Introduction
Septic shock is a complex pathophysiological process
characterized by hypotension, systemic inflammatory response
syndrome (SIRS) and widespread multiple organ dysfunction syndrome
(MODS), with a high mortality (1).
At present, a variety of pro-inflammatory cytokines are excessively
produced in the development of hypotension, tissue damage and organ
injury during sepsis/septic shock (2,3).
S100A8 is a member of S100 calcium-binding family of
proteins, and can combine with its binding partner S100A9 to form a
heterodimer of S100A8/A9 dimers (4). S100A8 protein is constitutively
expressed in neutrophils and can be induced in monocytes and
endothelial cells (5). S100A8
serves a variety of immune functions, including antibacterial
activity, cell death induction, pro- and anti-inflammatory
properties, and activation of endothelial cells (6–9). The
levels of S100A8 have been shown to be increased following septic
shock and decreased along recovery at both the protein and mRNA
expression levels (10).
In recent years, the vagus nerve and the release of
neurotransmitters acetylcholine demonstrate anti-inflammatory
effect and reduce the production of pro-inflammatory cytokines and
lethal effect of biological toxin (11–13).
Stimulation of the vagus nerve has potential protective effects on
the damaging effects of cytokine release, including endotoxemia,
sepsis, ischemia/reperfusion injury, arthritis and other
inflammatory syndromes (14).
Furthermore, vagus nerve stimulation demonstrated protective roles
against septic shock in rats (15). However, the precise mechanisms
underlying the anti-inflammatory effects of the vagus nerve remain
to be elucidated.
The present study established a septic shock model
in rats by cecal ligation and puncture (CLP), a model of
polymicrobial sepsis, and aimed to investigate the effects of vagus
nerve electrical stimulation on hemodynamics, liver function, the
serum level of S100A8 and advanced glycation end products (AGEs),
and the protein expression of hepatic receptor for advanced
glycation end products (RAGE) following CLP challenge.
α-bungarotoxin (α-BGT), an antagonist against nicotinic
acetylcholine receptor α7 (α7-nAChR) subunit, and anti-RAGE
antibody were applied in combination with vagus nerve electrical
stimulation to examine the precise target point of the vagus nerve
on septic shock and S100A8.
Materials and methods
CLP model
Male Sprague-Dawley rats (SLRC Laboratory Animal
Centre, Shanghai, China) weighing 200–250 g were used to establish
a polymicrobial sepsis model by the CLP procedure, which was
performed, as described previously (16). Briefly, the animals were
anesthetized with an intraperitoneal injection of ketamine (80
mg/kg; Henry Pharaceutical Co., Ltd., Jiangsu, China) and xylazine
(12 mg/kg; Sigma-Aldrich, St. Louis, MO, USA). A 2 cm incision was
made near the lower midline of the abdomen to isolate the cecum. To
ligate ~30% of the cecum just below the ileocecal valve, to avoid
bowel obstruction, 3.0 silk was used. This selected percentage of
cecum ligation revealed a reproducible rate of mortality in
Sprague-Dawley rats (17). An 18 g
needle was used to puncture twice the ligated portion of cecum,
causing a small quantity of contents expel into the abdominal
cavity. Following this, the cecum was returned to its original
location and the abdominal incision was closed in muscle and skin
layers. No antibiotics were administered; however, the animals
received normal saline (20 ml/kg) subcutaneously as fluid
resuscitation. The present study was performed according to the
protocol approved by Animal Care and Study Committee of Shanghai
Children's Hospital Affiliated to Shanghai Jiao-Tong University
(Shanghai, China).
Experimental protocols
A total of 36 rats were randomly divided into six
equal groups: i) Sham group, receiving sham operation; ii) CLP
group, subjected to CLP and the bilateral vagal trunks were
isolated but not transected; iii) VGX group, subjected to CLP and
bilateral cervical vagotomy; iv) STM group, subjected to CLP and
bilateral cervical vagotomy, followed by bipolar platinum
electrodes being connected to the left vagus nerve trunk in a
stimulation module and controlled by an acquisition system; v)
α-BGT group, administered α-BGT (1.0 µg/kg, i.v.;
Sigma-Aldrich) prior to vagus nerve electrical stimulation and
following CLP and bilateral cervical vagotomy; vi) anti-RAGE group,
administered with intraperitoneal injection of anti-RAGE
neutralizing antibody (1 mg/kg; Beijing Bioss Biotech Co., Ltd.,
Beijing, China) prior to stimulation of the vagus nerve and
following CLP and bilateral cervical vagotomy. The mean artery
pressure (MAP) was monitored by cannulating into the right carotid
artery.
Vagus nerve stimulation
The bipolar platinum electrodes were connected to
left vagus nerve trunk in a stimulation module and controlled by an
acquisition system. The vagus nerve was subjected to constant
electrical stimulation (left cervical vagus, 5 V, 2 ms, 1 Hz).
Immediately following CLP, stimulation was performed for 20 min.
Firstly, the left vagus nerve trunk was exposed and isolated
without transection. The animal was subsequently subjected to CLP
operation, followed by cutting off the left vagus nerve trunk and
electrical stimulation.
Measurement of liver function
markers
The serum was separated from blood samples of
Sprague-Dawley rats at 6 h after CLP by centrifugation (2,000 × g
per min for 10 min), and was analyzed within 24 h using a chemistry
analyzer (Hitachi 7170; Hitachi, Tokyo, Japan). Serum levels of
alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
were measured to assess the liver function.
Measuring serum levels of S100A8 and AGEs
by enzyme-linked immunosorbent assay (ELISA)
At 3 and 6 h after CLP or sham CLP operation, the
whole blood sample was drawn via the right carotid artery (2 ml
each). The serum was immediately separated by centrifugation at
2,000 × g for 15 min at 4°C, and was divided into aliquots and
stored at −70°C for assay. ELISA was performed to measure serum
levels of S100A8 (Hycult Biotechnology b.v., Uden, the Netherlands)
and AGEs (Xitang Bio Technology Co., Ltd., Shanghai, China),
according to the manufacturer's protocol. Ortho-phosphoric acid
(100 µl) was added to terminate the color reaction. A
microplate reader (Spectra MR; Dynex, Chantilly, VA, USA) was used
to acquire data. The concentrations of S100A8 or AGEs in the
samples were calculated based on standard curves.
Western blot analysis
The rat hepatic tissue was dissected for analyzing
protein levels by western blotting. The proteins from hepatic
tissue were extracted and their concentrations were determined by
bicinchoninic acid protein concentration assay kit (Beijing Biosea
Biotechnology Co., Ltd., Beijing China). The cell lysates (50
µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and the proteins were
subsequently electrophoretically transferred onto polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membrane was blocked in 5% non-fat dry milk for 60 min in
Tris-buffer saline, containing 0.05% Tween 20, at room temperature.
The blots were incubated with horseradish peroxidase
(HRP)-conjugated rabbit anti-rat antibodies against RAGE (cat. no.
sc-33662; 1:1,000) or β-actin (cat. no. sc-1616; 1:1,000) for 1 h
at 37°C (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The
protein bands were detected using enhanced chemiluminescence
(Pierce® ECL Plus Western Blotting Substrate; Pierce
Biotechnology, Inc., Rockford, IL, USA). Gel-Pro Analyzer software
(version 4.0; Media Cybernetics, Rockville, MD, USA) was applied to
analyze relative intensities of protein bands.
Statistical analysis
The data are expressed as mean ± standard deviation.
Statistical analysis was performed using commercially available
software (SPSS version 14.0; SPSS, Inc., Chicago, IL, USA). A
one-way analysis of variance was used to assess the differences
between groups at the same time point, and two-way analysis of
variance was used for repeated measurements with multiple
comparisons (Bonferroni test). P<0.05) was considered to
indicate a statistically significant difference.
Results
MAP
At baseline and 1 h after CLP, no significant
differences were observed in the MAP between any of the groups. In
the CLP group, MAP was significantly decreased compared with the
sham group 3 and 6 h after CLP challenge (P<0.05). At 3 and 6 h
after CLP challenge, the MAP increased significantly in the STM
group compared with the sham group (P<0.05). The inhibitory
effect on septic shock of vagus nerve electrical stimulation was
attenuated by α-BGT pre-treatment and enhanced by anti-RAGE
antibody pre-treatment. After CLP challenge, MAP was decreased in
the VGX group compared with the CLP group, with no significant
difference (Fig. 1).
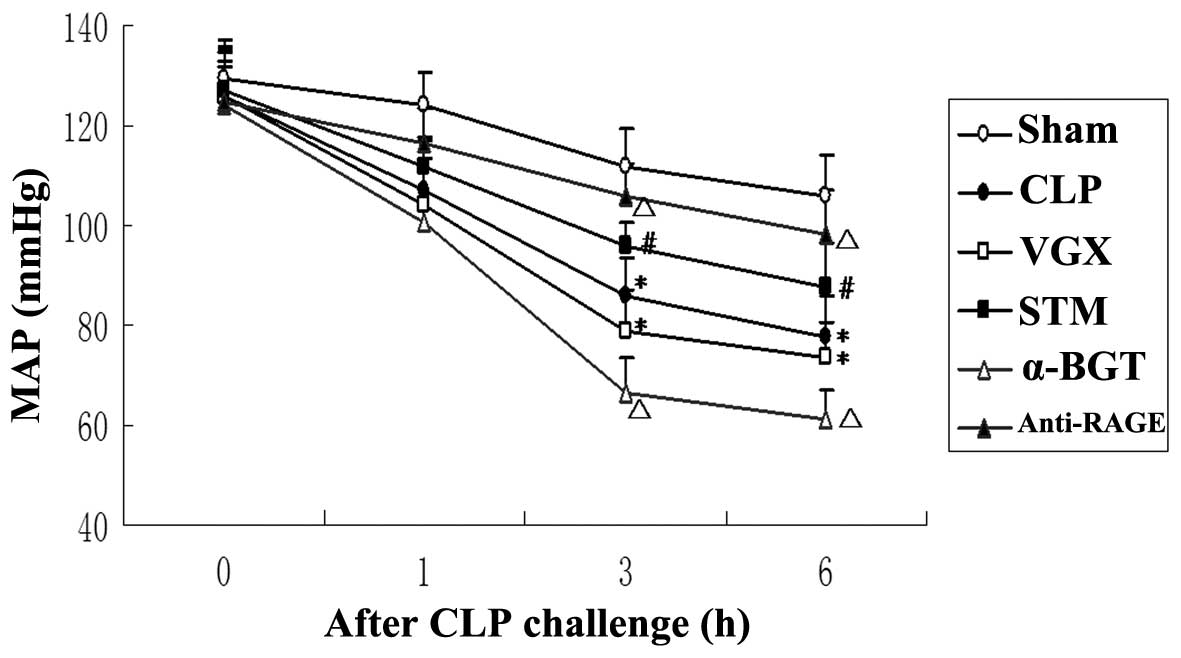 | Figure 1Changes of the MAP. The MAP following
CLP challenge was assessed for the Sham, CLP, VGX, STM, α-BGT and
anti-RAGE groups. The data are expressed as the mean ± standard
deviation (*P<0.05, compared with the Sham group;
#P<0.05, compared with the CLP group;
ΔP<0.05, compared with the STM group). MAP, mean
artery pressure; CLP, cecal ligation and puncture; VGX, CLP +
bilateral cervical vagotomy; STM, CLP + bilateral cervical vagotomy
+ electrical stimulation; BGT, bungarotoxin; RAGE, receptor for
advanced glycation end products. |
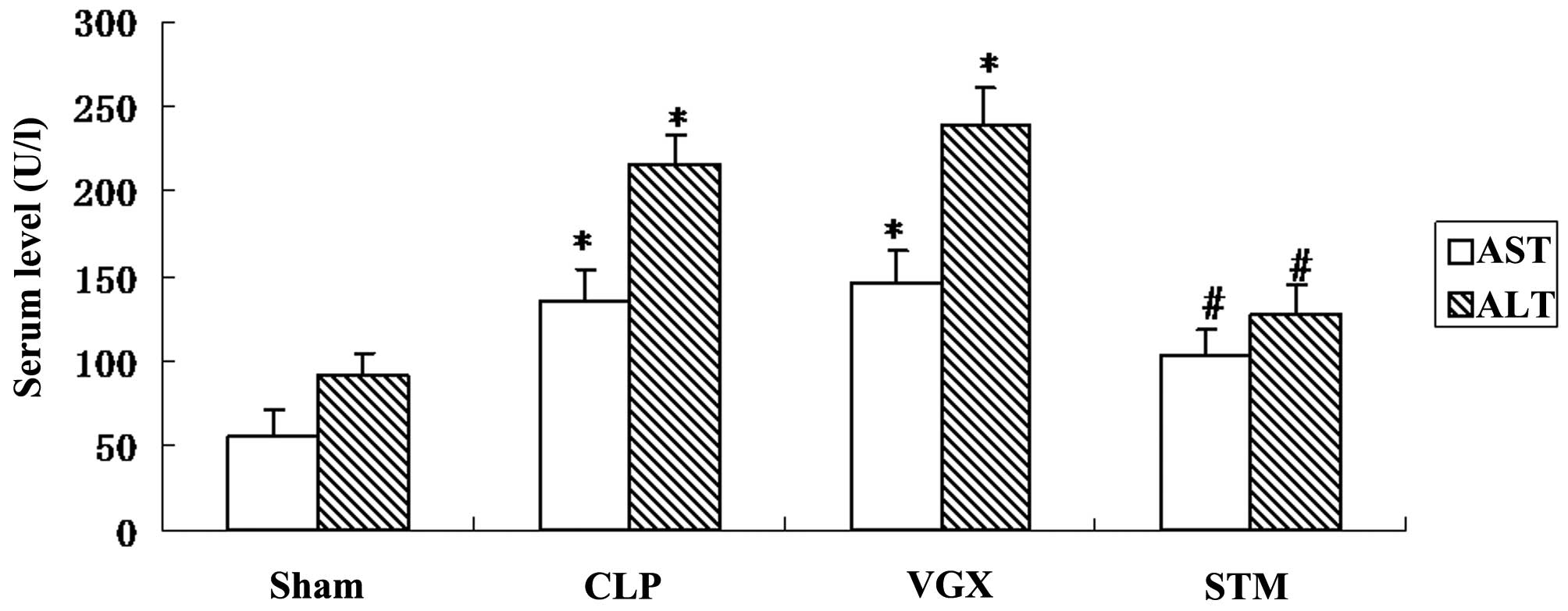
Blood biochemistry evaluation
To evaluate the hepatic injury by CLP induced septic
shock, the serum AST and ALT levels were measured in Sprague-Dawley
rats at 6 h after CLP. Serum AST and ALT levels were significantly
increased by CLP challenge compared with the sham group
(P<0.05). By contrast, the STM group exhibited significantly
decreased serum AST or ALT levels compared with that in the CLP
group (P<0.05; Fig. 2).
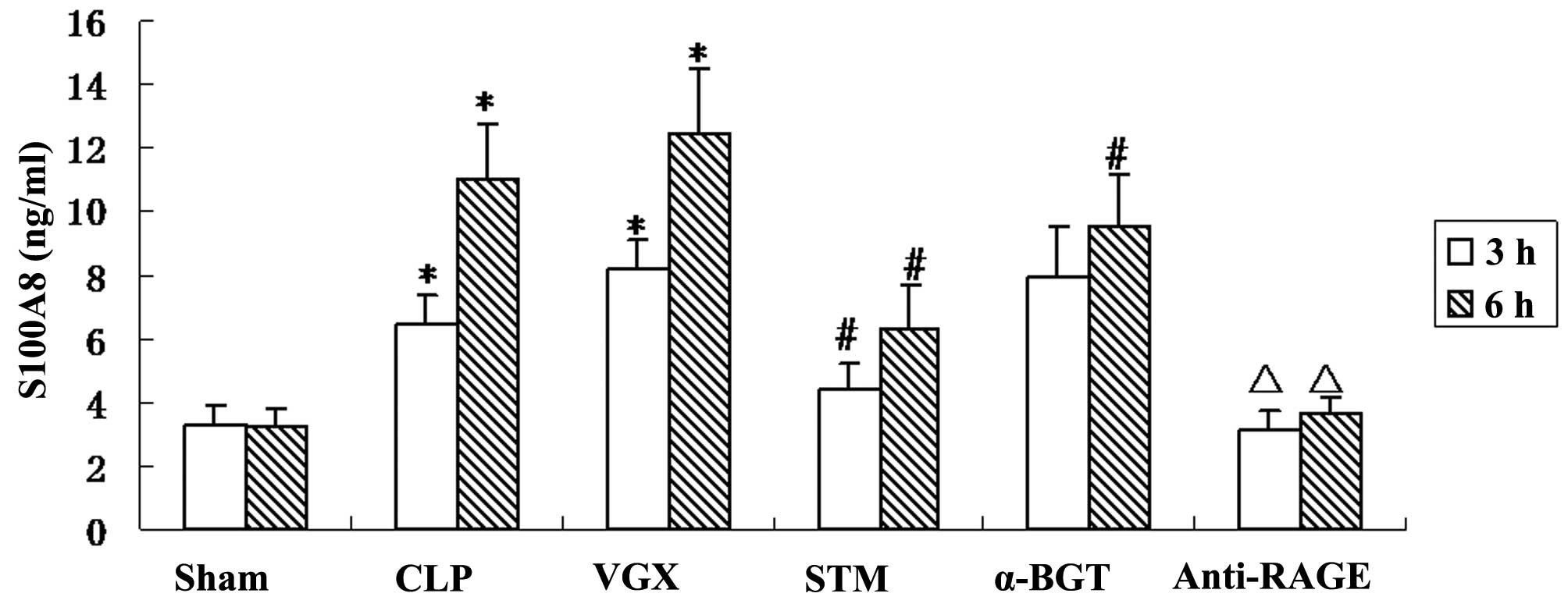
Serum levels of S100A8
To examine whether vagus nerve electrical
stimulation can modulate the production of S100A8 in septic shock
rats, ELISA was performed to measure serum S100A8 levels. The serum
levels of S100A8 were significantly increased at 3 and 6 h after
the CLP induction. More serum S100A8 production was observed after
bilateral cervical vagotomy compared with the CLP group, with no
significant difference. The left vagus nerve electrical stimulation
significantly reduced serum production of S100A8. α-BGT
pre-treatment significantly reversed, while anti-RAGE antibody
pre-treatment significantly enhanced the inhibitory effect of vagal
electrical stimulation in serum S100A8 level (Fig. 3).
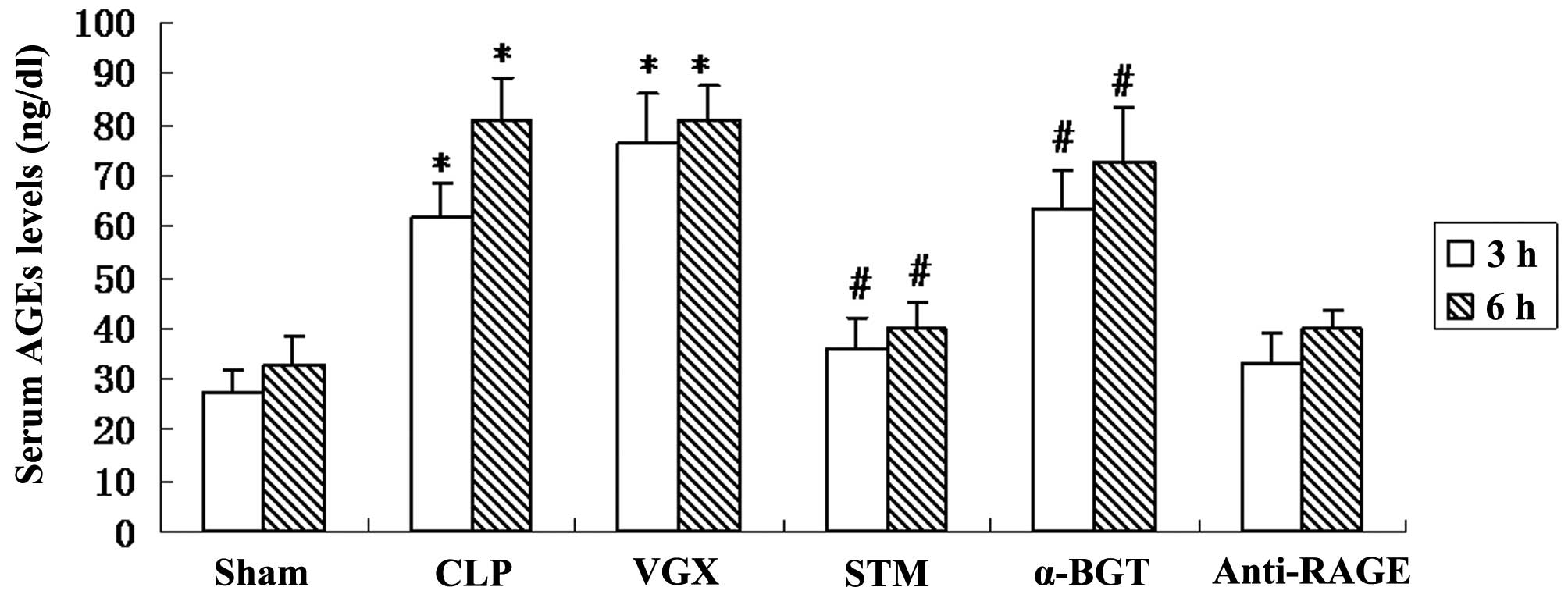
Serum levels of AGEs
The serum levels of AGEs were measure by ELISA at 3
and 6 h after CLP. Compared with the sham group, a significant
increase in the serum levels of AGEs was observed in the CLP group
(P<0.05). Vagus nerve electrical stimulation significantly
reduced the levels of AGEs compared with the CLP group. The
inhibitory effect on serum levels of AGEs was significantly
reversed by α-BGT pre-treatment and enhanced by anti-RAGE antibody
pre-treatment (Fig. 4).
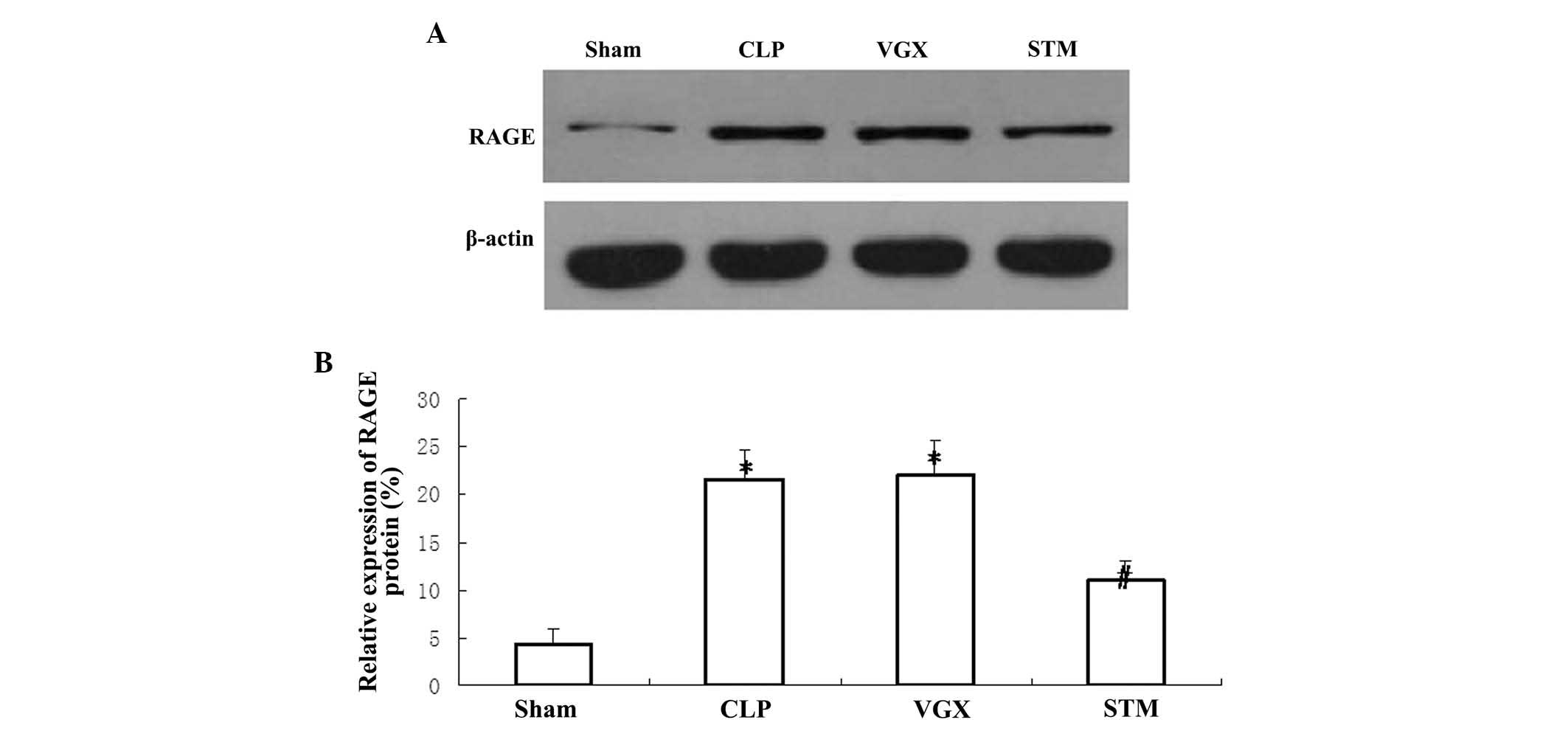
Hepatic protein expression of RAGE
Western blotting was performed to detect the hepatic
protein expression of RAGE at 6 h after CLP challenge. The protein
expression of hepatic RAGE was significantly increased in the CLP
group compared with the sham group (P<0.05). Vagus nerve
electrical stimulation significantly decreased the protein
expression of hepatic RAGE compared with the CLP group (P<0.05).
By contrast, no significant differences were observed in the
hepatic RAGE protein expression between the CLP and VGX groups
(Fig. 5).
Discussion
Previously, the cholinergic anti-inflammatory
pathway was identified to associate the central nervous system with
the immune system (18). In the
present study, a septic shock model was established in
Sprague-Dawley rats by CLP. This revealed that CLP provoked
progressive hypotension and liver damage, elevated serum S100A8 and
AGEs levels, and enhanced the protein expression of hepatic RAGE.
Application of electrical stimulation to the vagus nerve
significantly attenuated the CLP-induced hypotension and liver
damage, decreased serum S100A8 and AGEs levels, and reduced the
protein expression of hepatic RAGE. The present study indicated
that the cholinergic anti-inflammatory pathway can significantly
inhibit the production of the inflammatory cytokine, S100A8.
Following bilateral cervical vagotomy, α-BGT administration
reversed, while anti-RAGE antibody administration enhanced, the
effects of vagus nerve electrical stimulation. Furthermore, the
cholinergic anti-inflammatory pathway also inhibited serum AGEs
levels and hepatic RAGE protein, which may be associated with the
inhibition of S100A8 by vagus nerve electrical stimulation.
Therefore, the present study found that the cholinergic
anti-inflammatory pathway can inhibit serum S100A8 levels, and
serum AGEs and hepatic RAGE protein may mediate the mechanisms
underlying the inhibition of S100A8 production and cholinergic
anti-inflammatory pathway.
In patients of several acute or chronic inflammatory
diseases, S100A8 is increased. Higher concentrations of S100A8
protein has been identified in inflamed tissue of rheumatoid
arthritis, compared with healthy individuals (19). Furthermore, elevations of S100A8
protein have been found in a variety of diseases, including acute
pancreatitis (20), transplant
rejection (21), inflammatory
bowel disease (22), myocardial
infarction (23) and appendicitis
(24). Elevated protein expression
of S100A8 has also been described in sepsis and septic shock. Serum
S100A8 concentration was increased in patients with severe sepsis
(25). The present study also
revealed that the S100A8 protein is increased in CLP-induced septic
shock rats. Additionally, vagus nerve electrical stimulation
reduced the protein expression of S100A8, with attenuation of
septic shock. This indicated that the decreased protein expression
of S100A8 is associated with the therapeutic effects of vagus nerve
electrical stimulation on septic shock, since serum levels of
S100A8 protein were decreased during the recovery from septic shock
(10).
In the development of multiple organ dysfunction
syndrome, induced by septic shock, microvascular blood flow
disturbances are pivotal and lead to a decreased perfusion and
blood flow velocity, and formation of microthrombi in the liver
sinusoids, thereby enhancing liver tissue ischemia and damage
(26). In the present study, the
serum AST and ALT levels were significantly increased in septic
shock, and this indicated that CLP induced the hepatic damage.
Vagus nerve electrical stimulation decreased the serum AST and ALT
levels, and demonstrated that the cholinergic anti-inflammatory
pathway can alleviate the hepatic damage in septic shock rats.
Neutrophils are important immune effectors for inflammatory
responses, and the recruitment and infiltration of neutrophils are
associated with pathology of liver injury induced by a variety of
diseases and toxicities (27). In
circulating human blood neutrophils, S100A8 constitutes ~20% of the
cytosolic protein fraction (28).
S100A8 is an inflammatory factor of neutrophils and has strong
chemotactic effects on neutrophils around inflammatory foci. S100A8
can also combine with its partner S100A9 to regulate hepatic CXCL-2
expression and neutrophil recruitment in an injured liver (29). Therefore, in the present study, the
elevated S100A8 protein may promote the neutrophil recruitment in
injured liver and release of AST and ALT.
In the present study, serum levels of AGEs were
significantly increased in septic shock rats, and were decreased by
vagus nerve electrical stimulation. AGEs are non-enzymatic
glycation/oxidation products of proteins/lipids, and accumulate
during natural aging and are augmented in numerous disorders. In
septic shock patients, serum levels of AGEs are associated with
serum level of asymmetric dimethylarginine, which is significantly
higher in septic shock patients compared with in healthy volunteers
(30). The accumulation of AGEs is
also associated with inflammation, which is confirmed by the report
that AGEs increased S100A8 mRNA expression through the
RAGE-mitogen-activated protein kinase signaling pathway under
diabetic pulp conditions (31). In
the present study, the expression pattern of serum AGEs was similar
to that of S100A8, therefore, AGEs may be one regulator upstream of
S100A8 in septic shock. The present study also suggested that vagus
nerve electrical stimulation can inhibit AGEs production. Notably,
nicotine has been reported to inhibit the actions of two subtypes
of AGEs, AGE-2 and AGE-3, in human monocytes (32). Nicotine is a specific activator of
α7-nAChR and is reported to inhibit the activation of monocytes.
α7-nAChR is the receptor for acetylcholine, a neurotransmitter
released from the vagus nerve. In the present study, it remains
unknown whether vagus nerve electrical stimulation directly reduces
the production of AGEs or if decreased serum AGEs are the result of
attenuated septic shock. In the senescent mouse brain, declined
expression of muscarinic acetylcholine receptor M1 (mAChR1),
another receptor subtype for acetylcholine, is correlated with
increased expression of AGEs (33). Therefore, the regulation between
the vagus nerve and AGEs deserves further investigation.
RAGE can activate multiple inflammatory pathways of
numerous physiological and pathological processes. Recently, RAGE
has been observed to serve important roles in sepsis by
perpetuating inflammation (34).
In the present study, the protein expression of hepatic RAGE was
significantly increased in septic shock rats, and was decreased by
vagus nerve electrical stimulation. RAGE is a transmembrane
receptor of the immunoglobulin superfamily. Deletion of RAGE
inhibits the lethal effects of septic shock, induced by CLP
(35), and this indicates that
RAGE may serve a pro-inflammatory role and promote the development
of septic shock. Notably, compared with healthy volunteers, septic
patients exhibited elevated plasma soluble RAGE (sRAGE)
concentrations, and among septic patients, non-survivors had higher
plasma sRAGE concentrations compared with the survivors, indicating
that sRAGE may be a parameter for severity and outcome of septic
patients (36). Up until now, no
report about the regulation between the vagus nerve and RAGE has
been published. Therefore, it remains unknown whether vagus nerve
electrical stimulation directly reduces the protein expression of
hepatic RAGE or if decreased RAGE protein is the result of
attenuated septic shock. It was reported that AGEs upregulate the
expression of RAGE in various tissue types, and form a positive
feedback loop (37). The positive
association between serum AGEs and serum sRAGE was observed in
non-diabetic subjects and patients with type 2 diabetes (38,39).
Therefore, hepatic RAGE protein may be regulated by AGEs in septic
shock rats. In THP-1 human monocytes, AGE pre-incubation resulted
in upregulation of RAGE, and AGE pre-treatment, followed by
interleukin (IL)-6 or tumor necrosis factor (TNF)α stimulation
further increased the mRNA expression levels of S100A8 and S100A9
and increased the release of S100A8/A9 into the cell culture
supernatant. AGEs-mediated S100A8/A9 expression and release were
RAGE-dependent (40). Therefore,
the hepatic RAGE protein may be regulated by AGEs and RAGE protein
also may be a regulator upstream of S100A8 in septic shock rats.
Furthermore, since S100A8/A9 has been identified as a ligand of
RAGE (40), the protective effect
of S100A8 is mediated by RAGE in septic shock rats. This indicated
that inhibition of RAGE has potential therapeutic effect on septic
shock. Notably, in the present study, anti-RAGE antibody
pre-treatment significantly enhanced MAP elevation and S100A8
decrease induced by vagus nerve electrical stimulation. The present
result is in accordance with a previous report that anti-RAGE
monoclonal antibody served a protective effect and offered a
survival advantage to septic mice (41).
However, the precise mechanisms underlying the
inhibitory effects of vagus nerve electrical stimulation on S100A8
and RAGE proteins remains unclear. In addition, the roles of other
cytokines, including IL-6 and TNFα, require further investigation,
including their effect on septic shock and the expression of
S100A8.
In conclusion, vagus nerve electrical stimulation
attenuated hypotension and liver injury, decreased the production
of the pro-inflammatory cytokine, S100A8 in vivo. The vagus
nerve released acetylcholine, and may inhibit the release and
synthesis of AGEs significantly, which regulate the expression
levels of S100A8 and RAGE. The protective effect of S100A8 on
septic shock may be mediated by RAGE, as the anti-RAGE antibody can
enhanced the protective effects of vagus nerve electrical
stimulation. The present study demonstrated that S100A8 may be a
diagnostic marker for sepsis and septic shock. Further
investigation is required to evaluate the feasibility of S100A8 as
a therapeutic target in septic shock.
References
|
1
|
Pravda J: Metabolic theory of septic
shock. World J Crit Care Med. 3:45–54. 2014.PubMed/NCBI
|
|
2
|
Schulte W, Bernhagen J and Bucala R:
Cytokines in sepsis: Potent immunoregulators and potential
therapeutic targets-an updated view. Mediators Inflamm.
2013:1659742013. View Article : Google Scholar
|
|
3
|
King EG, Bauzá GJ, Mella JR and Remick DG:
Pathophysiologic mechanisms in septic shock. Lab Invest. 94:4–12.
2014. View Article : Google Scholar
|
|
4
|
Averill MM, Kerkhoff C and Bornfeldt KE:
S100A8 and S100A9 in cardiovascular biology and disease.
Arterioscler Thromb Vasc Biol. 32:223–229. 2012. View Article : Google Scholar :
|
|
5
|
Goyette J and Geczy CL:
Inflammation-associated S100 proteins: New mechanisms that regulate
function. Amino Acids. 41:821–842. 2011. View Article : Google Scholar
|
|
6
|
Sohnle PG, Hunter MJ, Hahn B and Chazin
WJ: Zinc-reversible antimicrobial activity of recombinant
calprotectin (migration inhibitory factor-related proteins 8 and
14). J Infect Dis. 182:1272–1275. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viemann D, Barczyk K, Vogl T, Fischer U,
Sunderkötter C, Schulze-Osthoff K and Roth J: MRP8/MRP14 impairs
endothelial integrity and induces a caspase-dependent and
-independent cell death program. Blood. 109:2453–2460. 2007.
View Article : Google Scholar
|
|
8
|
Ryckman C, Vandal K, Rouleau P, Talbot M
and Tessier PA: Proinflammatory activities of S100: Proteins
S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and
adhesion. J Immunol. 170:3233–3242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viemann D, Strey A, Janning A, Jurk K,
Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, et al:
Myeloid-related proteins 8 and 14 induce a specific inflammatory
response in human microvascular endothelial cells. Blood.
105:2955–2962. 2005. View Article : Google Scholar
|
|
10
|
Payen D, Lukaszewicz AC, Belikova I,
Faivre V, Gelin C, Russwurm S, Launay JM and Sevenet N: Gene
profiling in human blood leucocytes during recovery from septic
shock. Intensive Care Med. 34:1371–1376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martelli D, McKinley MJ and McAllen RM:
The cholinergic anti-inflammatory pathway: A critical review. Auton
Neurosci. 182:65–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mabley JG, Pacher P and Szabo C:
Activation of the cholinergic anti-inflammatory pathway reduces
ricin-induced mortality and organ failure in mice. Mol Med.
15:166–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnston GR and Webster NR: Cytokines and
the immunomodulatory function of the vagus nerve. Br J Anaesth.
102:453–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tracey KJ: Physiology and immunology of
the cholinergic anti-inflammatory pathway. J Clin Invest.
117:289–296. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song XM, Li JG, Wang YL, Hu ZF, Zhou Q, Du
ZH and Jia BH: The protective effect of the cholinergic
anti-inflammatory pathway against septic shock in rats. Shock.
30:468–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson J, Higgins D, Hutting H, Serkova N,
Baird C, Khailova L, Queensland K, Vu Tran Z, Weitzel L and
Wischmeyer PE: Early propranolol treatment induces lung
heme-oxygenase-1, attenuates metabolic dysfunction and improves
survival following experimental sepsis. Crit Care. 17:R1952013.
View Article : Google Scholar
|
|
17
|
Singleton KD and Wischmeyer PE: Distance
of cecum ligated influences mortality, tumor necrosis factor-alpha
and interleukin-6 expression following cecal ligation and puncture
in the rat. Eur Surg Res. 35:486–491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonaz B, Picq C, Sinniger V and Mayol JF:
From epilepsy to the cholinergic anti-inflammatory pathway.
Neurogastroenterol Motil. 25:208–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Odink K, Cerletti N, Brüggen J, Clerc RG,
Tarcsay L, Zwadlo G, Gerhards G, Schlegel R and Sorg C: Two
calcium-binding proteins in infiltrate macrophages of rheumatoid
arthritis. Nature. 330:80–82. 1987. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farkas G Jr, Tiszlavicz Z, Takács T,
Szabolcs A, Farkas G, Somogyvári F and Mándi Y: Analysis of plasma
levels and polymorphisms of S100A8/9 and S100A12 in patients with
acute pancreatitis. Pancreas. 43:485–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikemoto M, Matsumoto S, Egawa H, Okitsu T,
Iwanaga Y, Umemoto S, Itoh H, Murayama H and Fujita M: A case with
transient increases in serum S100A8/A9 levels implying acute
inflammatory responses after pancreatic islet transplantation. Ann
Clin Biochem. 44:570–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leach ST, Yang Z, Messina I, Song C, Geczy
CL, Cunningham AM and Day AS: Serum and mucosal S100 proteins,
calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis
in children with inflammatory bowel disease. Scand J Gastroenterol.
42:1321–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katashima T, Naruko T, Terasaki F, Fujita
M, Otsuka K, Murakami S, Sato A, Hiroe M, Ikura Y, Ueda M, et al:
Enhanced expression of the S100A8/A9 complex in acute myocardial
infarction patients. Circ J. 74:741–748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bealer JF and Colgin M: S100A8/A9: A
potential new diagnostic aid for acute appendicitis. Acad Emerg
Med. 17:333–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Zoelen MA, Vogl T, Foell D, Van Veen
SQ, van Till JW, Florquin S, Tanck MW, Wittebole X, Laterre PF,
Boermeester MA, et al: Expression and role of myeloid-related
protein-14 in clinical and experimental sepsis. Am J Respir Crit
Care Med. 180:1098–1106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spapen H: Liver perfusion in sepsis,
septic shock and multiorgan failure. Anat Rec (Hoboken).
291:714–720. 2008. View
Article : Google Scholar
|
|
27
|
Ramaiah SK and Jaeschke H: Role of
neutrophils in the pathogenesis of acute inflammatory liver injury.
Toxicol Pathol. 35:757–766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edgeworth J, Gorman M, Bennett R, Freemont
P and Hogg N: Identification of p8,14 as a highly abundant
heterodimeric calcium binding protein complex of myeloid cells. J
Biol Chem. 266:7706–7713. 1991.PubMed/NCBI
|
|
29
|
Moles A, Murphy L, Wilson CL, Chakraborty
JB, Fox C, Park EJ, Mann J, Oakley F, Howarth R, Brain J, et al: A
TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil
recruitment in acute and chronic liver injury in the mouse. J
Hepatol. 60:782–791. 2014. View Article : Google Scholar :
|
|
30
|
Nakamura T, Sato E, Fujiwara N, Kawagoe Y,
Suzuki T, Ueda Y, Yamada S, Shoji H, Takeuchi M, Ueda S, et al:
Circulating levels of advanced glycation end products (AGE) and
interleukin-6 (IL-6) are independent determinants of serum
asymmetric dimethylarginine (ADMA) levels in patients with septic
shock. Pharmacol Res. 60:515–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakajima Y, Inagaki Y, Kido J and Nagata
T: Advanced glycation end products increase expression of S100A8
and A9 via RAGE-MAPK in rat dental pulp cells. Oral Dis.
21:328–334. 2015. View Article : Google Scholar
|
|
32
|
Takahashi HK, Liu K, Wake H, Mori S, Zhang
J, Liu R, Yoshino T and Nishibori M: Effect of nicotine on advanced
glycation end product-induced immune response in human monocytes. J
Pharmacol Exp Ther. 332:1013–1021. 2010. View Article : Google Scholar
|
|
33
|
Zhang X, Jin C, Li Y, Guan S, Han F and
Zhang S: Catalpol improves cholinergic function and reduces
inflammatory cytokines in the senescent mice induced by
D-galactose. Food Chem Toxicol. 58:50–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bopp C, Bierhaus A, Hofer S, Bouchon A,
Nawroth PP, Martin E and Weigand MA: Bench-to-bedside review: The
inflammation-perpetuating pattern-recognition receptor RAGE as a
therapeutic target in sepsis. Crit Care. 12:2012008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liliensiek B, Weigand MA, Bierhaus A,
Nicklas W, Kasper M, Hofer S, Plachky J, Gröne HJ, Kurschus FC,
Schmidt AM, et al: Receptor for advanced glycation end products
(RAGE) regulates sepsis but not the adaptive immune response. J
Clin Invest. 113:1641–1650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bopp C, Hofer S, Weitz J, Bierhaus A,
Nawroth PP, Martin E, Büchler MW and Weigand MA: sRAGE is elevated
in septic patients and associated with patients outcome. J Surg
Res. 147:79–83. 2008. View Article : Google Scholar
|
|
37
|
Yamagishi S, Nakamura K and Matsui T:
Advanced glycation end products (AGEs) and their receptor (RAGE)
system in diabetic retinopathy. Curr Drug Discov Technol. 3:83–88.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamagishi S, Adachi H, Nakamura K, Matsui
T, Jinnouchi Y, Takenaka K, Takeuchi M, Enomoto M, Furuki K, Hino
A, et al: Positive association between serum levels of advanced
glycation end products and the soluble form of receptor for
advanced glycation end products in nondiabetic subjects.
Metabolism. 55:1227–1231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakamura K, Yamagishi S, Adachi H, Matsui
T, Kurita-Nakamura Y, Takeuchi M, Inoue H and Imaizumi T: Serum
levels of soluble form of receptor for advanced glycation end
products (sRAGE) are positively associated with circulating AGEs
and soluble form of VCAM-1 in patients with type 2 diabetes.
Microvasc Res. 76:52–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eggers K, Sikora K, Lorenz M, Taubert T,
Moobed M, Baumann G, Stangl K and Stangl V: RAGE-dependent
regulation of calcium-binding proteins S100A8 and S100A9 in human
THP-1. Exp Clin Endocrinol Diabetes. 119:353–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Christaki E, Opal SM, Keith JC Jr,
Kessimian N, Palardy JE, Parejo NA, Tan XY, Piche-Nicholas N,
Tchistiakova L, Vlasuk GP, et al: A monoclonal antibody against
RAGE alters gene expression and is protective in experimental
models of sepsis and pneumococcal pneumonia. Shock. 35:492–498.
2011. View Article : Google Scholar : PubMed/NCBI
|