Introduction
Umbilical cord mesenchymal stem cells (UC-MSCs) can
be easily isolated from the umbilical cord and expanded in
vitro, and are widely used in stem cells therapy (1,2).
However, the mechanisms behind their therapeutic benefits remain to
be elucidated. Initially, the promising effects of UC-MSCs were
based on their multipotent differentiation ability or paracrine
effects (3), however, the
retention of MSCs is poor, and their low survival rates in injured
tissues reduces their therapeutic effects (4). This suggests that the paracrine
effect of MSCs may be important in the replacement of damaged cells
(5–9). Therefore, it is essential to identify
strategies, which can enhance the effectiveness of MSC-based
therapies, which requires elucidation of the molecular pathways
responsible for MSC-mediated tissue repair.
The mechanisms by which the paracrine effects of
MSCs contribute to their therapeutic effects are at present,
unclear. It has been suggested that paracrine factors may mediate
regeneration via the activation and recruitment of
resident/circulating stem cells and progenitor cells to the site of
injury, where they collaborate to heal damaged tissues (10,11).
A number of studies have demonstrated that stromal cell-derived
factor-1 (SDF-1) is critical for stem/progenitor cell migration.
For example, the SDF-1/C-X-C chemokine receptor 4 (CXCR4) axis has
been reported to promote the recruitment of progenitor cells and
CXCR4-positive cells to lesions in the heart and brain (12,13).
Hepatocyte growth factor (HGF) is a chemokine, which exhibits
chemoattractive properties via interactions with its receptor
c-met, which can induce the proliferation and migration of
epithelial cells and MSCs (14,15).
Monocyte chemoattractant protein-1 (MCP-1) is a potent
chemoattractant, which recruits MSCs and induces the proliferation
of fibroblasts (16,17). However, the paracrine actions of
UC-MSCs remain to be elucidated. In particular, the involvement of
the SDF-1/CXCR4, MCP-1/C-C chemokine receptor 2 (CCR2) and
HGF/c-met axes in the therapeutic effects of MSCs as
chemoattractants has not been investigated. Several studies have
demonstrated that circulating MSCs are attracted to sites of
damage, where they undergo tissue-specific differentiation
(18). Progenitor cells possess
the capacity to differentiate into endothelial cells and are
considered to be relevant in revascularization (19). Fibroblasts are the predominant type
of stromal cell in tissues, and they contribute to scar healing in
injured tissues (20,21). Therefore, the present study
hypothesized that an increase in the level of paracrine factors
secreted from UC-MSCs in injured tissue may promote the recruitment
of circulating mesenchymal stem and progenitor cells to the injured
tissue.
Materials and methods
Isolation and culture of cells
The present study was approved by the ethics
committee of the West China Second University Hospital (Chengdu,
China). Umbilical cords were collected from patients who had
undergone full-term cesarean-section (n=5, 26–31 years old) with
their written informed consent at the West China Second University
Hospital. UC-MSCs were isolated, as described previously, with
certain modifications (1).
Briefly, the umbilical cords were sterilized by immersion in 1%
povidone-iodine (Sichuan Kelun Pharmaceutical Co., Ltd., Chengdu,
China) for 2 min and were rinsed three times with
phosphate-buffered saline (PBS; GE Healthcare Life Sciences, Logan,
UT, USA). Wharton’s jelly was cut into 30–40 small sections (2–5
mm) and was cultured in 5% CO2 at 37°C in Dulbecco’s
modified Eagle’s medium (DMEM; Basalmedia Technologies Co., Ltd.,
Shanghai, China) supplemented with 10% fetal bovine serum (FBS; GE
Healthcare Life Sciences), 100 U/ml penicillin G and 100 mg/ml
streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA). At
75% confluence, the cells were passaged with 0.25% trypsin (GE
Healthcare Sciences). The medium was replaced every 3 days. To
isolate the HUVECs, the cord vein was flushed with PBS and digested
with 100 mg/ml collagenase (Sigma-Aldrich, St. Louis, MO, USA) at
37°C for 15 min. The cells, which were isolated from the cord
veins, were cultured in endothelial growth media-2 (Lonza Group
Ltd., Basel, Switzerland) with 100 U/ml penicillin G and 100 mg/ml
streptomycin, at 37°C and 5% CO2. The medium was
replaced every 3 days. The fibroblasts used in the present study
were obtained from Dr J Chen (Cobaxer Biotechnology Co., Ltd.,
Chengdu, China). The cells were cultured in high-glucose DMEM
(Basalmedia Technologies Co., Ltd.) supplemented with 10% FBS, 100
U/ml penicillin G, 100 mg/ml streptomycin and 3 ng/ml basic
fibroblast growth factor (FGF; Invitrogen Life Technologies) and
were maintained at 37°C with 5% CO2. As the positive
control of expression of the CXCR4 and CCR2 genes, CD3-activated
peripheral blood mononuclear cells were kindly provided by Dr J
Chen (Cobaxer Biotechnology). 1×107 PBMCs were isolated
and cultured in RPMI 1640 medium (Basalmedia Technologies Co.,
Ltd.) containing 10% FBS and stimulated by 100 ng/ml mouse
anti-human CD3 monoclonal antibody (cat. no. 317315, BioLegend,
Inc., San Diego, CA, USA) and 100 IU/ml IL-2 (Invitrogen Life
Technologies). Fresh RPMI 1640 medium was added every 2 days.
Preparation of UC-CM
In order to obtain the UC-CM, UC-MSCs at passage
four were seeded at a density of 10,000 cells/cm2. At
80% confluence, the cells were washed three times with PBS and the
media were replaced with serum-free DMEM. After 72 h, the media
were centrifuged (Eppendorf, Hauppauge, NY, USA) at 300 × g for 5
min, filtered through a 0.22 μm filter (Pall Corporation,
Port Washington, NY, USA) and were then stored at −70°C until use.
For the in vivo assays the conditioned media were
concentrated 10-fold using an ultrafiltration membrane with a
molecular weight cut-off of 3 kDa (Pall Corporation, Port
Washington, NY, USA).
Growth factor assays
To analyze the types and levels of the accumulated
factors and cytokines released by the UC-MSCs, the conditioned
media were analyzed using ELISA and liquid chip assays. The levels
of insulin-like growth factor (IGF)-1, HGF, SDF-1, interleukin
(IL)-8, brain-derived neurotrophic factor (BDNF), vascular cell
adhesion protein (VCAM)-1 and transforming growth factor (TGF)-β in
UC-CM were measured using ELISA kits (Human IGF-1 ELISA, human BDNF
ELISA, human TGF-β ELISA, RayBiotech, Inc., Norcross, GA, USA; and
human CXCL12/SDF-1α quantikine ELISA kit, human HGF quantikine
ELISA kit, human VCAM-1 quantikine ELISA kit, R&D Systems,
Inc., Minneapolis, MN, USA). Briefly, 200 μl UC-CM or
serum-free DMEM was added to 96-well plates coated with monoclonal
antibodies specific to the factor of interest, and the plates were
incubated at 4°C for 3 h. Subsequent to washing with PBS, the
antibodies were added to each well, incubated for 1 h at 4°C, and
washed with wash buffer (PBS with 0.05% Tween-20). Substrate
solution (3,3′,5,5′-tetramethylbenzidine) was then added, followed
by stop solution (0.16 M sulfuric acid) after 45 min. The
concentrations of cytokines and growth factors were calculated by
measuring the absorbance at 450 nm using a microplate reader
(Multiskan; Thermo Fisher Scientific, Waltham, MA, USA). The levels
of stem cell factor, epidermal growth factor, FGF-2, TGF-α, IL-10,
platelet-derived growth factor-BB (PDGF-BB), interferon-inducible
protein-10, MCP-1 and vascular endothelial growth factor (VEGF)
were detected using liquid chip kits (Human Cytokine Magnetic kit;
EMD Millipore, Billerica, MA, USA) and the BeadXpress Reader system
(Illumina, Inc., San Diego, CA, USA), according to the
manufacturer’s instructions.
Tube formation assay
Tube formation was assessed, as described previously
(22) with certain modifications
using an in vitro angiogenesis assay kit (EMD Millipore).
The HUVECs and UC-MSCs (3×105 cells/well) were incubated
in 24-well plates coated with Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA) for 12 h in serum-free DMEM or UC-CM. Image J
version 1.45S software (National Institutes of Health, Bethseda,
MA, USA) was then used to measure the total tube length on the
captured images (magnification, ×40) by microscopy (CKX31; Olympus
Corporation, Tokyo, Japan).
In vivo migration assay
To investigate the chemotactic properties of UC-CM,
in vivo migration models were constructed, using stem cells
and other progenitor cells as targets to identify UC-CM-induced
cell migration. All animal experiments were performed in accordance
with the ethics committee of the West China Second University
Hospital. A total of 60 male 10-week-old C57BL/6 mice (weighing
25–30 mg; Experimental Animal Center of Sichuan University,
Chengdu, China) were maintained in an artificially ventilated
environment (temperature, 20–26°C; light intensity, 180–300 lux),
and were fed palatable and uncontaminated diets ad libitum.
The mice were anesthetized with 10% chloral hydrate (Tokyo Chemical
Industry Co., Ltd., Tokyo, Japan) (0.1 ml/10 g). A total of 300
μl ice-cold growth factor-reduced Matrigel was combined with
200 μl concentrated UC-CM or DMEM as a control, which was
subcutaneously injected into the left side of each mouse’s back
using an insulin syringe fitted with a 23G needle (BD Biosciences)
(n= 5). The injections were performed slowly, allowing the Matrigel
to polymerize and form a jelly-like implant under the skin. Prior
to cell implantation, the cultured fibroblasts, HUVECs and UC-MSCs
were detached using 0.25% trypsin, and stained with PKH26
(Sigma-Aldrich). The fibroblasts, HUVECs and UC-MSCs were diluted
(1×106 cells/100 μl saline) 2 h following
Matrigel implantation, and were then subcutaneously injected into
the 1 cm area surrounding the Matrigel implants.
Immunohistochemistry
To quantify the cell migration into the Matrigel
implants, the mice were sacrificed by isoflurane inhalation
(Sichuan Kelun Pharmaceutical Co., Ltd.) 8 and 16 days subsequent
to injection. The whole Matrigel was then isolated and fixed in 4%
paraformaldehyde (Sigma-Aldrich) overnight, followed by 30%
sucrose/phosphate buffer (Sigma-Aldrich) for 24 h, prior to being
embedded in optimum cutting temperature medium (Sakura Finetek
Europe B.V., Alphen aan den Rijn, The Netherlands). The frozen
Matrigel was cut into 10 mm sections using a cryostat (LEICA
CM3050S; Leica Microsystems Inc., Buffalo Grove, IL, USA), and
directly photographed (magnification, ×40) by fluorescence
microscopy (DMI3000 B; Leica Microsystems, Inc.). The total numbers
of migrated cells were then counted in three randomly selected
fields.
Flow cytometry
Fibroblasts, HUVECs and UC-MSCs were harvested using
0.25% trypsin, washed and resuspended in PBS containing 1% bovine
serum albumin (BSA; Sigma-Aldrich). Cells were stained with
PerCP/Cy5.5-conjugated mouse anti-human CCR2 (cat. no. 335303;
BioLegend, Inc.), PE-conjugated mouse anti-human CXCR4 (cat. no.
306505; BioLegend, Inc.) and fluorescein isothiocyanate-labeled
mouse anti-human c-met (cat. no. 11-8858; eBioscience, Inc., San
Diego, CA, USA), according to the manufacturer’s instructions. The
cells were then analyzed using flow cytometry (Gallios; Beckman
Coulter, Brea, CA, USA) and FlowJo software, version 7.6 (FlowJo,
LLC, Ashland, OR, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using an RNeasy mini kit
(Qiagen, Shanghai, China), according to the manufacturer’s
instructions. The RNA was incubated with DNase I (Invitrogen Life
Technologies) in order to eliminate any genomic DNA contamination.
The total RNA was then reverse transcribed using the SuperScript
III First-Strand Synthesis kit (Invitrogen Life Technologies). cDNA
was analyzed by PCR using 20 ng cDNA in a 50 μl reaction
volume containing primers and Ex-Taq DNA polymerase (Takara
Biotechnology Co., Ltd., Dalian, China). The PCR conditions
included 32 cycles of 94°C for 60 sec, 58°C for 60 sec and 72°C for
90 sec. GAPDH was used as the housekeeping control gene. The
following primers (Invitrogen Life Technologies) were used
(14,23): CXCR4, forward
ATGGAGGGGATCAGTATATACAC and reverse TGGAGTGTGCTATGTTGGCGTCT; c-met,
forward GGGTCGCTTCATGCAGGTTGTGGT and reverse
ATGGTCAGCCTTGTCCCTCCTTCA; CCR2, forward CCAACGAGAGCGGTGAAGAAGTC and
reverse TCCGCCAAAATAACCGATGTGAT; GAPDH, forward
GCCAAGGTCATCCATGACAACTTTGG and reverse
GCCTGCTTCACCACCTTCTTGATGTC.
Chemoinvasion assay
A chemoinvasion assay was performed to evaluate the
ability of cells to cross a Matrigel membrane. The upper chambers,
with 8 mm pores, were coated with 50 μl Matrigel diluted
1:10 (v:v) in DMEM and were incubated at 37°C for 4 h. The lower
chambers contained either DMEM supplemented with 1% BSA as a
control or UC-CM. For specific factor blocking assays, 20
μg/ml each of the monoclonal mouse anti-human anti-SDF-1
(cat. no. MAB350; R&D Systems, Inc.), anti-MCP-1 (cat. no.
16-7096; eBioscience, Inc.) and anti-HGF (eBioscience, Inc.)
antibodies were added to the lower chambers. The fibroblasts,
HUVECs and UC-MSCs were prepared in DMEM supplemented with 1% BSA,
and 5×104 cells in 0.5 ml suspension were added to each
upper chamber. Each experiment was performed in triplicate. The
chambers were placed in a 24-well plate and were incubated at 37°C,
with 5% CO2 for 24 h. The cells, which had not crossed
the membrane were removed with a wet cotton bud. The undersides of
the filters were then fixed in methanol (Sigma-Aldrich) for 10 min
and stained with 0.1% crystal violent (Sigma-Aldrich), and images
of the cells, which had invaded to the underside of the insert were
captured. Three random fields were selected (magnification, ×40) by
microscopy (CKX31; Olympus Corporation) and counted.
Scratch healing assay
A 24-well plate was coated with 8 mg/cm2
collagen I (Sigma-Aldrich) for 2 h at 37°C, excess fluid was
removed from the coated surface and the plate was dried overnight.
Following this, fibroblasts, HUVECs and UC-MSCs were incubated in
pre-coated plates (2×105 cells/well) and individually
maintained at 37°C with 5% CO2 for 24 h in serum-free
DMEM or UC-CM. A yellow pipette tip was then used to scratch the
confluent monolayers. The media were replaced with fresh medium and
the scratch was analyzed after 6 h using ImageJ software.
Statistical analyses
Data are expressed as the mean ± standard error of
the mean. Statistical comparisons were performed using Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference. One-way analysis of variance with
Bonferroni’s post hoc test was used to compare the migration of
cells in vivo. *P<0.05, **P<0.01
and ***P<0.001 vs. control group.
Results
Cytokine release from the UC-MSCs
To determine which migratory and angiogenic factors
were secreted by the UC-MSCs, the cytokine content of UC-CM was
measured using ELISA and liquid chip assays (Table I). Compared with the fibroblasts,
the UC-MSCs expressed markedly increased levels of chemoattractant
factors, including SDF-1, MCP-1, TGF-β, PDGF-BB, VEGF, VCAM-1 and
MCP-1. In particular, the levels of SDF-1, MCP-1 and HGF were
higher in the UC-MSCs compared with the fibroblasts. In addition,
UC-CM contained significantly increased levels of several
angiogenic factors, including IL-8, IGF-1 and VEGF. However, IL-10,
an immunoregulatory factor, was not detected in the UC-CM.
 | Table ICytokines and growth factor levels
present in conditioned medium derived from UC-MSCs and
fibroblasts. |
Table I
Cytokines and growth factor levels
present in conditioned medium derived from UC-MSCs and
fibroblasts.
| Cytokine | Assay | Conditioned medium
(pg/ml)
|
|---|
| UC-MSC (n=3) | Fibroblast
(n=3) |
|---|
| BDNF | ELISA |
13,900.25±2156.17 | ND |
| SDF-1 | ELISA |
770.63±45.36a | 74.44±8.23 |
| IGF | ELISA |
871.28±80.29a | 27±11.43 |
| VCAM-1 | ELISA | 549.44±63.32 | N/A |
| TGF-β | ELISA |
4,330.36±798.19a |
1,605.86±335.36 |
| HGF | ELISA | 643.05±31.91 | N/A |
| VEGF | LC | 224.06±47.42 |
340.75±117.09c |
| EGF | LC | <5.40±0.00 | <3.60±0.00 |
| FGF-2 | LC | 59.55±13.64b | 28.90±9.15 |
| PDGF-BB | LC | 38.05±9.05b | 29.10±12.21 |
| IL-10 | LC | <4.00±0.00 | 1.66±0.60 |
| IL-8 | LC |
1,444.60±225.33a | 285.61±172.00 |
| IP-10 | LC | 34.80±6.19 | 36.40±15.17 |
| TGF-α | LC | <0.4±0.00 | ND |
| MCP-1 | LC |
13,038.81±1134.06a | 914.23±213.06 |
| SCF | LC | <1.25±0.00 | ND |
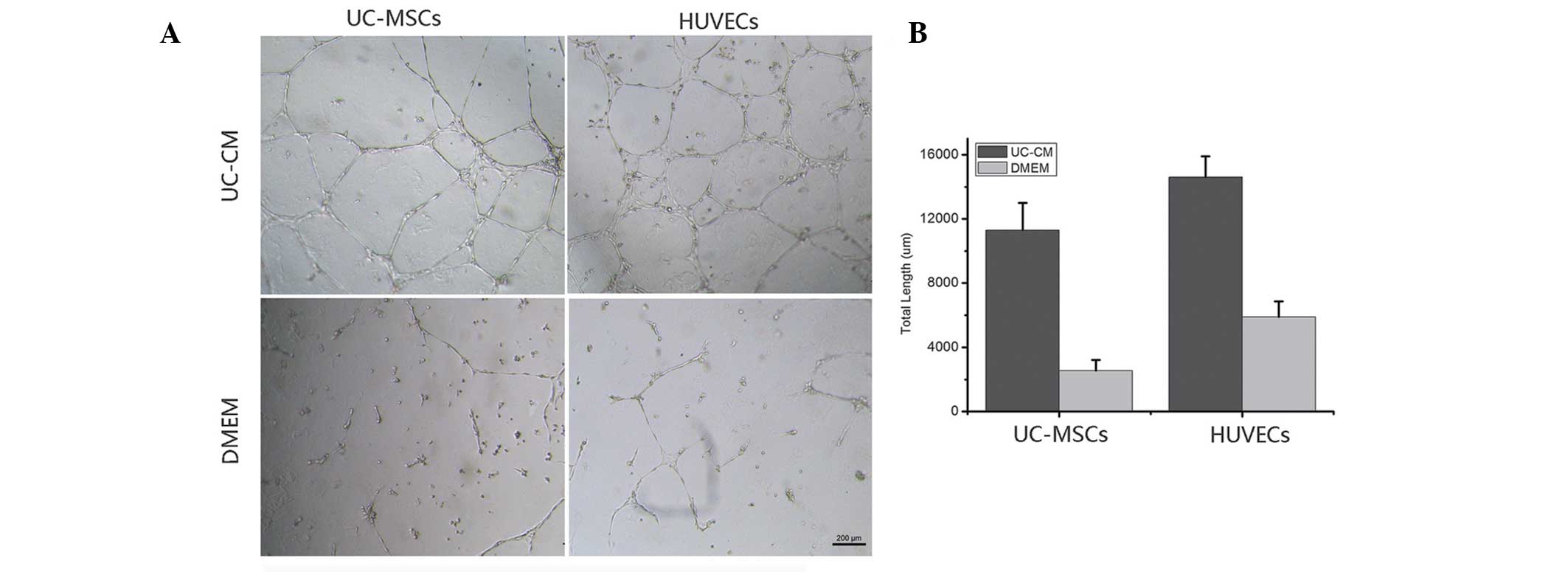
UC-CM enhances angiogenesis in vitro
To investigate the angiogenic effects of UC-CM, a
tube formation assay was performed to form vascular networks. The
UC-MSC and HUVEC tube formations were then quantified by counting
the total length of the formed networks (Fig. 1). The UC-MSCs and HUVECs grown in
DMEM only (control) did not form complex tubular structures,
whereas the cells cultured in UC-CM formed tubules and tubular
rings. The total length of the tubes was significantly increased in
the UC-MSCs and HUVECs incubated with UC-CM compared with those
incubated with DMEM. The UC-CM stimulated the formation of HUVEC
tubular networks as early as 4 h following seeding onto the matrix,
and the structures were maintained for a minimum of 36 h. The UC-CM
was less efficient at stimulating the growth of UC-MSC tubular
structures, which were visible after 10 h and lasted for 24 h.
UC-CM increases the in vivo migration of
transplanted cells
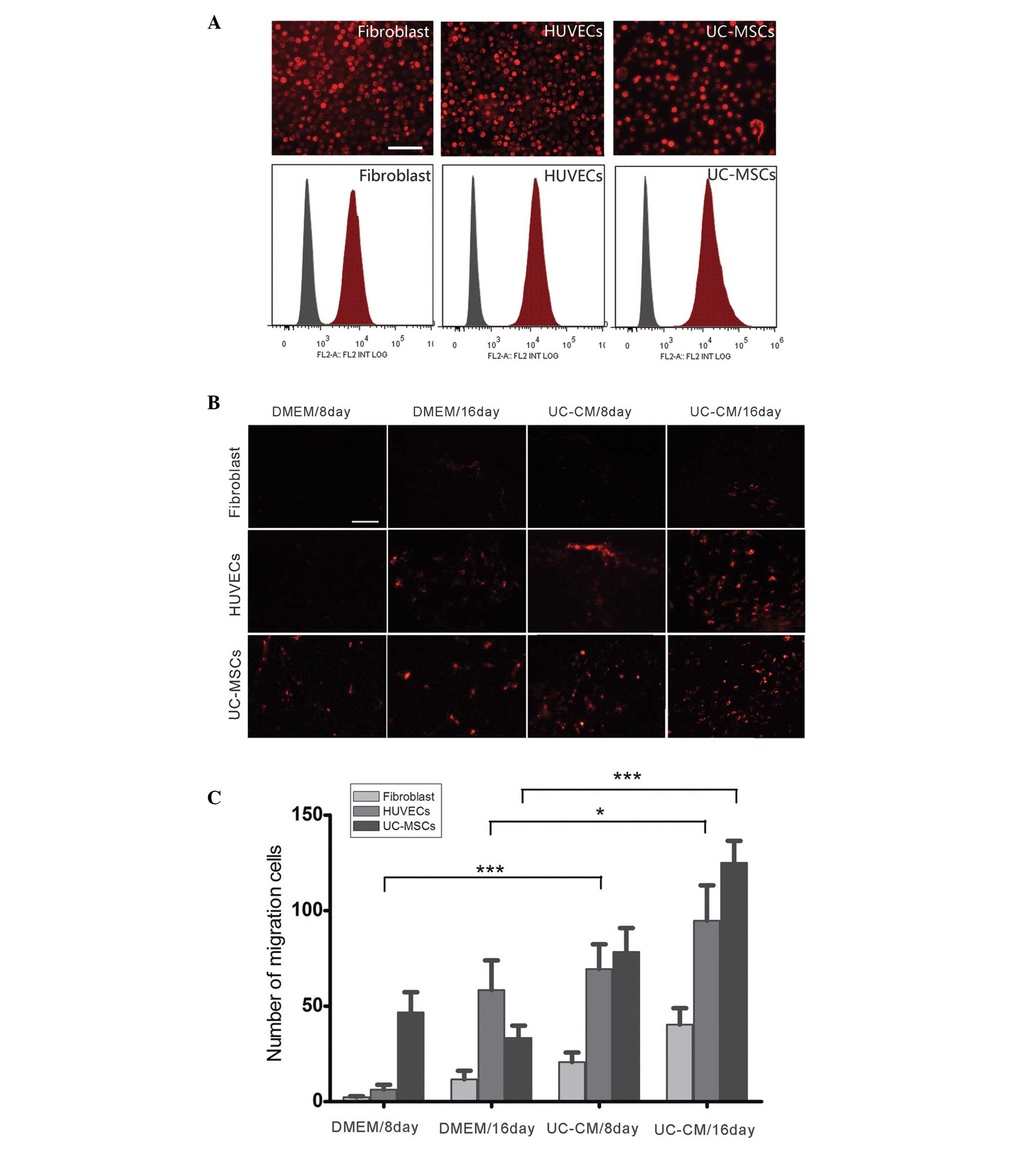
To investigate the ability of UC-CM to attract
UC-MSCs, HUVECs and fibroblasts in vivo, the recruitment of
cells into a Matrigel implant was analyzed in C57BL/6 mice. Flow
cytometry and fluorescent microscopy (Fig. 2A) demonstrated that >95% of the
UC-MSCs, HUVECs and fibroblasts were labeled with PKH26. The
abilities of the transplanted cells to invade through the Matrigel
in response to cytokines in the UC-CM were then assessed (Fig. 2B). At day 8 following implantation,
the UC-MSCs were only detected in the implants containing DMEM
[48±11 cells/high power field (HPF)]and UC-CM (80±14 cells/HPF).
HUVECs were detected in Matrigel containing UC-CM at day 8, but not
in the Matrigel containing DMEM. Starting from day 16, the number
of HUVECs inside the implants increased significantly when induced
by UC-CM (93±8 cells/HPF) compared with the DMEM-induced migration
(61±10 cells/HPF; P<0.05). By contrast, the UC-CM did not
significantly increase the invasive ability of fibroblasts compared
with DMEM on days 8 or 16. The increased UC-MSC migration in
response to UC-CM (127±9 cells/HPF) was significantly greater
compared with that observed with DMEM (38±6 cells/HPF; P<0.001;
Fig. 2C). These findings suggested
that UC-CM affected the local microenvironment, which facilitated
the migration of resident stem/progenitor cells in response to the
chemoattractants and may reinforce tissue repair.
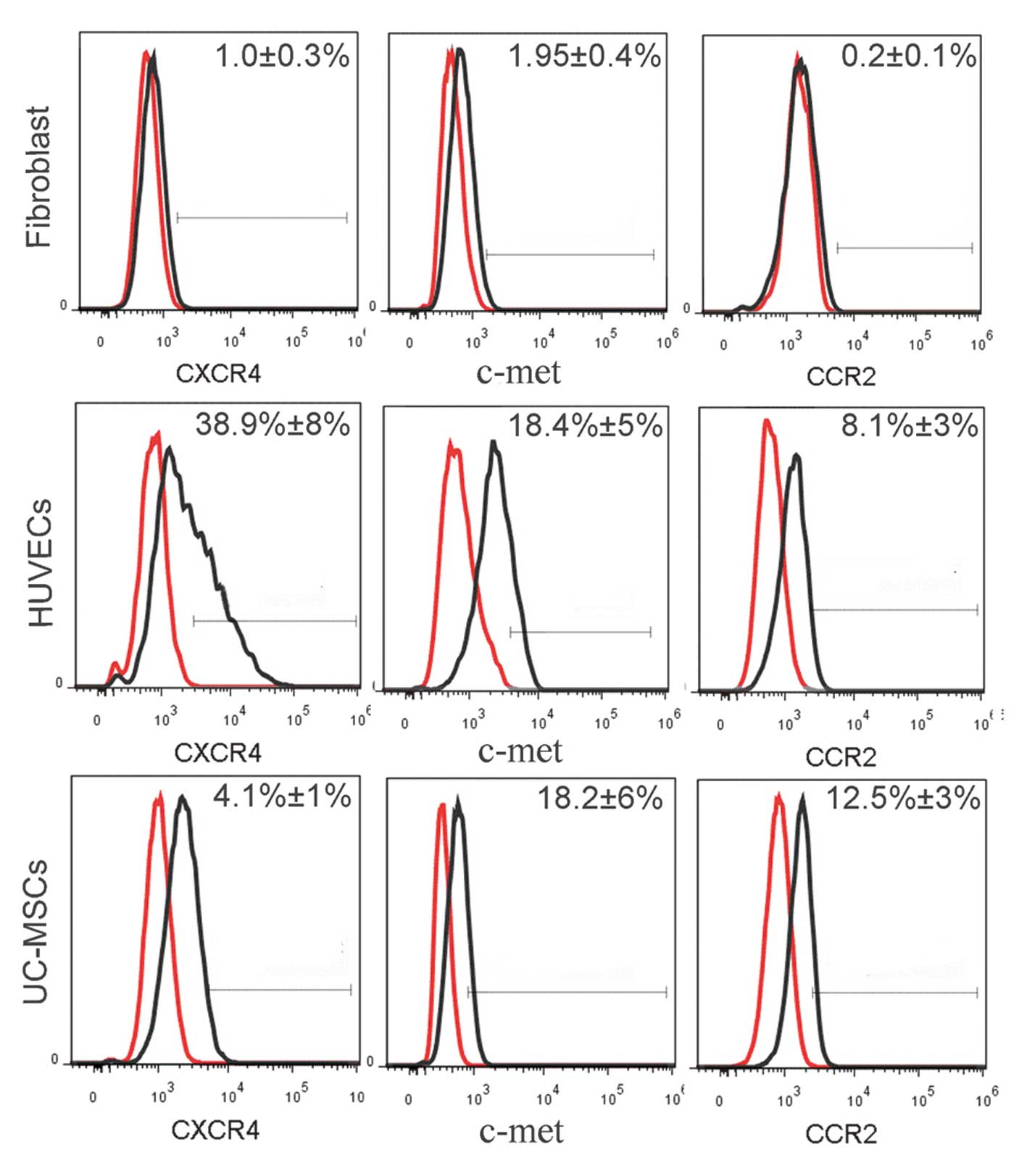
Expression of CXCR4, CCR2 and c-met
receptors in the UC-MSCs, HUVECs and fibroblasts
The in vivo migration assay demonstrated that
the UC-CM contributed to the recruitment of transplanted cells. To
investigate the effect of the SDF-1/CXCR4, MCP-1/CCR2 and HGF/c-met
axes on the migration of UC-MSCs, HUVECs and fibroblasts, the
expression levels of the CXCR4, CCR2 and c-met receptors were
measured (Fig. 3). The GAPDH gene
was used as an internal control for the expression of mRNA. The
expression of CXCR4 was significantly higher in the HUVECs compared
with the UC-MSCs, and was not detected in the fibroblasts. RT-qPCR
demonstrated that the expression of c-met was positive in the
UC-MSCs, HUVECs and fibroblasts. By contrast, the expression of
CCR2 was positive in the UC-MSCs and HUVECs, but negative in the
fibroblasts. These results were confirmed using flow cytometry
(Fig. 4). The data collected
indicated that 38.9±8% of the HUVECs expressed CXCR4, which was
10-fold higher compared with the UC-MSCs. In addition, >18% of
the UC-MSCs and HUVECs expressed c-met, although the fibroblasts
expressed significantly lower levels compared with the UC-MSCs. A
total of 12.5±3% of the UC-MSCs and 8.1±3% of the HUVECs expressed
CCR2, however, this receptor was almost undetectable in the
fibroblasts.
 | Figure 3Reverse transcription-quantitative
polymerase chain reaction of the expression levels of CXCR4, c-met
and CCR2. Lane 1, CD3-activated PBMCs; lane 2, fibroblasts; lane 3,
UC-MSCs; lane 4, HUVECs. CXCR4, C-X-C chemokine receptor 4; CCR2,
C-C chemokine receptor 2; PBMCs, peripheral blood mononuclear
cells; UC-MSCs, umbilical cord mesenchymal stem cells; HUVECs,
human umbilical vein endothelial cells. |
UC-CM increases the migratory capacity of
cells
A migration assay was used to investigate the role
of the cytokines in UC-CM in promoting cell migration and to
determine whether the cells receptors were involved. The migration
of UC-MSCs, HUVECs and fibroblasts from the upper chamber across
the membrane was significantly higher in the UC-CM group compared
with the DMEM group (Fig. 5A and
B). As the investigation identified the expression of CXCR4,
CCR2 and c-met, receptors involved in cell migration toward SDF-1,
MCP-1 and HGF, on the cell surface, an antibody-based blocking
assay was performed. The UC-CM significantly increased the
migration of HUVECs, which was blocked by the anti-SDF-1
(P<0.001), anti-MCP-1 (P<0.001) and anti-HGF antibodies
(P<0.001). The UC-CM-induced migration of UC-MSCs was almost
eradicated by blocking SDF-1 with an anti-SDF-1 antibody, or MCP-1
with an anti- MCP-1 antibody. These results suggested that SDF-1,
MCP-1 and HGF were involved in the UC-CM-induced migration of
HUVECs via the SDF-1-CXCR4, MCP-1-CCR2 and HGF-c-met axes. Similar
to the HUVECs, the SDF-1-CXCR4 and MCP-1-CCR2 axes may also be
involved in the migration of UC-MSCs. By contrast, no significant
alteration in migratory activity was observed in the fibroblasts in
response to the neutralized antibodies.
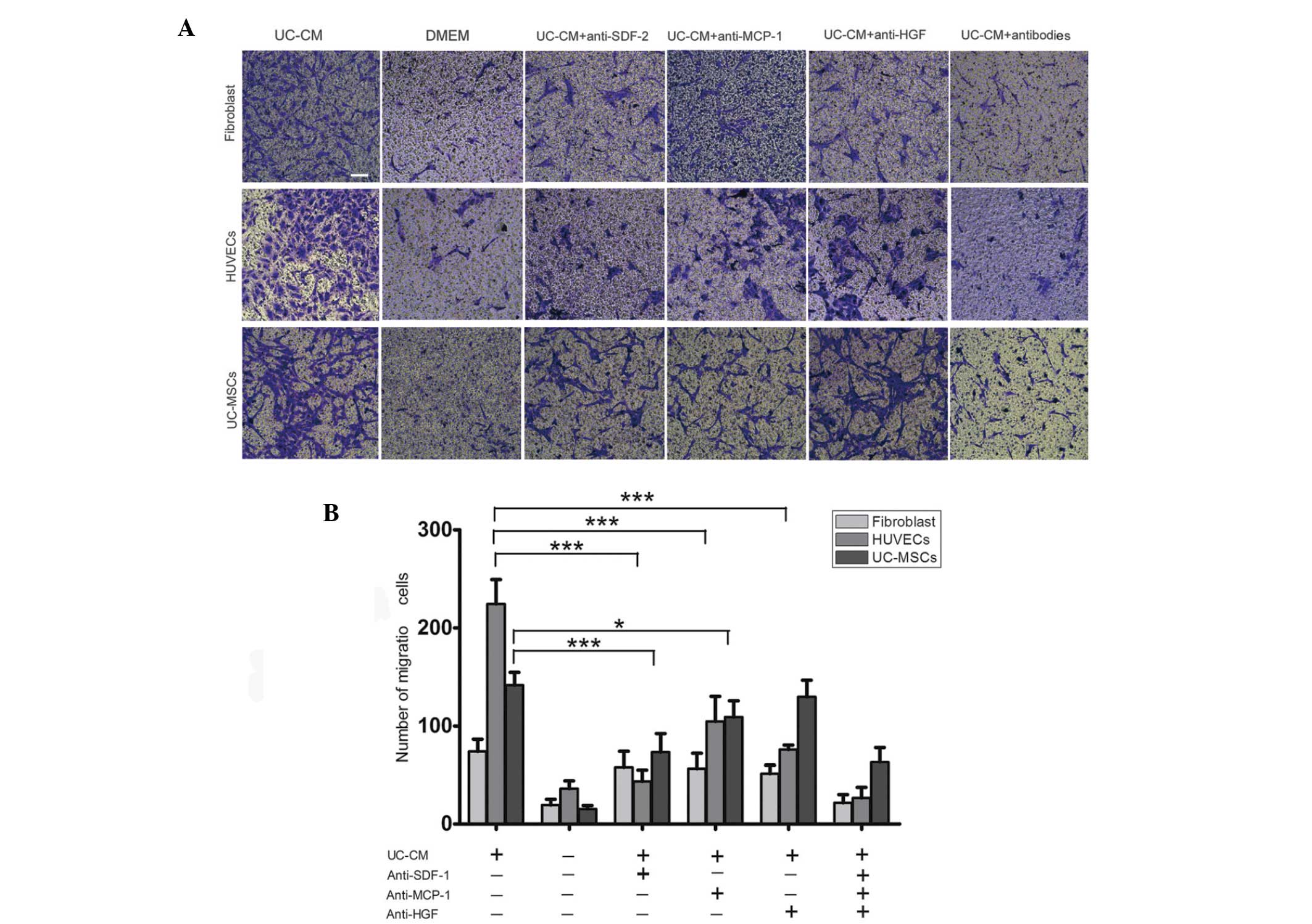 | Figure 5Migration of fibroblasts, HUVECs and
UC-MSCs in response to UC-CM. (A) A total of 5×104 cells
were collected and allowed to migrate. Lane 1, UC-CM; lane 2, DMEM;
lanes 3–6, in the presence or absence of anti-SDF-1 (20
μg/ml), anti-MCP-1 (20 μg/ml) or anti-HGF (20
μg/ml), respectively. Results are from a representative
experiment and are expressed as the mean number of migrated cells
in three random fields, scale bar=200 μm. Cells that crossed
the matrigel membrane were stained with crystal violet
(magnification, ×40). (B) Graphical presentation of the quantified
data, presented as the number of migrated cells and expressed as
the mean ± standard error of the mean. HUVECs, human umbilical vein
endothelial cells; UC-MSCs, umbilical cord mesenchymal stem cells;
UC-CM, UC-MSCs conditioned medium; DMEM, Dulbecco’s modified
Eagle’s medium; SDF-1, stromal cell-derived factor 1; MCP-1,
monocyte chemotactic protein 1; HGF, hepatocyte growth factor. |
Subsequently, a wound-healing assay was performed,
in which cell monolayers were scratched and cell growth and
migration were quantified (Fig.
6A). The results demonstrated that incubation with UC-CM
enhanced the migration of cells toward the wound, reducing its
surface area (Fig. 6B). The
ability of cells to migrate towards a cytokine gradient was
determined using antibody blocking assays. Notably, the fibroblasts
migrated in response to different concentrations of MCP-1 and HGF
in a dose-dependent manner (Fig.
6C). The UC-MSCs treated with cytokine antibodies exhibited
significantly reduced cell migration and wound recovery in response
to the inhibition of SDF-1, MCP-1 and HGF (P<0.01). In addition,
the UC-CM-induced migration of HUVECs was markedly inhibited in the
presence of the anti-SDF-1, anti-HGF or MCP-1 antibodies,
confirming that SDF-1, MCP-1 and HGF in the UC-CM were important
for cell proliferation and/or migration.
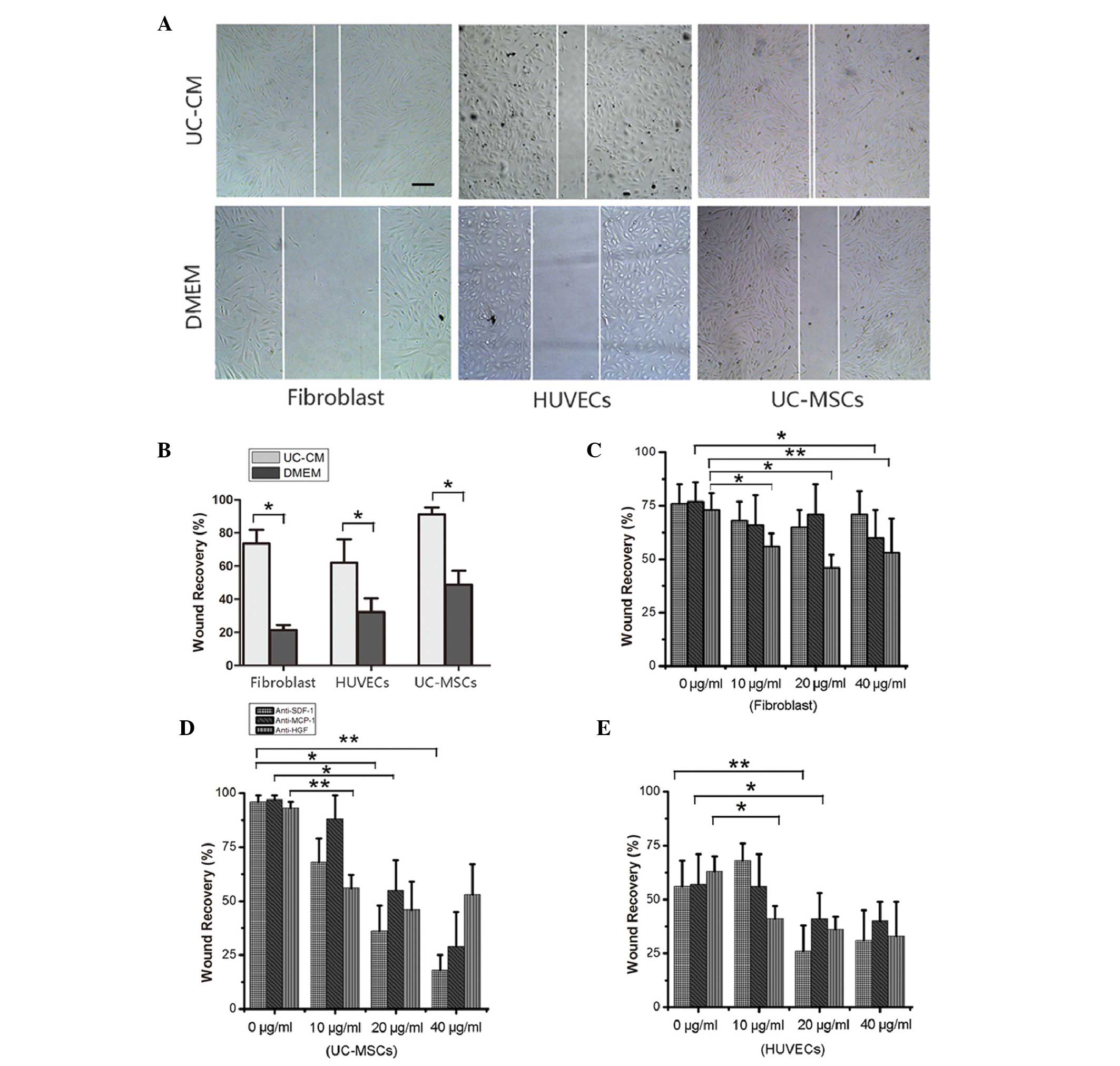 | Figure 6Cell migration analyzed using
wound-healing assays. (A) Representative images of in vitro
wound-healing assays in fibroblasts, HUVECs and UC-MSCs in the
presence of UC-CM, vs. DMEM. Scale bar=200 μm. (B)
Quantification of in vitro wound healing, There was a
significant increase in the wound closure in fibroblasts, HUVECs
and UC-MSCs exposed to UC-CM compared with DMEM at 6 h
(*P<0.05). The migration of (C) fibroblasts, (D)
UC-MSCs and (E) HUVECs in response to UC-CM were inhibited by
specific antibodies against known receptors. Anti-SDF-1, -MCP-1 and
-HGF antibodies were added to UC-CM at concentrations of 10, 20 and
40 μg/ml. A concentration-dependent reduction in cell
migration was observed. *P<0.05,
**P<0.01 and ***P<0.001, vs. UC-CM,
determined with analysis of variance followed by Bonferroni’s
post-hoc test (n=3 per group). Data are presented as the mean ±
standard error of the mean. HUVECs, human umbilical vein
endothelial cells; UC-MSCs, umbilical cord mesenchymal stem cells;
UC-CM, UC-MSCs conditioned medium; DMEM, Dulbecco’s modified
Eagle’s medium; SDF-1, stromal cell-derived factor 1; MCP-1,
monocyte chemotactic protein 1; HGF, hepatocyte growth factor. |
Discussion
The clinical application of UC-MSCs has been
reported for several diseases, and the paracrine effects of UC-MSCs
may contribute to these beneficial effects (24,25).
In the present study, in vitro experiments demonstrated that
UC-CM supported tube formation and stimulated the migration of
UC-MSCs and HUVECs. Therefore, CM harvested from UC-MSCs may
enhance the positive effects of cellular-based therapy. However,
the factors and mechanisms responsible for stimulating the
migration of cells towards wounded microenvironments, remain to be
fully elucidated. Tissue repair is a complex process, which
requires the collaboration of various factors and cells (26). It is likely that UC-CM contains
high levels of growth factors and chemokines, which may contribute
to a chemoattractive environment to circulating progenitor and stem
cells in adjacent tissues (27). A
previous study demonstrated that MSCs are likely to possess
chemotactic properties in injury tissue (28), and HUVECs are involved in blood
vessel remodeling (29).
Fibroblasts however, contribute to the maintenance and regeneration
of connective tissues (30). In
the present study, the expression levels of specific cell surface
receptors were assessed in UC-MSCs, HUVECs and fibroblasts, and the
cells were labeled with PKH26 for in vivo cell tracking and
chemoinvasion assays.
Previous studies have reported that UC-MSCs secrete
certain cytokines and factors (14,31–33),
similar to other stem cells. However, the relative expression
levels of these factors and the importance of UC-MSC-derived
cytokines in tissue repair remain to be elucidated. In the present
study, seven factors, known for their angiogenic and chemotactic
properties, were investigated (Table
I). The data revealed that UC-MSCs secreted significantly
increased the levels of IGF-1 (871±80 pg/ml), IL-8 (1,444±225
pg/ml) and HGF (643±31 pg/ml), however, markedly lower levels of
the two angiogenic factors, PDGF-BB (38.5±9 pg/ml) and FGF-2 (59±13
pg/ml) were observed. This suggested that IGF-1, IL-8 and HGF,
rather than PDGF-BB and FGF-2, may be responsible for the
angiogenic potential of UC-CM. Notably, the UC-MSCs produced higher
levels of BDNF (13,900±2156 pg/ml), which can enhance the growth,
differentiation and survival of neurons (34) This suggested additional potential
applications for UC-CM. SDF-1, MCP-1 and HGF can be isolated from
the UC-MSCs in large quantities compared with other chemotactic
factors in UC-CM. Several studies have demonstrated that SDF-1,
MCP-1 and HGF are able to induce the homing and migration of
various types of cells (14,35).
Therefore, it was hypothesized that SDF-1, MCP-1 and HGF may be key
regulators in UC-CM, and that growth factors and chemokines
secreted by the UC-MSCs injected into an injured area, attract
circulating progenitor/stem cells, which migrate and infiltratd
into the tissue and initiate regeneration (Fig. 7). The present study demonstrated
that SDF-1, MCP-1 and HGF were secreted by the UC-MSCs and were
able to mediate productive repair by recruiting reparative cells
with specific cell receptors. Specifically, UC-MSCs and HUVECs were
able to migrate in vitro and in vivo, in response to
chemotactic factors secreted by the UC-MSCs.
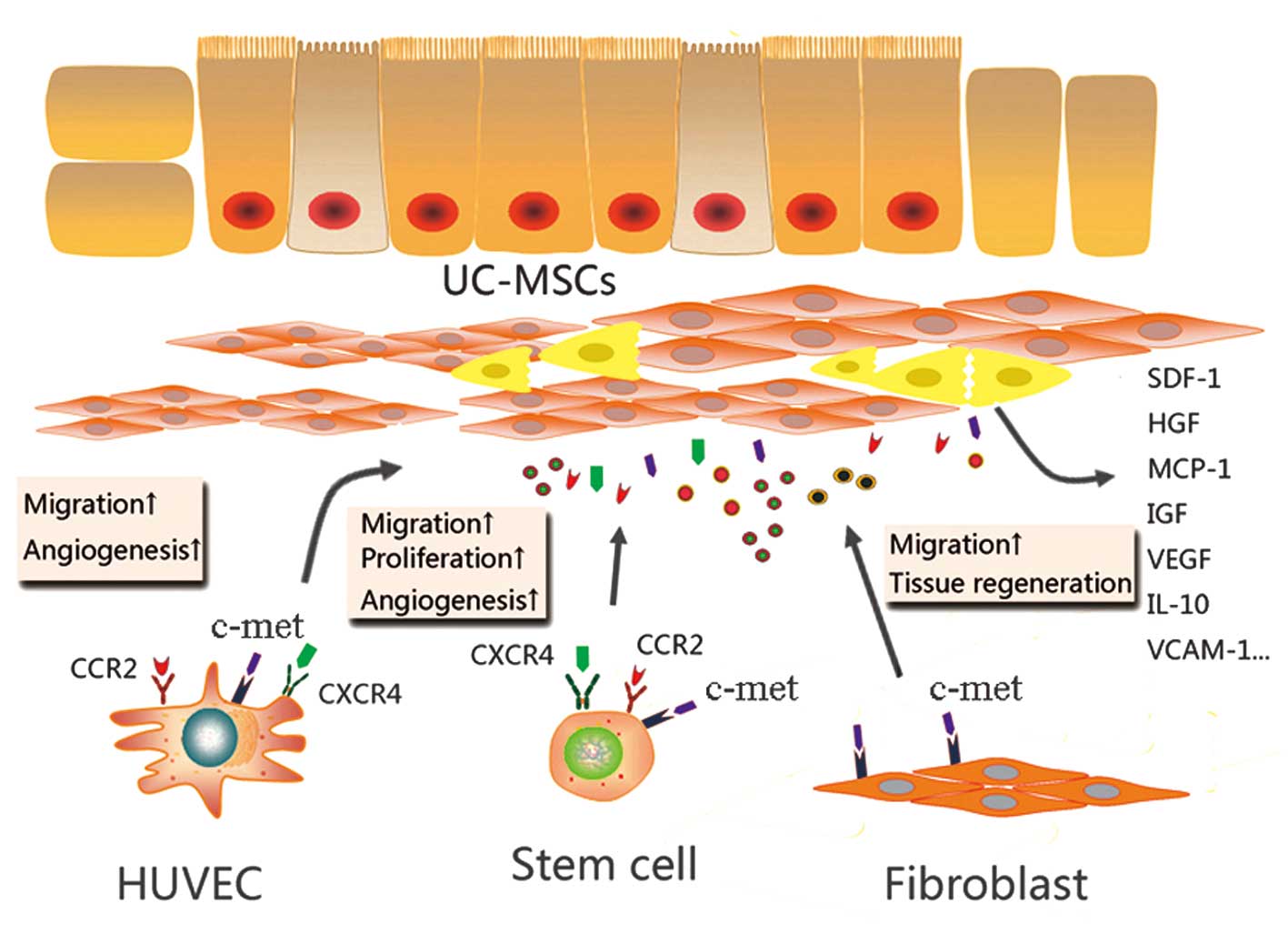 | Figure 7A model of the paracrine mechanisms
of UC-MSCs in tissue repair. In damaged tissues, UC-MSCs attract
stem/progenitor cells via paracrine activity involving SDF-1/CXCR4
and MCP-1/CCR2 interaction. Potent paracrine chemoattractant and
angiogenic factors affect the microenvironment by acting on
different cell types, leading to tissue repair and angiogenesis.
UC-MSCs, umbilical cord mesenchymal stem cells; SDF-1, stromal
cell-derived factor 1; CXCR4, C-X-C chemokine receptor 4; c-met,
MCP-1, monocyte chemotactic protein 1; HGF, hepatocyte growth
factor; CCR2, C-C chemokine receptor 2; HUVECs, human umbilical
vein endothelial cells; IGF, insulin-like growth factor; VEGF,
vascular endothelial growth factor; IL, interleukin; VCAM, vascular
cell adhesion protein. |
Based on the expression levels of CXCR4, the role of
the SDF-1-CXCR4 axis in chemotactic actions of UC-CM was analyzed
using a Matrigel migration assay. The UC-CM was incubated with
neutralizing antibodies against SDF-1, which suppressed the
chemotactic response of the HUVECs (P<0.001) and UC-MSCs
(P<0.01) to UC-CM (Fig. 5).
When the antibodies were added to inhibit the effects of the
SDF-1-CXCR4 axis in the wound-healing assay, the data revealed that
HUVECs exhibited a greater migratory ability in the presence of 20
μg/ml anti-SDF-1 (P<0.01) compared with the UC-MSCs
(P<0.05;Fig. 6), suggesting
that the SDF-1 in the UC-CM was responsible for chemotaxis.
Consistent with these results, the UC-MSCs and HUVECs expressed
detectable levels of CXCR4, as determined by flow cytometry and
RT-qPCR, wheras CXCR4 was not detected in the fibroblasts. SDF-1
stimulates the recruitment of progenitor cells to ischemic tissue
(32,36). The present study demonstrated that
SDF-1 not only induced the concentration-dependent migration of
UC-MSCs and HUVECs, but promoted cell proliferation.
MCP-1 is also important in cell migration. Previous
studies identified the expression of the MCP-1 receptor, CCR2, in
BM-MSCs, however, reports describing MSC migration in response to
MCP-1 are conflicting (33,37).
In the present study, assays were performed in the presence of an
MCP-1 neutralizing antibody. As expected, the numbers of migrated
UC-MSCs (P<0.05) and HUVECs (P<0.001) were significantly
reduced in the presence of the antibody. The wound-healing assay
confirmed these findings; the repair of scratch wounds in the
UC-MSCs and HUVECs was significantly slower following treatment
with 20 μg/ml anti-MCP-1. Therefore, it is likely that the
signal transduction pathways involved in MCP-1/CCR2-mediated cell
migration are cell type-specific, and that the expression of CCR2
on the cell surface was critical in this process.
HGF is a pleiotropic cytokine, which promotes
epithelial and endothelial cell proliferation and invasion through
the extracellular matrix (38,39).
The HGF-c-met axis is important in enhancing the engraftment of
MSCs in the injured heart (40).
To further investigate whether the cell migration was mediated by
HGF-c-met signaling, Matrigel migration and scratch wound healing
assays were performed. The chemotactic effects of UC-CM treated
with anti-HGF on the HUVECs were significantly inhibited compared
with the control (P<0.001). By contrast, the migration of
UC-MSCs and fibroblasts were equivalent to those of the control
(Fig. 5). Notably, the
extracellular expression of c-met was detected on the UC-MSCs and
HUVECs, but not on the fibroblasts (Figs. 3 and 4), suggesting that the HGF-c-met axis was
only responsible for the migration of HUVECs. In addition, the
scratch wound healing assay indicated that wound closure in the
UC-MSCs, HUVECs and fibroblasts was significantly slower in the
presence of anti-HGF antibodies. It was hypothesized that HGF
enhanced the rate of wound closure by promoting cell proliferation
(41,42).
The present study hypothesized that at least two
factors within the UC-CM induced chemotaxis. UC-CM may also attract
and enhance the proliferation of target cells and induce tube
formation. The antibody blocking experiment resulted in
significantly reduced cell migration compared with single antibody
experiments (Figs. 5 and 6). Therefore, the present study concluded
that UC-CM induced the migratory activity of cells via the
SDF-1/CXCR4 axis and via the binding of MCP-1 to CCR2. It is likely
that there are multiple complex paracrine factors within UC-CM,
rather than one single molecule (Fig.
7).
Taken together, the data presented in the present
study revealed a mechanism, whereby UC-CM exerted significant
angiogenic abilities and chemoattractant effects on progenitor
cells, fibroblasts and stem cells. These results suggest a role for
the SDF-1/CXCR4 and MCP-1/CCR2 axes in UC-CM-induced migration. The
local delivery of UC-CM may induce the recruitment of cells from
the surrounding tissues and enhance the proliferation of these
cells in injured tissue. Therefore, the use of UC-CM may be
suitable for regenerative medicine.
Acknowledgments
The authors would like to thank Dr ZH Wang and Dr YC
Yao (Guangzhou Institutes of Biomedicine and Health, Guangzhou,
China) for their technical assistance. The current study was
supported by the National Natural Science Foundation of China (nos.
30973232 and 81170606), the Program for Changjiang Scholars and
Innovative Research Team in University (no. IRT0935) and the Ph.D.
Programs Foundation of Ministry of Education of China (no.
20120181110087). The authors would also like to thank LetPub for
its linguistic assistance during the preparation of this
manuscript.
References
|
1
|
Wang HS, Hung SC, Peng ST, et al:
Mesenchymal stem cells in the Wharton’s jelly of the human
umbilical cord. Stem Cells. 22:1330–1337. 2004. View Article : Google Scholar
|
|
2
|
Madlambayan G and Rogers I: Umbilical
cord-derived stem cells for tissue therapy: current and future
uses. Regen Med. 1:777–787. 2006. View Article : Google Scholar
|
|
3
|
Ruan ZB, Zhu L, Yin YG and Chen GC: The
mechanism underlying the differentiation of human umbilical
cord-derived mesenchymal stem cells into myocardial cells induced
by 5-azacytidine. Indian J Med Sci. 64:402–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider RK, Püllen A, Kramann R, et al:
Long-term survival and characterisation of human umbilical
cord-derived mesenchymal stem cells on dermal equivalents.
Differentiation. 79:182–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rabb H: Paracrine and differentiation
mechanisms underlying stem cell therapy for the damaged kidney. Am
J Physiol Renal Physiol. 289:F29–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deschepper M, Oudina K, David B, et al:
Survival and function of mesenchymal stem cells (MSCs) depend on
glucose to overcome exposure to long-term, severe and continuous
hypoxia. J Cell Mol Med. 15:1505–1514. 2011. View Article : Google Scholar
|
|
7
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Müller-Ehmsen J, Krausgrill B, Burst V, et
al: Effective engraftment but poor mid-term persistence of
mononuclear and mesenchymal bone marrow cells in acute and chronic
rat myocardial infarction. J Mol Cell Cardiol. 41:876–884. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao JJ, Liu JL, Liu L and Jia HY:
Protection of mesenchymal stem cells on acute kidney injury. Mol
Med Rep. 9:91–96. 2014.
|
|
10
|
Chen Y, Xiang LX, Shao JZ, et al:
Recruitment of endogenous bone marrow mesenchymal stem cells
towards injured liver. J Cell Mol Med. 14:1494–1508. 2010.
View Article : Google Scholar
|
|
11
|
Tasso R, Augello A, Boccardo S, et al:
Recruitment of a host’s osteoprogenitor cells using exogenous
mesenchymal stem cells seeded on porous ceramic. Tissue Eng Part A.
15:2203–2212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tögel F, Isaac J, Hu Z, Weiss K and
Westenfelder C: Renal SDF-1 signals mobilization and homing of
CXCR4-positive cells to the kidney after ischemic injury. Kidney
Int. 67:1772–1784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Ge J, Zhang S, et al: Time course of
myocardial stromal cell-derived factor 1 expression and beneficial
effects of intravenously administered bone marrow stem cells in
rats with experimental myocardial infarction. Basic Res Cardiol.
100:217–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Son BR, Marquez-Curtis LA, Kucia M, et al:
Migration of bone marrow and cord blood mesenchymal stem cells in
vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte
growth factor-c-met axes and involves matrix metalloproteinases.
Stem Cells. 24:1254–1264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Somerset DA, Li XF, Afford S, et al:
Ontogeny of hepatocyte growth factor (HGF) and its receptor (c-met)
in human placenta: reduced HGF expression in intrauterine growth
restriction. Am J Pathol. 153:1139–1147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao WT, Yu HS, Arbiser JL, et al:
Enhanced MCP-1 release by keloid CD14+ cells augments fibroblast
proliferation: role of MCP-1 and Akt pathway in keloids. Exp
Dermatol. 19:e142–e150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furuichi K, Wada T, Iwata Y, et al: CCR2
signaling contributes to ischemia-reperfusion injury in kidney. J
Am Soc Nephrol. 14:2503–2515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shyu WC, Lee YJ, Liu DD, Lin SZ and Li H:
Homing genes, cell therapy and stroke. Front Biosci. 11:899–907.
2006. View Article : Google Scholar
|
|
19
|
Ashrafpour H, Huang N, Neligan PC, et al:
Vasodilator effect and mechanism of action of vascular endothelial
growth factor in skin vasculature. Am J Physiol Heart Circ Physiol.
286:H946–H954. 2004. View Article : Google Scholar
|
|
20
|
Morimoto N, Saso Y, Tomihata K, et al:
Viability and function of autologous and allogeneic fibroblasts
seeded in dermal substitutes after implantation. J Surg Res.
125:56–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamme EN, van Leeuwen RT, Mekkes JR and
Middelkoop E: Allogeneic fibroblasts in dermal substitutes induce
inflammation and scar formation. Wound Repair Regen. 10:152–160.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cavallari G, Olivi E, Bianchi F, et al:
Mesenchymal stem cells and islet cotransplantation in diabetic
rats: improved islet graft revas-cularization and function by human
adipose tissue-derived stem cells preconditioned with natural
molecules. Cell Transplant. 21:2771–2781. 2012. View Article : Google Scholar
|
|
23
|
Wynn RF, Hart CA, Corradi-Perini C, et al:
A small proportion of mesenchymal stem cells strongly expresses
functionally active CXCR4 receptor capable of promoting migration
to bone marrow. Blood. 104:2643–2645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai W, Hale SL and Kloner RA: Role of a
paracrine action of mesen-chymal stem cells in the improvement of
left ventricular function after coronary artery occlusion in rats.
Regen Med. 2:63–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Guo J, Chang Q and Zhang A:
Paracrine role for mesen-chymal stem cells in acute myocardial
infarction. Biol Pharm Bull. 32:1343–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohnishi S, Sumiyoshi H, Kitamura S and
Nagaya N: Mesenchymal stem cells attenuate cardiac fibroblast
proliferation and collagen synthesis through paracrine actions.
FEBS Lett. 581:3961–3966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakanishi C, Yamagishi M, Yamahara K, et
al: Activation of cardiac progenitor cells through paracrine
effects of mesenchymal stem cells. Biochem Biophys Res Commun.
374:11–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dwyer RM, Potter-Beirne SM, Harrington KA,
et al: Monocyte chemotactic protein-1 secreted by primary breast
tumors stimulates migration of mesenchymal stem cells. Clin Cancer
Res. 13:5020–5027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Z, Htay A, Dos Santos W, et al: In
vitro angiogenesis by human umbilical vein endothelial cells
(HUVEC) induced by three-dimensional co-culture with glioblastoma
cells. J Neurooncol. 92:121–128. 2009. View Article : Google Scholar
|
|
30
|
Jacob M, Chang L and Puré E: Fibroblast
activation protein in remodeling tissues. Curr Mol Med.
12:1220–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mbalaviele G, Orcel P, Bouizar Z,
Jullienne A and De Vernejoul MC: Transforming growth factor-beta
enhances calcitonin-induced cyclic AMP production and the number of
calcitonin receptors in long-term cultures of human umbilical cord
blood monocytes in the presence of 1,25-dihydroxychole-calciferol.
J Cell Physiol. 152:486–493. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peled A, Kollet O, Ponomaryov T, et al:
The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5
on immature human CD34(+) cells: role in transendothelial/stromal
migration and engraftment of NOD/SCID mice. Blood. 95:3289–3296.
2000.PubMed/NCBI
|
|
33
|
Wang L, Li Y, Chen J, et al: Ischemic
cerebral tissue and MCP-1 enhance rat bone marrow stromal cell
migration in interface culture. Exp Hematol. 30:831–836. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patel AV and Krimm RF: BDNF is required
for the survival of differentiated geniculate ganglion neurons. Dev
Biol. 340:419–429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sohni A and Verfaillie CM: Mesenchymal
stem cells migration homing and tracking. Stem Cells Int.
130763:20132013.
|
|
36
|
Lapidot T and Kollet O: The essential
roles of the chemokine SDF-1 and its receptor CXCR4 in human stem
cell homing and repopulation of transplanted immune-deficient
NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 16:1992–2003. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ringe J, Strassburg S, Neumann K, et al:
Towards in situ tissue repair: human mesenchymal stem cells express
chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon
stimulation with CXCL8 but not CCL2. J Cell Biochem. 101:135–146.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Duan HF, Wu CT, Wu DL, et al: Treatment of
myocardial ischemia with bone marrow-derived mesenchymal stem cells
overexpressing hepatocyte growth factor. Mol Ther. 8:467–474. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oyagi S, Hirose M, Kojima M, et al:
Therapeutic effect of transplanting HGF-treated bone marrow
mesenchymal cells into CCl4-injured rats. J Hepatol. 44:742–748.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burgazli KM, Bui KL, Mericliler M,
Albayrak AT, Parahuleva M and Erdogan A: The effects of different
types of statins on proliferation and migration of HGF-induced
Human Umbilical Vein Endothelial Cells (HUVECs). Eur Rev Med
Pharmacol Sci. 17:2874–2883. 2013.PubMed/NCBI
|