBG-RCA groups were significantly lower than that
observed in the low-dose MBG-RCA group, indicating a negative
correlation between the blood ammonia and the increasing dose of
MBG-RCA (Fig. 4).
Intestinal ammonia level
Rats in the therapeutic treatment groups were
observed to have lower levels of intestinal ammonia compared with
the HE group, with a level of 410±56 μmol/l. The high-, medium- and
low-dose MBG-RCA groups exhibited levels of 251±69 (P=0.001),
270±88 (P=0.003) and 317±47 μmol/l (P=0.013), respectively. The
lactulose and acetic acid control groups exhibited levels of 336±72
(P=0.11) and 246±70 μmol/l (P=0.051), respectively. Intestinal
ammonia in the high-dose MBG-RCA group was observed to be
significantly lower than in the lactulose and acetic acid control
groups, by 75 and 102%, respectively. Compared with the medium-dose
MBG-RCA group, the lactulose and acetic acid control groups
demonstrated higher amounts by 124 and 91%, respectively. No
significant differences among the acetic acid and lactulose control
groups and the low-dose MBG-RCA group were observed. Table I shows details of the intestinal
ammonia levels in each group. The correlation between blood ammonia
and the administered dose of MBG-RCA was negative (Fig. 4).
ALT and TBIL levels
Rats in the therapeutic treatment groups exhibited
lower ALT levels compared with the HE group, with an ALT level of
252±22 U/l and a TBIL level of 55±12 μmol/l. The high-, medium- and
low-dose MBG-RCA groups exhibited ALT levels of 196±24 (P=0.001),
202±22 (P=0.002) and 219±23 U/l (P=0.01), respectively, and TBIL
levels of 36±6 (P=0.002), 38±5 (P=0.002) and 45±10 μmol/l
(P=0.138), respectively. Lactulose and acetic acid control groups
exhibited ALT levels of 222±20 (P=0.038) and 226±18 U/l (P=0.045),
respectively, and TBIL levels of 46±5 (P=0.041) and 48±10 μmol/l
(P=0.325), respectively. The ALT and TBIL levels in the high-dose
MBG-RCA group were significantly lower than those in the lactulose
and acetic acid control groups, by 88 and 87% for ALT and by 80 and
77% for TBIL, respectively. Compared with the medium-dose MBG-RCA
group ALT and TBIL levels, the lactulose and acetic acid control
groups demonstrated elevated levels, by 110 and 112% for ALT and by
121 and 126% for TBIL, respectively. No significant differences
were observed among the acetic acid and lactulose control groups
and the low-dose MBG-RCA groups in ALT or TBIL levels. Finally, ALT
and TBIL levels in the high- and medium-dose MBG-RCA groups as well
as in the medium- and low-dose MBG-RCA groups demonstrated no
significant differences. Conversely, the high-dose MBG-RCA group
demonstrated lower levels than the low-dose MBG-RCA group for both
ALT and TBIL, as shown in Fig. 4.
The correlation between the ALT and TBIL levels and the increasing
dosage of MBG-RCA was determined to be negative based on these
findings (Table I and Fig. 4).
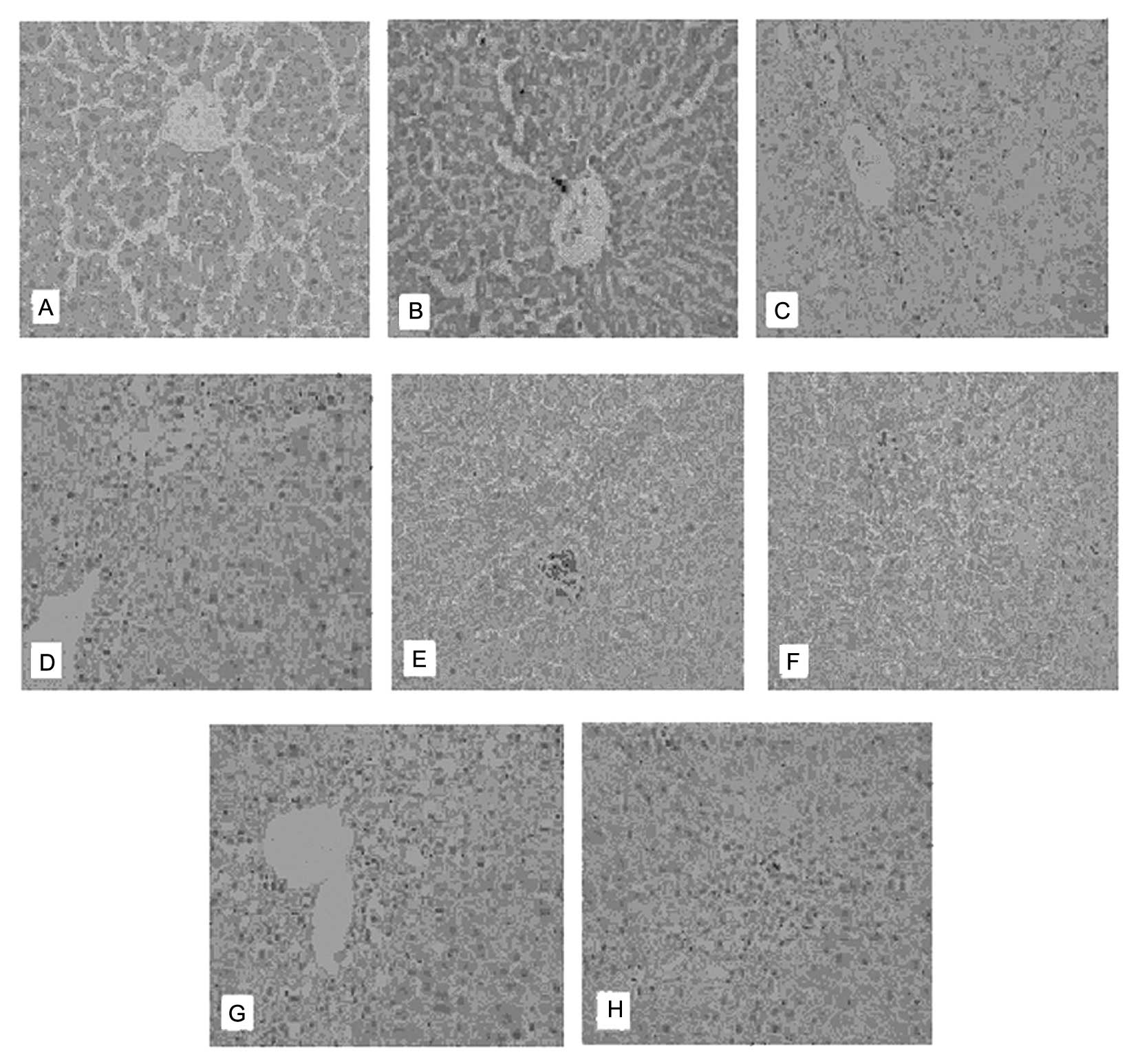
Liver pathology
The structure of the excised hepatic tissue examined
with H&E staining exhibited unclear results in the HE group,
with a characteristic disordered architecture of the hepatic lobule
containing edema and ballooning degeneration. Necrosis coupled with
the presence of infiltrating inflammation cells was extensively
observed. The portal area was notably infiltrated by inflammatory
cells, and the liver sinusoids were extended and congested. The
acetic acid and lactulose control groups, as well as the
therapeutic groups, exhibited varying levels of the pathological
changes described above (Fig.
5).
Discussion
Rat models provide a viable point of reference for
the in vivo treatment of HE by administration of oral
medications, including the pH-sensitive microemulsion gel proposed
in this study. Rat models of type A HE, as modeled in the current
study, were induced by intraperitoneal injection of TAA, a method
known for its outstanding repeatability, shorter completion time
and higher success rate compared with other established methods of
HE treatment. Furthermore, the model has been widely accepted
internationally due to its similarity with human liver damage
(34,35). Thus, this study provides an initial
step in the development of a novel and clinically viable treatment
and prevention of HE in humans.
MBG-RCA molecules are designed to survive the pH
variation of normal oral administration without releasing their
ammonia-reducing contents until reaching the specific pH levels
found in the colon. In order to exclude the possibility of the
influence of the microemulsion molecules on rats directly, the
normal control group was compared with the blank control group. The
treatment effect of MBG-RCA on removing colonic ammonia was further
verified by establishing therapeutic controls, including acetic
acid (enteroclysis for acidifying the colonic tract) and lactulose
(commonly used to reduce colonic ammonia). Furthermore, high,
medium and low doses of MBG-RCA were applied to observe the dose
correlation.
In the present study, MBG-RCA was shown to reduce
blood ammonia by removing intestinal ammonia directly from the
colon, its primary center of production. The results of ammonia
removal included significant improvements in sleep duration
(including stupor), neural reflex, EEG, food intake, weight and
mortality in HE model rats. High- and medium-level dosages of
MBG-RCA demonstrated superior results to treatment with either
acetic acid, lactulose or low dosages of MBG-RCA, indicating a
positive correlation of symptom cessation with increasing dosage of
MBG-RCA. Notably, high and medium MBG-RCA dosages were shown to be
more effective than the lactulose treatments commonly used in
clinical settings. These treatments also have the benefit of being
able to directly remove intestinal ammonia without the involvement
of colonic bacteria or enzymes, producing fewer restrictions
compared with contemporary lactulose treatments.
Acetic acid may also be influenced by the pH of the
GI tract, and may thus be neutralized, diluted or consumed prior to
reaching the colon. This effect is likely responsible for the few
documented accounts of oral administration of acetic acid for
therapeutic purposes (36). The
current study, however, indicates that direct oral intake of acetic
acid may serve to reduce blood and intestinal levels of ammonia
when dosages are sufficient to overcome the ability of the GI tract
to neutralize the compound, thus reducing acute liver failure and
HE symptoms. The remaining acetic acid that reaches the colon may
be enough to acidify the colonic tract, removing ammonia from the
body as ammonium ions. However, mortality in the acetic acid
control group was relatively high in the present study, with
abnormalities including edema common in hepatic cells. These
findings are concurrent with studies suggesting the use of acetic
acid solution as a coloclyster to treat HE by acidifying the
colonic tract (37). This method,
however, is not suitable for preventing HE as it is relatively
complex and requires significant patient cooperation during an
uncomfortable treatment.
In rat models, food intake and sleep were influenced
by MBG-RCA administration, indicating that this treatment is likely
to improve the quality of life by increasing food intake and
reducing sleep duration. Subjects administered MBG-RCA demonstrated
similar mortality rates to those treated with lactulose, and both
rates were significantly lower than those observed with low-dose
MBG-RCA and acetic acid treatments as well as those observed among
the untreated (HE group) subjects. While these results are
encouraging, a larger sample size is required to further study the
effectiveness and optimal dosage of MBG-RCA treatment. MBG-RCA
treatment was shown to produce notably reduced levels of ALT and
TBIL, superior to lactulose treatment.
Whether MBG-RCA ameliorates pathological findings
remains unknown. As lactulose combined with antibiotic
administration has often been suggested to improve liver
performance, it is possible that combined treatment may also
demonstrate positive effects with microemulsions (38–40).
Other drugs that improve the metabolism and increase survival rates
by reducing toxin absorption, thus protecting the brain and liver
tissues, may also be beneficial when administered together with
MBG-RCA (41,42). The optimal mechanism and
administration technique for MBG-RCA to enhance liver function
requires further study; however, it is likely that the removal of
intestinal ammonia is only one aspect of MBG-RCA’s potential
benefits in HE treatment.
While several indirect methods exist, the majority
with significant side effects, no method of directly decreasing the
intestinal level of ammonia by oral medication has been developed.
The MBG-RCA designed in this experiment is the first to remove
intestinal ammonia without the involvement of colonic bacteria or
enzymes, reducing side effects and discomfort during treatment. It
has the advantages of higher clearance, stability of the GI tract
and elimination of ammonia from the body without producing harmful
metabolic byproducts.
The MBG-RCA designed in the current study has
demonstrated excellent potential for in vivo treatment of HE
by direct ammonia reduction; however, numerous questions remain
unanswered with regard to the mechanism, action and molecular
structure. These issues include optimal surfactant/co-surfactant
determination and proportioning, prevention of adverse reactions,
and methods to reduce acid wastage during GI transport upon oral
administration. Although no enzymes along the course of the GI
tract have been determined to influence this molecule, it is yet to
be ascertained whether the molecule has an effect on digestive
enzyme molecules as it travels through the body. These observations
may play a significant role in assessing drug interactions,
improving compound stability and reducing side effects. Additional
pharmacological research is required to determine the optimal
formula for maximal removal of intestinal ammonia with minimal side
effects. Although MBG-RCA remains experimental, its novel
characteristics merit future investigation as a potential clinical
alternative for removal of ammonia in humans.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 30970886) and the
Science and Technology Project of Dalian (grant no.
2008E13SF193).
References
|
1
|
Butterworth RF, Norenberg MD, Felipo V,
Ferenci P, Albrecht J and Blei AT; Members of the ISHEN Commission
on Experimental Models of HE. Experimental models of hepatic
encephalopathy: ISHEN guidelines. Liver Int. 29:783–788. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang HC, Chang CC, Wang SS, Chan CY, Lee
FY, et al: Pravastatin for thioacetamide-induced hepatic failure
and encephalopathy. Eur J Clin Invest. 42:139–145. 2012. View Article : Google Scholar
|
|
3
|
Rama Rao KV, Reddy PV, Tong X and
Norenberg MD: Brain edema in acute liver failure: inhibition by
L-histidine. Am J Pathol. 176:1400–1408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butterworth RF: Altered glial-neuronal
crosstalk: cornerstone in the pathogenesis of hepatic
encephalopathy. Neurochem Int. 57:383–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strekalova OS, Uchaĭkin VF, Ipatova OM,
Torkhovskaia TI, Medvedeva NV, et al: Comatose states:
etiopathogenesis, experimental studies, treatment of hepatic coma.
Biomed Khim. 55:380–396. 2009.(In Russian). PubMed/NCBI
|
|
6
|
Hassanein T, Blei AT, Perry W, Hilsabeck
R, Stange J, et al: Performance of the hepatic encephalopathy
scoring algorithm in a clinical trial of patients with cirrhosis
and severe hepatic encephalopathy. Am J Gastroenterol.
104:1392–1400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kochar DK, Agarwal P, Kochar SK, Jain R,
Rawat N, et al: Hepatocyte dysfunction and hepatic encephalopathy
in Plasmodium falciparum malaria. QJM. 96:505–512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bémeur C, Vaquero J, Desjardins P and
Butterworth RF: N-acetylcysteine attenuates cerebral complications
of non-acetaminophen-induced acute liver failure in mice:
antioxidant and anti-inflammatory mechanisms. Metab Brain Dis.
25:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharma P, Sharma BC and Sarin SK: Critical
flicker frequency for diagnosis and assessment of recovery from
minimal hepatic encephalopathy in patients with cirrhosis.
Hepatobiliary Pancreat Dis Int. 9:27–32. 2010.PubMed/NCBI
|
|
10
|
Zafirova Z and O’Connor M: Hepatic
encephalopathy: current management strategies and treatment,
including management and monitoring of cerebral edema and
intracranial hypertension in fulminant hepatic failure. Curr Opin
Anaesthesiol. 23:121–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al Sibae MR and McGuire BM: Current trends
in the treatment of hepatic encephalopathy. Ther Clin Risk Manag.
5:617–626. 2009.PubMed/NCBI
|
|
12
|
Zheng Z, Li X, Li Z and Ma X: Artificial
and bioartificial liver support systems for acute and
acute-on-chronic hepatic failure: A meta-analysis and
meta-regression. Exp Ther Med. 6:929–936. 2013.PubMed/NCBI
|
|
13
|
Choi JW, Yoon KT, Park JY, Kim JK, Ahn SH,
et al: Usefulness and safety of extracorporeal liver support
therapy using MARSR for patients with liver failure: a preliminary
report. Korean J Gastroenterol. 54:28–35. 2009.(In Korean).
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phongsamran PV, Kim JW, Cupo Abbott J and
Rosenblatt A: Pharmacotherapy for hepatic encephalopathy. Drugs.
70:1131–1148. 2009. View Article : Google Scholar
|
|
15
|
Sharma P and Sharma BC: Disaccharides in
the treatment of hepatic encephalopathy. Metab Brain Dis.
28:313–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma BC, Sharma P, Agrawal A and Sarin
SK: Secondary prophylaxis of hepatic encephalopathy: an open-label
randomized controlled trial of lactulose versus placebo.
Gastroenterology. 137:885–891. 891.e12009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malaguarnera M, Gargante MP, Malaguarnera
G, Salmeri M, Mastrojeni S, et al: Bifidobacterium combined with
fructo-oligosaccharide versus lactulose in the treatment of
patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol.
22:199–206. 2010. View Article : Google Scholar
|
|
18
|
Weber FL Jr: Effects of lactulose on
nitrogen metabolism. Scand J Gastroenterol Suppl. 222:83–87.
1997.PubMed/NCBI
|
|
19
|
Sotelo N, de los Angeles Durazo M,
Gonzalez A and Dhanakotti N: Early treatment with N-acetylcysteine
in children with acute liver failure secondary to hepatitis A. Ann
Hepatol. 8:353–358. 2009.PubMed/NCBI
|
|
20
|
Sundaram V and Shaikh OS: Hepatic
encephalopathy: pathophysiology and emerging therapies. Med Clin
North Am. 93:819–836. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang AH, Duan ZJ, Tian G, Lu D, Zhang WJ,
et al: The addition of a pH-sensitive gel improves microemulsion
stability for the targeted removal of colonic ammonia. BMC
Gastroenterol. 11:502011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tønnesen HH and Karlsen J: Alginate in
drug delivery systems. Drug Dev Ind Pharm. 28:621–630. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan MD, Li JC, Lin Q, Wang XH and Wang LH:
Progress on sodium alginate application to drug controlled release.
China Pharmaceuticals. 17:3–5. 2008.(In Chinese).
|
|
24
|
Chen SC, Wu YC, Mi FL, et al: A novel
pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and
alginate crosslinked by genipin for protein drug delivery. J
Control Release. 96:285–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moroni A, Drefko W and Thone G:
Formulations of zero-order, pH-dependent, sustained release matrix
systems by ionotropic gelation of alginate-containing mixtures.
Drug Dev Ind Pharm. 37:216–224. 2011. View Article : Google Scholar
|
|
26
|
El-Sherbiny IM, Salama A and Sarhan AA:
Ionotropically cross-linked pH-sensitive IPN hydrogel matrices as
potential carriers for intestine-specific oral delivery of protein
drugs. Drug Dev Ind Pharm. 37:121–130. 2011. View Article : Google Scholar
|
|
27
|
Xiong W, Gao X, Zhao Y, Xu H and Yang X:
The dual temperature/pH-sensitive multiphase behavior of poly
(N-isopropylacrylamide-co-acrylic acid) microgels for potential
application in in situ gelling system. Colloids Surf B
Biointerfaces. 84:103–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin YH, Yang YJ and Xu BH: Swelling
kinetics of hydrogels for colonic-site drug delivery. Acta
Polymerica Sinica. 2001.650–655. 2001.(In Chinese).
|
|
29
|
Yin YH, Yang YJ and Xu BH: Hydrogels
containing 4,4′-di(methacryloylmino)azobenzenes: Synthesis and
animal experiment for colonic-site drug release. Acta Polymerica
Sinica. 2002.408–413. 2002.(In Chinese).
|
|
30
|
Kim S, Kim JH and Kim D: pH sensitive
swelling and releasing behavior of nano-gels based on
polyaspartamide graft copolymers. J Colloid Interface Sci.
356:100–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kono K, Tabata F and Takagishi T:
pH-responsive permeability of poly (acrylic acid)-poly
(ethylenimine) complex capsule membrane. J Memb Sci. 76:233–243.
1993. View Article : Google Scholar
|
|
32
|
Li XQ, Dong L, Liu ZH and Luo JY:
Expression of gamma-aminobutyric acid A receptor subunits alpha1,
beta1, gamma2 mRNA in rats with hepatic encephalopathy. World J
Gastroenterol. 11:3319–3322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zimmermann C, Ferenci P, Pifl C, Yurdaydin
C, Ebner J, et al: Hepatic encephalopathy in thioacetamide-induced
acute liver failure in rats: characterization of an improved model
and study of amino acid-ergic neurotransmission. Hepatology.
9:594–601. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chu CJ, Lee FY, Wang SS, Chang FY, Lin HC,
et al: Establishment of an animal model of hepatic encephalopathy.
Zhonghua Yi Xue Za Zhi (Taipei). 63:263–269. 2000.
|
|
35
|
Yurdaydin C, Walsh TJ, Engler HD, Ha JH,
Li Y, et al: Gut bacteria provide precursors of benzodiazepine
receptor ligands in a rat model of hepatic encephalopathy. Brain
Res. 679:42–48. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bongaerts G, Severijnen R and Timmerman H:
Effect of antibiotics, prebiotics and probiotics in treatment for
hepatic encephalopathy. Med Hypotheses. 64:64–68. 2005. View Article : Google Scholar
|
|
37
|
Häberle J: Clinical practice: the
management of hyperammonemia. Eur J Pediatr. 170:21–34. 2011.
View Article : Google Scholar
|
|
38
|
Kowdley KV and Burman BE: ACP Journal
Club. Adding rifaximin to lactulose increased reversal and
decreased mortality in hepatic encephalopathy. Ann Intern Med.
159:JC82013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sharma BC, Sharma P, Lunia MK, et al: A
randomized, double-blind, controlled trial comparing rifaximin plus
lactulose with lactulose alone in treatment of overt hepatic
encephalopathy. Am J Gastroenterol. 108:1458–1463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Debray D, Yousef N and Durand P: New
management options for end-stage chronic liver disease and acute
liver failure: potential for pediatric patients. Paediatr Drugs.
8:1–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharma P, Sharma BC and Sarin SK:
Predictors of nonresponse to lactulose for minimal hepatic
encephalopathy in patients with cirrhosis. Liver Int. 29:1365–1371.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maclayton DO and Eaton-Maxwell A:
Rifaximin for treatment of hepatic encephalopathy. Ann
Pharmacother. 43:77–84. 2009. View Article : Google Scholar
|