Introduction
Among the mechanisms that mediate cancer
progression, cell mobility is a significant factor necessary for
liberation from the primary focus and infiltration. Various cell
growth factors (1–5), including epidermal growth factor,
transforming growth factor β (6,7) and
hepatocyte growth factor (HGF) (8), are known to facilitate cell
mobility.
HGF, which was first isolated and cloned by Nakamura
et al (9–12), performs various biological
activities in cells, including stimulation of cell growth,
promotion of migration, induction of morphogenesis and
anti-apoptotic activities, via the c-Met receptor, which is a
transmembrane protein containing a tyrosine kinase domain (13–15).
The involvement of HGF in the infiltration/metastasis of cancer
cells was first suggested in 1991, in a study in which the scatter
factor, isolated as a fibroblast-derived bioactive factor with cell
stimulatory activities in various cultured epithelial and cancer
cells, was found to share an identical structure to that of the HGF
molecule (16,17). The functions of HGF were further
elucidated by in vitro and in vivo analyses using
various types of cancer cell (18,19).
Activation of the HGF/c-Met pathway leads to simultaneous
activation of multiple signal transduction pathways that promote
the infiltration of cancer cells and is considered to underlie the
potent infiltrative/stimulatory effect of HGF (20–25).
Genetic mutations of the c-Met receptor have been reported in
various cancer types, including papillary renal (20–21),
hepatic (22), gastric (23) and pulmonary cancer (24,25),
and the overexpression of c-Met has also been reported in numerous
cancer tissues (26). Therefore,
if the c-Met receptor is present in cancer cells, HGF antagonists
should be able to inhibit multiple signal transduction pathways
that lead to cancer cell infiltration, thereby exerting potential
anti-cancer effects (27).
In a previous study by our group, an association
between elevated pre-operative serum HGF levels and advanced
disease stages in colon cancer was identified, mainly regarding the
depth of tumor invasion into the wall and liver metastasis, which
suggested the expression of the HGF/c-Met pathway as a potential
predictive factor of colon cancer progression (8). In the present study, serological and
immunohistological analyses were conducted in order to evaluate the
clinical significance of the expression of the HGF/c-Met pathway in
assessing the stage of gastric cancer progression.
Materials and methods
Patients
Subjects (n=110) were randomly selected from a
cohort of patients with gastric cancer who underwent surgical
resection at the Department of Surgery II, Tokyo Women’s Medical
University (Tokyo, Japan) between April 1999 and March 2003. Verbal
consent was obtained from all patients upon hospitalization and
written consent was obtained on the inpatient treatment plan. The
study was conducted in 2005 in accordance with the ethical
guidelines established by the updated Declaration of Helsinki and
Tokyo Women’s Medical University. Preoperative serum HGF levels in
these subjects were measured and various pathological factors were
analyzed. For 50 of these patients, immunohistochemical staining of
tissue preparations for HGF and c-Met was additionally performed in
order to analyze various factors identified in serological
analysis.
The subjects comprised 83 males and 27 females aged
29–84 years [mean ± standard deviation (SD), 62.8±9.9 years]. The
tissue samples were histologically classified as follows: Four as
papillary adenocarcinoma, 51 as tubular adenocarcinoma (25 as
well-differentiated and 26 as moderately differentiated), 45 as
poorly differentiated adenocarcinoma, six as signet-ring cell
carcinoma and three as mucinous adenocarcinoma. The histological
classification of invasion depth was as follows: Mucosa (m) in 28
patients, submucosa (sm) in 31 patients, muscularis propria (mp) in
11 patients, subserosa (ss) in 18 patients and serosa (se) in 22
patients. The stage classification was IA in 55 patients, IB in 18
patients, II in 16 patients, IIIA in nine patients, IIIB in six
patients and IV in six patients (Table
I). Data obtained from 200 healthy individuals were used as the
control. Healthy individuals comprised patients undergoing surgery
for benign diseases, including inguinal hernia or hemorrhoid, and
healthy volunteers. Classification of infiltrative growth pattern
(INF) was performed according to the General Rules for the Gastric
Cancer Society, by the Japanese Research Society for Gastric
Cancer, which is based on the Union for International Cancer
Control criteria (28).
 | Table IClinicopathological factors and serum
HGF. |
Table I
Clinicopathological factors and serum
HGF.
| Factor | n | Serum HGF
(pg/ml) | P-value |
|---|
| Gastric cancer | 110 | 391.02±68.44 | <0.0001 |
| Control | 200 | 193.30±52.00 | |
| Stagea |
| IA | 55 | 381.21±65.92 | NS |
| IB | 18 | 414.41±82.48 | |
| II | 16 | 378.28±47.01 | |
| IIIA | 9 | 404.64±65.95 | |
| IIIB | 6 | 402.38±93.44 | |
| IV | 6 | 412.94±71.45 | |
| Depth |
| m | 28 | 378.89±58.81 | NS |
| sm | 31 | 384.87±69.89 | |
| mp | 11 | 386.97±98.72 | |
| ss | 18 | 403.36±54.88 | |
| s | 22 | 407.04±71.75 | |
| INFa |
| α | 22 | 369.65±68.93 | <0.001 (α.β vs.
γ) |
| β | 37 | 367.34±59.68 | |
| γ | 44 | 418.42±67.72 | |
| Histological
type |
| well | 25 | 387.23±54.56 | NS |
| mod | 26 | 379.56±80.35 | |
| poor | 45 | 399.25±70.48 | |
| sig | 6 | 404.95±74.10 | |
| muc | 3 | 361.36±77.65 | |
| pap | 4 | 396.12±54.23 | |
| Macroscopic
type |
| 0 | 59 | 382.03±64.39 | NS |
| 0-advanced | 9 | 364.13±68.61 | |
| 1 | 4 | 426.32±66.53 | |
| 2 | 16 | 404.70±87.33 | |
| 3 | 10 | 402.00±43.63 | |
| 4 | 11 | 423.53±72.43 | |
| 5 | 1 | 335.54±0.000 | |
| Lymphatic
invasion |
| ly0 | 48 | 384.71±63.69 | NS |
| ly1 | 37 | 391.22±70.67 | |
| ly2 | 21 | 398.01±76.63 | |
| ly3 | 4 | 428.13±68.74 | |
| Venous
invasion |
| v0 | 89 | 387.92±67.32 | NS |
| v1 | 20 | 404.99±75.03 | |
| v2 | 1 | 387.52±0.000 | |
| Lymph node
metastasis |
| n0 | 78 | 391.17±70.37 | NS |
| n1 | 17 | 382.79±58.94 | |
| n2 | 13 | 394.60±68.08 | |
| n3 | 0 | | |
| n4 | 2 | 431.73±116.00 | NS |
| Peritoneal
dissemination |
| p0 | 105 | 389.37±68.19 | NS |
| p1 | 5 | 425.58±71.99 | |
| Tumor size, mm |
| ≤–70 | 97 | 384.64±66.22 | NS |
| >70 | 10 | 428.09±61.82 | |
The 50 subjects that were subjected to
immunostaining comprised 38 males and 12 females, with a mean age
of 61.8±10.6 years (range, 29–81 years). The tissue samples were
histologically classified as follows: One as papillary
adenocarcinoma, 23 as tubular adenocarcinoma (12
well-differentiated and 11 moderately differentiated), 20 as poorly
differentiated adenocarcinoma, five as signet-ring cell carcinoma
and one as mucinous adenocarcinoma. The histological classification
of invasion depth was as follows: m in 13 patients, sm in 15
patients, mp in five patients, ss in eight patients and se in nine
patients. The stage classification was IA in 25 patients, IB in
seven patients, II in nine patients, IIIA in four patients, IIIB in
two patients and IV in three patients (Table II).
 | Table IICorrelation between HGF/c-Met
overexpression and clinicopathological factors. |
Table II
Correlation between HGF/c-Met
overexpression and clinicopathological factors.
| | | HGF expression | c-Met
expression |
|---|
| | |
|
|
|---|
| Factor | n | subtotal | (−) | (+) | P-value | (−) | (+) | P-value |
|---|
| All | 50 | | 14 | 36 | | 25 | 25 | |
| Gender |
| Male | 38 | | | | | | | |
| Female | 12 | | | | | | | |
| Stage |
| IA | 25 | 41 | 12 | 29 | NS | 23 | 18 | NS |
| IB | 7 | | | | | | | |
| II | 9 | | | | | | | |
| IIIA | 4 | 9 | 2 | 7 | | 2 | 7 | |
| IIIB | 2 | | | | | | | |
| IV | 3 | | | | | | | |
| Depth |
| m | 13 | 13 | 5 | 8 | NS | 9 | 4 | NS |
| sm | 15 | 37 | 9 | 28 | | 16 | 21 | |
| mp | 5 | | | | | | | |
| ss | 8 | | | | | | | |
| se | 9 | | | | | | | |
| INF |
| α | 10 | 32 | 10 | 22 | NS | 16 | 16 | NS |
| β | 22 | | | | | | | |
| γ | 17 | 17 | 3 | 14 | | 8 | 9 | |
| Histological
type |
| well | 12 | 23 | 5 | 18 | NS | 11 | 12 | NS |
| mod | 11 | | | | | | | |
| poor | 20 | 25 | 8 | 17 | | 13 | 12 | |
| sig | 5 | | | | | | | |
| muc | 1 | | | | | | | |
| pap | 1 | | | | | | | |
| Lymphatic
invasion |
| ly0 | 27 | 43 | 12 | 31 | NS | 24 | 19 | 0.0416 |
| ly1 | 16 | | | | | | | |
| ly2 | 4 | 7 | 2 | 5 | | 1 | 6 | |
| ly3 | 3 | | | | | | | |
| Venous
invasion |
| v0 | 43 | 43 | 11 | 32 | NS | 23 | 20 | NS |
| v1 | 7 | 7 | 3 | 4 | | 2 | 5 | |
| v2 | 0 | | | | | | | |
| Lymph node
metastasis |
| n0 | 34 | 45 | 12 | 33 | NS | 25 | 20 | 0.0184 |
| n1 | 11 | | | | | | | |
| n2 | 5 | 5 | 2 | 3 | | 0 | 5 | |
| n3 | 0 | | | | | | | |
| n4 | 0 | | | | | | | |
| Peritoneal
dissemination |
| p0 | 47 | 47 | 14 | 33 | NS | 24 | 23 | NS |
| p1 | 3 | 3 | 0 | 3 | NS | 1 | 2 | NS |
| Tumor size
(mm) |
| ≤50 | 38 | 38 | 12 | 26 | NS | 22 | 16 | 0.0469 |
| >50 | 12 | 12 | 2 | 10 | | 3 | 9 | |
| Serum HGF
(pg/ml) |
| ≤400 | 36 | 36 | 10 | 26 | NS | 17 | 19 | NS |
| >400 | 14 | 14 | 4 | 10 | | 8 | 6 | |
Serological analysis
Serum was obtained by centrifugation of venous blood
collected prior to surgery at 1,000–2,000 × g for 10 min, which was
stored frozen at −80°C and thawed at the time of measurement. HGF
levels were measured using a two-step sandwich HGF ELISA kit
(Otsuka, Tokyo, Japan), which included the antibodies and
o-Phenylenediamine substrate solution, according to the
manufacturer’s instructions. In the first reaction, 50 μl
phosphate-buffered saline (PBS; Wako Pure Chemical Industries, Ltd,
Osaka, Japan) and 50 μl sample were added to each well of a
microtiter plate, which was sealed and incubated at room
temperature for 1 h with agitation. Following removal of the
reaction mixture, the plate was washed five times with wash buffer
(Wako Pure Chemical Industries, Ltd). Subsequently, 100 μl/well
rabbit polyclonal anti-HGF primary antibody was added for the
second reaction and incubated for 1 h at room temperature.
Following aspiration and washing five times, 100 μl/well of the
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
secondary antibody was added for the third reaction and incubated
for 1 h at room temperature. Following aspiration and washing five
times, 100 μl/well o-Phenylenediamine substrate solution was added.
Following incubation at room temperature for 10 min, the reaction
was stopped by adding 100 μl of stop solution. Absorbance was
measured at 420 nm using a microplate reader (SpectraMax Plus 384;
Molecular Devices, Sunnyvale, CA, USA), and HGF levels were
determined using a standard curve.
Immunohistological analysis
HGF: Following deparaffinization with petroleum
benzene (Kanto Chemical Co., Inc., Tokyo, Japan) of the 20%
formalin-fixed (Wako Pure Chemical Industries, Ltd)
paraffin-embedded (Junsei Chemical Co., Ltd, Tokyo, Japan) sections
(4 μm), which included the innermost tumor portion of each gastric
cancer primary focus, the sections were immersed in PBS and exposed
to microwaves at 95°C for 15 min to activate the antigens.
Subsequently, the tissue sections were treated with 3%
H2O2 (Sankyo Kagaku Yakuhin Co., Ltd,
Kanagawa, Japan) for 20 min to remove the intrinsic peroxidase
activity and immunohistochemical staining was performed using the
avidin-biotin-peroxidase complex (ABC) method. Following dilution
of the reaction with normal horse serum at room temperature for 10
min, rabbit polyclonal anti-human HGF antibody (dilution, 1:20; IBL
Co., Ltd, Gunma, Japan) was used as the primary antibody and
incubation was continued at room temperature for 60 min. This was
followed by reaction with a biotin-conjugated anti-mouse
immunoglobulin G secondary antibody (DAKO Japan, Kyoto, Japan) at
room temperature for 30 min and reaction with the ABC reagent
(DAKO, Glostrup, Denmark) at room temperature for 30 min. The color
was developed by addition of 20% 3,3′-diaminobenzidine
tetrahydrochloride (Dojindo Laboratories, Kumamoto, Japan), the
nuclei were stained with hematoxylin (Merck Millipore KGaA,
Darmstadt, Germany) and the sections were dehydrated.
c-Met: c-Met was assayed in a similar manner to HGF,
except that the antigen was activated by autoclaving at 95°C for 15
min and a rabbit polyclonal anti-human c-Met primary antibody
(dilution, 1:20; IBL Co., Ltd.) was allowed to react at room
temperature for 1 h.
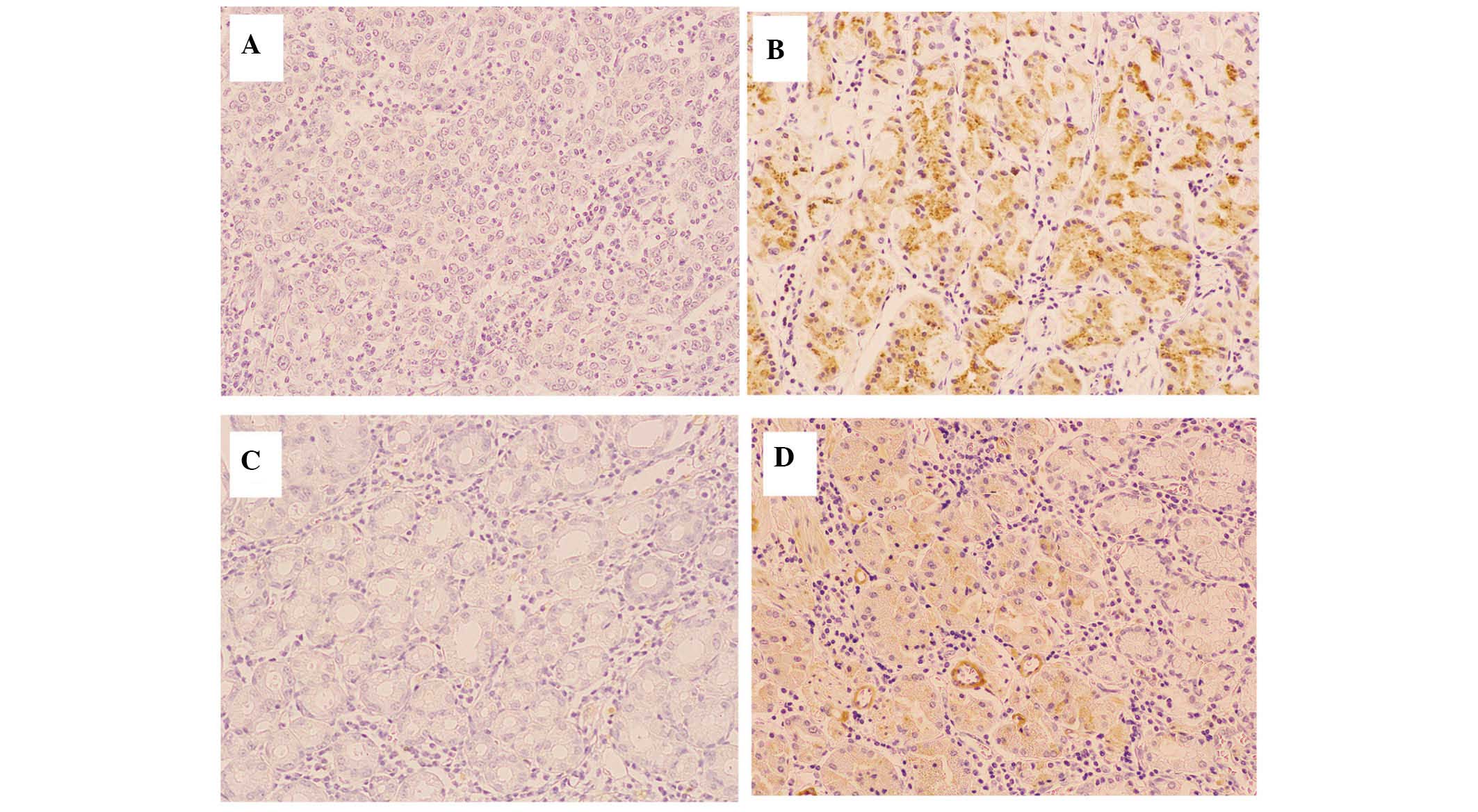
Microscopic examination of HGF and c-Met was
performed on the tip of the tumor, particularly the innermost
section. Three fields of each section were observed at 200×
magnification using a BHS/System Living microscope (Olympus Corp.,
Tokyo, Japan) and the results were classified as positive when the
ratio of stained cancer cells was >25%, according to previous
studies that were analyzed for comparison (Fig. 1) (8,29–31).
Statistical analysis
JMP version 9.0.2 statistical software (SAS
Institute, Inc., Cary, NC, USA) was used for statistical analyses.
Values are presented as the mean ± SD. The Mann-Whitney U
test was used to compare differences between two independent
groups. Cumulative survival rates were calculated using the
Kaplan-Meier method and distributions were identified using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference between values.
The terminology used in this report is in accordance
with the General Rules of the Gastric Cancer Society by the
Japanese Research Society for Gastric Cancer (28).
Results
Serological analysis of HGF
Significant differences were detected in
preoperative HGF levels between the gastric cancer and control
groups (391.0±68.4 vs. 193.3±52.0 pg/ml, respectively;
P<0.0001). There was no correlation between preoperative serum
HGF levels and patient age or gender. The results of analyses to
identify correlations between serum HGF levels and
clinicopathological factors are shown in Table I. Advanced progression in the
INFα/β vs. INFγ was correlated with elevated preoperative serum HGF
levels (P<0.001). Although there was no significant difference
in tumor diameter, invasion depth or lymphatic vessel invasion
(ly), preoperative serum HGF levels increased as the disease
progressed. In patients with peritoneal dissemination, serum HGF
levels were frequently increased.
Immunohistological analysis
Of the 50 cases analyzed, 36 (72%) were HGF-positive
and 14 (28%) were HGF-negative, whereas 25 (50%) were
c-Met-positive and 25 (50%) were c-Met-negative. No correlation was
found between serum HGF levels in either staining. There was no
correlation between the pathological factors analyzed and HGF
levels, whereas a significant correlation was found between c-Met,
which is a receptor of HGF, and lymphatic vessel invasion (ly0.1
vs. 2.3; P=0.0416), lymph node metastasis (n0.1 vs. 2; P=0.0184)
and maximum tumor diameter (≤50 mm vs. >50 mm; P=0.0469)
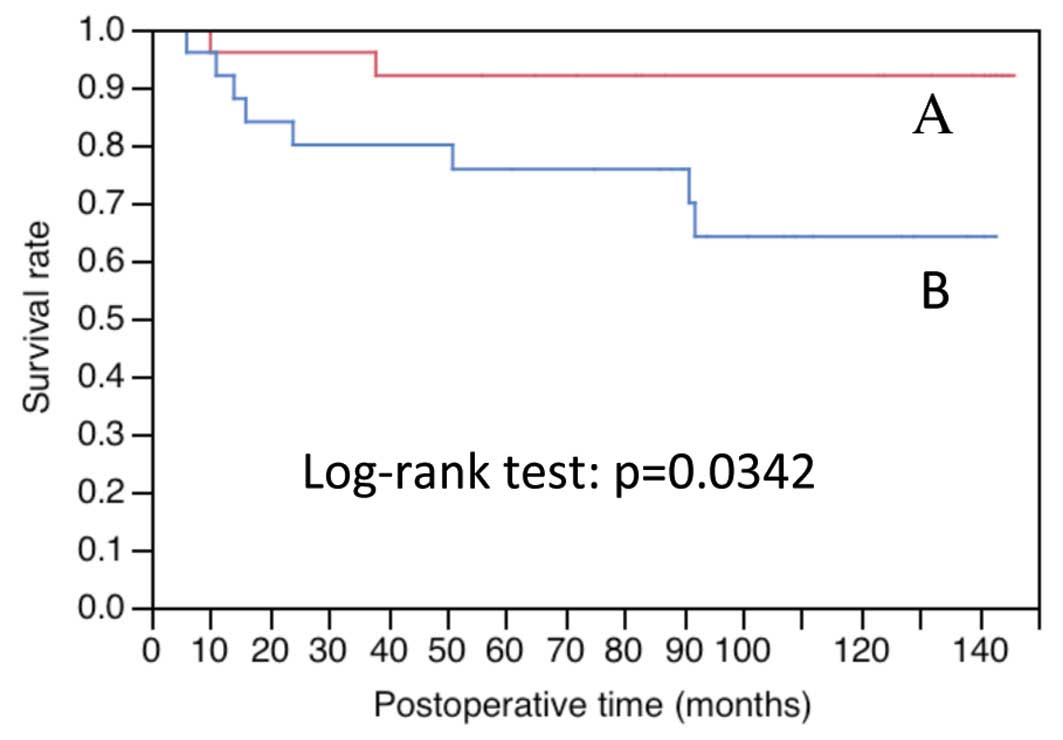
(Table II). The overall survival
(OS) was significantly lower in c-Met-positive cases than that in
c-Met-negative cases (P=0.0342; Fig.
2 and Table III).
 | Table IIIFive-year survival rate and P-value
for overall survival. |
Table III
Five-year survival rate and P-value
for overall survival.
| Clinicopathological
factor | n | Five-year survival
rate | P-value |
|---|
| Peritoneal
dissemination |
| p0 | 47 | 0.893 | <0.0001 (p0 vs.
1) |
| p1 | 3 | 0.000 | |
| Stage |
| I/II | 41 | 0.975 | <0.0001 (I/II
vs. III/IV) |
| III/IV | 9 | 0.222 | |
| Tumor size
(mm) |
| <50 | 38 | 0.920 | 0.0001 (<50 vs.
>50) |
| >50 | 12 | 0.583 | |
| Venous
invasion |
| v0 | 43 | 0.906 | 0.0011 (v0 vs.
1/2) |
| v1/2 | 7 | 0.429 | |
| Lymphatic
invasion |
| ly0/1 | 43 | 0.906 | 0.0017 (ly0/1 vs.
2/3) |
| ly2/3 | 7 | 0.429 | |
| Lymph node
metastasis |
| n0/1 | 45 | 0.888 | 0.0056 (n0/1 vs.
2) |
| n2 | 5 | 0.400 | |
| Infiltrative growth
pattern |
| IFNα/β | 32 | 0.937 | 0.0083 (IFNα/β vs.
γ) |
| IFNγ | 17 | 0.647 | |
| c-Met
expression |
| (−) | 25 | 0.920 | 0.0342 [(−) vs.
(+)] |
| (+) | 25 | 0.758 | |
| serum HGF
(pg/ml) |
| <400 | 36 | 0.887 | 0.0558 (<400 vs.
>400) |
| >400 | 14 | 0.714 | |
| Histological
type |
| well/mod | 23 | 0.920 | 0.1793 (well/mod
vs. por/sig) |
| poor/sig | 25 | 0.756 | |
| Depth |
| m | 13 | 0.917 | 0.2649 (m vs.
sm/mp/ss/se) |
| sm/mp/ss/se | 37 | 0.811 | |
| HGF expression |
| (−) | 14 | 0.929 | 0.5385 [(−) vs.
(+)] |
| (+) | 36 | 0.806 | |
Discussion
Cell growth factors, including HGF, constitute a
significant group of molecules that regulate cell proliferation,
migration and apoptosis in the dynamic organization of cell
populations during embryogenesis, organogenesis and regeneration.
Numerous factors amongst these additionally promote cell migration.
It has been previously reported that HGF has the most potent effect
on the promotion of cancer cell infiltration, the cell migration
associated with the degradation of extracellular matrix components,
including the basement membrane and collagen (9–16).
Therefore, activation of the HGF/c-Met pathway results in the
simultaneous activation of multiple signal transduction pathways
that promote cancer cell infiltration. Antagonists of the HGF/c-Met
pathway represent potential anti-cancer agents to inhibit cancer
infiltration and metastasis, and therefore, the development of such
antagonists is currently underway (27).
In the present study, serological and
immunohistological analyses of the expression of the HGF/c-Met
pathway in gastric cancer were performed in order to establish its
clinical significance in the assessment of disease progression. To
the best of our knowledge, no previous studies analyzing serum HGF
levels and immunostaining for HGF and c-Met simultaneously with
pathological factors were available in the literature.
Although elevated serum HGF levels in patients with
gastric cancer had been previously reported (32–35),
the present study aimed to determine whether this factor may be
used in the assessment of disease progression. The results
indicated that pre-operative serum HGF levels were significantly
higher in patients with gastric cancer than those in the control
group (P<0.0001), and that high HGF levels above the cut-off
value (297.3 pg/ml; mean in the control+2 SD) were observed in
93.75% of patients, similar to that reported previously. However,
the correlation between HGF levels and disease stage previously
reported by Wu et al (32)
and Han et al (33) was not
observed in the present study, the results of which were similar to
those reported by Taniguchi et al (34).
Conversely, advanced progression in the infiltrating
growth pattern (INFα/β vs. INFγ) was significantly correlated with
high preoperative serum HGF levels (P<0.001). Although this
effect may be associated with the involvement of HGF in the
infiltrating growth of cancer cells, this factor could not be
evaluated because, to the best of our knowledge, no other study on
infiltrating growth patterns was available in the literature.
HGF levels were not significantly correlated with
certain parameters, including tumor diameter, invasion depth and ly
factors; however, preoperative serum HGF levels were elevated as
the disease progressed. Regarding the association between HGF
levels and invasion depth (pT factor), Niki et al (35) identified a significant difference
between pT1 and pT2–4 tumors.
Although a significant difference in HGF levels was
not detected in patients with peritoneal dissemination, there was a
tendency towards high HGF levels among these patients.
Subjects for the present study were selected
randomly; therefore no patient with liver metastasis was included.
Niki et al (35) reported a
significant elevation in serum HGF levels in patients diagnosed
with liver metastasis, whereas Taniguchi et al (34) reported that there was no
significant difference in serum HGF levels in patients with relapse
independent of liver metastasis. Therefore, the preoperative serum
HGF levels in patients with gastric cancer represent a potential
predictive factor for disease progression, as observed in colon
cancer (6).
In the present study, no correlation was identified
between serum HGF levels and immunostaining for HGF or c-Met in
tissue preparations; this was potentially due to the complex
paracrine and autocrine mechanisms of HGF in cancer cells (36,37).
Therefore, the significance of HGF expression in the
microenvironment surrounding tumors requires further
investigation.
Although there was no correlation between
pathological factors and immunostaining for HGF, a significant
correlation was identified between c-Met, which is a receptor of
HGF, and lymphatic vessel invasion (ly0.1 vs. 2.3, P=0.0416), lymph
node metastasis (n0.1 vs. 2, P=0.0184) and maximum tumor diameter
(<50 mm vs. >50 mm, P=0.0469). Correlations between
immunostaining for c-Met and various pathological factors,
particularly invasion depth and disease stage, have been reported
in previous studies (38–46). In the present study, cases were
selected randomly for immunostaining analysis, as for serological
analysis. It was demonstrated that 41 (82%) of the 50 cases
analyzed were stage I or II, and 28 (56%) had an invasion depth of
m or sm, indicating that the majority of the cohort comprised
relatively early stage cancer cases. Only three (6%) cases that
were Peritoneum dissemination-factor-positive were stage IV. These
results likely explain the absence of statistically significant
differences between immunostaining and invasion depth or disease
stage.
However, in the present study, which included
numerous relatively early cancer cases, the OS of c-Met
immunostaining-positive cases was significantly lower than that of
negative cases (P=0.0342), indicating that c-Met positivity may be
a prognostic factor for gastric cancer.
In chemotherapy for unresectable recurrent gastric
cancer, the efficacy of trastuzumab was demonstrated in
HER2-positive cases, which subsequently led to the use of
personalized drug treatments with molecularly targeted drugs
(47). Rilotumumab, which is a
fully human monoclonal antibody against HGF and a ligand of the
c-Met receptor, suppresses c-Met downstream signaling (47). In pre-clinical models, rilotumumab
was shown to inhibit tumor progression in a HGF/c-Met- dependent
manner, and its tolerability was verified in early clinical trials
(48,49). If future phase II/III trials are
implemented under clinical trial designs that allow sufficient
verification of the potential of c-Met expression as a biomarker to
aid the identification of cases in which rilotumumab is effective,
a field of c-Met-positive gastric cancer may be established,
similarly to that of HER2-positive gastric cancer. Therefore,
further basic studies regarding c-Met expression are required,
particularly to improve quality control in immunostaining.
In conclusion, the results of the present study
revealed that elevated pre-operative serum HGF levels were
indicative of invasive growth of tumor foci, categorized as IFNγ,
and characterized by high-grade tumors with an unclear border
between the tumor and the surrounding tissue. c-Met-positive
immunostaining indicated a tumor with a large diameter, advanced
lymphatic vessel invasion and a high degree of lymph node
metastasis, and may therefore be a factor indicating poor
prognosis. Based on the results described above, the expression of
the HGF/c-Met pathway in gastric cancer is a potential predictive
factor for disease progression, as previously established for colon
cancer.
Acknowledgements
The authors would like to thank Miho Tabe (SRL
Laboratory, Tokyo, Japan) for providing the instrumentation. The
authors would also like to thank Minoru Sakurada and Mizuho Karita
(Department of Pathology, Tokyo Women’s Medical University, Tokyo,
Japan) for their technical advice.
References
|
1
|
Yamada A, Saito N, Kameoka S and Kobayashi
M: Clinical significance of epidermal growth factor (EGF)
expression in gastric cancer. Hepatogastroenterology. 54:1049–1052.
2007.PubMed/NCBI
|
|
2
|
Hirosawa T, Saito N, Kameoka S and
Kobayashi M: Clinical significance of epidermal growth factor (EGF)
expression for assessing the spreading of human colon cancer.
Nippon Daicho Komonbyo Gakkai Zasshi. 55:402–412. 2002.(In
Japanese). View Article : Google Scholar
|
|
3
|
Soyama K, Saito N and Kameoka S: Study on
adhesion molecule beta1 integrin in colorectal
cancer-quantification of blood levels and immunohistological
staining. Nippon Daicho Komonbyo Gakkai Zasshi. 52:119–127.
1999.(In Japanese). View Article : Google Scholar
|
|
4
|
Saito N, Nishimura H and Kameoka S:
Clinical significance of fibronectin expression in colorectal
cancer. Mol Med Rep. 1:77–81. 2008.PubMed/NCBI
|
|
5
|
Tani H, Saito N, Kobayashi M and Kameoka
S: Clinical significance of keratinocyte growth factor and K-sam
gene expression in gastric cancer. Mol Med Rep. 7:1381–1386.
2013.PubMed/NCBI
|
|
6
|
Suda A, Saito N, Seshimo A, Kameoka S and
Kobayashi M: Examination of transforming growth factor beta1
expression in the serum and tumor tissue of gastric cancer. Int
Surg. 94:182–188. 2009.
|
|
7
|
Daiko W, Saito N and Kameoka S: Clinical
significance of TGF-beta1 expression in evaluation of the
malignancy of colorectal cancer. Nippon Daicho Komonbyo Gakkai
Zasshi. 58:377–382. 2005.(In Japanese). View Article : Google Scholar
|
|
8
|
Hashimoto T, Saito N, Kameoka S, Shibata N
and Kobayashi M: Clinical significance of hepatocyte growth factor
and its specific receptor c-Met expression in colorectal cancer
progression. Acta Histochem Cytochem. 37:139–146. 2004. View Article : Google Scholar
|
|
9
|
Nakamura T, Nawa K and Ichihara A: Partial
purification and characterization of hepatocyte growth factor from
serum of hepatectomized rats. Biochem Biophys Res Commun.
122:1450–1459. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura T, Teramoto H and Ichihara A:
Purification and characterization of a growth factor from rat
platelets for mature parenchymal hepatocytes in primary cultures.
Proc Natl Acad Sci USA. 83:6489–6493. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura T, Nawa K, Ichihara A, Kaise N
and Nishino T: Purification and subunit structure of hepatocyte
growth factor from rat platelets. FEBS Lett. 224:311–316. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura T, Nishizawa T, Hagiya M, et al:
Molecular cloning and expression of human hepatocyte growth factor.
Nature. 342:440–443. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higashio K, Shima N, Goto M, et al:
Identity of a tumor cytotoxic factor from human fibroblasts and
hepatocyte growth factor. Biochem Biophys Res Commun. 170:397–404.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stoker M and Gherardi E: Regulation of
cell movement: the motogenic cytokines. Biochim Biophys Acta.
1072:81–102. 1991.PubMed/NCBI
|
|
15
|
Nakamura T: Structure and function of
hepatocyte growth factor. Prog Growth Factor Res. 3:67–85. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Botraro DP, Rubin JS, Faletro DL, et al:
Identification of the hepatocyte growth factor receptor as the
c-met proto-oncogene product. Science. 251:802–804. 1991.
View Article : Google Scholar
|
|
17
|
Naldini L, Weidner KM, Vigna E, et al:
Scatter factor and hepatocyte growth factor are indistinguishable
ligands for the MET receptor. EMBO J. 10:2867–2878. 1991.PubMed/NCBI
|
|
18
|
Conrotto P, Valdembri D, Corso S, et al:
Sema4D induces angiogenesis through Met recruitment by Plexin B1.
Blood. 105:4321–4329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi S and Tsao MS: Activation of hepatocyte
growth factor-met autocrine loop enhances tumorigenicity in a human
lung adenocarcinoma cell line. Neoplasia. 2:226–234. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt L, Duh FM, Chen F, et al: Germline
and somatic mutations in the tyrosine kinase domain of the MET
proto-oncogene in papillary renal carcinomas. Nat Genet. 16:68–73.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmidt L, Junker K, Weirich G, et al: Two
North American families with hereditary papillary renal carcinoma
and identical novel mutations in the MET proto-oncogene. Cancer
Res. 58:1719–1722. 1998.PubMed/NCBI
|
|
22
|
Park WS, Dong SM, Kim SY, et al: Somatic
mutations in the kinase domain of the Met/hepatocyte growth factor
receptor gene in childhood hepatocellular carcinomas. Cancer Res.
59:307–310. 1999.PubMed/NCBI
|
|
23
|
Lee JH, Han SU, Cho H, et al: A novel germ
line juxtamembrane Met mutation in human gastric cancer. Oncogene.
19:4947–4953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma PC, Kijima T, Maulik G, et al: c-MET
mutational analysis in small cell lung cancer: novel juxtamembrane
domain mutations regulating cytoskeletal functions. Cancer Res.
63:6272–6281. 2003.PubMed/NCBI
|
|
25
|
Ma PC, Jagdeesh S, Jagadeeswaran R, et al:
c-MET expression/activation, functions and mutations in non-small
cell lung cancer. Proc Am Assoc Cancer Res. 44:18752004.
|
|
26
|
Christensen JG, Burrows J and Salgia R:
c-Met as a target for human cancer and characterization of
inhibitors for therapeutic intervention. Cancer Lett. 225:1–26.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura T, Sakai K, Nakamura T and
Matsumoto K: Hepatocyte growth factor twenty years on: Much more
than a growth factor. J Gastroenterol Hepatol. 26:188–202. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Japanese Gastric Cancer Association.
Gastric cancer. 1. Japanese Classification of Gastric Carcinoma.
2nd English Edition. June. 1998, pp. 10–24
|
|
29
|
Hattori Y, Itoh H, Uchino S, et al:
Immunohistochemical detection of K-sam protein in stomach cancer.
Clin Cancer Res. 2:1373–1381. 1996.PubMed/NCBI
|
|
30
|
Maeda K, Chung YS, Ogawa Y, et al:
Prognostic value of vascular endothelial growth factor expression
in gastric carcinoma. Cancer. 77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishimoto N: Identification of
clinicopathological and molecular prognostic factors in patients of
gastric cancer by multivariate analyses. Hiroshima J Med Sci.
49:171–181. 2001.(In Japanese).
|
|
32
|
Wu CW, Chi CW, Su TL, Liu TY, Lui WY and
P’eng FK: Serum hepatocyte growth factor level associate with
gastric cancer progression. Anticancer Res. 18:3657–3659.
1998.PubMed/NCBI
|
|
33
|
Han SU, Lee JH, Kim WH, Cho YK and Kim MW:
Significant correlation between serum level of hepatocyte growth
factor and progression of gastric carcinoma. World J Surg.
23:1176–1180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taniguchi T, Kitamura M, Arai K, Iwasaki
Y, Yamamoto Y, Igari A and Toi M: Increase in the circulating level
of hepatocyte growth factor in gastric cancer patients. Br J
Cancer. 75:673–677. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niki M, Okajima K, Isozaki H, et al:
Clinical evaluation of preoperative measurement of the serum human
hepatocyte growth factor levels in patients of gastric cancer. Jpn
J Gastroenterol Surg. 28:2139–2144. 1995.(In Japanese). View Article : Google Scholar
|
|
36
|
Ide T, Kitajima Y, Miyoshi A, et al:
Tumor-stromal cell interaction under hypoxia increases the
invasiveness of pancreatic cancer cells through the hepatocyte
growth factor/c-Met pathway. Int J Cancer. 119:2750–2759. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang WG, Martin TA, Parr C, Davies G,
Matsumoto K and Nakamura T: Hepatocyte growth factor, its receptor,
and their potential value in cancer therapies. Crit Rev Oncol
Hematol. 53:35–69. 2005. View Article : Google Scholar
|
|
38
|
Catenacci DV, Cervantes G, Yala S, et al:
RON (MST1R) is a novel prognostic marker and therapeutic target for
gastroesophageal adenocarcinoma. Cancer Biol Ther. 12:9–46. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Chen CQ, He YL, Cai SR, Yang DJ, et
al: Abnormal expression of e-cadherin in tumor cells is associated
with poor prognosis of gastric carcinoma. J Surg Oncol.
106:304–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taniguchi K, Yonemura Y, Nojima N, et al:
The relation between the growth patterns of gastric carcinoma and
the expression of hepatocyte growth factor receptor (c-met),
autocrine motility factor receptor, and urokinase-type plasminogen
activator receptor. Cancer. 82:2112–2122. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakajima M, Sawada H, Yamada Y, et al: The
prognostic significance of amplification and overexpression of
c-met and c-erb B-2 in human gastric carcinomas. Cancer.
85:1894–1902. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang TJ, Wang JY, Lin SR, Lian ST and
Hsieh JS: Overexpression of the c-met protooncogene in human
gastric carcinoma: Correlation to clinical features. Acta Oncol.
40:638–643. 2001. View Article : Google Scholar
|
|
43
|
Kubicka S, Claas C, Staab S, et al: p53
mutation pattern and expression of c-erbB2 and c-met in gastric
cancer: relation to histological subtypes, Helicobacter pylori
infection, and prognosis. Dig Dis Sci. 47:114–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han SU, Lee HY, Lee JH, et al: Modulation
of E-cadherin by hepatocyte growth factor induces aggressiveness of
gastric carcinoma. Ann Surg. 242:676–683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Drebber U, Baldus SE, Nolden B, et al: The
overexpression of c-met as a prognostic indicator for gastric
carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep.
19:1477–1483. 2008.PubMed/NCBI
|
|
46
|
Zhao J, Zhang X and Xin Y: Up-regulated
expression of Ezrin and c-Met proteins are related to the
metastasis and prognosis of gastric carcinomas. Histol Histopathol.
26:1111–1120. 2011.PubMed/NCBI
|
|
47
|
Iveson T, Donehower RC, Davidenko I, et
al: Rilotumumab in combination with epirubicin, cisplatin, and
capecitabine as first-line treatment for gastric or
oesophagogastric junction adenocarcinoma: an open-label, dose
de-escalation phase 1b study and a double-blind, randomised phase 2
study. Lancet Oncol. 15:1007–1018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Davidenko I, Iveson T, Donehower RC, et
al: Updated efficacy, biomarker, and exposure-response data form a
phase 2 study of rilotumumab (R) plus epirubicin, cisplatin, and
capecitabine (ECX) in gastric (G) or esophagogastric junction (EGJ)
cancer. In: European Society for Medical Oncology Congress; Vienna.
pp. 687P2012
|
|
49
|
Yamada Yasuhide: Molecular therapy for
gastric cancer. Chin Clin Oncol. 2:52013.
|