Introduction
Increasing numbers of radioactive sources have been
widely used as nuclear-generated energy for engineering and in
nuclear weapons (1). As a medical
diagnosis and treatment method, radiotherapy is ubiquitously
applied in cancer treatment, owing to its significant elevation in
patient survival rates (2–4). However, radiotherapy presents latent
hazards, which lead to severe toxic effects on the healthy tissues
of patients (5). Due to its
harmful side-effects, it is necessary to investigate the cellular
reaction caused by irradiation at the genetic level.
Cell senescence is one of the common side-effects of
lung cancer treatment by radiotherapy. Cell senescence may lead to
irreversible cell cycle arrest, which maintains cell viability and
metabolically activity, but resistance to apoptosis and
proliferation occurs (6). Cell
senescence may also result in various pathological changes in
different types of cell (7–11). A
previous study demonstrated that pneumocyte senescence induced by
irradiation may promote severe radiation-induced lung injury
(RILI), which is a progressive, life-threatening complication
characterized by interstitial infiltrates, dyspnoea and pulmonary
dysfunction that can result in respiratory failure (12). At present, there are few
potentially effective therapeutics for the treatment of RILI
(13). Therefore, elucidating the
genetic pathophysiology of pneumocyte senescence may be a useful
strategy to prevent patients from developing severe RILI during
lung cancer treatment.
Gene expression microarrays allow the measurement of
alterations in genetic expression patterns, and facilitate the
identification of genes, which are crucial to diseases induced by
irradiation (14). Xie et
al (15) used a cDNA
microarray to analyze miRNA and mRNA expression levels in rat lung
tissue samples 3, 12 and 26 weeks following exposure to 24-Gy X-ray
irradiation. The results confirmed that the miRNA expression levels
were negatively correlated with the mRNA expression levels
(16). They also demonstrated that
RILI did not develop in a single linear process (16). Chauhan et al (16) identified 67 upregulated and 141
downregulated genes in human lung fibroblast cells 24 h following
0–1.5-Gy X-ray irradiation, compared with the expression profile of
untreated lung fibroblasts cells. These genes were involved in cell
cycle control/mitosis, chromosome instability and cell
differentiation (16). Gene
Ontology and pathway enrichment analyses of genes enable the
molecular pathogenesis of irradiation to be elucidated.
It is necessary to extract available information by
discarding redundant or 'noisy' information from high-throughput
data sets. With a systemic biological view, WGCNA is a novel
approach, which quantitatively measures the interconnectivity of
genes, and reveals the importance of genes within networks. WGCNA
is a useful tool for detecting gene modules that maintain genes
with similar expression patterns, as well as for identifying
disease biomarkers and the functions of genes (17). Furthermore, due to the fact that
less false positive correlations are found between genes using
WGCNA, it is widely utilized to investigate complex diseases,
including endometrial cancer (18), schizophrenia (19) and breast cancer (20).
In the present study, based on the microarray data
of pneumocyte senescence induced by irradiation, WGCNA was used to
construct a scale-free weighted genetic interaction network
comprising specific gene modules that maintain common biological
roles in the process of pneumocyte senescence. Moreover, in a given
gene module, the present study attempted to identify hub genes as
candidate biomarkers and as therapeutic targets for pneumocyte
senescence.
Materials and methods
Microarray data, processing and
differentially expressed gene filtering
The high-throughput data was deposited in the Gene
Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/), which is the
predominant public repository for microarray data (21). The transcription profiles of
GSE41789 (Affymetrix mouse 430_2 GeneChips; Affymetrix, Inc., Santa
Clara, CA, USA), submitted by Citrin et al and updated in
2014 (2), were downloaded from the
GEO database. A total of 30 mouse lung tissue samples from the
dataset were selected and divided into non-senescence (n=15) and
senescence (n=15) subgroups. The non-senescence group (n=15)
pneumocytes exhibited no signature features of senescence
(senescence-associated β-galactose) following thorax X-ray
irradiation (Precision X-Ray, North Branford, CT, USA) at a dose of
0 Gy, whereas the senescence group (n=15) exhibited the signature
of pneumocyte senescence following thorax X-ray irradiation at
doses of 5 or 17.5 Gy (2).
The multi-microarray raw data of the CEL files were
then corrected, quantile normalized, and log2 transformed with the
rma function using the Affy package in R 3.0.3 software in
Bioconductor (http://www.bioconductor.org/) (22,23).
Only the perfectly matched probes were maintained for further
analysis, and mismatched probes were discarded. The collapseRows
and intersect functions of the WGCNA package were used to combine
multiple probes by the highest intensity values. The differentially
expressed genes between the non-senescence and senescence groups
were identified using Student's t-test with R software, and
only genes with P≤0.05 (n=2,916 genes) were considered for
subsequent network analysis (24).
In addition, the annotation information of the GeneChip was
obtained from the GPL1216 microarray platform (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL1261).
Pneumocyte-weighted gene co-expression
network construction
The differentially expressed genes were used for
weighted gene co-expression network construction in the WGCNA
package (25). In the WGCNA
package, the discrete adjacency matrix is replaced with the
weighted adjacency matrix considering a continuous connection
strength ([0,1]) with respect to the β parameter. In the present
study, β=9 was selected, according to the scale-free topology
criterion proposed by Zhang and Horvath (17). Following the definition of the
weighted adjacency matrix for each group (senescence and
non-senescence), the co-expression matrix and the topological
overlap matrix (TOM) were established. The TOM reflects the
relative interconnectivity between two genes according to their
degree of shared neighbors across whole network (17). The gene co-expression networks were
constructed using the blockwiseModules function in the WGCNA
package of the R software.
Pneumocyte module analysis
Module detection is the primary strategy for
reducing high-dimensional microarray data (26). In the present study, the
differentially expressed genes were considered in performing module
detection. Modules were defined as groups of genes with high
topological overlap (TO). Using the average linkage hierarchical
clustering coupled with the TO, the present study detected the
modules of the senescence and non-senescence groups, respectively.
The intramodular connectivity, known as the intramodule degree, of
the genes was also evaluated (27). The module eigengene (ME), which is
defined as the first principal component of a given module, was
then calculated. The ME can also be considered as a representative
of the gene expression profiles in a module (28). The intramodular Connectivity and
signed KME functions in the WGCNA package were used to compute
intramodular connectivity and the ME.
Module preservation evaluation
Module preservation statistics supply information
about whether the properties of a module in a network are altered
under different conditions. For the correlation network, the
following composite module preservation statistic:
Zsummary score [Zsummary =
(Zdensity + Zconnectivity)/2)] was
recommended by Langfelder et al (29), and has been used extensively in
previous studies (30,31). The Zdensity emphasizes
whether the genes in each of the defined modules in the reference
network remain highly connected in the experimental network,
whereas Zconnectivity identifies whether the
connectivity patterns between the genes in the experimental network
remain similar, compared with the reference network. Simulations or
permutation tests are used to determine the thresholds for
Zsummary. A Zsummary value <2 suggests
that there is no evidence for module preservation,
Zsummary values >2 and <10 indicate weak to
moderate evidence of module preservation, and Zsummary
values >10 indicate substantial evidence for module
preservation. Notably, the Zsummary score is
size-dependent, and tends to increase with module size. Another,
less size-dependent, preservation statistic is the medianRank, as
follows: (medianRankdensity +
median-Rankconnectivity)/2, which is recommended to
assess module preservation (29).
Generally, a module with a lower median rank tends to exhibit more
marked preservation than a module with a high median rank.
Combining the Zsummary with preservation medianRank was
considered in the assessment of module preservation in the present
study (29,32). Module preservation was evaluated
using the modulePreservation function of the WGCNA package.
Measurement of gene significance (GS) and
module membership (MM)
The GS of a gene is defined as the differential
expression between non-senescence and senescence groups, determined
using a t-test, and is evaluated using the P-value obtained
from the t-test, as GSi =
-log(pi) (33).
The GS for each gene in a module was calculated to quantitatively
assess how connected the gene was with senescence. Using the ME,
the MM was measured, and was determined as the correlation between
the gene expression profile and the ME. The MM is characterized by
correlating the expression profile of the i-th gene with the
ME of a given module, as follows: MMi = |cor
(x(i), ME)|. The
i-th gene is not part of the module if MMi
is close to 0. In addition, the i-th gene is deemed to have
a high level of connectivity to a given module if the
MMi is close to 1. The MM is highly correlated
with intramodular connectivity, and the highly connected
intramodular genes tend to have high MM values correlated with
their respective module (17,33).
Hub gene analyses
Certain terminology may be considered prior to
identifying the hub genes. Generally, intramodular hub genes
exhibit higher biologically significance, compared with global
network hub genes. Intramodular connectivity assesses the
connection strength among the genes, and intramodular connectivity
is considered more reliable than differential expression assessed
using Student's t-test (17). The GS incorporates external
information into the co-expression network; higher absolute values
of GSi indicate higher biological significance of
the i-th gene. The MM assesses the correlation between a
gene and the ME in a given module. Therefore, an ideal hub gene
possesses the highest intra-modular connectivity, highest MM and
highest GS within a given module (19,30).
Results
Weighted co-expression network
construction with respect to the senescence and non-senescence
groups
Network construction was restricted to 2,916
differentially expressed genes between the non-senescence and
senescence groups, which were determined using t-tests (P≤0.05).
The weighted gene co-expression networks were constructed with
respect to each group using the WGCNA package of the R software.
Based on the TOM, a hierarchical average linkage clustering method
was used to cluster the genes into modules. The number of modules
was detected using Dynamic Tree Cut (deep split = 2, cut height =
0.99; http://www.genetics.ucla.edu/labs/horvath/CoexpressionNetwork/BranchCut),
which is a novel cluster detection technique which uses an
iteration of an adaptive process of cluster decomposition and
combination until the number of clusters becomes stable. In the
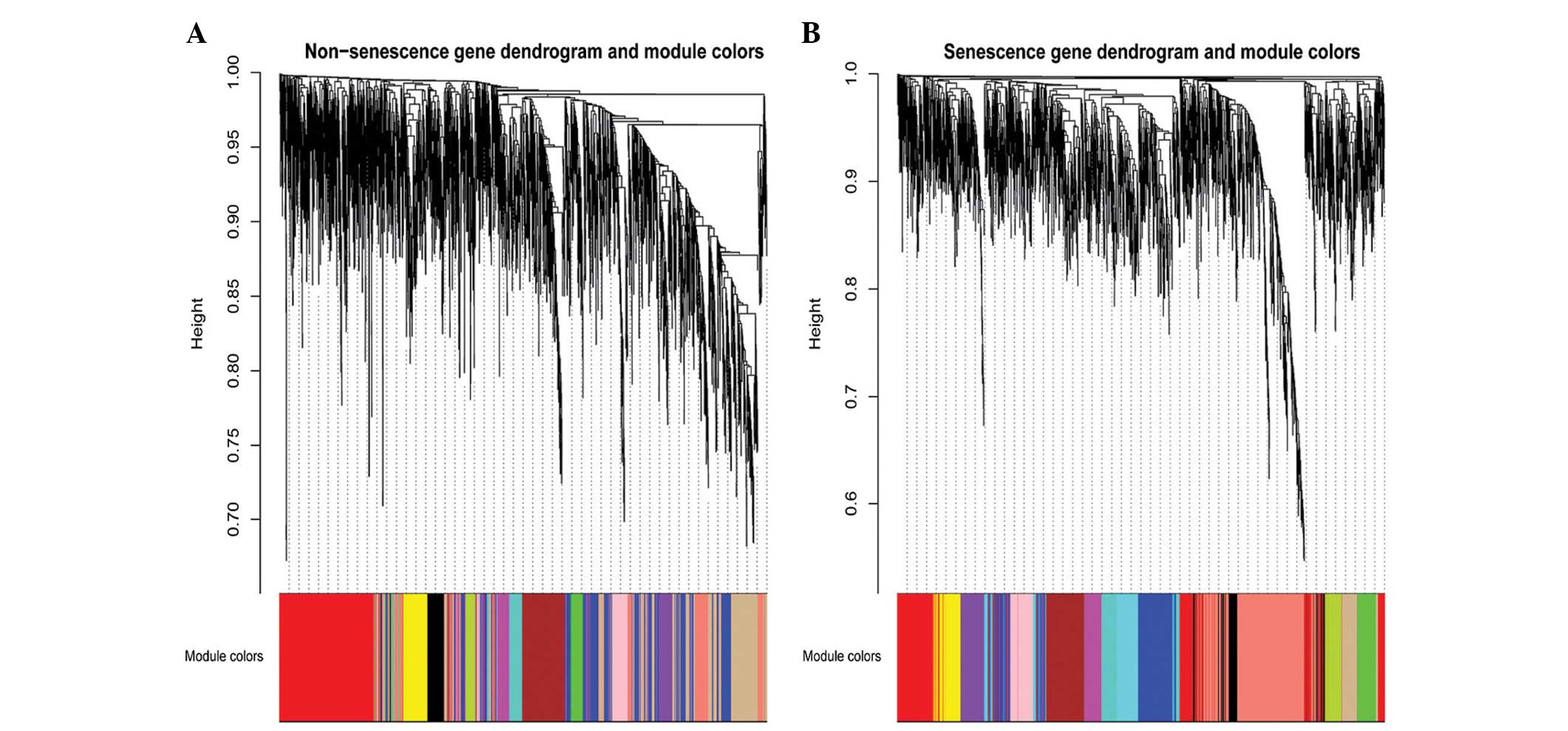
senescence group, 13 modules were detected, and in the
non-senescence group, 12 modules were detected (Fig. 1). Genes that did not cluster into
any of the modules were retained in the grey module in the WGCNA
package.
Screening of specific modules associated
with pneumocyte senescence
The strategy for screening the gene modules of
interest associated with pneumocyte senescence depended on the
preservation statistics. Module preservation statistics are based
on a permutation test implemented in the modulePreser-vation
function of the WGCNA package. The non-senescence network was
referred to as the reference network in the preservation
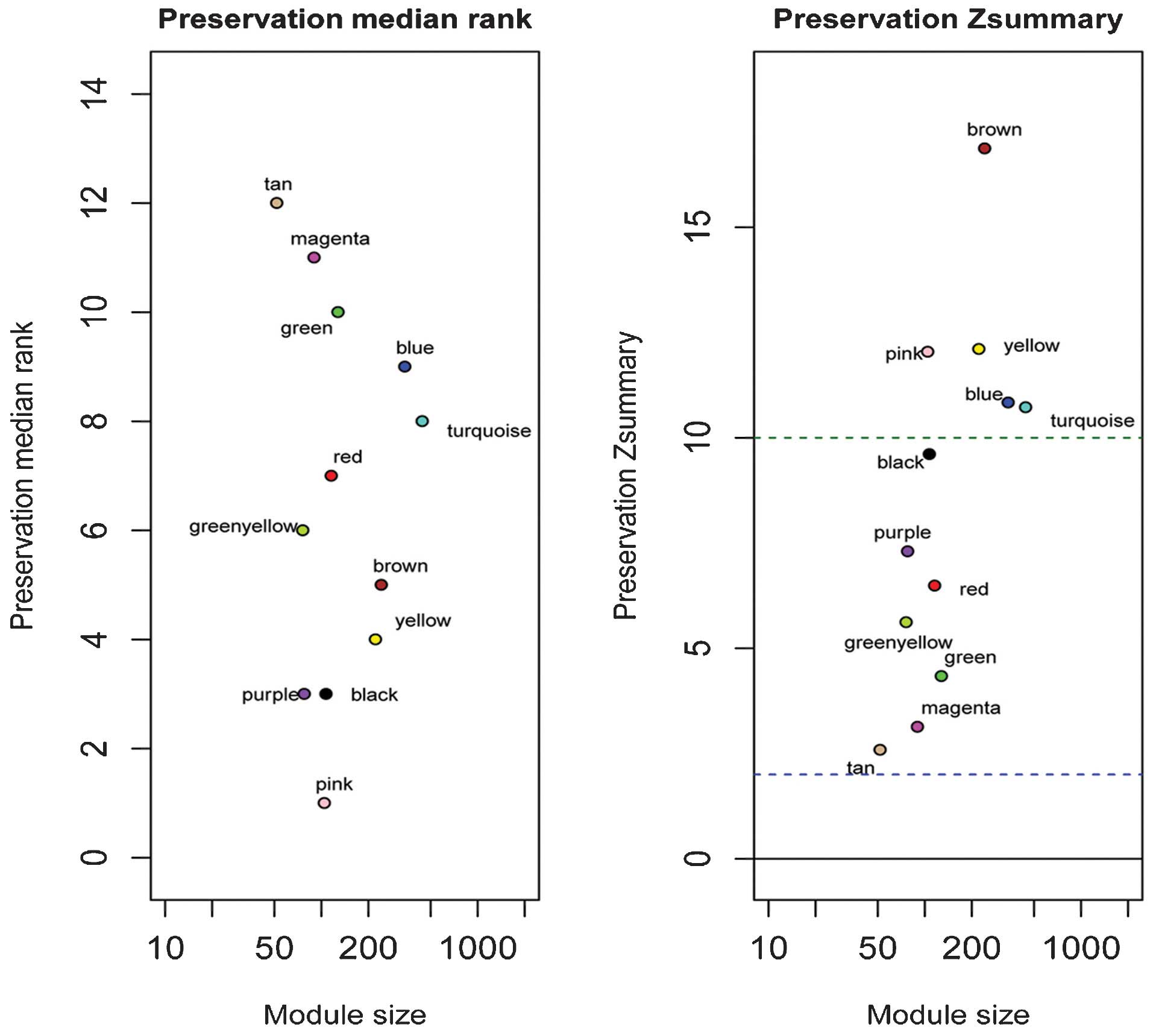
statistics. In total, nine gene modules were identified with small
Zsummary values and large preservation median rank
values, including the blue, brown, green, green/yellow, magenta,
red, tan, turquoise and yellow modules. These nine modules
exhibited significantly altered intramodular connectivity in the
senescence group, compared with the non-senescence group following
irradiation exposure (29)
(Fig. 2). The salmon-colored
module was detected only in the senescence group, and this module
was specific to the senescence group exposed to irradiation.
Therefore, a total of 10 modules were selected as modules that may
be important in cellular senescence. The remaining black, pink and
purple gene modules were well-preserved in the two groups, and were
excluded from the following analysis.
Candidate genes associated with
senescence
Another aim of the weighted network analysis was to
identify the hub genes associated with irradiation-induced
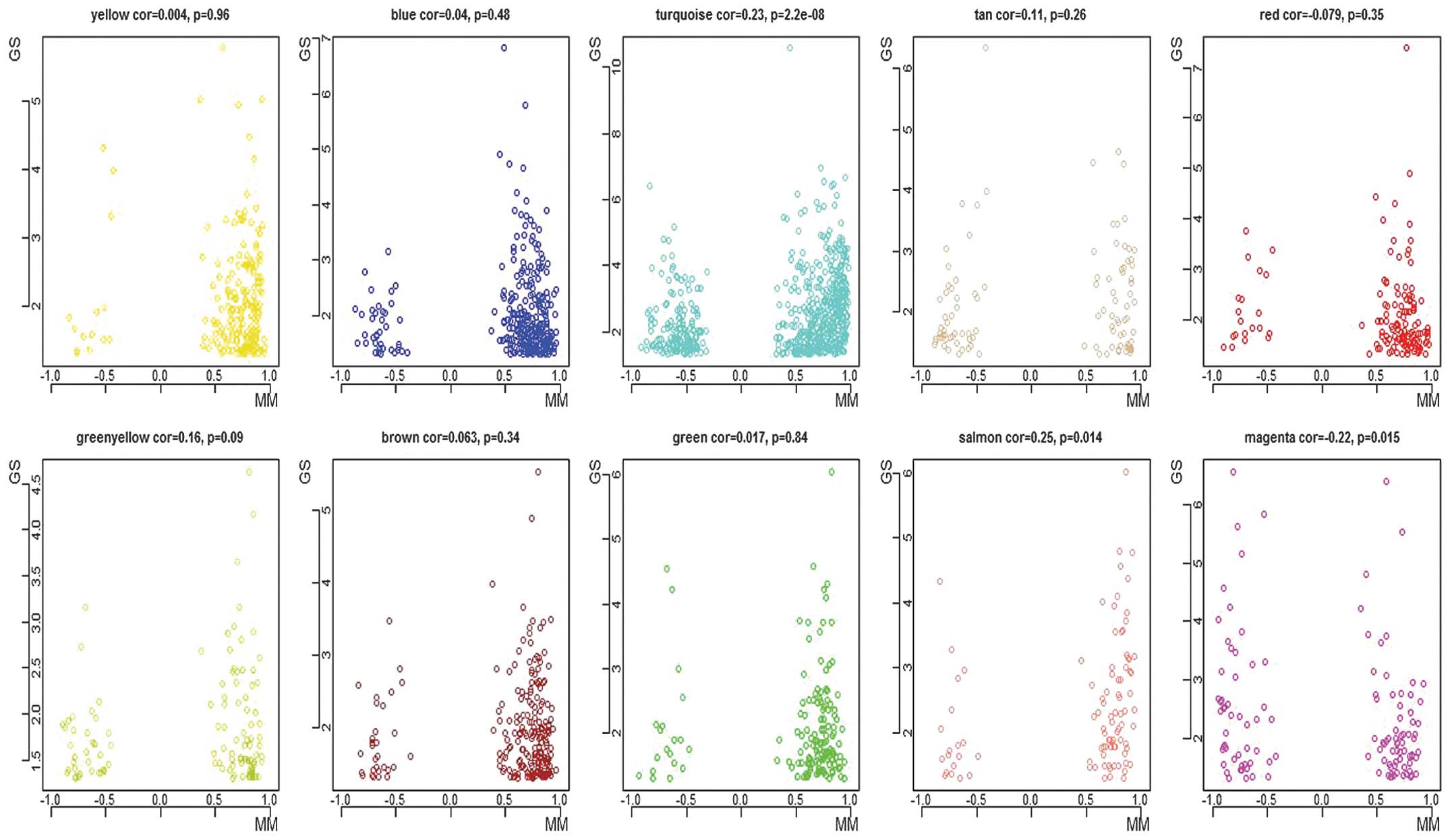
senescence. It is well-established that the MM measures the
importance of a node (gene) within a network, and that the GS
indicates the differential degree of the node under different
conditions. A node with maximum connectivity strength is centrally
located in the network (17). A
marked positive correlation was observed between MM and
intramodular connectivity in the modules of interest. However, no
correlation was observed between GS and intramodular connectivity,
or between GS and MM in the modules of interest (Fig. 3). Therefore, the hub genes were
identified in each module of interest, predominantly by relying on
high MM values, owing to the weak correlation between the GS and
MM. Accordingly, 10 hub genes were identified with respect to the
modules of interest, including translocase of outer mitochondrial
membrane 70 homolog A (Tomm70a; MM.blue = 0.96; P=0.02),
actin filament-associated protein 1 (Afap1; MM.brown = 0.97;
P=0.04), Zfp518b (MM.green = 0.94; P=0.03), Tbc1d9b
(MM. greenyellow = 0.93; P=0.03), Cd84 (MM.magenta = -0.95;
P=0.002), Nuf2 (MM.red = 0.98; P=0.03), B-cell scaffold
protein with ankyrin repeats 1 (Bank1; MM.salmon = 0.94;
P=0.001), Gm6377 (MM.tan = 0.94; P=0.0009), nuclear factor
erythroid 2 (NFE2; MM.turquoise = 0.98; P=0.001), and
Slc25a15 (MM.yellow = 0.95; P=0.04).
Annotation and functional enrichment
analysis of co-expressed modules
Gene ontology (GO) functional annotation and
enrichment analyses were used to identify the significantly
enriched biological terms for genes in the modules of interest
(26). GO enrichment analysis was
performed using Database for Annotation, Visualization and
Integrated Discovery (DAVID) software (http://david.abcc.ncifcrf.gov/) by uploading a probe
set to DAVID and initiating the Annotation Tool. Functional
annotations enriched in the modules of interest are shown in
Table I. A total of 10 biological
processes were involved in the modules of interest: RNA processing
(blue), mesoderm development (brown), apoptotic mitochondrial
changes (green), regulation of the hippo signaling cascade
(green/yellow), regulation of cell development (magenta), cell
division (red), the immune system process (salmon), signal
transduction (tan), the immune system process (turquoise) and the
phenol-containing compound metabolic process (yellow).
 | Table ITop functional annotations enriched
in the cellular senescence-specific modules for term ontology
'Biological process'. |
Table I
Top functional annotations enriched
in the cellular senescence-specific modules for term ontology
'Biological process'.
| Module | Term name | P-value |
|---|
| Blue | RNA processing |
4.42×10−9 |
| Blue | mRNA metabolic
process |
1.19×10−6 |
| Brown | Mesoderm
development |
6.72×10−5 |
| Brown | Protein
localization |
2.18×10−3 |
| Green | Apoptotic
mitochondrial changes |
3.20×10−5 |
| Green | Regulation of
mitochondrion organization |
5.09×10−5 |
| Green/yellow | Hippo signaling
cascade |
6.27×10−5 |
| Green/yellow | Regulation of hippo
signaling cascade |
1.59×10−3 |
| Magenta | Regulation of cell
development |
2.90×10−4 |
| Magenta | Positive regulation
of catalytic activity |
3.64×10−4 |
| Red | Cell division |
5.48×10−11 |
| Red | Cell cycle |
4.78×10−9 |
| Salmon | Immune system
process |
5.34×10−7 |
| Salmon | Cell surface
receptor signaling pathway |
6.48×10−7 |
| Tan | Signal
transduction |
1.48×10−7 |
| Tan | Immune
response |
5.23×10−7 |
| Turquoise | Immune system
process |
1.56×10−22 |
| Turquoise | Response to
wounding |
9.28×10−20 |
| Yellow | Phenol-containing
compound metabolic process |
3.20×10−3 |
| Yellow | Neurotransmitter
catabolic process |
5.06×10−3 |
Pathway enrichment analysis of
co-expression modules
The Kyoto Encyclopaedia of Genes and Genomes (KEGG)
(http://www.genome.jp/kegg/) database was
used to enrich the biological signaling pathways for the 10
identified modules. The pathway enrichment analysis was implemented
using DAVID software by assigning a probe set to KEGG metabolic
processes and testing the statistical enrichment of the target gene
in KEGG pathways. The most highly enriched pathways in each module
were as follows: Cell cycle (P=1.60×10−6) in the blue
module; cell cycle (P=1.67×10−3) in the brown module;
glycolysis/gluconeogenesis (P=1.50×10−2) in the green
module; spliceosome (P=2.22×10−2) in the green/yellow
module; steroid biosynthesis (P=6.38×10−3) in the
magenta module; proteasome (P=9.40×10−3) in the red
module; melanoma (P=9.63×10−3) in the salmon module;
renal cell carcinoma (P=1.43×10−2) in the tan module;
protein processing in endoplasmic reticulum (ER;
P=3.79×10−4) in the turquoise module and linoleic acid
metabolism (P=2.92×10−3) in the yellow module (Table II).
 | Table IIMost enriched pathways in the
cellular senescence-specific modules. |
Table II
Most enriched pathways in the
cellular senescence-specific modules.
| Module | Pathway name | Number of
genes | P-value |
|---|
| Blue | Cell cycle | 12 |
1.60×10−6 |
| Blue | DNA
replication | 5 |
3.02×10−4 |
| Brown | Cell cycle | 7 |
1.67×10−3 |
| Brown | mTOR signaling
pathway | 4 |
5.73×10−3 |
| Green |
Glycolysis/Gluconeogenesis | 3 |
1.50×10−2 |
| Green | Oxidative
phosphorylation | 4 |
3.45×10−2 |
| Green/yellow | Spliceosome | 4 |
2.22×10−2 |
| Green/yellow | Pentose and
glucuronate interconversions | 2 |
2.41×10−2 |
| Magenta | Steroid
biosynthesis | 2 |
6.38×10−3 |
| Magenta | Alzheimer's
disease | 4 |
3.73×10−2 |
| Red | Proteasome | 3 |
9.40×10−3 |
| Red | Oxidative
phosphorylation | 5 |
1.40×10−2 |
| Salmon | Melanoma | 3 |
9.63×10−3 |
| Salmon | Prostate
cancer | 3 |
1.71×10−2 |
| Tan | Renal cell
carcinoma | 3 |
1.43×10−2 |
| Tan | Rheumatoid
arthritis | 3 |
2.02×10−2 |
| Turquoise | Protein processing
in endoplasmic reticulum | 16 |
3.79×10−4 |
| Turquoise | PPAR signaling
pathway | 10 |
5.66×10−4 |
| Yellow | Linoleic acid
metabolism | 4 |
2.92×10−3 |
| Yellow | Cell cycle | 6 |
6.12×10−3 |
Discussion
In the present study, 10 specific modules and 10 hub
genes associated with pneumocyte senescence were identified, of
which the salmon module was only observed in the senescence group.
The GO enrichment of the salmon module suggested that the immune
process may be involved in senescence. Accordingly, Bank1
was identified as a hub gene of this module, and was significantly
upregulated (P=0.0007) in the senescence group. Bank1
encodes a B cell-specific scaffold protein, which functions in the
B cell receptor (35).
Bank1 has been associated with systemic lupus erythematosus
and diffuse systemic sclerosis (36,37).
Using Bank1 deficient-mice, it has been previously
demonstrated that BANK1 acts as a negative regulator of
CD40-mediated protein kinase B activation to prevent hyperactive
responses. The absence of BANK1 reduced the secretion of
interleukin-6 via the p38/mitogen-activated protein kinase 1/2
signaling pathway and decreased the expression levels of
translation initiation factor eIF4e in B cells. Notably, high
expression levels of eIF4E promotes cell proliferation in carcinoma
(38). Whether BANK1 is associated
with cellular senescence remains to be fully elucidated, however,
these results provide a novel research direction for further
investigations to clarify the importance of Bank1 during the
cellular senescence process.
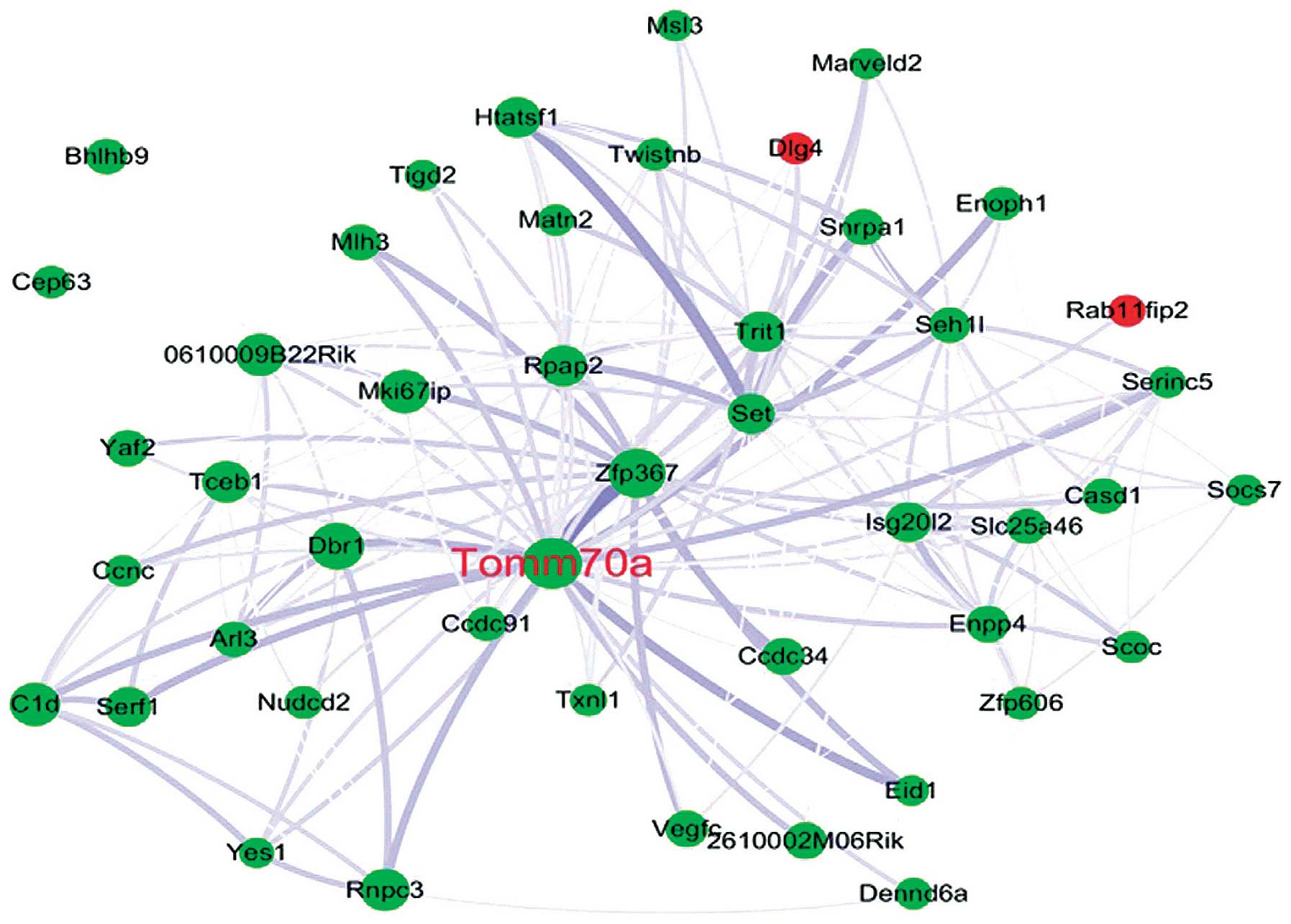
In the present study, the blue module was determined
as the RNA-modifying module, based on the GO analysis.
Tomm70a, which encodes the TOM70 protein in humans, was
selected as the hub gene in the blue module and was downregulated
during senescence (P=0.019). TOM70 is a subunit of the outer
mitochondrial membrane translocase, which acts as a receptor for
hydrophobic pre-proteins targeted to the mitochondria and is
involved in importing the majority of mitochondrial proteins from
the cytosol into the mitochondria (39). For example, ribosomal protein S3
(rpS3) may be effectively transported into the mitochondria via the
interaction between TOM70, heat shock protein (HSP)90 and HSP70.
When rpS3 accumulates in the mitochondria, it repairs damaged
mitochondrial DNA and decreases the levels of reactive oxygen
species (ROS) (40). rpS3 not only
mediates cellular anti-apoptosis by binding the p65 protein
(41), it also induces interferon
(IFN)-β production (42,43). It is well-established that IFN-β
inhibits cell proliferation and arrests the cell cycle by targeting
the p53 signaling pathway (44).
In addition, TOM70 is a member of the TOM machinery, a molecular
switch of the phosphatase and tensin homolog-induced putative
kinase 1-PARKIN signaling pathway, which clears cell remnants in an
autophagy-dependent manner in mitochondria (45). Once this signaling pathway is
interrupted, cellular senescence is induced via ROS and the p53
signaling pathway (46). Based on
these results, Tomm70a may be a novel hub gene associated
with pneumocyte senescence induced by thoracic irradiation
(Fig. 4).
In the present study, the brown module was
predominantly enriched in the protein localization process by GO
analysis. Afap1 was selected as the hub gene of the brown
module and was significantly downregulated (P=0.04) in the
senescence group. AFAP1 regulates actin filament integrity,
podosome formation, focal contacts and cell migration (47). AFAP1 is an essential adaptor
protein, which activates c-Src tyrosine kinase by binding and
interacting with SH3 (48).
c-Src is a proto-oncogene with a wide range of substrates
and various functions in processes, including cell proliferation,
differentiation, cell cycle, adhesion, invasion and motility
(49). Zhang et al
(50) demonstrated that the loss
of AFAP1 in PC3 prostate cancer cells reduces cell proliferation
in vitro. Using an AFAPΔABD expression vector, a previous
study reported that AFAP1 activates c-Src and subsequently
elevates the expression levels of transcriptional factor activator
protein 1 (AP-1) (51). AP-1
modulates a wide range of cellular processes, including
proliferation, apoptosis, differentiation and survival (52). Therefore, the present study
hypothesized that AFAP1 regulates cell senescence through c-Src or
AP-1, however, further investigations are required in order to
confirm this hypothesis.
The magenta module was predominantly enriched in the
cell development and protein transport GO terms. Cd84
encodes the type I transmembrane glycoprotein CD84, and was
significantly upregulated (P=0.002) in the senescence group. CD84
is a member of the signaling lymphocyte activation molecule family,
which is expressed in the majority of immune cell populations
(53,54). A previous study has indicated that
CD84 promotes early-stage chronic lymphocytic leukemia (CLL)
survival, and that cell death is induced in CLL following the
inhibition of CD84 in vitro and in vivo (55). By measuring the incorporation of
3H thymidine during the final 8 h of a 72-h culture
period, the investigators demonstrated that CD84 regulated the
proliferation of anti-CD3 monoclonal antibody-stimulated human T
cells (56). These studies
suggested that CD84 may be associated with the cell cycle and
survival of lymphocytes.
Based on the GO analysis in the present study, the
red module was denoted the cell cycle module. The evolutionarily
conserved gene Nuf2 was significantly downregulated in the
senescence group (P=0.03). Nuf2, also known as cell division cycle
associated 1 (CDCA1) is a kinetochore protein, which forms a
subcomplex with Hec1 and belongs to the larger Ndc80 complex
(57). The Nuf2-Hec1 complex
connects the plus ends of spindle microtubules to centromeres, and
is essential for correct chromosome segregation and genomic
stability during mitosis (57,58).
In HeLa cells, it has been confirmed that prometaphase is prolonged
during the mitosis, if Nuf2 or Hec1 was silenced by RNA
interference (59). The
interaction of Nuf2 and PTPIP51 recruits microtubules to the
kinetochore and ensures proper cellular proliferation,
differentiation and apop-tosis (60). In addition, the interaction of
CDCA1 and KNTC2 has been reported to induce cell cycle arrest in
non-small cell lung carcinoma, as well as in colorectal and gastric
cancer (61,62). Therefore, Nuf2 may be a hub
gene, as well as a therapeutic target of irradiation-induced tumor
and pneumocyte senescence.
In the present study, the turquoise module was
associated with immune system and wound healing in the GO analysis.
NFE2 was considered the hub gene and was significantly
upreg-ulated in the senescence group (P=0.001). NFE2 is a member of
the basic-leucine zipper family of heterodimeric transcriptional
activators, and comprises p45 and Maf subunits (63). Studies have confirmed that NFE2 is
an important transcriptional regulator of hemoglobin biosynthesis,
normal platelet function and erythroid differentiation (64,65).
In addition, NFE2 increases the expression levels of IL-8 to
stimulate CD34+ proliferation and survival in bone
marrow stromal cells, and ensure megakaryocyte proliferation and
differentiation in primary myelofibrosis (66). In red blood cells (RBCs), p45NFE2
decreases ROS levels in order to protect RBCs from oxidative stress
damage (67). Therefore,
NFE2 may be involved in inflammatory cell proliferation or
senescence during irradiation damage.
The four hub genes identified in the remaining
modules included Zfp518b (green), Tbc1d9b
(greenyellow), Gm6377 (tan) and Slc25a15 (yellow). To
date, no reports regarding the roles of these four hub genes in the
cell cycle, proliferation or senescence are available.
The results of the present stead revealed a total of
70 KEGG signaling pathways in the senescence group, which were
enriched in the 10 modules of interest. Cell cycle, DNA replication
and lysine biosynthesis were most highly enriched in the blue
module, whereas the cell cycle and mammalian target of rapamycin
(mTOR) signaling pathways were the most highly enriched in the
brown module. mTOR is highly conserved across species. Rapamycin, a
specific inhibitor of mTOR, mitigates senescence progression in
HT-p21 cells (68), normal human
fibroblast WI-38 cells (69) and
ARPE-19 cells (70). Furthermore,
the oncogenic proteins RAF and RAS, which are known to activate the
mTOR signaling pathway, have been reported to mediate cellular
senescence (71,72). The glycolysis/gluconeogenesis and
oxidative phosphorylation signaling pathways were enriched in the
green module. Dichloroacetate mitigated senescence by suppressing
glycol-ysis, and a study by Liao et al (73) demonstrated the activation of
glyceraldehyde-3-phosphate dehydrogenase and the upregu-lation of
glycolysis in radiation-induced human breast cancer cell
senescence. The spliceosome biological pathway was the most highly
enriched in the green-yellow module. In WI-38, WiDr, HeLa and
HEK239 cells, the cell cycle is blocked in the G1 and
G2/M phases upon the deletion or inhibition of splicing
factors by small interfering RNA or inhibitors (74-76).
A previous investigation also reported the impairment of
spliceosome assembly-promoted cell cycle arrest in the S and
G2/M phases (76). The
proteasome and oxidative phos-phorylation signaling pathways were
the most highly enriched pathways in the red module. The
ubiquitination/proteasome signaling pathway is crucial for
determining protein fate post-translation (77). p53 is a substrate of Mdm2, which is
an E3 ubiquitin ligase of the proteasome signaling pathway, and
Mdm2 regulates the stability and activity of p53, ultimately
contributing to the process of cellular senescence (76). The mitogen-activated protein kinase
(MAPK) signaling pathway was significantly enriched in the salmon
module. The MAPK signaling pathway has long been associated with
cell senescence. For example, ROS or oncogene-induced senescence
enables activation of the p38 MAPK signaling pathway through the
activation of McKusick-Kaufman syndrome 3/6, which subsequently
promotes the expression of p16 and p53-p21, and increases the DNA
damage response (78). This
response suppresses cyclin-dependent kinase and ultimately
generates cell senescence (79).
In the tan module, the ribosome biogenesis pathway was the most
significantly enriched pathway. A previous study investigating
dyskeratosis congenita demonstrated that the failure of ribosome
biogenesis and the induction of DNA damage activates the p53
signaling pathway and induces cell senescence in X-DC or AD-DC
fibroblasts (80). In the
turquoise module in the present study, protein processing in the ER
was the most highly enriched signaling pathway. The ER is the
principal organelle for several cellular functions, including
protein folding, maturation and the maintenance of cellular
homeostasis (81). Wiel et
al (82) reported that the
calcium channel, inositol 1,4,5-trisphosphate receptor type 2 and
mitochondrial calcium uniporter led to calcium release from the ER
and mitochondrial, respectively, resulting in the induction of
cellular senescence. This pathway is independent of the
retinoblastoma and p53 pathways (82). In addition, a previous study
reported the repression of ER stress and the promotion of
oncogene-induced cellular senescence by peroxisome
proliferator-activated receptor β/δ in mouse keratinocytes
(83). The linoleic acid
metabolism signaling pathway was enriched in the yellow module.
Linoleic acid is a polyunsaturated essential fatty acid, which
maintains normal physiological functions and is involved in disease
prevention (84). It has been
demonstrated that linoleic acid affects the cell cycle and
proliferation. For example, in mouse embryonic stem cells, Kim
et al (85) reported that
linoleic acid induces the cell cycle through multiple signaling
pathways, including Ca2+/protein kinase C,
phosphoinositide 3 kinase and MAPKs. In addition, in mouse
pancreatic β-cells, others have demonstrated that linoleic acid
elevates the expression levels of cell cycle inhibitors, p16 and
p18, in vivo and in vitro.
The results of the present study enabled the
identification of the gene modules and hub genes which exhibit
crucial biological activity in pneumocyte senescence induced by
thoracic irradiation. The modules with different activities were
involved in the progress of senescence, including immune system
responses, post-transcriptional modifications, post-translational
modifications, cell signal transduction pathways, cellular
motility, hormone regulation and biomolecule metabolism. The
analyses demonstrated that various significant responses arose
during irradiation-induced pneumocyte senescence. Hub genes
associated with cellular senescence may offer an important source
of novel hypotheses, experimental directions and novel therapeutic
targets. The results of the present study not only further current
understanding of the mechanism of senescence, but also provide
considerable information for understanding RILI and for generating
innovative therapies to treat this life-threatening disease.
It is increasingly evident that biological functions
arise from complex interactions among macromolecules, including
proteins, DNA and RNA. Microarray techniques and gene co-expression
profile network analyses are becoming necessary to examine these
complex interactions. The novel WGCNA method is a popular
microarray data analysis approach due to its lower rate of false
positive connections and its impressive module detection ability.
From a systemic perspective, the results of the present study
provide a comprehensive summary of irradiation-induced pneumocyte
senescence analyzed using the WGCNA method.
Acknowledgments
The present study was supported by grants from the
National Program on Key Basic Research Project (Program 973; grant
no. 2011CB964800-G) and the National Natural Science Foundation of
China (grant nos. 81129020 and 81372928).
References
|
1
|
Wang J, Feng J, Jia W, Chang S, Li S and
Li Y: Lignin engineering through laccase modification: A promising
field for energy plant improvement. Biotechnol Biofuels. 8:1452015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Citrin DE, Shankavaram U, Horton JA,
Shield W III, Zhao S, Asano H, White A, Sowers A, Thetford A and
Chung EJ: Role of type II pneumocyte senescence in
radiation-induced lung fibrosis. J Natl Cancer Inst. 105:1474–1484.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schabath MB, Nguyen A, Wilson P, Sommerer
KR, Thompson ZJ and Chiappori AA: Temporal trends from 1986 to 2008
in overall survival of small cell lung cancer patients. Lung
Cancer. 86:14–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pedicini P, Strigari L, Benassi M, Caivano
R, Fiorentino A, Nappi A, Salvatore M and Storto G: Critical dose
and toxicity index of organs at risk in radiotherapy: analyzing the
calculated effects of modified dose fractionation in non-small cell
lung cancer. Med Dosim. 39:23–30. 2014. View Article : Google Scholar
|
|
6
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linton PJ, Gurney M, Sengstock D, Mentzer
RM Jr and Gottlieb RA: This old heart: Cardiac aging and autophagy.
J Mol Cell Cardiol. 83:44–54. 2015. View Article : Google Scholar
|
|
8
|
Park YH: Stem cell therapy for
sensorineural hearing loss, still alive? J Audiol Otol. 19:63–67.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durham AL and Adcock IM: The relationship
between COPD and lung cancer. Lung Cancer. 90:121–127. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farup J, Madaro L, Puri PL and Mikkelsen
UR: Interactions between muscle stem cells, mesenchymal-derived
cells and immune cells in muscle homeostasis, regeneration and
disease. Cell Death Dis. 6:e18302015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mikhed Y, Daiber A and Steven S:
Mitochondrial oxidative stress, mitochondrial DNA damage and their
role in age-related vascular dysfunction. Int J Mol Sci.
16:15918–15953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hill RP: Radiation effects on the
respiratory system. Brit J Radiol Suppl. 27:75–81. 2005. View Article : Google Scholar
|
|
13
|
Ley B, Brown KK and Collard HR: Molecular
biomarkers in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell
Mol Physiol. 307:L681–L691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernandez Bort JA, Hackl M, Höflmayer H,
Jadhav V, Harreither E, Kumar N, Ernst W, Grillari J and Borth N:
Dynamic mRNA and miRNA profiling of CHO-K1 suspension cell
cultures. Biotechnol J. 7:500–515. 2012. View Article : Google Scholar
|
|
15
|
Xie L, Zhou J, Zhang S, Chen Q, Lai R,
Ding W, Song C, Meng X and Wu J: Integrating microRNA and mRNA
expression profiles in response to radiation-induced injury in rat
lung. Radiat Oncol. 9:1112014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chauhan V and Howland M: Gene expression
responses in human lung fibroblasts exposed to alpha particle
radiation. Toxicol In Vitro. 28:1222–1229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B and Horvath S: A general framework
for weighted gene co-expression network analysis. Stat Appl Genet
Mol Biol. 4:2005.
|
|
18
|
Chou WC, Cheng AL, Brotto M and Chuang CY:
Visual gene-network analysis reveals the cancer gene co-expression
in human endometrial cancer. BMC Genomics. 15:3002014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Jong S, Boks MP, Fuller TF, Strengman
E, Janson E, de Kovel CG, Ori AP, Vi N, Mulder F, Blom JD, et al: A
gene co-expression network in whole blood of schizophrenia patients
is independent of antipsychotic-use and enriched for
brain-expressed genes. PLoS One. 7:e394982012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clarke C, Madden SF, Doolan P, Aherne ST,
Joyce H, O'Driscoll L, Gallagher WM, Hennessy BT, Moriarty M, Crown
J, et al: Correlating transcriptional networks to breast cancer
survival: A large-scale coexpression analysis. Carcinogenesis.
34:2300–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrett T, Troup DB, Wilhite SE, Ledoux P,
Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Edgar
R, et al: NCBI GEO: Mining tens of millions of expression
profiles-database and tools update. Nucleic Acids Res. 35D760–D765.
(Database issue)2007. View Article : Google Scholar
|
|
22
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gentleman RC, Carey VJ, Bates DM, Bolstad
B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al:
Bioconductor: Open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tejera E, Bernardes J and Rebelo I:
Co-expression network analysis and genetic algorithms for gene
prioritization in preeclampsia. BMC Med Genomics. 6:512013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yip AM and Horvath S: Gene network
interconnectedness and the generalized topological overlap measure.
BMC Bioinformatics. 8:222007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lecca P and Re A: Detecting modules in
biological networks by edge weight clustering and entropy
significance. Front Genet. 6:2652015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langfelder P, Zhang B and Horvath S:
Defining clusters from a hierarchical cluster tree: The Dynamic
tree cut package for R. Bioinformatics. 24:719–720. 2008.
View Article : Google Scholar
|
|
28
|
Langfelder P and Horvath S: Eigengene
networks for studying the relationships between co-expression
modules. BMC Syst Biol. 1:542007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Langfelder P, Luo R, Oldham MC and Horvath
S: Is my network module preserved and reproducible? PLoS Comput
Biol. 7:e10010572011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan N, Chung MK, Smith JD, Hsu J, Serre D,
Newton DW, Castel L, Soltesz E, Pettersson G, Gillinov AM, et al:
Weighted gene coexpression network analysis of human left atrial
tissue identifies gene modules associated with atrial fibrillation.
Circ Cardiovasc Genet. 6:362–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peña-Castillo L, Mercer RG, Gurinovich A,
Callister SJ, Wright AT, Westbye AB, Beatty JT and Lang AS: Gene
co-expression network analysis in Rhodobacter capsulatus and
application to comparative expression analysis of Rhodobacter
sphaeroides. BMC Genomics. 15:7302014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Filteau M, Pavey SA, St-Cyr J and
Bernatchez L: Gene coexpression networks reveal key drivers of
phenotypic divergence in lake whitefish. Mol Biol Evol.
30:1384–1396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Yang XY and Shi WJ: Identifying
differentially expressed genes and pathways in two types of
non-small cell lung cancer: Adenocarcinoma and squamous cell
carcinoma. Genet Mol Res. 13:95–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aiba Y, Yamazaki T, Okada T, Gotoh K,
Sanjo H, Ogata M and Kurosaki T: BANK negatively regulates Akt
activation and subsequent B cell responses. Immunity. 24:259–268.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kozyrev SV, Abelson AK, Wojcik J, Zaghlool
A, Linga Reddy MV, Sanchez E, Gunnarsson I, Svenungsson E, Sturfelt
G, Jönsen A, et al: Functional variants in the B-cell gene BANK1
are associated with systemic lupus erythematosus. Nat Genet.
40:211–216. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rueda B, Gourh P, Broen J, Agarwal SK,
Simeon C, Ortego-Centeno N, Vonk MC, Coenen M, Riemekasten G,
Hunzelmann N, et al: BANK1 functional variants are associated with
susceptibility to diffuse systemic sclerosis in Caucasians. Ann
Rheum Dis. 69:700–705. 2010. View Article : Google Scholar :
|
|
38
|
Wu M, Liu Y, Di X, Kang H, Zeng H, Zhao Y,
Cai K, Pang T, Wang S, Yao Y and Hu X: EIF4E over-expresses and
enhances cell proliferation and cell cycle progression in
nasopharyngeal carcinoma. Med Oncol. 30:4002013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blesa JR, Hernández JM and Hernández-Yago
J: NRF-2 transcription factor is essential in promoting human
Tomm70 gene expression. Mitochondrion. 3:251–259. 2004. View Article : Google Scholar
|
|
40
|
Kim Y, Kim HD and Kim J: Cytoplasmic
ribosomal protein S3 (rpS3) plays a pivotal role in mitochondrial
DNA damage surveillance. Biochim Biophys Acta. 1833:2943–2952.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sen N, Paul BD, Gadalla MM, Mustafa AK,
Sen T, Xu R, Kim S and Snyder SH: Hydrogen sulfide-linked
sulfhydration of NF-kB mediates its antiapoptotic actions. Mol
Cell. 45:13–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu XY, Wei B, Shi HX, Shan YF and Wang C:
Tom70 mediates activation of interferon regulatory factor 3 on
mitochondria. Cell Res. 20:994–1011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kasama Y, Saito M, Takano T, Nishimura T,
Satoh M, Wang Z, Ali SN, Harada S, Kohara M and Tsukiyama-Kohara K:
Translocase of outer mitochondrial membrane 70 induces interferon
response and is impaired by hepatitis C virus NS3. Virus Res.
163:405–409. 2012. View Article : Google Scholar
|
|
44
|
Resnitzky D, Yarden A, Zipori D and Kimchi
A: Autocrine beta-related interferon controls c-myc suppression and
growth arrest during hematopoietic cell differentiation. Cell.
46:31–40. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bertolin G, Ferrando-Miguel R, Jacoupy M,
Traver S, Grenier K, Greene AW, Dauphin A, Waharte F, Bayot A,
Salamero J, et al: The TOMM machinery is a molecular switch in
PINK1 and PARK2/PARKIN-dependent mitochondrial clearance.
Autophagy. 9:1801–1817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang HT, Lee KB, Kim SY, Choi HR and Park
SC: Autophagy impairment induces premature senescence in primary
human fibroblasts. PLoS One. 6:e233672011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiao H, Han B, Lodyga M, Bai XH, Wang Y
and Liu M: The actin-binding domain of actin filament-associated
protein (AFAP) is involved in the regulation of cytoskeletal
structure. Cell Mol Life Sci. 69:1137–1151. 2012. View Article : Google Scholar
|
|
48
|
Snyder BN1, Cho Y, Qian Y, Coad JE, Flynn
DC and Cunnick JM: AFAP1L1 is a novel adaptor protein of the AFAP
family that interacts with cortactin and localizes to invadosomes.
Eur J Cell Biol. 90:376–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Frame MC: Newest findings on the oldest
oncogene; how activated src does it. J Cell Sci. 117:989–998. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang J, Park SI, Artime MC, Summy JM,
Shah AN, Bomser JA, Dorfleutner A, Flynn DC and Gallick GE:
AFAP-110 is overex-pressed in prostate cancer and contributes to
tumorigenic growth by regulating focal contacts. J Clin Invest.
117:2962–2973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han B, Xiao H, Xu J, Lodyga M, Bai XH, Jin
T and Liu M: Actin filament associated protein mediates c-Src
related SRE/AP-1 transcriptional activation. FEBS Lett.
585:471–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye N, Ding Y, Wild C, Shen Q and Zhou J:
Small molecule inhibitors targeting activator protein 1 (AP-1). J
Med Chem. 57:6930–6948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hofmann S, Vögtle T, Bender M, Rose-John S
and Nieswandt B: The SLAM family member CD84 is regulated by ADAM10
and calpain in platelets. J Thromb Haemost. 10:2581–2592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang A, Batteux F and Wakeland EK: The
role of SLAM/CD2 polymorphisms in systemic autoimmunity. Curr Opin
Immunol. 22:706–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Binsky-Ehrenreich I, Marom A, Sobotta MC,
Shvidel L, Berrebi A, Hazan-Halevy I, Kay S, Aloshin A, Sagi I and
Goldenberg DM: CD84 is a survival receptor for CLL cells. Oncogene.
33:1006–1016. 2014. View Article : Google Scholar
|
|
56
|
Tangye SG, Nichols KE, Hare NJ and van de
Weerdt BC: Functional requirements for interactions between CD84
and Src homology 2 domain-containing proteins and their
contribution to human T cell activation. J Immunol. 171:2485–2495.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Asakawa H, Hayashi A, Haraguchi T and
Hiraoka Y: Dissociation of the Nuf2-Ndc80 complex releases
centromeres from the spindle-pole body during meiotic prophase in
fission yeast. Mol Biol Cell. 16:2325–2338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sundin LJ, Guimaraes GJ and Deluca JG: The
NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to
kinetochore-microtubule attachment in mitosis. Mol Biol Cell.
22:759–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
DeLuca JG, Dong Y, Hergert P, Strauss J,
Hickey JM, Salmon ED and McEwen BF: Hec1 and nuf2 are core
components of the kinetochore outer plate essential for organizing
microtubule attachment sites. Mol Biol Cell. 16:519–531. 2005.
View Article : Google Scholar :
|
|
60
|
Brobeil A, Graf M, Eiber M and Wimmer M:
Interaction of PTPIP51 with Tubulin, CGI-99 and Nuf2 During cell
cycle progression. Biomolecules. 2:122–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kaneko N, Miura K, Gu Z, Karasawa H,
Ohnuma S, Sasaki H, Tsukamoto N, Yokoyama S, Yamamura A, Nagase H,
et al: siRNA-mediated knockdown against CDCA1 and KNTC2, both
frequently overexpressed in colorectal and gastric cancers,
suppresses cell proliferation and induces apoptosis. Biochem
Biophys Res Commun. 390:1235–1240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Suzuki H, Fukuhara M, Yamaura T, et al:
Multiple therapeutic peptide vaccines consisting of combined novel
cancer testis antigens and anti-angiogenic peptides for patients
with non-small cell lung cancer. J Transl Med. 11:972013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jung KA and Kwak MK: The Nrf2 system as a
potential target for the development of indirect antioxidants.
Molecules. 15:7266–7291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fujita R, Takayama-Tsujimoto M, Satoh H,
Gutiérrez L, Aburatani H, Fujii S, Sarai A, Bresnick EH, Yamamoto M
and Motohashi H: NF-E2 p45 is important for establishing normal
function of platelets. Mol Cell Biol. 33:2659–2670. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gasiorek JJ, Nouhi Z and Blank V: Abnormal
differentiation of erythroid precursors in p45 NF-E2(−/−) mice. Exp
Hematol. 40:393–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yigit N, Covey S, Barouk-Fox S, Turker T,
Geyer JT and Orazi A: Nuclear factor-erythroid 2, nerve growth
factor receptor, and CD34-microvessel density are differentially
expressed in primary myelofibrosis, polycythemia vera, and
essential thrombocythemia. Hum Pathol. 46:1217–1225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chan JY, Kwong M, Lo M, Emerson R and
Kuypers FA: Reduced oxidative-stress response in red blood cells
from p45NFE2-deficient mice. Blood. 97:2151–2158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Leontieva OV and Blagosklonny MV: Tumor
promoter-induced cellular senescence: Cell cycle arrest followed by
geroconversion. Oncotarget. 5:12715–12727. 2014. View Article : Google Scholar
|
|
69
|
Demidenko ZN and Blagosklonny MV: Growth
stimulation leads to cellular senescence when the cell cycle is
blocked. Cell Cycle. 7:3355–3361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Demidenko ZN, Zubova SG, Bukreeva EI,
Pospelov VA, Pospelova TV and Blagosklonny MV: Rapamycin
decelerates cellular senescence. Cell Cycle. 8:1888–1895. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Serrano M, Lin AW, McCurrach ME, Beach D
and Lowe SW: Oncogenic ras provokes premature cell senescence
associated with accumulation of p53 and p16INK4a. Cell. 88:593–602.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhu J, Woods D, McMahon M and Bishop JM:
Senescence of human fibroblasts induced by oncogenic Raf. Genes
Dev. 12:2997–3007. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liao EC, Hsu YT, Chuah QY, Lee YJ, Hu JY,
Huang TC, Yang PM and Chiu SJ: Radiation induces senescence and a
bystander effect through metabolic alterations. 5:e12552014.
|
|
74
|
Ghosh AK and Li J: A stereoselective
synthesis of (+)-herbox-idiene/GEX1A. Org Lett. 13:66–69. 2011.
View Article : Google Scholar :
|
|
75
|
Yokoi A, Kotake Y, Takahashi K, Kadowaki
T, Matsumoto Y, Minoshima Y, Sugi NH, Sagane K, Hamaguchi M, Iwata
M and Mizui Y: Biological validation that SF3b is a target of the
antitumor macrolide pladienolide. Febs J. 278:4870–4880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pawellek A, McElroy S, Samatov T, Mitchell
L, Woodland A, Ryder U, Gray D, Lührmann R and Lamond AI:
Identification of small molecule inhibitors of pre-mRNA splicing. J
Biol Chem. 289:34683–34698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hegde AN, Haynes KA, Bach SV and Beckelman
BC: Local ubiquitin-proteasome-mediated proteolysis and long-term
synaptic plasticity. Front Mol Neurosci. 7:962014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xu Y, Li N, Xiang R and Sun P: Emerging
roles of the p38 MAPK and PI3K/AKT/mTOR pathways in
oncogene-induced senescence. Trends Biochem Sci. 39:268–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carrillo J, González A, Manguán-García C,
Pintado-Berninches L and Perona R: p53 pathway activation by
telomere attrition in X-DC primary fibroblasts occurs in the
absence of ribosome biogenesis failure and as a consequence of DNA
damage. Clin Transl Oncol. 16:529–538. 2014. View Article : Google Scholar
|
|
81
|
Wang Y, Yu H, Zhang J, Gao J, Ge X and Lou
G: Hesperidin inhibits HeLa cell proliferation through apoptosis
mediated by endoplasmic reticulum stress pathways and cell cycle
arrest. BMC Cancer. 15:6822015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wiel C, Lallet-Daher H, Gitenay D, Gras B,
Le Calvé B, Augert A, Ferrand M, Prevarskaya N, Simonnet H,
Vindrieux D and Bernard D: Endoplasmic reticulum calcium release
through ITPR2 channels leads to mitochondrial calcium accumulation
and senescence. Nat Commun. 5:37922014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhu B, Ferry CH, Markell LK, Blazanin N,
Glick AB, Gonzalez FJ and Peters JM: The nuclear receptor
peroxisome proliferator-activated receptor-β/Δ (PPARβ/Δ) promotes
oncogene-induced cellular senescence through repression of
endoplasmic reticulum stress. J Biol Chem. 289:20102–20119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Choque B, Catheline D, Rioux V and Legrand
P: Linoleic acid: Between doubts and certainties. Biochimie.
96:14–21. 2014. View Article : Google Scholar
|
|
85
|
Kim MH, Kim MO, Kim YH, Kim JS and Han HJ:
Linoleic acid induces mouse embryonic stem cell proliferation via
Ca2+/PKC, PI3K/Akt, and MAPKs. Cell Physiol Biochem. 23:53–64.
2009. View Article : Google Scholar : PubMed/NCBI
|