Introduction
Myocardial ischemia/reperfusion (I/R) injury is the
most common cause of cardiac morbidity and mortality and can lead
to arrhythmia, heart hypofunction, cardiocyte apoptosis and other
disorders (1). Therefore,
identification of therapeutic approaches and molecular mechanisms
responsible for I/R injury are important for the improvement of
ischemic heart diseases associated with I/R.
It has been suggested that elevation of the
intracellular chloride ion concentration
([Cl−]i) is an important pathophysiological
factor of I/R injury. Increased [Cl−]i can
activate Cl−-OH− exchange activity (2,3),
thereby increasing the intracellular concentration of
OH−, which is an important member of the reactive oxygen
species (ROS) family. Alternatively, the increased
[Cl−]i may induce the opening of the
mitochondrial permeability transition pore (mPTP), which results in
a ROS burst and subsequent ROS-dependent apoptosis (4,5).
Therefore, the inhibition of the elevation of
[Cl−]i induced by I/R has been considered to
be a reasonable therapeutic strategy to alleviate I/R injury.
Sasanquasaponin (SQS) is an active component of
Camellia oleifera Abel, which is used in Traditional Chinese
Medicine. SQS is a triterpenoid with structural similarity to
certain ginseng saponins (6,7), and
is known to exert potent cardioprotective effects against I/R
injury (8). A previous study by
our group indicated that these beneficial effects of SQS may be
attributed to the inhibition of I/R-induced elevation of
[Cl−]i (9);
however, the intracellular signal transduction mechanisms
underlying these effects have yet to be elucidated.
Protein kinase C epsilon (PKCε), a novel PKC isotype
characterized as a calcium-independent and phorbol
ester/diacylglycerol-sensitive serine/threonine kinase (10,11),
has been well documented to have an important role in
cardioprotection (12–15). Several recent studies have reported
that PKCε signaling is involved in maintaining intracellular
chloride homeostasis (2,16).
Considering that PKCε signaling has important roles
in the regulation of [Cl−]i and in
cardioprotection, and that SQS can effectively inhibit I/R-induced
elevation of [Cl−]i, the present study
hypothesized that the cardioprotective effects of SQS against I/R
injury are mediated by PKCε via attenuation of
[Cl−]i increases following I/R. To verify
this hypothesis neonatal rat cardiomyocytes were subjected to
simulated (s) I/R as an in vitro I/R model, and the effects
of SQS on cell viability, PKCε phosphorylation levels,
[Cl−]i, mitochondrial membrane potential
(Δψm) and ROS production were assessed.
Materials and methods
Reagents
SQS was kindly provided by Professor Yongming Luo
from Jiangxi Chinese Medical University (Nanchang, China), and its
identity and purity (>99%) were determined by nuclear magnetic
resonance spectroscopy and high-performance liquid chromatography
tandem mass spectroscopy analyses. εV1–2, a specific inhibitor of
PKCε, was purchased from Anaspec (cat. no. AS-62186; Fremont, CA,
USA). Anti-PKCε and anti-phosphorylated (p)-PKCε (Ser 729)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-β-actin antibody was purchased from the
Jiancheng Bioengineering Institute of Nanjing (Nanjing, China). The
horseradish peroxidase-labeled immunoglobulin G secondary antibody
was purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell culture
Cardiomyocytes from 30 Sprague-Dawley rats (weight,
10–15 g; age, 1–3 days; Nanchang University School of Medicine,
Nanchang, China) were prepared according to the protocol of a
previous study (17). The rats
were treated in accordance with the National Institute of Health's
Guide for the Care and Use of Laboratory Animals, and all
procedures were approved by the ethics committee of Nanchang
University (Nanchang, China). The rats were anesthetized by
intraperitoneal injection with 10% chloral hydrate (0.03 ml/kg;
Sigma-Aldrich, St. Louis, MO, USA), after which they were
sacrificed by cervical dislocation prior to the removal of the rat
hearts. The hearts were removed and placed in phosphate-buffered
saline (PBS). The ventricles were digested with 0.1% trypsin
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and then collected by centrifugation at 60 × g for 10 min,
re-suspended in plating medium, including 85% Eagle's minimum
essential medium, 15% fetal calf serum and 100 U/ml penicillin and
streptomycin (Beijing Solarbio Science & Technology Co., Ltd.),
seeded in a culture dish and incubated for 2 h in an atmosphere
containing 95% air and 5% CO2 to remove non-myocytes.
The supernatant was collected and plated in 60-mm gelatin-coated
culture dishes at 1×106 cells per dish. After 24 h,
cardiomyocytes were washed and the medium was replaced, followed by
incubation for three days prior to the experiments.
Experimental groups and treatments
The neonatal primary rat cardiomyocytes were divided
into various experimental groups as follows: i) In the control
group, cardiomyocytes were incubated under normal conditions for an
additional 24 h; ii) in the sI/R group, cardiomyocytes were
subjected to anoxia by incubating them in fresh anoxic medium [0.9
mM NaH2PO4, 6.0 mM NaHCO3, 1.8 mM
CaCl2, 1.2 mM MgSO4, 40 mM sodium lactate, 20
mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 98.5
mM NaCl, 10.0 mM KCl, pH 6.8; Sigma-Aldrich] at 37°C in a chamber
containing 95% N2 and 5% CO2 for 3 h.
Subsequently, the medium was replaced with re-oxygenation medium
(129.5 mM NaCl, 5.0 mM KCl, 0.9 mM NaH2PO4,
20 mM NaHCO3, 1.8 mM CaCl2, 1.2 mM
MgSO4, 5.5 mM glucose, 20 mM HEPES, pH 7.4;
Sigma-Aldrich) and the cells were incubated at 37°C in an
atmosphere of 95% air and 5% CO2 for 2 h; iii) In the
SQS+sI/R group, cells were pre-treated with SQS at 10 µM for
24 h prior to sI/R; iv) In the SQS+εV1–2+sI/R group, cells were
pre-treated with εV1–2 (1 µM) and SQS (10 µM) for 24
h prior to sI/R. The doses of SQS and εV1–2 used were selected on
the basis of a preliminary study by our group and previous reports
(8,18,19).
Cell viability assay
A colorimetric MTS assay was used to assess the
viability of cardiomyocytes subjected to sI/R. MTS is a pale yellow
substrate that is converted into a dark blue formazan product by
living cells. Primary cardiomyocytes were seeded into 96-well
plates at 1×104 cells/well. After sI/R treatment, cells
were incubated with 20 µl MTS (5 mg/ml; Promega Corp.,
Madison, WI, USA) in 100 µl medium at 37°C for 2 h. The
absorbance of each well at a wavelength of 490 nm was then measured
using a microplate reader (no. 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The results were expressed as a percentage of
the control.
Biochemical parameters
Following sI/R treatment, the culture media of the
control groups and the supernatants of cell lysates from each group
were collected to the activities of creatine phosphokinase (CPK)
and lactate dehydrogenase (LDH) were assessed using kits (A020-2
and A032, respectively; Jiancheng Bioengineering Institute of
Nanjing), according to the manufacturer's instructions.
Measurement of intracellular ROS
ROS levels were determined using the fluorescent
probe 2′7′-dichlorodihy-drofluorescein diacetate (DCFH-DA;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
which is cleaved by cellular esterases to non-fluorescent DCFH and
oxidized by intracellular ROS to the fluorescent product
dichlorofluorescein. ROS production is proportional to the
fluorescence ratio of the treatment vs control group. After the
indicated treatments, the cells were incubated with 10 µM
DCFH-DA for 20 min at 37°C prior to being harvested and analyzed
for green and red fluorescence intensity using a flow cytometer
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) at
wavelengths of 485 and 528 nm, respectively.
Determination of
[Cl−]i
The measurement of [Cl−]i was
performed according to the method described in a previous study by
our group with minor modifications (8). Briefly, after the indicated
treatments, cardiomyocytes were washed twice with
Cl−-free solution (NaCl was replaced by an equimolar
amount of D-glucuronic acid, MgCl2 by MgSO4
and KCl by potassium gluconate), and incubated with 10 mM
N-(ethoxycarbonylmethyl)-6-methoxyquinolinium (MQAE; Thermo
Fisher Scientific, Inc.) in the dark for 20 min at 37°C.
Cl−-free solution was then used to remove any excess
dye. Cells were suspended in Cl−-free solution
immediately subjected to flow cytometric analysis
(FACSCalibur).
Assessment of Δψm
The fluorescent dye JC-1 was used to assess the
mitochondrial membrane potential as described in the study by Tang
et al (18). Briefly, after
the indicated treatments, cardiomyocytes were incubated with JC-1
(200 µM; Beijing Solarbio Science & Technology Co.,
Ltd.) at 37°C for 20 min. Cells were then washed with ice-cold PBS
to remove remaining dye. Fluorescence was measured by flow
cytometry (FACSCalibur) with excitation/emission wavelengths of
530/580 nm (red) and at 485/530 nm (green). The ratio of red to
green fluorescence intensity of cells reflected the Δψm.
Western blot analysis
Western blotting was performed as described
previously (18). The protein
concentrations were measured using the DC Protein Assay kit II
(cat. no. 500-0112; Bio-Rad Laboratories, Inc.). Equal quantities
of protein (30 µl/lane) were then separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto polyvinylidene difluoride membranes. After blocking with 5%
non-fat milk in Tris-buffered saline containing 0.2% Tween 20
(TBST), the blots were probed with rabbit anti-PKCε (C-15; 1:500;
sc-214), goat anti-p-PKCε (Ser 729; 1:500; sc-12355) and rabbit
anti-β-actin (1:1,000; #21338) polyclonal antibodies overnight at
4°C. After washing with TBST, the blots were incubated with
horseradish peroxidase-conjugated goat anti-rabbit (1:5,000; #7074)
and rabbit anti-goat (1:5,000; #7075) secondary antibodies for 1 h
at room temperature. After washing, the blots were saturated with
enhanced chemiluminescence mixture (Pierce Biotechnology, Inc.,
Rockford, IL, USA) for 1 min, and images of the blot were captured
by exposure to pre-flashed X-ray film (Kodak, Rochester, NY, USA).
for 300 sec. Densitometric scanning was performed using Quantity
One software, version 4.62 (Bio-Rad Laboratories, Inc.). Results
were expressed as a percentage of the control. The bands were
normalized to β-actin.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using SPSS software,
version 11.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was applied to test the significance of differences
between groups, followed by post-hoc testing for individual
differences. P<0.05 was considered to indicate a statistically
significant difference between values. For each assessment, at
least three independent experiments were performed.
Results
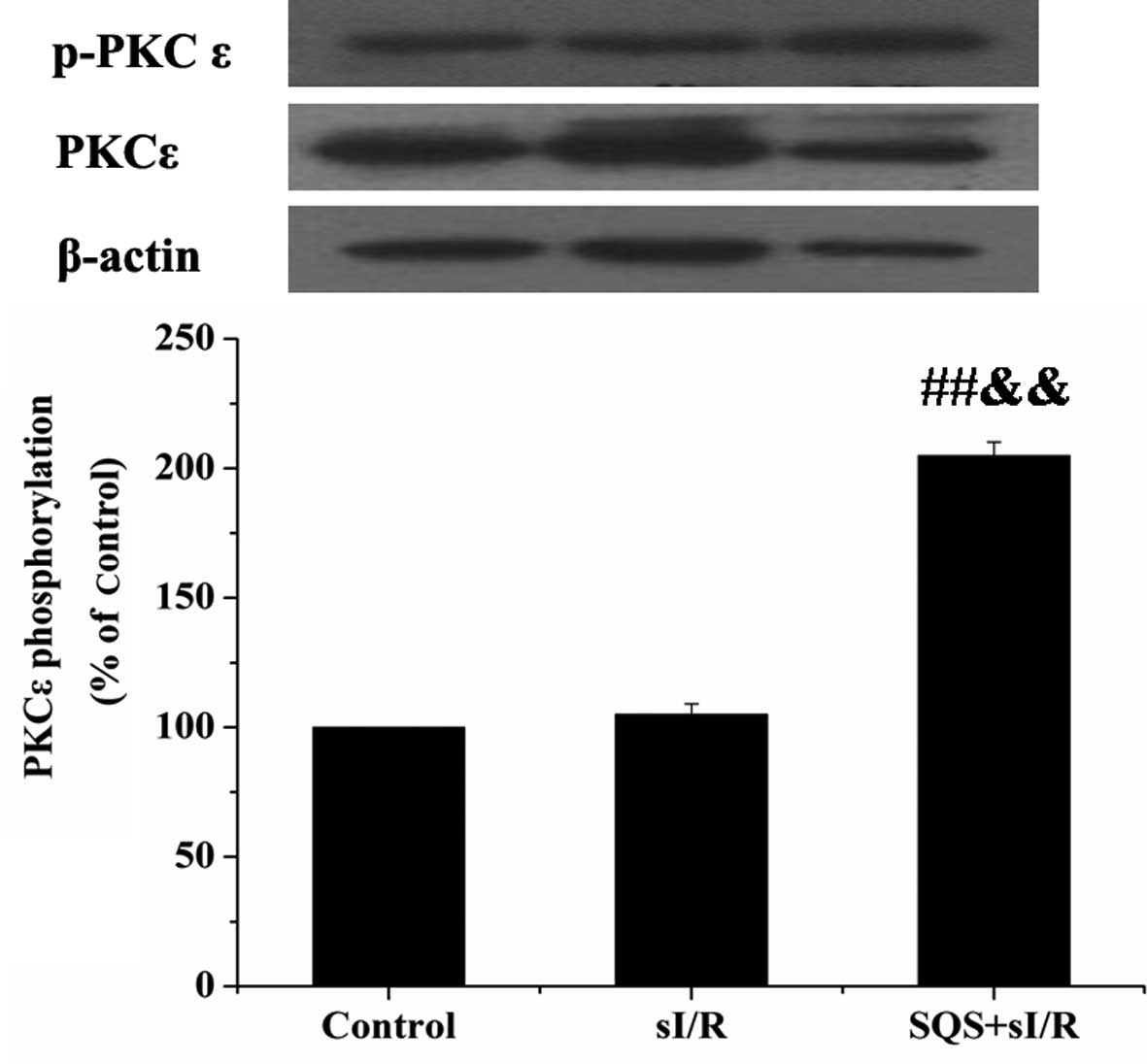
SQS activates PKCε in cardiomyocytes
following sI/R
Primary cardiomyocytes were incubated with or
without 10 µM SQS for 24 h, followed by sI/R, and the levels
of total and phosphorylated PKCε (Ser 729) were assessed by western
blotting. As shown in Fig. 1, the
levels of p-PKCε Ser 729 were significantly increased in the SQS
pre-treated group (P<0.01 vs. control group; P<0.01 vs. sI/R
group), whereas the levels of total PKCε remained unchanged. These
results suggested that SQS treatment led to the activation of PKCε
in cardiomyocytes following sI/R.
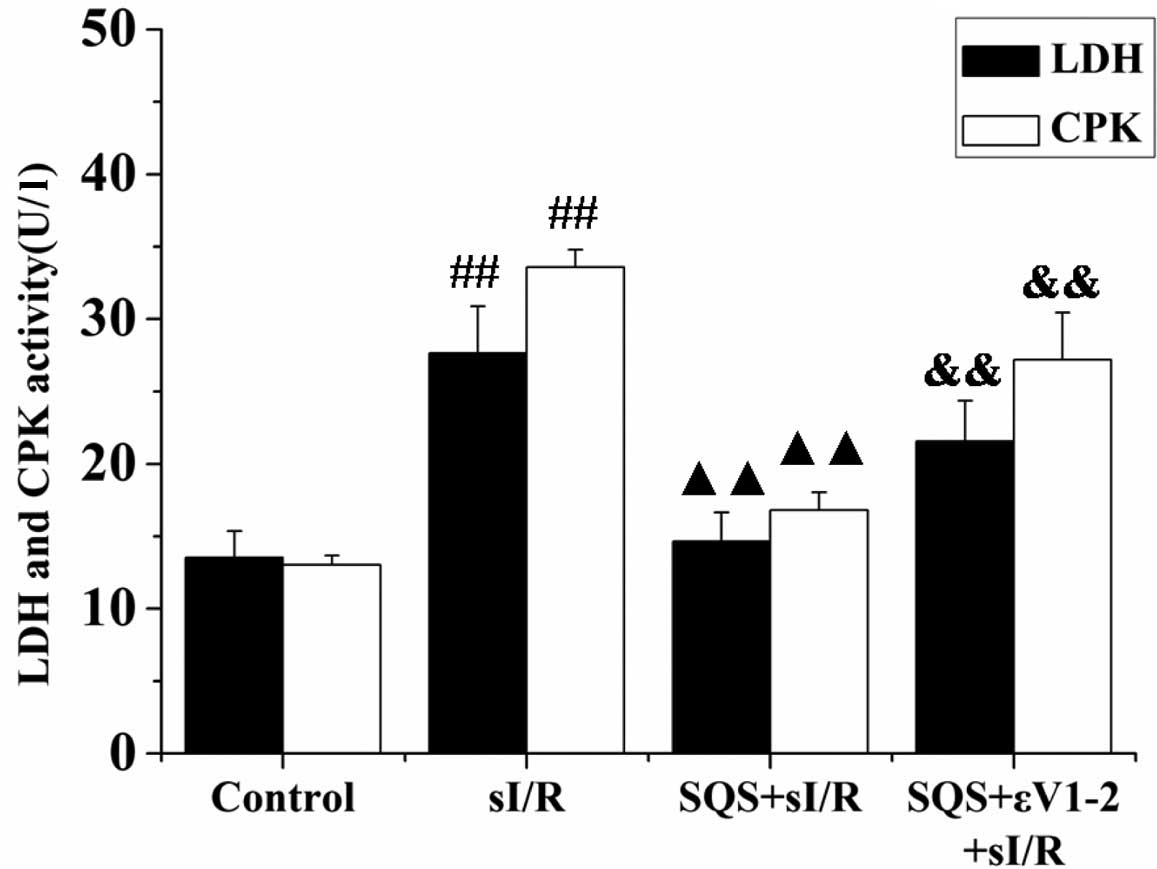
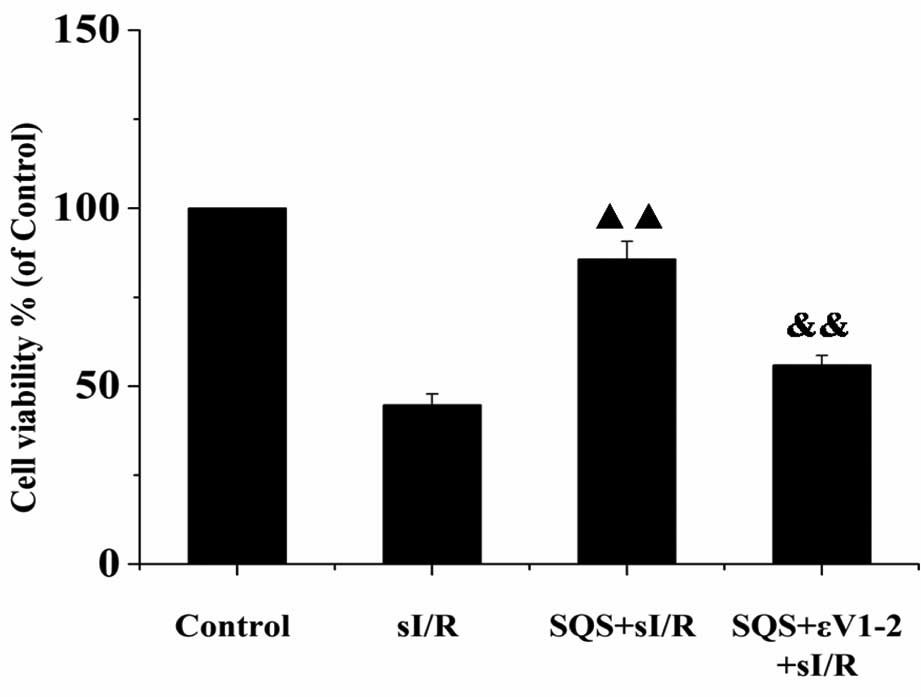
SQS reduces cardiomyocyte death following
sI/R via PKCε
The release of CPK and LDH into the culture medium
was analyzed to evaluate the effects of SQS and PKCε inhibitor
εV1–2 on cardiomyocyte death. As shown in Fig. 2, a significant increase in CPK and
LDH levels was observed in the sI/R group compared to that in the
control group (P<0.01), which was significantly inhibited by
pre-treatment with SQS (P<0.01 vs. sI/R group). Of note, the
release of CPK and LDH in the group additionally pre-treated with
εV1–2 prior to sI/R was enhanced when compared with that in the
SQS+sI/R group (P<0.01) (Fig.
2), indicating that SQS may exert its protective effects via
PKCε. Furthermore, the results of the MTS assay showed that SQS
significantly increased cardiomyocyte viability following sI/R
(P<0.01), which was significantly inhibited by co-treatment with
εV1–2 (P<0.01 vs. SQS+sI/R group) (Fig. 3). These data suggested that PKCε is
required for SQS to elicit its cardioprotective effects.
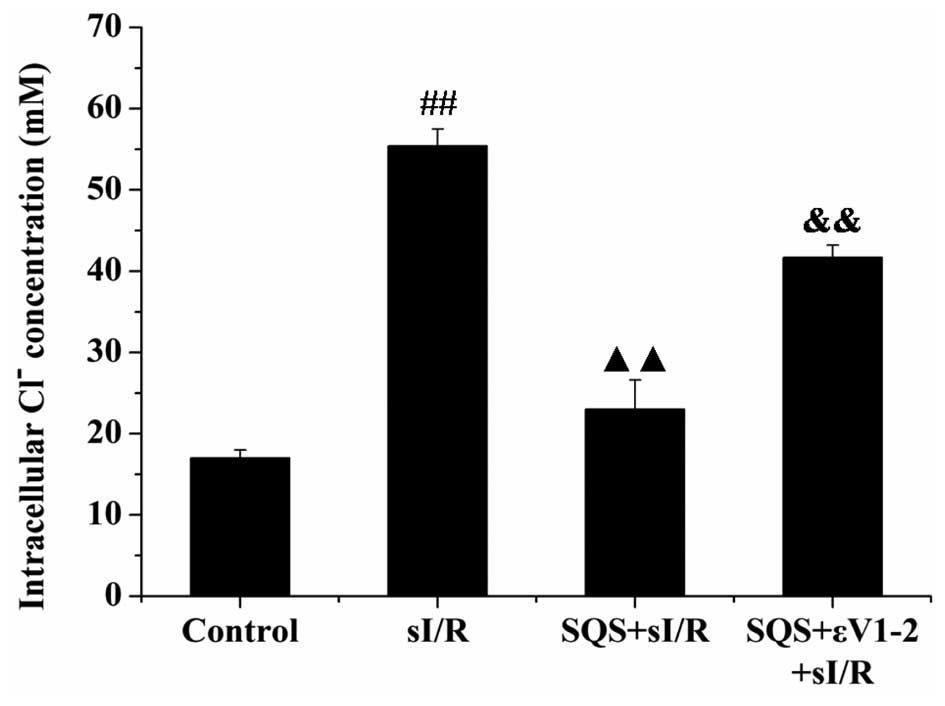
SQS reduces sI/R-induced increases in
[Cl−]i in cardiomyocytes via PKCε
To investigate whether PKCε is implicated in the
inhibitory effects of SQS on the elevation of
[Cl−]i induced by sI/R, cardiomyocytes were
treated with PKCε inhibitor εV1–2 in combination with SQS and
[Cl−]i was assessed via MQAE staining and
flow cytometric analysis. As shown in Fig. 4, SQS pre-treatment abrogated the
increases in [Cl−]i in cardiomyocytes
following sI/R (P<0.01 vs. sI/R group), which was inhibited by
the selective PKCε inhibitor εV1–2 (P<0.01 vs. SQS+sI/R group).
These results indicated that activation of PKCε is required for SQS
to inhibit sI/R-induced elevation of
[Cl−]i.
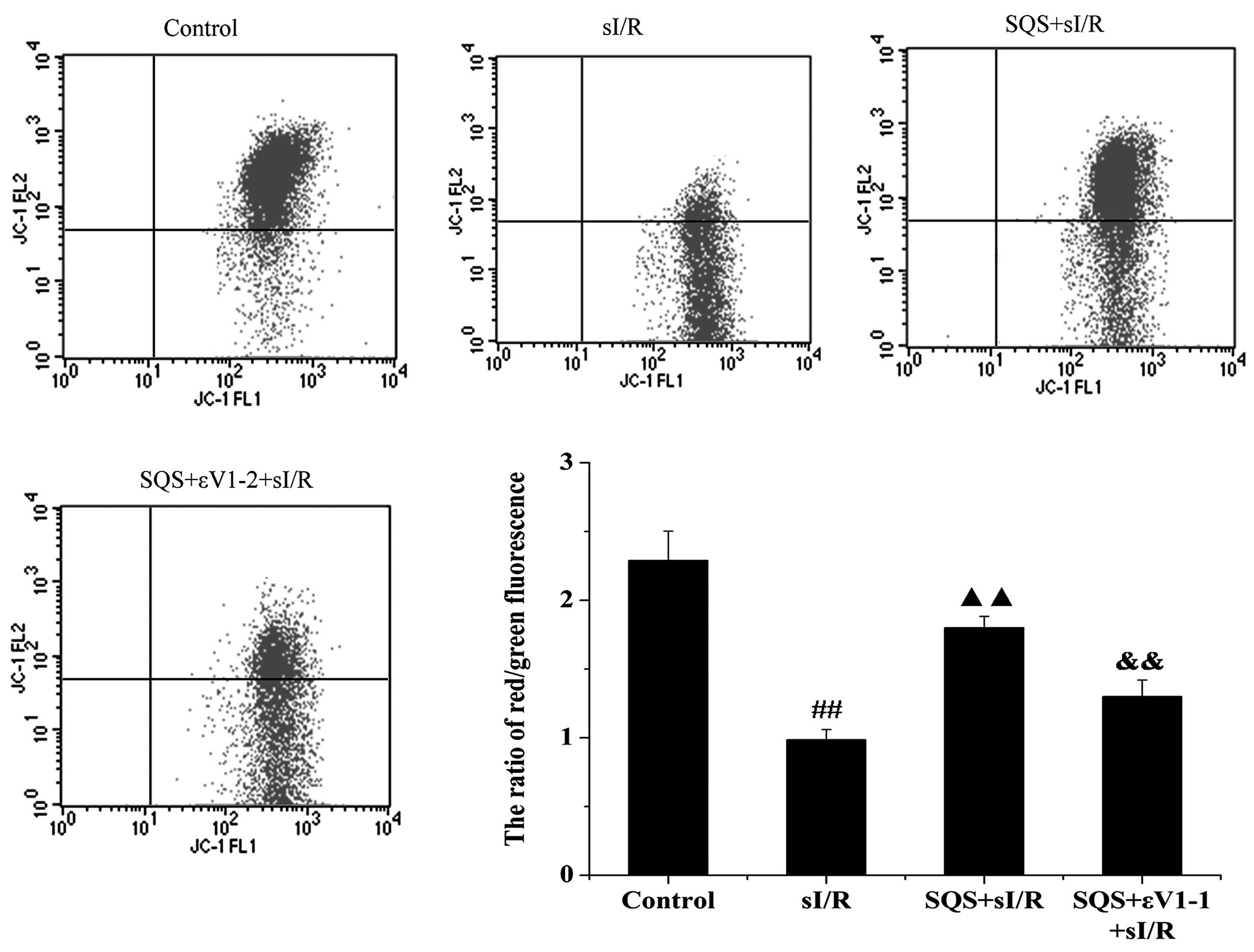
SQS attenuates increases of the Δψm in
cardiomyocytes following sI/R via PKCε
As the decrease of Δψm induced by Cl−
influx reflects the opening of the mPTP, which results in a ROS
burst, the present study assessed the effects of SQS on
sI/R-induced changes in Δψm in cardiomyocytes as well as the
possible involvement of PKCε. As shown in Fig. 5, sI/R decreased the Δψm as
indicated by a decrease in the ratio of red to green fluorescence
intensity (P<0.01 vs. control group). However, pre-treatment
with SQS significantly attenuated the loss of Δψm (P<0.01 vs.
sI/R group). As expected, the restorative effect of SQS on the Δψm
was inhibited by εV1–2 (P<0.01 vs. SQS+sI/R group), indicating
that PKCε is required for SQS-mediated attenuation of Δψm loss
following sI/R in cardiomyocytes.
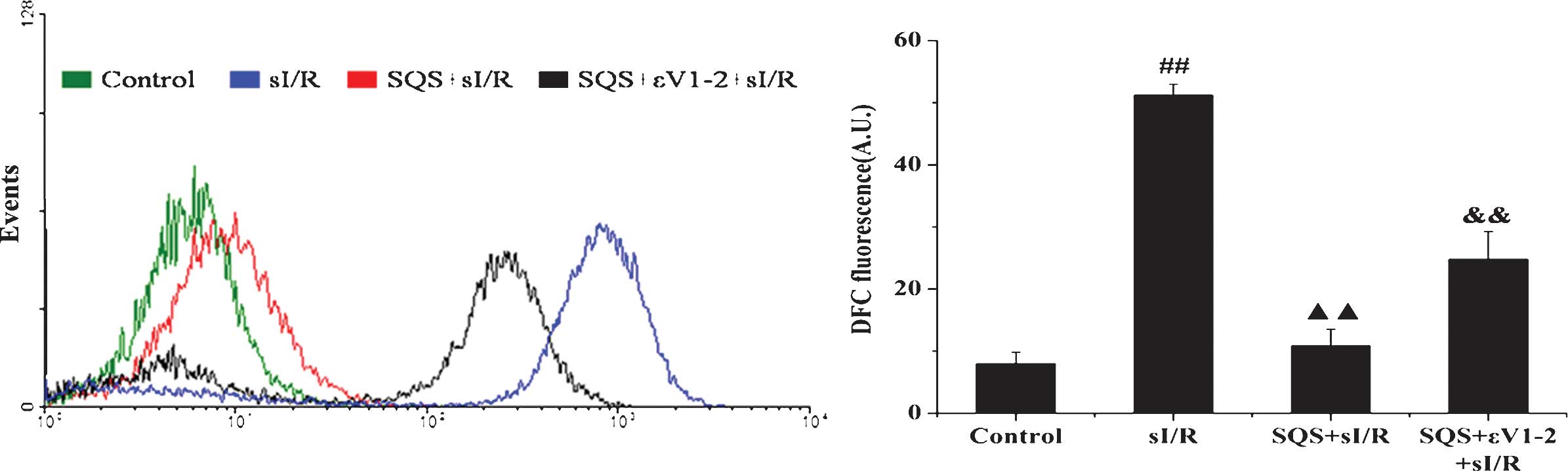
SQS inhibits ROS generation in
cardiomyocytes undergoing sI/R via PKCε
Intracellular ROS levels were assessed by measuring
cDCF fluorescence intensity. As shown in Fig. 6, sI/R induced marked intracellular
ROS production, while pre-treatment with SQS inhibited ROS
production induced by sI/R. Co-treatment with εV1–2 attenuated the
inhibitory effects of SQS on ROS production induced by sI/R,
indicating that PKCε is required for SQS to inhibit sI/R-induced
ROS production.
Discussion
SQS is an active component extracted from the
Chinese medicinal herb Camellia oleifera Abel and has gained
considerable attention due to its wide range of biological and
pharmacological properties (19–21).
Previous studies by our group showed that SQS effectively protected
against myocardial injury in an isoproterenol-induced rat model of
ischemia in vivo and in a hypoxia and reoxygenation model in
Langendorff-perfused rat hearts in vitro (8,22,23).
Another previous study by our group revealed that SQS exerts its
cardioprotective effects by suppressing intracellular
Cl− accumulation induced by sI/R (9). The present study investigated the
molecular mechanisms underlying SQS-induced effects, focusing on
the link between the PKCε signalling pathway and the variations of
[Cl−]i.
It is well known that Cl− is the primary
intracellular anion and that changes in
[Cl−]i can affect a variety of basic cellular
functions, including ionic conductances, membrane potential,
intracellular pH, apoptosis, cell volume and Ca2+
homeostasis (2,24). Studies on ischemic myocardial
injury have revealed that I/R-induced elevation of
[Cl−]i is an important pathophysiological
factor. Huang et al (4)
found that increases in [Cl−]i can lead to
the opening of the mPTP, which results in ROS burst and subsequent
ROS-dependent cell injury. In line with this result, the present
study found that cardiomyocytes subjected to sI/R show a rapid and
significant increase in intracellular Cl− levels
accompanied by loss of Δψm, mPTP opening and ROS production. This
was also accompanied by cardiomyocyte injury as indicated by a
marked increase of LDH and CPK release as well as reduction of cell
viability. These results further suggested that dysregulation of
intracellular Cl− homeostasis has a significant role in
myocardial cell injury induced by ischemia/reperfusion. In
addition, SQS was shown to suppress the elevation of
[Cl−]i induced by sI/R and induce
cardioprotection from sI/R injury in an in vitro cell model.
As the underlying molecular mechanisms had remained elusive, the
present study assessed the possible role of PKCε in the inhibition
of sI/R-induced [Cl−]i by SQS in
cardiomyocytes.
PKCε is a member of a novel group of the PKC family
of serine and threonine kinases, which are involved in a wide range
of physiological processes, including cell survival under stressful
conditions, mitogenesis, transcriptional regulation and metastasis
(12). It has been confirmed that
PKCε-associated signaling exerts cardioprotective functions
(25–27), and that the PKCε pathway is
involved in maintaining intracellular chloride homeostasis
(2,16). On this basis, the present study
hypothesized that activation of the PKCε pathway may be responsible
for the cardioprotective effects of SQS and inhibition of
I/R-induced increases in [Cl−]i by SQS. To
test this hypothesis, the present study first determined the
effects of SQS on the phosphorylation of PKCε in cardiomyocytes. Of
note, pre-treatment with SQS significantly increased the levels of
p-PKCε, indicating that SQS can activate the PKCε pathway in
cardiomyocytes subjected to sI/R. To further explore the
association between PKCε activation and the cardioprotective
effects of SQS, the selective PKCε inhibitor εV1–2 was employed.
The results revealed that SQS reduced cardiomyocyte death following
sI/R injury, which was inhibited by εV1–2. Furthermore, SQS
inhibited sI/R-induced elevation of [Cl−]i,
which was attenuated by εV1–2. These results suggested that
activation of PKCε is required for SQS to elicit its
cardioprotective effects.
Due to the fact that the elevation of
[Cl−]i induced by sI/R contributes to loss of
Δψm, mPTP opening and ROS production, the present study further
detected the effects of SQS on Δψm and ROS production in the
absence or presence of εV1–2. As expected, it was observed that SQS
attenuated the sI/R-induced loss of Δψm as well as ROS production
in cardiomyocytes, which was inhibited by εV1–2, suggesting that
the activation of PKCε is also required for SQS to attenuate
sI/R-induced Δψm loss and ROS production.
Although the present study demonstrated that
activation of PKCε is required for the inhibitory effects of SQS on
the elevation of [Cl−]i following sI/R
injury, the detailed molecular interactions deserve further
investigation. Anion exchanger 3 (AE3), a member of the solute
carrier 4 protein family, mediates the reversible electroneutral
exchange of Cl− for HCO3− across
the plasma membrane (28). A
previous study by our group showed that AE3 is closely linked with
the inhibitory effects of SQS on sI/R-induced elevation of
[Cl−]i (9).
Of note, Alvarez et al (29) showed that AE3 is the PKC-sensitive
anion exchange protein of the heart, and that the PKCε-dependent
phosphorylation of serine 67 on AE3 can cause an increase in anion
transport. Therefore, it may be speculated that SQS inhibits
sI/R-induced elevation of [Cl−]i and induces
cardioprotection through activation of the PKCε pathway and
consequent PKCε-dependent phosphorylation of serine 67 on AE3.
However, this notion requires experimental validation.
In conclusion, the present study was the first to
report that activation of PKCε is required for SQS to exert its
cardioprotective effects against sI/R injury. It was demonstrated
that activation of PKCε is crucial for SQS-mediated inhibition of
sI/R-induced elevation of [Cl−]i, Δψm loss
and ROS production. The present study enhanced the current
understanding of the molecular mechanisms of the cardioprotective
effects of SQS, suggesting that it may be efficient for reducing
I/R-induced injury.
Acknowledgments
The present study was supported by grants from the
Natural Scientific Foundation of China (nos. 30660209 and
81260491).
References
|
1
|
Lee YM, Cheng PY, Chen SY, Chung MT and
Sheu JR: Wogonin suppresses arrhythmias, inflammatory responses and
apoptosis induced by myocardial ischemia/reperfusion in rats. J
Cardiovasc Pharmacol. 58:133–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hume JR, Duan D, Collier ML, Yamazaki J
and Horowitz B: Anion transport in heart. Physiol Rev. 80:31–81.
2000.PubMed/NCBI
|
|
3
|
Alvarez BV, Kieller DM, Quon AL, Markovich
D and Casey JR: Slc26a6: A cardiac chloride-hydroxyl exchanger and
predominant chloride-bicarbonate exchanger of the mouse heart. J
Physiol. 561:721–734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang QR, Li Q, Chen YH, Li L, Liu LL, Lei
SH, Chen HP, Peng WJ and He M: Involvement of anion exchanger-2 in
apoptosis of endothelial cells induced by high glucose through an
mPTP-ROS-Caspase-3 dependent pathway. Apoptosis. 15:693–704. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu CX and Xiao PG: Recent advances on
ginseng research in China. J Ethnopharmacol. 36:27–38. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai ZF, Shao Z, Chen YZ, He M, Huang Q and
Nishi K: Effects of sasanquasaponin on ischemia and reperfusion
injury in mouse hearts. J Pharmacol Sci. 94:313–324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HP, He M, Mei ZJ, Huang QR, Peng W
and Huang M: Anion exchanger 3 is required for sasanquasaponin to
inhibit ischemia/reperfusion-induced elevation of intracellular
Cl-concentration and to elicit cardioprotection. J Cell Biochem.
112:2803–2812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ono Y, Fujii T, Ogita K, Kikkawa U,
Igarashi K and Nishizuka Y: The structure, expression and
properties of additional members of the protein kinase C family. J
Biol Chem. 263:6927–6932. 1988.PubMed/NCBI
|
|
11
|
Ohno S, Akita Y, Konno Y, Imajoh S and
Suzuki K: A novel phorbol ester receptor/protein kinase, nPKC,
distantly related to the protein kinase C family. Cell. 53:731–741.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akita Y: Protein kinase C-epsilon
(PKC-epsilon): Its unique structure and function. J Biochem.
132:847–852. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu GS, Cohen MV, Mochly-Rosen D and
Downey JM: Protein kinase C-epsilon is responsible for the
protection of preconditioning in rabbit cardiomyocytes. J Mol Cell
Cardiol. 31:1937–1948. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogbi M and Johnson JA: Protein kinase
Cepsilon interacts with cytochrome c oxidase subunit IV and
enhances cytochrome c oxidase activity in neonatal cardiac myocyte
preconditioning. Biochem J. 393:191–199. 2006. View Article : Google Scholar :
|
|
15
|
Ping PP, Takano H, Zhang J, Tang XL, Qiu
Y, Li RC, Banerjee S, Dawn B, Balafonova Z and Bolli R:
Isoform-selective activation of protein kinase c by nitric oxide in
the heart of conscious rabbits: A signaling mechanism for both
nitric oxide-induced and ischemia-induced preconditioning. Circ
Res. 84:587–604. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budas GR, Churchill EN, Disatnik MH, Sun L
and Mochly-Rosen D: Mitochondrial import of PKCepsilon is mediated
by HSP90: A role in cardioprotection from ischaemia and reperfusion
injury. Cardiovasc Res. 88:83–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watkins SJ, Borthwick GM and Arthur HM:
The H9C2 cell line and primary neonatal cardiomyocyte cells show
similar hypertrophic responses in vitro. In Vitro Cell Dev Biol
Anim. 47:125–131. 2011. View Article : Google Scholar
|
|
18
|
Tang L, Peng Y, Xu T, Yi X, Liu Y, Luo Y,
Yin D and He M: The effects of quercetin protect cardiomyocytes
from A/R injury is related to its capability to increasing
expression and activity of PKCε protein. Mol Cell Biochem.
382:145–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HP, He M, Huang QR, Liu D and Huang
M: Sasanquasaponin protects rat cardiomyocytes against oxidative
stress induced by anoxia-reoxygenation injury. Eur J Pharmacol.
575:21–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akagi M, Fukuishi N, Kan T, Sagesaka YM
and Akagi R: Anti-allergic effect of tea-leaf saponin (TLS) from
tea leaves (Camellia sinensis var. sinensis). Biol Pharm Bull.
20:565–567. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sur P, Chaudhuri T, Vedasiromoni JR, Gomes
A and Ganguly DK: Antiinflammatory and antioxidant property of
saponins of tea [Camellia sinensis (L) O. Kuntze] root extract.
Phytother Res. 15:174–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu D, He H, Li GL, Chen J, Yin D, Liao
ZP, Tang L, Huang QR, Lai ZF and He M: Mechanisms of chloride in
cardiomyocyte anoxia-reoxygenation injury: The involvement of
oxidative stress and NF-kappaB activation. Mol Cell Biochem.
355:201–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao Z, Yin D, Wang W, Zeng G, Liu D, Chen
H, Huang Q and He M: Cardioprotective effect of sasanquasaponin
preconditioning via bradykinin-NO pathway in isolated rat heart.
Phytother Res. 23:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiraoka M, Kawano S, Hirano Y and Furukawa
T: Role of cardiac chloride currents in changes in action potential
characteristics and arrhythmias. Cardiovasc Res. 40:23–33. 1998.
View Article : Google Scholar
|
|
25
|
Ping P, Song C, Zhang J, Guo Y, Cao X, Li
RC, Wu W, Vondriska TM, Pass JM, Tang XL, et al: Formation of
protein kinase C (epsilon)-Lck signaling modules confers
cardioprotection. J Clin Invest. 109:499–507. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schwanke U, Konietzka I, Duschin A, Li X,
Schulz R and Heusch G: No ischemic preconditioning in heterozygous
connexin43-deficient mice. Am J Physiol Heart Circ Physiol.
283:H1740–H1742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schulz R, Cohen MV, Behrends M, Downey JM
and Heusch G: Signal transduction of ischemic preconditioning.
Cardiovasc Res. 52:181–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alper SL, Darman RB, Chernova MN and Dahl
NK: The AE gene family of Cl/HCO3 exchangers. J Nephrol.
15(Suppl 5): S41–S53. 2002.
|
|
29
|
Alvarez BV, Fujinaga J and Casey JR:
Molecular basis for angiotensin II-induced increase of
chloride/bicarbonate exchange in the myocardium. Circ Res.
89:1246–1253. 2001. View Article : Google Scholar : PubMed/NCBI
|