Introduction
Retinitis pigmentosa (RP) is an pathological
condition leading to progressive visual decline resulting from
continual loss of photoreceptor cells and outer nuclear layers of
the retina (1). At present, no
cures for this degenerative retinal disease are available.
Experimental approaches for the development of treatments of RP
have advanced in recent years, which include drug treatments
(2,3), stem-cell transplantation (4), gene therapy (5) and light sensitivity-modulating
proteins (6).
Treatment approaches using light
sensitivity-enhancing proteins such as channel rhodopsins (ChR) and
melanopsin have recently emerged as a hotspot of RP research. ChR2,
which was first described in green algae, is a light-gated ion
channel which evokes ultrafast membrane depolarization and mediates
an action potential (7,8). Of note, ChR2 has been demonstrated to
be able to restore the function of degenerative retinal diseases.
Unlike ChR2, the photopigment melanopsin is expressed by
intrinsically photosensitive retinal ganglion cells (ipRGC) in the
human retina (9). Melanopsins
mediate a broad range of physiological responses, including
circadian rhythm, modulation of sleep and alertness (10–12).
The light-evoked response mediated by melanopsin is slower but more
durable than that of ChR2 in the absence of photoreceptor-based
input (12). However, whether
melanopsin has the potential to restore visual function similarly
to ChR2 has remained elusive. Therefore, the present study
evaluated the effects of melanopsin in Royal College of Surgeons
(RCS) rats, which are widely used as a model of inherited retinal
degeneration. These animals carry a mutation in the Mertk
gene expressed in retinal pigment epithelial cells, which results
in progressive photoreceptor cell death and loss of
electrophysiological response (13,14).
The present study aimed to determine whether sub-retinal injection
of melanopsin-overexpressing adenovirus may restore visual function
in RCS rats in order to explore the feasibility of using melanopsin
as a novel therapeutic for retinal degenerative diseases.
Materials and methods
Experimental animals
A total of 18 healthy neonatal RCS rats (4-week-old)
were provided by the Experimental Animal Center of Southwest
Hospital, Third Military Medical University (Chongqing, China) of
mixed gender. Of these rats, 10 were used for behavioral testing
and 8 were used for flash electroretinography (FERG). Prior to the
start of the experiments, the rats were acclimatized for 1 week.
The rats were reared in a light-controlled room with a 12-h
light/dark cycle at 22–25°C and 50–60% humidity. All experimental
protocols were in accordance with the Guidance Suggestions for the
Care and Use of Laboratory Animals, formulated by the Ministry of
Science and Technology of the People's Republic of China (15), and approved by the ethics committee
of the Third Military Medical University (Chongqing, China).
Experiments were performed in the laboratory of Southwest Eye
Hospital from August 2012 to December 2013.
Viral vectors
Two viral vectors were used: Adenovirus - opsin4 -
green fluorescent protein (AV-Opn4-GFP) and the empty vector
AV-GFP. Full-length mouse melanopsin (GenBank accession no.
6693702) was cloned into pAV vector under the transcriptional
control of the murine cytomegalovirus promoter. These two
constructs were packaged at the GeneChem Biological Company virus
production core (Shanghai, China). The packaged viruses were
concentrated and dissolved in phosphate-buffered saline (PBS) to
1.08 or 2.2×1013 genome copies per milliliter for
AV-Opn4-GFP or AV-GFP, respectively.
Subretinal injection of adenovirus
For anesthesia, animals at the age of five weeks
were subjected to intramuscular thigh injection with a mixture of
10 mg/kg ketamine (Sigma-Aldrich, St. Louis, MO, USA) and 1 mg/kg
xylazine (Sigma-Aldrich). Oxybuprocaine 0.4% (Santen Pharmaceutical
Co., Ltd., Osaka, Japan) eye drops were used for superficial
anesthesia. Under aseptic conditions, the RCS rats were
individually moved to an animal operating table under a microscope
on a biological super-clean bench. 2 µl viral suspension was
injected into the sub-retinal space in the superior hemisphere of
the eyes. In the group subjected to behavioral testing, five rats
received AV-Opn4-GFP injection into both eyes in the experimental
group, while the control group consisted of five untreated RCS
rats. In the group subjected to FERG testing, in order to avoid
individual differences, the right eye of the rats received
AV-Opn4-GFP injection, while the left eye was injected with a
control viral vector (AV-GFP; n=8).
Immunohistochemistry
At 3, 5 and 7 weeks following injection with the
vectors, the eyes were immediately enucleated from RCS rats
sacrificed by decapitation under deep anesthesia via intramuscular
injection of 20 mg/kg ketamine and 2 mg/kg xylazine. For
immunohistochemical analysis, the whole-mount retina was isolated
and blocked with 5% bovine serum albumin (Beyotime Institute of
Biotechnology, Haimen, China) diluted in 10% goat serum (Beyotime
Institute of Biotechnology) in PBS for 1 h at room temperature
(25°C). The blocked retina was washed with PBS for 5 min for times.
Next, the retina was incubated with rabbit anti-mouse
anti-melanopsin polyclonal antibody (1:500; cat. no. ab19306;
Abcam, Cambridge) overnight at 4°C and washed in PBS three times
for 15 min each. Subsequently, the retinas were incubated with
Cy3-conjugated goat anti-rabbit immunoglobulin G (1:500; cat. no.
A0516; Beyotime Institute of Biotechnology) for 4 h at room
temperature and washed three times in PBS for 15 min each. A
fluorescence microscope (Zeiss LSM 510, Carl Zeiss AG, Oberkochen,
Germany) was used to capture images. The number of ipRGCs was
counted by fluorescence microscopy under ×200 magnification. For
each sample, nine fields of view with the same size (200×300
µm) were randomly selected. The length of the axons was also
measured under a fluorescence microscope (×400 magnification) and
27 cells were randomly selected (16,17).
Western blot analysis
The retina was isolated using the liquid nitrogen
method (17) and lysed using a
cell lysis solution [50 mM Tris-HCl buffer (pH 7.4), 150 mM NaCl,
1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium
orthovanadate, sodium fluoride, EDTA and leupeptin; P0013B;
Beyotime Institute of Biotechnology]. The cell lysates were
separated by 12% SDS-PAGE and electrotransferred onto
nitrocellulose (NC) membranes (Whatman, Fairfield, CT, USA). After
blocking with 5% de-fatted milk in PBS containing 0.05% Tween-20
overnight at 4°C, the membranes were incubated with the
anti-melanopsin antibody overnight at 4°C. After washing, all
membranes were incubated with rat polyclonal GAPDH antibody
(1:1,000; cat. no. CW0101; CWBio, Beijing, China) for 1 h at room
temperature and horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:2,000; cat. no. A0208; Beyotime Institute of Biotechnology)
at room temperature for 1 h. The NC membranes were visualized using
an enhanced chemiluminescence kit (Applied Biosystems; Thermo
Fisher Scientific, Waltham, MA, USA) and scanned using the Odyssey
infrared imaging system with the Odyssey Application software
(V1.2.15; LI-COR Biosciences, Lincoln, NE, USA). Quantification of
melanopsin was performed by densitometric analysis of the protein
bands (16,17).
Open field test
The open field test box was 45×30×40 cm in size and
divided into a white open field and a dark zone with a door (10×10
cm) between them. To test rats in the open field (5 weeks
post-injection), the rats were placed in the dark zone for 2 min
for adaptation and the door was then opened to observe the behavior
of the rats. The time spent in the dark zone and white open field
was recorded. The open field test was performed under 300 lux light
intensity and recorded using a video camera to enable subsequent
evaluation. The bottom surface of the box was cleaned with 70%
ethanol prior to testing of each animal (18,19).
FERG recording
FERG recording was performed on animals at 3, 5 and
7 weeks following injection with melanopsin-overexpression/empty
vector. Animals were allowed to adapt to darkness for almost 12 h
and prepared for recording under dim red light. After anesthesia,
pupils were dilated with tropicamide and phenylephrine eye drops
(Santen Pharmaceutical Co., Ltd). FERG responses of both eyes were
recorded simultaneously using goldwire loops (Roland Consult,
Brandenburg a.d. Havel Germany). The cornea was frequently treated
with 0.9% saline to prevent its dehydration and allow electrical
contact with the recording electrode. Two needle electrodes were
inserted under the skin of the angulus oculi temporal to serve as
reference electrodes, while the other electrodes were placed in the
tail to serve as the grounding electrodes. The b-waves were
acquired using the Retiscan system (Roland Consult, Brandenburg
a.d. Havel, Germany). Dark-adapted intensity responses to stimuli
with intensities of −0.5, −0.02, 0.5 and 1 Log(cd
sec/m2) were measured. To avoid any adapting effect of
the previous flash, the flash interval was set as 60–120 sec
depending on stimulus intensity.
Statistical analysis
Data analysis was performed using the SPSS 13.0
statistical package (SPSS, Inc., Chicago, IL, USA). Values are
expressed as the mean ± standard deviation. Data were analyzed
using the independent-samples t test to compare the
differences between two treatment modalities/groups in the FERG and
behavioral experiments. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
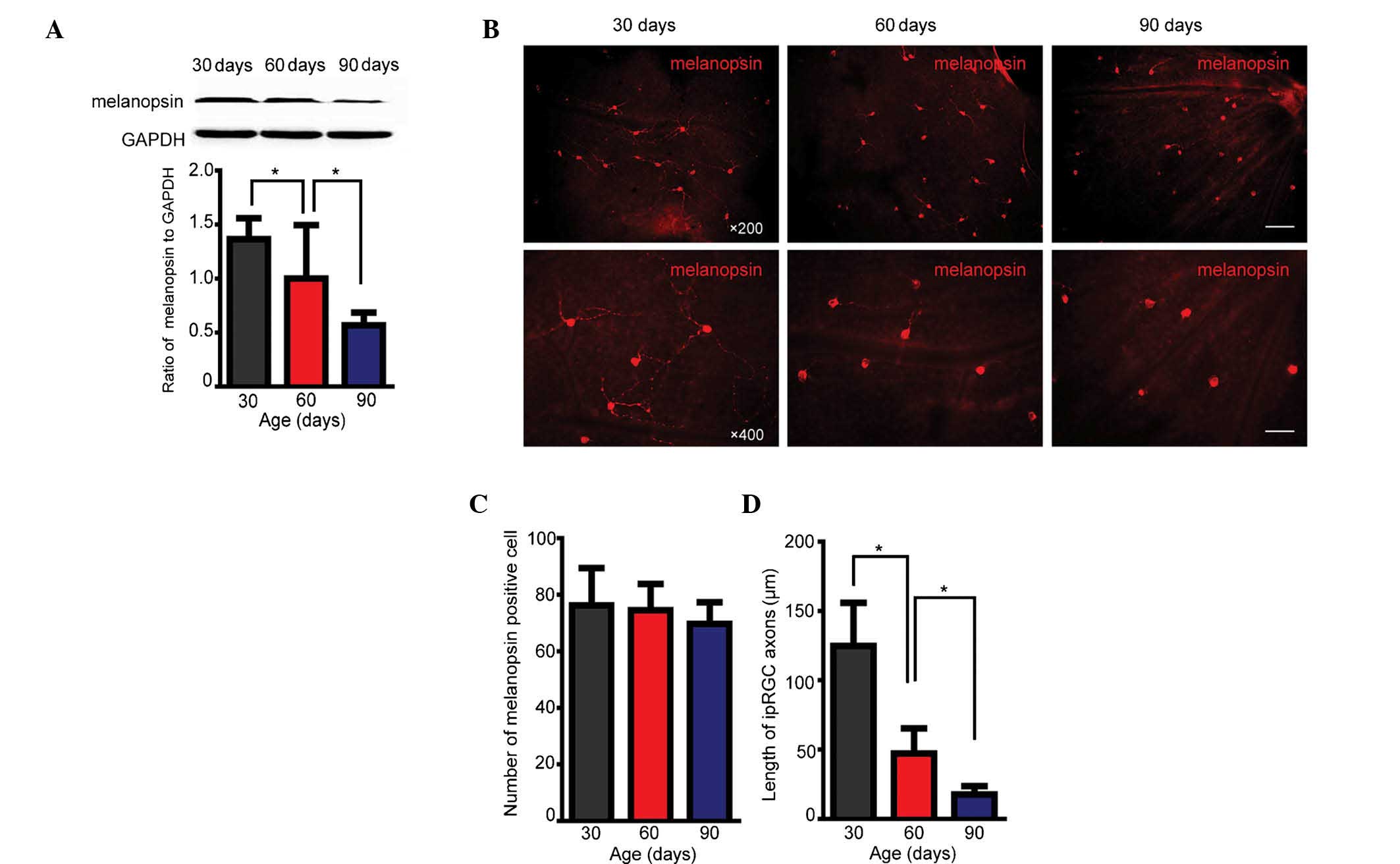
Melanopsin expression is decreased during
retinal degradation due to loss of dendritic axons
Western blot analysis demonstrated that in the
retinal degeneration model, melanopsin expression levels in the
retinas of rats aged 30, 60 and 90 days decreased with increasing
age (Fig. 1A). However, during
this time, the number of melanopsin-positive cells did not
decrease, as indicated by immunohistochemistry (Fig. 1B and C). Assessment of the average
axon length of ipRGCs revealed significant shortening after 60 and
90 days compared with axon length at 30 postnatal days (Fig. 1D). These results confirmed that in
RCS rats as a model of chronic retinal degeneration, melanopsin
expression is gradually decreased with increasing age. This
diminished expression of melanopsin was demonstrated to result from
the decreased length of dendritic axons of ipRGCs, but not the loss
of the number of ipRGCs.
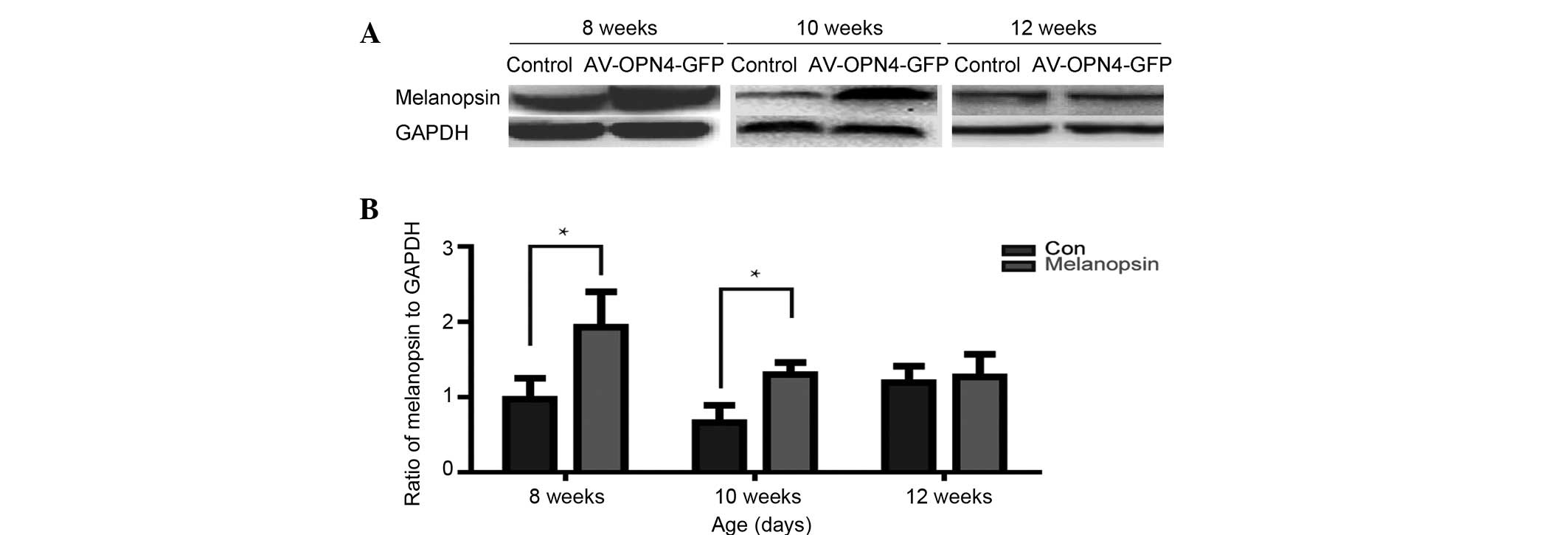
Vector-mediated overexpression of
melanopsin
The retinas of rats at 3, 5 and 7 weeks following
injection of AV-OPN4-GFP (at the age of 8, 10 and 12 weeks,
respectively) were subjected to western blot analysis of
melanopsin. The results confirmed that at 3 and 5 weeks following
sub-retinal injection of AV-OPN4-GFP, a significant overexpression
of melanopsin was achieved in the retina, compared with its
expression in the control vector-injected eyes (P<0.05)
(Fig. 2).
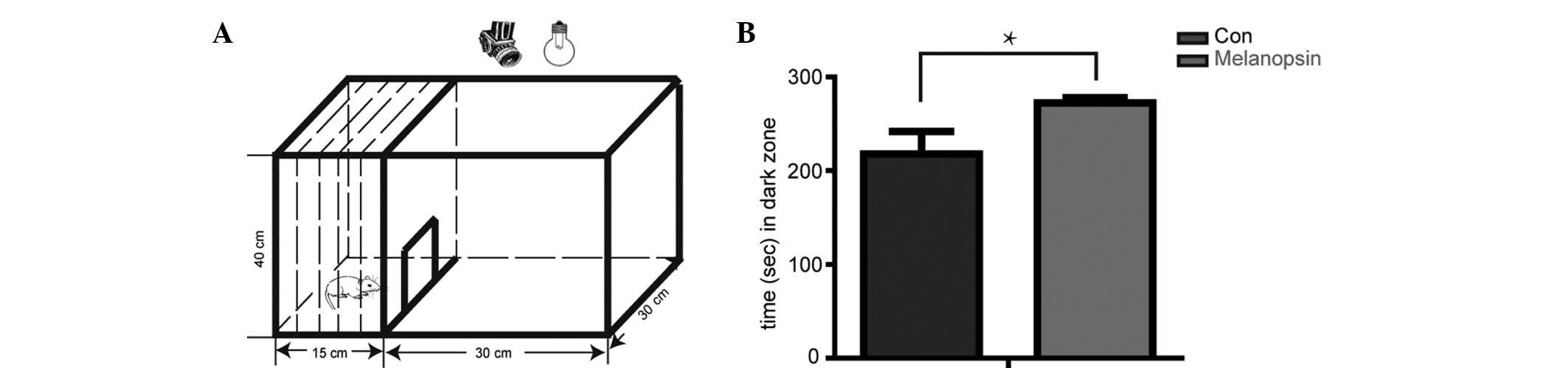
Melanopsin overexpression in retina
reduces loss of light sensitivity in RCS rats
Previous studies have demonstrated that normal rats
avoid open, bright spaces, and that this innate tendency can be
utilized as the basis of a simple test of their ability to detect
light (18,19). In the open-field test, rats are
placed in an illuminated open field with access to a dark zone. The
time spent by the rats in the open space is recorded using a
camera. The total time of the test is 300 sec. The results showed
that the RCS rats at 10 weeks of age injected with the empty vector
AV-GFP did not possess a sufficient number of photoreceptors to
mediate light avoidance, resulting in 217.8±24.13 sec (n=5) in the
dark zone, whereas RCS rats injected with AV-OPN4-GFP spent
292.4±5.59 sec (n=5) in the dark zone (P<0.05) (Fig. 3). This result indicated that
melanopsin overexpression in the retina inhibited the loss of light
sensitivity in a rat model of ongoing retinal degradation.
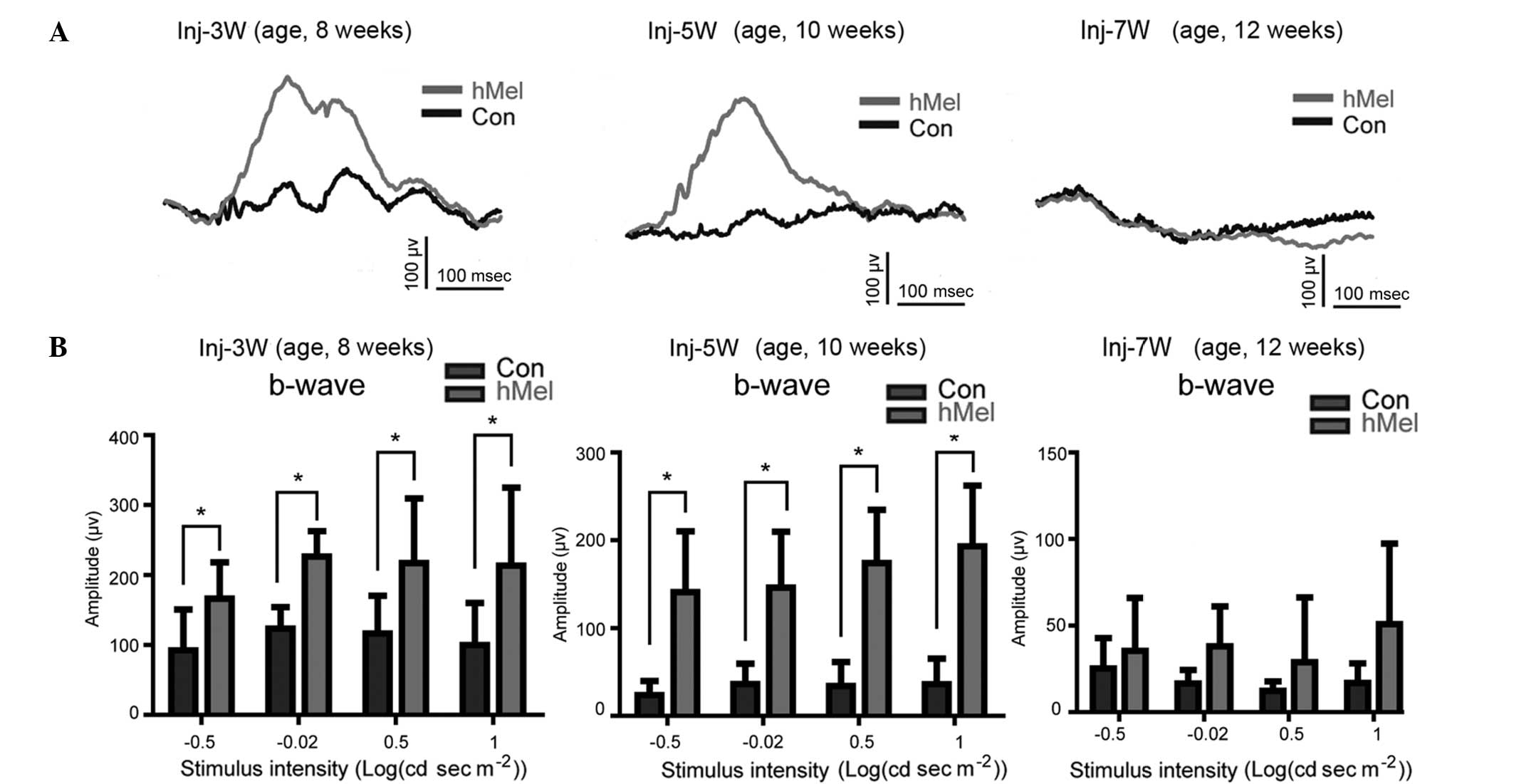
Melanopsin enhances the retinal b-wave
and response to flash light stimuli
FERG is a means of measuring the entire function of
the retina in response to stimulation with flashed light. It has
been previously reported that the b-wave amplitude of RCS rats
decreases gradually and is almost completely abolished at 60 days
of age (20). In the present
study, the b-wave amplitude at 10 weeks of age was reduced compared
with that at 8 weeks of age, and was completely abolished at 12
weeks of age in the rat eyes injected with empty vector (Fig. 4A). However, the eyes of RCS rats
which had received sub-retinal injections of AV-OPN4-GFP at the age
of five weeks showed an enhanced b-wave amplitude compared with
that of empty vector-injected eyes at 8 and 10 weeks of age (n=8)
(Fig. 4A). However, at 12 weeks,
the amplitude of the b-waves in the melanopsin-overexpressing eyes
could not be restored. Similarly, the response to stimuli with
dosed light flashes at various intensities was decreased in the
empty vector-injected eyes at weeks 10 and 12 of age compared with
that at 8 weeks. In the eyes injected with AV-OPN4-GFP, the
response was significantly increased compared with that of empty
vector-injected eyes at 8 and 10 weeks of age (P<0.05); however,
differences were not significant at 12 weeks of age (P>0.05)
(Fig. 4B).
Discussion
The structure of the mammalian retina is highly
organized and all light detection is performed by two visual
pathways: The image-forming and the non-image-forming systems
(21). In the traditional model,
the retina contains two types of photoreceptor - the rod and the
cone. These perform the first step of the visual process by
capturing light and transducing it into electrical signals.
However, in recent years, a novel type of specialized retinal
ganglion cell, namely the ipRGC, was discovered, which expresses
the photopigment melanopsin and is intrinsically photosensitive
(21,22). Upon their discovery, ipRGCs were
first considered to be involved in the circadian rhythm, and within
the last few years it has become evident that these photoreceptors
mediate light detection to regulate behavioral and physiological
responses to light (23,24). It has now been suggested that the
ipRGC family has up to five distinct cell types (termed M1-5) with
various morphological characteristics and locations of their
dendritic arbors (25). M1 ipRGCs
are well established as predominantly being responsible for
circadian photoentrainment and the pupillary light reflex (26,27).
The non-M1 ipRGCs may be responsible for image-forming behaviors
via projection to the lateral geniculate nucleus (28). In addition, a study on bipolar
cells showed that bipolar axons communicate with outer stratifying
melanopsin cells in the retina of primates (29).
FERG is a widely used method for detecting the
entire function of the retina. With the aggravation of retinal
degeneration, RCS rats are depleted of almost all of their rods and
cones, and in spite of a few surviving cells, retinal function is
almost completely lost, followed by the complete abolishment of the
electrophysiological response (20). In the present study, western blot
and immunofluorescence analysis were used to assess melanopsin
expression during retinal degeneration, revealing that its protein
expression was gradually decreased, particularly at 60 postnatal
days. The length of retinal axons was significantly shortened after
30 postnatal days. A viral vector was used to deliver melanopsin to
the retina in order to supplement the reduced melanopsin levels
with the aggravation of retinal degeneration; this led to a delay
in the time-dependent loss of the b-wave response. Of note, these
findings indicated a role for melanopsin and ipRGCs in the
image-forming systems of the eye.
Behavioral studies have shown that normal rat avoids
open, bright spaces, while the rats with retinal degeneration spend
a decreased amount of time in a dark area (18,19,30).
Of note, in the present study RCS rats sub-retinally injected with
AV-OPN4-GFP spent a longer duration in the dark zone compared with
untreated RCS rats. This indicated that melanopsin may be able to
delay retinal degradation or enhance the efficiency of the
remaining photoreceptors in the retina, leading to an increased
electrical signal and light sensitivity. In addition, the FERG
assay showed that melanopsin rescued the electrophysiological
response to the flashed light, which was transmitted to the
image-forming system. Therefore, modulation of melanopsin
expression may be a novel therapeutic method for treating patients
with retinal degeneration. However, the life time of the AV used to
deliver melanopsin into the sub-retinal region was relatively low,
as in the present study, its effects were diminished at 7 weeks
following injection in terms of protein expression of melanopsin
and the b-wave response. Thus, a further study by our group will
use adeno-associated virus instead of AV for the delivery of
melanopsin, which may be more suitable for use in vivo.
Future studies should be performed to enhance the current
understanding of the molecular mechanisms of action of the
endogenous opsin melanopsin, including regulation of the
electrophysiological response to flash light and the effects of its
modulation on visual performance.
Acknowledgments
The authors would like to thank Professor Yaochen Li
(Shantou University Medical College, Shantou, China) for his advice
on the design of the present study. This work was supported by a
grant from the Science Foundation of Chongqing, Major International
(Regional) Joint Research Project (no. CSTC2013GJH210004 to
ZY).
References
|
1
|
Sung CH, Davenport CM, Hennessey JC,
Maumenee IH, Jacobson SG, Heckenlively JR, Nowakowski R, Fishman G,
Gouras P and Nathans J: Rhodopsin mutations in autosomal dominant
retinitis pigmentosa. Proc Natl Acad Sci USA. 88:6481–6485. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rayapudi S, Schwartz SG, Wang X and Chavis
P: Vitamin A and fish oils for retinitis pigmentosa. Cochrane
Database Syst Rev. 12:CD0084282013.PubMed/NCBI
|
|
3
|
Zarbin MA, Arlow T and Ritch R:
Regenerative nanomedicine for vision restoration. Mayo Clin Proc.
88:1480–1490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamao H, Mandai M, Okamoto S, Sakai N,
Suga A, Sugita S, Kiryu J and Takahashi M: Characterization of
human induced pluripotent stem cell-derived retinal pigment
epithelium cell sheets aiming for clinical application. Stem Cell
Reports. 2:205–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang L, Frederick JM and Baehr W: RNA
interference gene therapy in dominant retinitis pigmentosa and
cone-rod dystrophy mouse models caused by GCAP1 mutations. Front
Mol Neurosci. 7:252014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan ZH, Ganjawala TH, Lu Q, Ivanova E and
Zhang Z: ChR2 Mutants at L132 and T159 with improved operational
light sensitivity for vision restoration. PLoS One. 9:e989242014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagel G, Szellas T, Huhn W, Kateriya S,
Adeishvili N, Berthold P, Ollig D, Hegemann P and Bamberg E:
Channelrhodopsin-2, a directly light-gated cation-selective
membrane channel. Proc Natl Acad Sci USA. 100:13940–13945. 2013.
View Article : Google Scholar
|
|
8
|
Bi A, Cui J, Ma YP, Olshevskaya E, Pu M,
Dizhoor AM and Pan ZH: Ectopic expression of a microbial-type
rhodopsin restores visual responses in mice with photoreceptor
degeneration. Neuron. 50:23–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hattar S, Liao HW, Takao M, Berson DM and
Yau KW: Melanopsin-containing retinal ganglion cells: Architecture,
projections, and intrinsic photosensitivity. Science.
295:1065–1070. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramsey DJ, Ramsey KM and Vavvas DG:
Genetic advances in ophthalmology: The role of
melanopsin-expressing, intrinsically photosensitive retinal
ganglion cells in the circadian organization of the visual system.
Semin Ophthalmol. 28:406–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roecklein KA, Wong PM, Miller MA, Donofry
SD, Kamarck ML and Brainard GC: Melanopsin, photosensitive ganglion
cells and seasonal affective disorder. Neurosci Biobehav Rev.
37:229–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koizumi A, Tanaka KF and Yamanaka A: The
manipulation of neural and cellular activities by ectopic
expression of melanopsin. Neurosci Res. 75:3–5. 2013. View Article : Google Scholar
|
|
13
|
Kyger M, Worley A and Adamus G: Autoimmune
responses against photoreceptor antigens during retinal
degeneration and their role in macrophage recruitment into retinas
of RCS rats. J Neuroimmunol. 254:91–100. 2013. View Article : Google Scholar :
|
|
14
|
McGill TJ, Prusky GT, Douglas RM, Yasumura
D, Matthes MT, Lowe RJ, Duncan JL, Yang H, Ahern K, Daniello KM, et
al: Discordant anatomical, electrophysiological and visual
behavioral profiles of retinal degeneration in rat models of
retinal degenerative disease. Invest Ophthalmol Vis Sci.
53:6232–6244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
The Ministry of Science and Technology of
the People's Republic of China: Guidance suggestions for the care
and use of laboratory animals. Beijing, China: 2006
|
|
16
|
Tao Z, Dai J, He J, Li C, Li Y and Yin ZQ:
The influence of NaIO(3)-induced retinal degeneration on
intra-retinal layer and the changes of expression
profile/morphology of DA-ACs and mRGCS. Mol Neurobiol. 47:241–260.
2013. View Article : Google Scholar
|
|
17
|
Li YC, Li CS, Chen ZS, He JR, Tao Z and
Yin ZQ: A MicroRNA, mir133b, suppresses melanopsin expression
mediated by failure dopaminergic amacrine cells in RCS rats. Cell
Signal. 24:685–698. 2012. View Article : Google Scholar
|
|
18
|
Go RE, Hwang KA, Kim SH, Lee MY, Kim CW,
Jeon SY, Kim YB and Choi KC: Effects of anti-obesity drugs,
phentermine and mahuang, on the behavioral patterns in
Sprague-Dawley rat model. Lab Anim Res. 30:73–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin B, Koizumi A, Tanaka N, Panda S and
Masland RH: Restoration of visual function in retinal degeneration
mice by ectopic expression of melanopsin. Proc Natl Acad Sci USA.
105:16009–16014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinilla I, Lund RD and Sauvé Y:
Contribution of rod and cone pathways to the dark-adapted
electroretinogram (ERG) b-wave following retinal degeneration in
RCS rats. Vision Res. 44:2467–2474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Münch M and Kawasaki A: Intrinsically
photosensitive retinal ganglion cells: Classification, function and
clinical implications. Curr Opin Neurol. 26:45–51. 2013. View Article : Google Scholar
|
|
22
|
Pickard GE and Sollars PJ: Intrinsically
photosensitive retinal ganglion cells. Rev Physiol Biochem
Pharmacol. 162:59–90. 2012.
|
|
23
|
Ramsey DJ, Ramsey KM and Vavvas DG:
Genetic advances in ophthalmology: The role of
melanopsin-expressing, intrinsically photosensitive retinal
ganglion cells in the circadian organization of the visual system.
Semin Ophthalmol. 28:406–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Semo M, Gias C, Ahmado A and Vugler A: A
role for the ciliary marginal zone in the melanopsin-dependent
intrinsic pupillary light reflex. Exp Eye Res. 119:8–18. 2014.
View Article : Google Scholar
|
|
25
|
Hu C, Hill DD and Wong KY: Intrinsic
physiological properties of the five types of mouse ganglion-cell
photoreceptors. JNeurophysiol. 109:1876–1889. 2013. View Article : Google Scholar
|
|
26
|
Güler AD, Ecker JL, Lall GS, Haq S,
Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, et al:
Melanopsin cells are the principal conduits for rod-cone input to
non-image-forming vision. Nature. 453:102–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gamlin PD, McDougal DH, Pokorny J, Smith
VC, Yau KW and Dacey DM: Human and macaque pupil responses driven
by melanopsin-containing retinal ganglion cells. Vision Res.
47:946–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ecker JL, Dumitrescu ON, Wong KY, Alam NM,
Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM and Hattar S:
Melanopsin-expressing retinal ganglion-cell photoreceptors:
Cellular diversity and role in pattern vision. Neuron. 67:49–60.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grünert U, Jusuf PR, Lee SC and Nguyen DT:
Bipolar input to melanopsin containing ganglion cells in primate
retina. Vis Neurosci. 28:39–50. 2011. View Article : Google Scholar
|
|
30
|
Padilla E, Shumake J, Barrett DW, Holmes
G, Sheridan EC and Gonzalez-Lima F: Novelty-evoked activity in open
field predicts susceptibility to helpless behavior. Physiol Behav.
101:746–754. 2010. View Article : Google Scholar : PubMed/NCBI
|