Introduction
The corpus luteum is a temporary endocrine structure
in mammals, which is important in the female reproductive cycle and
is formed temporarily from a ruptured and ovulated follicle with
rapid angiogenesis (1–5). The ruptured follicle, which occurs
immediately following ovulation is considered to be under hypoxia
conditions due to bleeding and immature vasculature (6). Previous studies have indicated that
hypoxia is important for establishing the vascular system during
luteal development (5), which
induces the expression of hypoxia-inducible factor (HIF)-1α in
luteal cells (7–11). When vascular endothelial factor
(VEGF) is regulated by hypoxia, there is an upregulation of
specific transcription factors, notably HIF-1α (7,8,12).
However, the physical role of HIF-1α in this process of luteal
development remains to be fully elucidated.
Notably, VEGF is important in the regulation of
normal and abnormal angiogenesis in the ovary (3,4,13–18),
particularly in the newly formed corpus luteum (19–22).
Hypoxia is a potent stimulus for the expression of VEGF (14,23),
as ovulation causes a decline of local oxygen concentration,
producing a hypoxic environment, and may be the predominant
stimulator for VEGF production in the developing corpus luteum
(5,24). However, there have been no reports
regarding the contribution of HIF-1α signaling to VEGF-dependent
angiogenesis and luteal function during ovarian luteal formation
in vivo.
Due to the important role of HIF-1α in regulating
the expression of VEGF in luteal cells cultured in vitro
(9,25), the present study hypothesized that
the HIF-1α signaling pathway contributes to VEGF-dependent
angiogenesis and the following functions of corpus luteum in the
ovary in vivo. Therefore, the present study investigated the
expression levels of HIF-1α and VEGF during luteal development in
pregnant rats, determined by measuring serum hormone levels during
luteal development. In addition, the effects of the HIF-1α
small-molecule inhibitor echinomycin (Ech) on luteal function and
HIF-1α-mediated expression of VEGF in the early corpus luteum of
pregnant rats was investigated. The present study aimed to
demonstrate that HIF-1α is an important mediator of luteal
functions in vivo, which may be an important mechanism
regulating VEGF-dependent angiogenesis during luteal development in
mammals.
Materials and methods
Animals
A total of 78 female Sprague-Dawley (SD) rats (body
weight, 50–70 g; age, 25 days), and 21 male SD rats (age, 2–3
months) Wushi Experimental Animal Supply Co., Ltd. (Fuzhou, China).
The animals were maintained under a 14 h light/10 h dark schedule
at 20–25°C, with a continuous supply of chow and water. The study
was approved by the Ethics Committee on Animal Experimentation of
the Fujian Normal University (Fuzhou, China). All efforts were made
to minimize animal discomfort and to reduce the number of animals
used.
Experimental design
The rats were allowed to accommodate for 1–2 weeks
prior to mating with males, which occurred at 2–3 months of age
(179–250 g body weight). Previously unmated female rats (three per
cage) were mated with an unvasectomized male (one per cage) and
were examined every morning for the presence of a vaginal plug. Day
2 of pregnancy was defined as the day at which a vaginal plug was
recovered. The pregnant females were removed and used in the
subsequent experiments.
To further confirm the pregnant rat model,
measurements of the serum levels of progesterone, testosterone and
estradiol in the animals were measured on days 2, 5, 8, 11, 14, 17
and 20 of pregnancy. The rats were anesthetized with atropine
(Sigma-Aldrich, St. Louis, MO, USA) on the days of sample
collection prior to opening of the abdomen, and ≥3.0 ml blood was
collected from the abdominal aorta, and centrifuged at 1,000 x g at
4°C for 10 min. The ovaries were rapidly excised and chilled in
ice-cold 0.154 M NaCl with 14.0 µM indo-methacin
(Sigma-Aldrich) immediately following perfusion for measuring the
expression levels of HIF-1α and VEGF.
For estimating the possible role of HIF-1α/VEGF
signaling during the formation of the corpora lutea in the pregnant
rats, 1 mg/kg body weight Ech (BioViotic, Dransfelg, Germany), a
small-molecule inhibitor of HIF-1α, was intra-peritoneally injected
on day 1 of pregnancy. DMSO (dimethyl sulphoxide; Sigma-Aldrich)
served as a the control/vehicle. The plasma levels of progesterone
and the expression levels of HIF-1α and VEGF in the perfused
ovaries were then determined on days 2, 8 and 14.
For histological analysis, one ovary from each rat
was fixed in 4% paraformaldehyde (Sigma-Aldrich), and the other
ovary was snap-frozen and used for the remaining experiments.
Ovarian perfusion
To avoid the effects of the vascular system, ovarian
perfusion was performed prior to collection of the ovaries. The
female rats were perfused in vivo through the abdominal
aorta with 0.154 M NaCl. The animals were anesthetized with 0.05 mg
atropine/kg body weight subcutaneously, 2.5 mg diazepam/kg body
weight intraperitoneally. (Sigma-Aldrich). The abdomen was opened
via a mid-ventral incision and an intravenous cannula (1.4 mm outer
diameter) was inserted via the aortic bifurcation. The abdominal
aorta was clamped caudally to the celiac artery, and the inferior
vena cava was severed. Subsequently, 40 µl of the perfusion
solution was perfused at ambient temperature through the lower
abdominal vascular system for ~5 min at constant pressure using a
hand-held syringe. Perfusion was discontinued when the ovaries,
particularly the corpora lutea, had become completely pale. The
ovaries were then rapidly removed for subsequent analysis of gene
expression.
Radioimmunoassay of the levels of
progesterone, testosterone and estradiol
The levels of serum progesterone, testosterone and
estradiol were determined using specific radioimmunoassay kits,
according to the manufacturer's instructions. The progesterone RIA
kit [intra-assay coefficient of variation (cv) <4.3%;
inter-assay cv <7.1%], testosterone RIA kit (intra-assay cv
<5.0%; inter-assay cv <10.0%) and estradiol RIA kit
(intra-assay cv <3.5%; inter-assay cv <7.0%) were purchased
from Atomic Gaoke Co., Ltd., Department of Isotope, China Institute
of Atomic Energy (Beijing, China). Protein concentrations were
determined using a Bio-Rad protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), with bovine serum albumin standards.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis of the mRNA levels of VEGF and HIF-1α
Total RNA was extracted from the ovaries using
TRIzol solution (Invitrogen Life Technologies, Carlsbad, CA, USA)
and then reverse-transcribed using a cDNA Synthesis kit; Bio-Rad,
Laboratories, Inc.). The reverse-transcribed products were
amplified using a TaqMan Gene Expression Assay kit (Applied
Biosystems Life Technologies, Foster City, CA, USA), with TaqMan
Universal PCR Master Mix (4304437), HIF-1α primer (Rn00577560_m1)
and VEGF primer (cat. no. Rn01511601_m1). A kit for detecting the
levels of 18S ribosomal RNA (Hs99999901_s1) was used as an
endogenous control. The 20 µl PCR reaction mix contained
10.0 µl 2X TaqMan Gen Expression mix (Applied Biosystems
Life Technologies), 1.0 ul 20X TaqMan Gene Expression Assay
(Applied Biosystems Life Technologies), 4.0 µl cDNA template
and 5.0 µl RNase-free water. The PCR conditions of the
RT-qPCR system (Applied Biosystems Life Technologies), were as
follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at
95°C for 15 s, and 60°C for 1 min. The relative gene expression
levels were calculated in accordance with the ΔΔCt
method (26–29). Relative mRNA levels were expressed
as 2−ΔΔCt values (26–29).
Western blot analysis of the protein
expression levels of VEGF and HIF-1α
The protein samples were obtained using a Nuclear
and Cytoplasmic Protein Extraction kit (Beyotime Institute of
Biotechnology, Haimen, China). Protein concentrations were
determined using a Bio-Rad assay with bovine serum albumin
standards. Following determination, 20 µg of the protein
samples were subjected to 8% SDS-PAGE gel electrophoresis and then
electrophoretically transferred onto a polyvinylidene difluoride
membrane (Pall Life Sciences, Port Washington, NY, USA). The
membrane was washed with phosphate-buffered saline with 0.2% Tween
20 (PBST; Sigma-Aldrich), blocked with 5% non-fat dried milk in
PBST, and probed with mouse monoclonal anti-HIF-1α (1:500; cat. no.
ab1; Abcam, Cambridge, MA, USA) and rabbit polyclonal anti-β-actin
(1:5000; cat. no. NB600501H; Novus Biologicals, Littleton, CO, USA)
primary antibodies overnight at 4°C. Following washing, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse immunoglobulin (Ig)G (1:5,000; cat. no. NB7570;
Novus Biologicals) or goat anti rabbit IgG (1:5,000; cat. no.
NBP230348H; Novus Biologicals) secondary antibodies for 1 h at room
temperature. β-actin was used as a loading control. To detect the
immunoblotting signal, 2 ml enhanced chemiluminescence detection
solution was used (Thermo Fisher Scientific, Inc., Rockford, IL,
USA), and the membrane was exposed to Kodak OMAT film (Kodak,
Rochester, NY, USA). The blots were quantified using ImageJ 1.49
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The significance of differences in the mean values within
and between multiple groups were evaluated using one-way analysis
of variance, followed by Tukey's multiple range test. Statistical
analysis was conducted using Sigmastat 3.02 (Sigma-Aldrich).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes in the levels of serum
progesterone, testosterone and estradiol during luteal development
in pregnant rats
In order to confirm successful generation of a rat
model of pregnancy in the present study, the levels of serum
progesterone, testosterone and estradiol were examined during
luteal development. The results demonstrated that serum
progesterone concentration was significantly increased on day 5
(P<0.05; Fig. 1A), however, no
significant change was observed in the remaining two hormones
(Fig. 1B and C), suggesting that
the corpus luteum had already formed. The level of serum
progesterone was highest on day 17 (P<0.05; Fig. 1A), indicating that the corpus
luteum had matured. A decrease of serum progesterone was observed
(P<0.05; Fig. 1A) and suggested
luteolysis had occurred. At the same time, serum estradiol
concentrations increased significantly (Fig. 1B) and stimulated follicular
development during the subsequent follicular wave in the ovary.
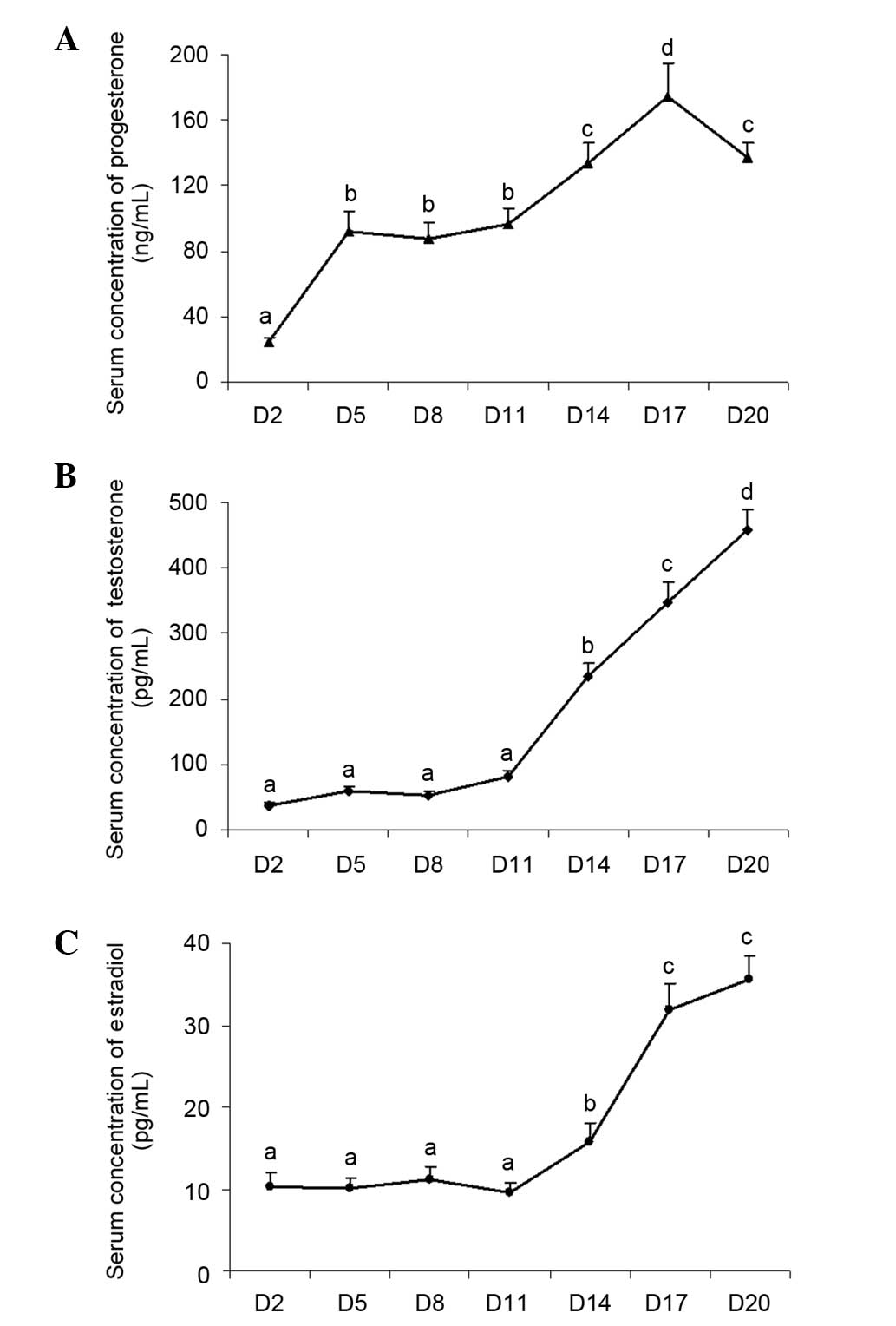 | Figure 1Changes in the serum levels of
progesterone, testosterone and estradiol during luteal development
in pregnant rats Adult female rats were mated with males to induce
pregnancy. Day 1 of pregnancy was defined as the day when a vaginal
plug was recovered. Animals were sacrificed by decapitation and
trunk blood was collected for hormone determination on days 2, 5,
8, 14, 17 and 20. The serum levels of (A) progesterone, (B)
testosterone and (C) estradiol were examined. The abdomen was
opened, and the ovaries were rapidly excised and chilled in
ice-cold NaCl (0.154 M) with 14.0 µM indomethacin
immediately following perfusion to determine the expression of
HIF-1 and VEGF. Data are presented as the mean ± standard error of
the mean. The different letters indicate statistically significant
differences in the mean values within and between multiple groups,
which was evaluated using a one-way analysis of variance, followed
by Tukey's multiple range test. P<0.05 was considered to
indicate a statistically significant difference. VEGF, vascular
endothelial growth factor; HIF, hypoxia-inducible factor; D, day of
pregnancy. |
Changes in the mRNA levels of VEGF and
HIF-1a during luteal development in pregnant rats
To investigate the possible role of the HIF-1α/VEGF
signaling pathway during the development of the corpus luteum in
pregnant rats, the present study also detected the mRNA expression
levels of VEGF and HIF-1α in the ovaries during luteal development.
The mRNA expression level of VEGF increased significantly between
days 5 and 17 (Fig. 2A), and then
decreased markedly on day 20 (Fig.
2A). Notably, the changes in the mRNA expression levels of
HIF-1α were similar to those of VEGF (Fig. 2B), indicating that the HIF-1α/VEGF
signaling pathway may be involved in luteal development and the
following endocrine function.
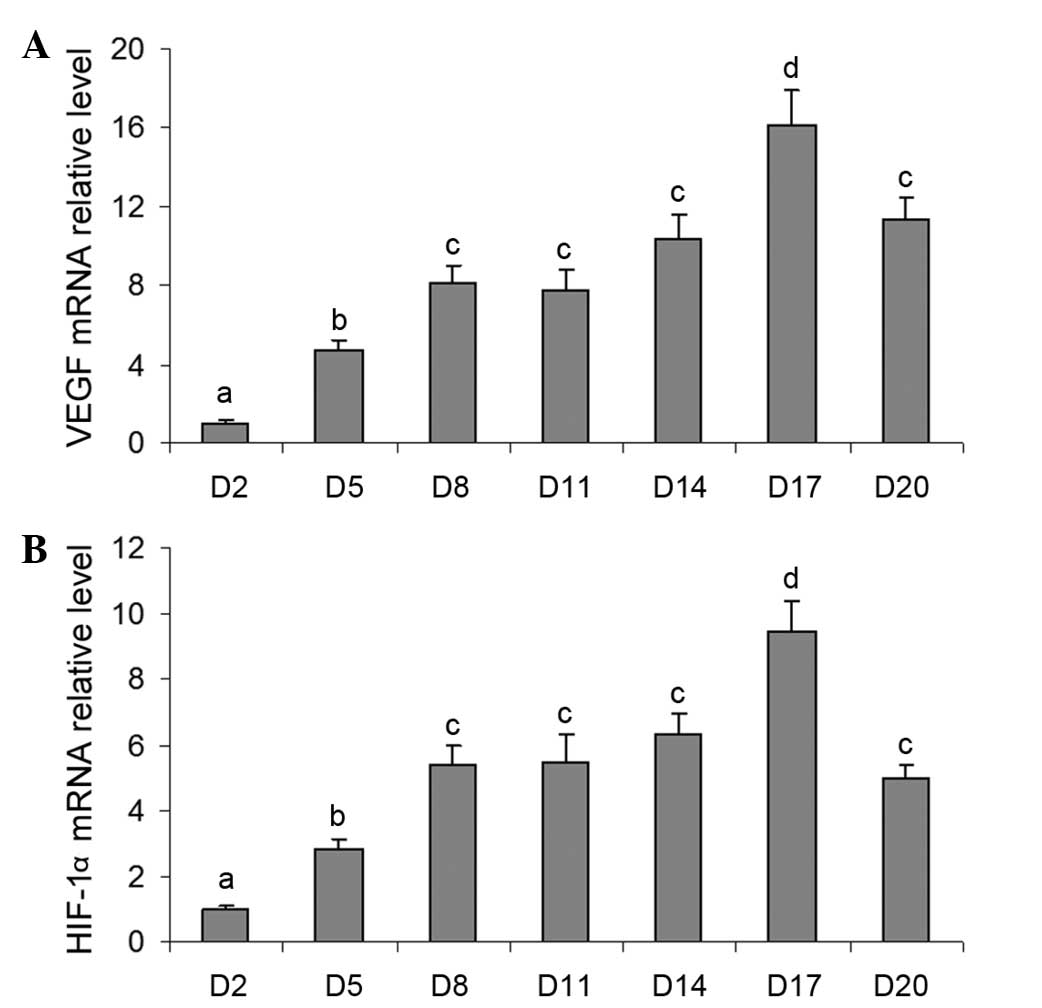 | Figure 2Changes in the mRNA expression of
VEGF and HIF-1α during luteal development in pregnant rats.
Relative mRNA levels of (A) VEGF and (B) HIF-1α were detected using
reverse transcription-quantitative polymerase chain reaction
analysis on days 2, 5, 8, 11, 14, 17 and 20. Data are presented as
the mean ± standard error of the mean. The different letters
indicate statistically significant differences in the mean values
within and between multiple groups, which was evaluated using a
one-way analysis of variance, followed by Tukey's multiple range
test. P<0.05 was considered to indicate a statistically
significant difference. VEGF, vascular endothelial growth factor;
HIF, hypoxia-inducible factor; D, day of pregnancy. |
Effects of Ech on luteal development in
pregnant rats
In order to further clarify the involvement of
HIF-1α in luteal development, the pregnant rats were injected with
Ech, a small-molecule inhibitor of HIF-1α, on day 2 of pregnancy,
and the levels of serum progesterone were determined on days 2, 8
and 14 of pregnancy in rats treated with or without Ech (Fig. 3). The results revealed that Ech
significantly decreased the levels of serum progesterone, compared
with the untreated control group, further demonstrating the
importance of HIF-1α in the formation and development of the corpus
luteum in vivo.
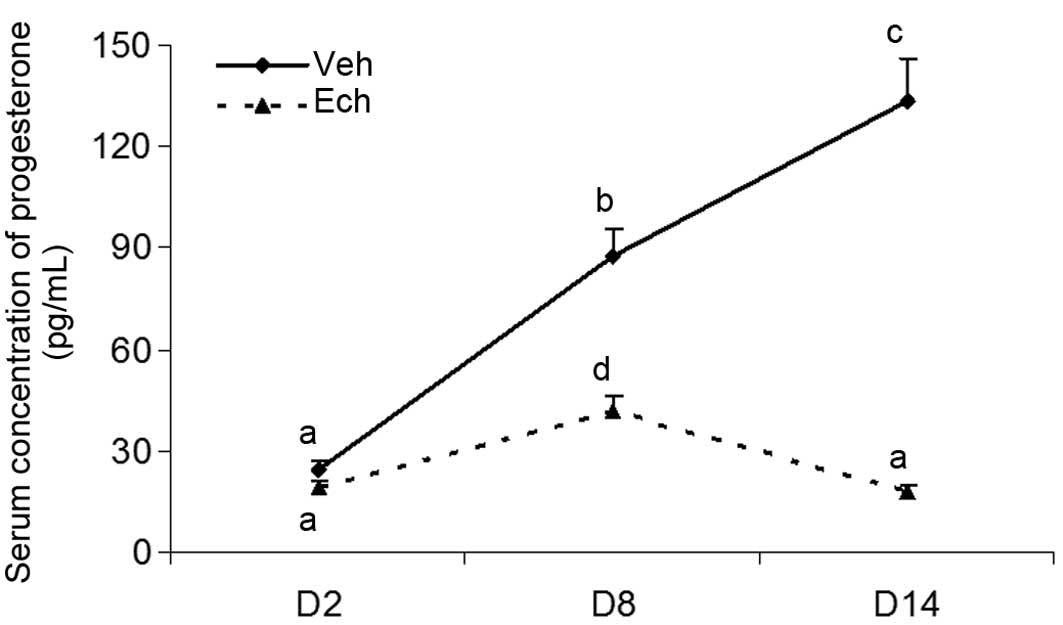 | Figure 3Effects of Ech on luteal development
in pregnant rats Adult female rats were mated with males to induce
pregnancy. Day 1 of pregnancy was defined as the day when a vaginal
plug was recovered. The pregnant rats were intraperitoneally
injected with Ech (1 mg/kg body weight), a small-molecule inhibitor
of HIF-1α, on Day 2 of pregnancy. Animals were sacrificed and trunk
blood was collected for progesterone determination on days 2, 8 and
14. The abdomen was opened, and then the ovaries were rapidly
excised and chilled in ice-cold NaCl (0.154 M) with 14.0 uM
indomethacin immediately following perfusion for measuring the
expression of HIF-1 and VEGF. Each Data are presented as the mean ±
standard error of the mean. The different letters indicate
statistically significant differences in the mean values within and
between multiple groups, which was evaluated using a one-way
analysis of variance, followed by Tukey's multiple range test.
P<0.05 was considered to indicate a statistically significant
difference. Dimethyl sulfoxide served as the vehicle. VEGF,
vascular endothelial growth factor; HIF, hypoxia-inducible factor;
Ech, echinomycin; Veh, vehicle; D, day of pregnancy. |
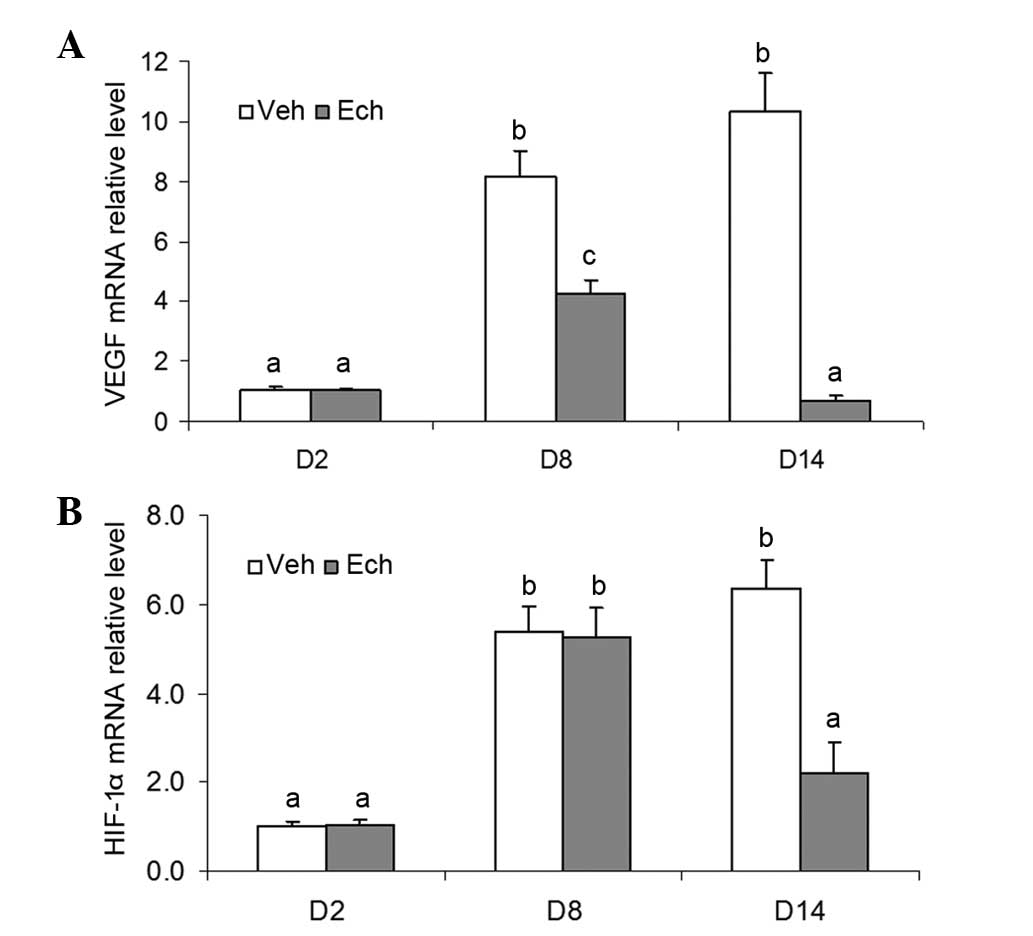
Effects of Ech on the mRNA expression
levels of VEGF and HIF-1α during luteal development in pregnant
rats
To determine the possible role of HIF-1α/VEGF
signaling during luteal development in vivo, the mRNA
expression levels of VEGF and HIF-1α were also assessed in the
ovaries of the pregnant rats treated with or without Ech. The
results demonstrated that Ech inhibited the mRNA expression of VEGF
in the ovary of the pregnant rats (Fig. 4A), suggesting that HIF-1α affected
the expression of VEGF during this developmental process. However,
no significant changes in the mRNA expression of HIF-1α were
observed in the ovaries following Ech treatment on day 8 of
pregnancy, although a marked decrease was observed on day 14
(Fig. 4B). Therefore, the present
study investigated the protein expression of HIF-1α.
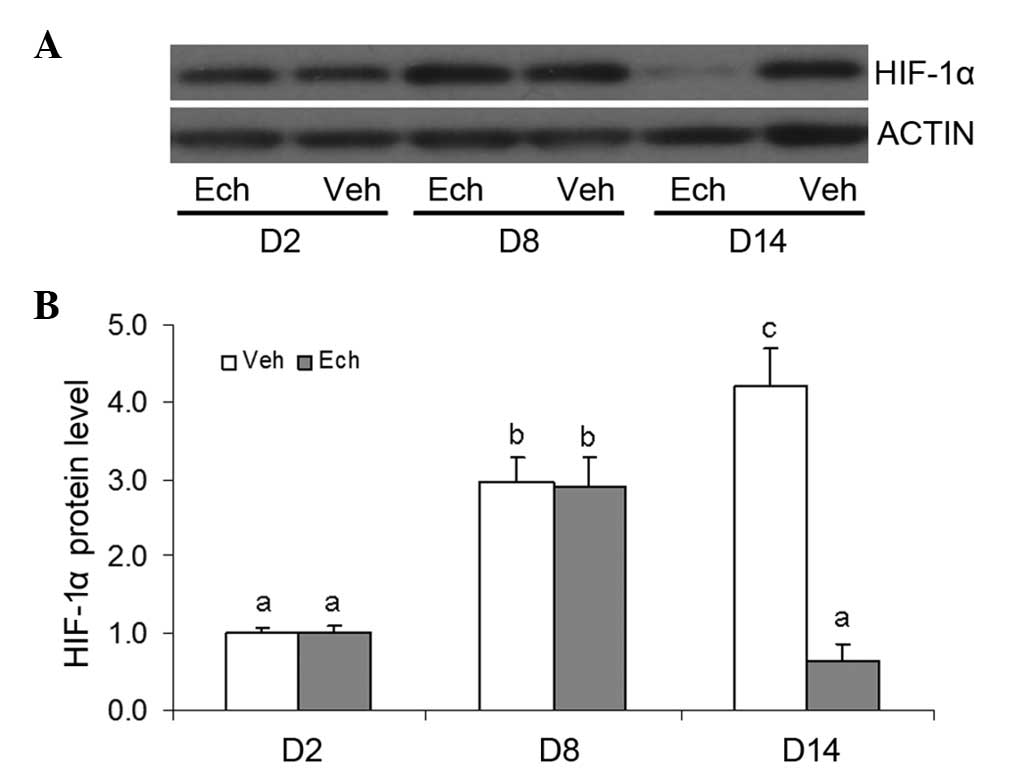
Effects of Ech on the protein expression
of HIF-1α during luteal development in pregnant rats
The results of examination of the protein expression
levels of HIF-1α in the pregnant ovaries indicated no significant
change following Ech treatment on day 8 of pregnancy (Fig. 5), which may be due to Ech being an
inhibitor of HIF-1α activity. A marked decrease in the protein
expression of HIF-1α was observed on day 14, which may have been
caused by luteolysis. Therefore, the binding activity of
HIF-1required further analysis.
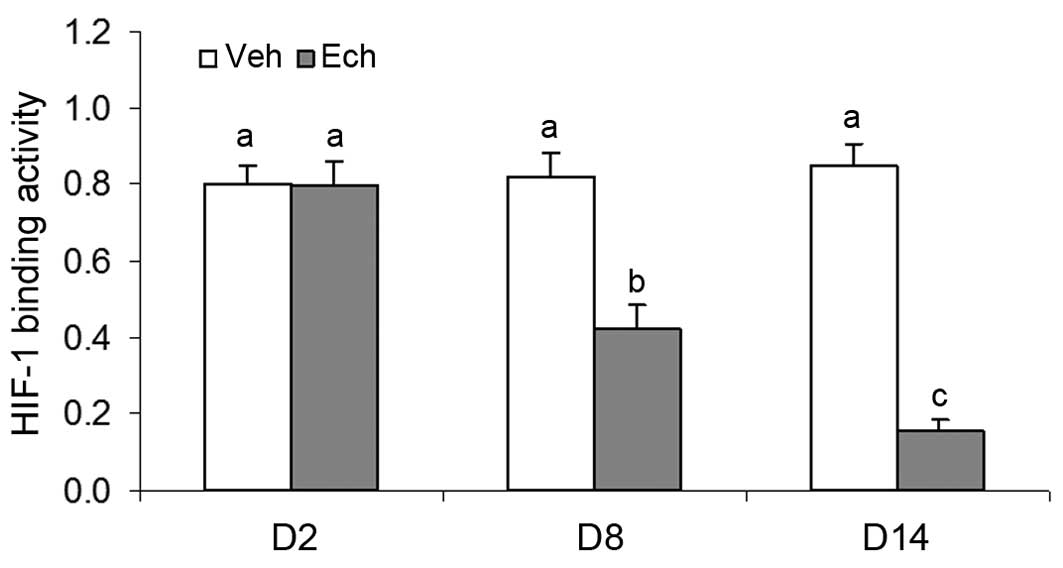
Effects of Ech on HIF-1 binding activity
during luteal development in pregnant rats
To further investigate the regulatory role of HIF-1α
on the mRNA expression of VEGF, the present experiment examined the
effects of Ech on HIF-1 binding activity during luteal development
in pregnant rats. The results revealed that Ech significantly
inhibited the binding activity of HIF-1 (Fig. 6), which contributed to the decrease
in the mRNA expression of VEGF in the ovaries of the pregnant rats
treated with Ech, and inhibition of the subsequent luteal functions
on days 8 and 14 of pregnancy.
Discussion
The results of the present study demonstrated that
HIF-1a and VEGF were expressed in the ovaries, which was similar to
serum progesterone secretion in a luteal development-dependent
manner in the pregnant rats. This suggested that HIF-1α/VEGF
signaling may have an important regulatory role in corpus luteum
formation and development in vivo in mammals.
It is well-known that the corpus luteum is a
temporary endocrine structure, which is important in the female
reproductive cycle and is formed from a ruptured and ovulated
follicle, with rapid angiogenesis, in mammals (1–3,5,9,25).
VEGF is considered to be critical in the regulation of normal and
abnormal angiogenesis in the ovary (3,9,13–17,25,30),
particularly in the newly formed corpus luteum. In primates, VEGF
protein is localized in the hormone-producing cells of the corpus
luteum, and is highest in the granulosaderived cells (14–16,30–35).
VEGF is fundamental in the physiological angiogenesis and
vascularization of the follicular luteinizing granulosa layer
during corpus luteum formation (15,16,30,32).
Whereas the inhibition of VEGF in vivo during the luteal
phase prevents luteal angiogenesis and subsequent progesterone
secretion (2–4,36,37),
excess VEGF generation during the vascularization of multiple
follicles is also considered to cause ovarian hyperstimulation
syndrome (13,38). Furthermore, if VEGF is inhibited,
the corpus luteum has a rudimentary vascular bed with poor
functions (37,39). VEGF is also required for the
ongoing function and vasculature maintenance of the mature corpus
luteum (3,4,36).
These observations are consistent with the those observed in the
present study of changes in the mRNA expression levels of VEGF in
the luteal development of pregnant rats.
Our previous studies demonstrated that VEGF is
transcriptionally activated by an HIF-1α-mediated mechanism in
luteal cells cultured in vitro under hypoxia (9,25),
which is caused by ovulation of a ruptured follicle with bleeding
and immature vasculature in vivo (5,30).
However, several reports have also revealed that reproductive
hormones, including human chorionic gonadotropim (HCG) are involved
in the primary regulation of the expression of VEGF in the ovary.
For example, the mRNA expression of VEGF in human luteinized
granulosa cells has been observed to increase in a dose- and
time-dependent manner by HCG in vitro (13,38).
Chronic or acute exposure to HCG directly stimulates the production
and secretion of VEGF in monkeys (32) and human luteinized granulosa cells
(2,13,14,38).
The administration of a gonadotropin-releasing hormone antagonist
decreases the mRNA expression of VEGF in the monkey corpus luteum
(31). In addition, luteal
vascularization and the development of ovarian hyperstimulation
syndrome are dependent on luteinizing hormone/HCG stimulation
(13,38). Furthermore, in the fully formed,
highly vascular corpus luteum, HCG also upregulates the expression
of VEGF (2). Therefore, the
present study examined changes in the mRNA experssion levels of
HIF-1α during development of the corpus luteum in pregnant rats.
The results demonstrated that the expression of HIF-1α changed in a
stage-specific manner and was correlated with the expression of
VEGF, suggesting that HIF-1α may be vital to the VEGF-dependent
development of the corpus luteum and its functions in
vivo.
HIF-1, a helix-loop-helix transcriptional factor,
which consists of HIF-1α and HIF-1β, has been cloned and
characterized as a transcriptional activator of several
oxygen-sensitive genes, including erythropoietin, heme oxygenases,
transferrin and several glycolytic enzymes (27–29,40),
whose protein products are important in developmental and
physiological processes, including angiogenesis, erythropoiesis,
glycolysis, iron transport and cell proliferation/survival
(2,5,11,41–43).
It has been reported that HIF-1α protein is inducible by a decrease
of O2 concentration in tissue or cells. HIF-1β is not
inducible, however, it can be bound to HIF-1α to form a dimer to
activate the transcription of several genes containing cis
hypoxia-response element in their promoter or enhancer regions
(10,44,45).
Therefore, the present study treated pregnant rats with a HIF-1α
specific small-molecule inhibitor, Ech, and then examined the
levels of gene expression and progesterone secretion. A significant
decrease in the level of serum progesterone was found following Ech
treatment. Further analysis revealed that the ovarian mRNA level of
VEGF was also markedly inhibited, which may contribute to
insufficient luteal development and function. However, no
significant change in the mRNA expression of HIF-1α was observed in
the ovaries. On subsequent examination of the protein expression of
HIF-1α, Ech also had no effect. The following analysis of HIF-1
binding activity suggested that this decrease in the mRNA
expression of VEGF may have been caused by the inhibition of Ech on
HIF-1 binding activity during luteal development in the pregnant
rats. Together, these results suggested that HIF-1α was involved in
luteal function through the VEGF signaling pathway in the ovary of
pregnant rats.
In conclusion, the present study demonstrated that
changes in the expression of VEGF occurred in a stage-dependent
manner during the development of the corpus luteum in the ovary.
Further investigation revealed that the change in the expression of
VEGF was regulated by HIF-1α signaling. This HIF-1α-mediated
expression of VEGF may be an important mechanism regulating luteal
development and function in the mammalian ovary. HIF-1α antagonism
may be useful for the development of novel treatments for fertility
control and for certain types of ovarian dysfunction (11,43,46),
particularly those conditions characterized by pathological
angiogenesis and excessive vascular permeability, including
polycystic ovarian syndrome, ovarian hyperstimulation syndrome and
ovarian neoplasia.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant. nos. 31101032 and
31271255), the Program for New Century Excellent Talents in
University of Ministry of Education of China (grant. no.
NCET-120614), the Doctoral Foundation of the Ministry of Education
in China (grant. no. 20113503120002) and the Fujian Provincial
Science and Technology Projects of the Department of Education
(grant. no. JB14041).
References
|
1
|
Young FM, Rodger FE, Illingworth PJ and
Fraser HM: Cell proliferation and vascular morphology in the
marmoset corpus luteum. Hum Reprod. 15:557–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wulff C, Dickson SE, Duncan WC and Fraser
HM: Angiogenesis in the human corpus luteum: Simulated early
pregnancy by HCG treatment is associated with both angiogenesis and
vessel stabilization. Hum Reprod. 16:2515–2524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fraser HM, Bell J, Wilson H, Taylor PD,
Morgan K, Abderson RA and Duncan WC: Localization and
quantification of cyclic changes in the expression of endocrine
gland vascular endothelial growth factor in the human corpus
luteum. J Clin Endocrinol Metab. 90:427–434. 2005. View Article : Google Scholar
|
|
4
|
Fraser HM and Duncan WC: Vascular
morphogenesis in the primate ovary. Angiogenesis. 8:101–116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishimura R and Okuda K: Hypoxia is
important for establishing vascularization during corpus luteum
formation in cattle. J Reprod Dev. 56:110–116. 2010. View Article : Google Scholar
|
|
6
|
Amselgruber WM, Schäfer M and Sinowatz F:
Angiogenesis in the bovine corpus luteum: An immunocytochemical and
ultra-structural study. Anat Histol Embryol. 28:157–166. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: HIF-1: Mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol. 88:1474–1480. 2000.PubMed/NCBI
|
|
8
|
Semenza GL: Expression of
hypoxia-inducible factor 1: Mechanisms and consequences. Biochem
Pharmacol. 59:47–53. 2000. View Article : Google Scholar
|
|
9
|
Zhang Z, Yin D and Wang Z: Contribution of
hypoxia-inducible factor-1α to transcriptional regulation of
vascular endothelial growth factor in bovine developing luteal
cells. Anim Sci J. 82:244–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molitoris KH, Kazi AA and Koos RD:
Inhibition of oxygen-induced hypoxia-inducible factor-1alpha
degradation unmasks estradiol induction of vascular endothelial
growth factor expression in ECC-1 cancer cells in vitro.
Endocrinology. 150:5405–5414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyazawa M, Yasuda M, Fujita M,
Hirabayashi K, Hirasawa T, Kajiwara H, Muramatsu T, Miyazaki S,
Harasawa M, Matsui N, et al: Granulosa cell tumor with activated
mTOR-HIF-1alpha-VEGF pathway. J Obstet Gynaecol Res. 36:448–453.
2000. View Article : Google Scholar
|
|
12
|
Critchley HO, Osei J, Henderson TA,
Boswell L, Sales KJ, Jabbour HN and Hirani N: Hypoxia-inducible
factor-1alpha expression in human endometrium and its regulation by
prostaglandin E-series prostanoid receptor 2 (EP2). Endocrinology.
147:744–753. 2006. View Article : Google Scholar
|
|
13
|
Neulen J, Yan Z, Raczek S, Weindel K, Keck
C, Weich HA, Marmé D and Breckwoldt M: Human chorionic
gonadotropin-dependent expression of vascular endothelial growth
factor/vascular permeability factor in human granulosa cells:
Importance in ovarian hyperstimulation syndrome. J Clin Endocrinol
Metab. 80:1967–1971. 1995.PubMed/NCBI
|
|
14
|
Lee A, Christenson LK, Patton PE, Burry KA
and Stouffer RL: Vascular endothelial growth factor production by
human luteinized granulosa cells in vitro. Hum Reprod.
12:2756–2761. 1997. View Article : Google Scholar
|
|
15
|
Shimizu T, Jayawardana BC, Tetsuka M and
Miyamoto A: Differential effect of follicle-stimulating hormone and
estradiol on expressions of vascular endothelial growth factor
(VEGF) 120, VEGF164 and their receptors in bovine granulosa cells.
J Reprod Dev. 53:105–112. 2007. View Article : Google Scholar
|
|
16
|
Shimizu T and Miyamoto A: Progesterone
induces the expression of vascular endothelial growth factor (VEGF)
120 and Flk-1, its receptor, in bovine granulosa cells. Anim Reprod
Sci. 102:228–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van den Driesche S, Myers M, Gay E, Thong
KJ and Duncan WC: HCG up-regulates hypoxia inducible factor-1 alpha
in luteinized granulosa cells: Implications for the hormonal
regulation of vascular endothelial growth factor A in the human
corpus luteum. Mol Hum Reprod. 14:455–464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khandrika L, Lieberman R, Koul S, Kumar B,
Maroni P, Chandhoke R, Meacham RB and Koul HK: Hypoxia-associated
p38 mitogen-activated protein kinase-mediated androgen receptor
activation and increased HIF-1alpha levels contribute to emergence
of an aggressive phenotype in prostate cancer. Oncogene.
28:1248–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Redmer DA and Reynolds LP: Angiogenesis in
the ovary. Rev Reprod. 1:182–192. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fraser HM and Wulff C: Angiogenesis in the
primate ovary. Reprod Fertil Dev. 13:557–566. 2001. View Article : Google Scholar
|
|
21
|
Fraser HM and Wulff C: Angiogenesis in the
corpus luteum. Reprod Biol Endocrinol. 1:882003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamanini C and De Ambrogi M: Angiogenesis
in developing follicle and corpus luteum. Reprod Domest Anim.
39:206–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koos RD: Increased expression of vascular
endothelial growth/permeability factor in the rat ovary following
an ovulatory gonadotropin stimulus: Potential roles in follicle
rupture. Biol Reprod. 52:1426–1435. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishimura R, Sakumoto R, Tatsukawa Y,
Acosta TJ and Okuda K: Oxygen concentration is an important factor
for modulating progesterone synthesis in bovine corpus luteum.
Endocrinology. 147:4273–4280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Yu D, Yin D and Wang Z:
Activation of PI3K/mTOR signaling pathway contributes to induction
of vascular endothelial growth factor by hCG in bovine developing
luteal cells. Anim Reprod Sci. 125:42–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Pang X, Tang Z, Yin D and Wang Z:
Overexpression of hypoxia-inducible factor prolyl hydoxylase-2
attenuates hypoxia-induced vascular endothelial growth factor
expression in luteal cells. Mol Med Report. 12:3809–3814. 2015.
|
|
27
|
Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li
PL and Li N: Hypoxia-inducible factor-1α contributes to the
profibrotic action of angiotensin II in renal medullary
interstitial cells. Kidney Int. 79:300–310. 2011. View Article : Google Scholar :
|
|
28
|
Wang Z, Zhu Q, Li PL, Dhaduk R, Zhang F,
Gehr TW and Li N: Silencing of hypoxia-inducible factor-1α gene
attenuates chronic ischemic renal injury in two-kidney, one-clip
rats. Am J Physiol Renal Physiol. 306:1236–1242. 2014. View Article : Google Scholar
|
|
29
|
Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ and
Li N: Hypoxia-inducible factor prolylhydroxylase 2 senses high-salt
intake to increase hypoxia inducible factor 1alpha levels in the
renal medulla. Hypertension. 55:1129–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaczmarek MM, Schams D and Ziecik AJ: Role
of vascular endothelial growth factor in ovarian physiology-An
overview. Reprod Biol. 5:111–136. 2005.PubMed/NCBI
|
|
31
|
Ravindranath N, Little-Ihrig L, Phillips
HS, Ferrara N and Zeleznik AJ: Vascular endothelial growth factor
messenger ribonucleic acid expression in the primate ovary.
Endocrinology. 131:254–260. 1992.PubMed/NCBI
|
|
32
|
Christenson LK and Stouffer RL:
Follicle-stimulating hormone and luteinizing hormone/chorionic
gonadotropin stimulation of vascular endothelial growth factor
production by macaque granulosa cells from pre- and periovulatory
follicles. J Clin Endocrinol Metab. 82:2135–2142. 1997.PubMed/NCBI
|
|
33
|
Endo T, Kitajima Y, Nishikawa A, Manase K,
Shibuya M and Kudo R: Cyclic changes in expression of mRNA of
vascular endothelial growth factor, its receptors Flt-1 and
KDR/Flk-1 and Ets-1 in human corpora lutea. Fertil Steril.
76:762–768. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tesone M, Stouffer RL, Borman SM,
Hennebold JD and Molskness TA: Vascular endothelial growth factor
(VEGF) production by the monkey corpus luteum during the menstrual
cycle: Isoform-selective messenger RNA expression in vivo and
hypoxia-regulated protein secretion in vitro. Biol Reprod.
73:927–934. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tropea A, Miceli F, Minici F, Tiberi F,
Orlando M, Gangale MF, Romani F, Catino S, Mancuso S, Navarra P, et
al: Regulation of vascular endothelial growth factor synthesis and
release by human luteal cells in vitro. J Clin Endocrinol Metab.
91:2303–2309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fraser HM, Wilson H, Wulff C, Rudge JS and
Wiegand SJ: Administration of vascular endothelial growth factor
Trap during the 'post-angiogenic' period of the luteal phase causes
rapid functional luteolysis and selective endothelial cell death in
the marmoset. Reproduction. 132:589–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duncan WC, van den Driesche S and Fraser
HM: Inhibition of vascular endothelial growth factor in the primate
ovary up-regulates hypoxia-inducible factor-1alpha in the follicle
and corpus luteum. Endocrinology. 149:3313–3320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nastri CO, Ferriani RA, Rocha IA and
Martins WP: Ovarian hyperstimulation syndrome: Pathophysiology and
prevention. J Assist Reprod Genet. 27:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fraser HM and Lunn SF: Angiogenesis and
its control in the female reproductive system. Br Med Bull.
56:787–797. 2000. View Article : Google Scholar
Duncan WC, van den Driesche S and Fraser
HM: Inhibition of vascular endothelial growth factor in the primate
ovary up-regulates hypoxia-inducible factor-1alpha in the follicle
and corpus luteum. Endocrinology. 149:3313–3320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhong H, Chiles K, Feldser D, Laughner E,
Hanrahan C, Georgescu MM, Simons JW and Semenza GL: Modulation of
hypoxia-inducible factor 1alpha expression by the epidermal growth
factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human
prostate cancer cells: Implications for tumor angiogenesis and
therapeutics. Cancer Res. 60:1541–1545. 2000.PubMed/NCBI
|
|
42
|
Yaba A, Bianchi V, Borini A and Johnson J:
A putative mitotic checkpoint dependent on mTOR function controls
cell proliferation and survival in ovarian granulosa cells. Reprod
Sci. 15:128–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyazawa M, Yasuda M, Fujita M, Kajiwara
H, Hirabayashi K, Takekoshi S, Hirasawa T, Murakami M, Ogane N,
Kiguchi K, et al: Therapeutic strategy targeting the
mTOR-HIF-1alpha-VEGF pathway in ovarian clear cell adenocarcinoma.
Pathol Int. 59:19–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kazi AA, Jones JM and Koos RD: Chromatin
immunoprecipitation analysis of gene expression in the rat uterus
in vivo: Estrogen-induced recruitment of both estrogen receptor
alpha and hypoxia-inducible factor 1 to the vascular endothelial
growth factor promoter. Mol Endocrinol. 19:2006–2019. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kazi AA and Koos RD: Estrogen-induced
activation of hypoxia-inducible factor-1alpha, vascular endothelial
growth factor expression, and edema in the uterus are mediated by
the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology.
148:2363–2374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chan DA and Giaccia AJ: PHD2 in tumour
angiogenesis. Br J Cancer. 103:1–5. 2010. View Article : Google Scholar : PubMed/NCBI
|