Introduction
Acute pancreatitis (AP) is a common abdominal
inflammatory disease for which a specific clinical treatment
remains elusive (1,2). The majority of patients with AP
exhibit a mild form of the disease; however, 20–25% of patients
suffer a severe episode and consequently may develop multiple organ
dysfunction syndrome, a main cause of AP-associated mortality
(3,4). Alcoholism and gallstones are the most
common etiological factors, which lead to intrapancreatic
trypsinogen activation and cellular injury of the pancreas
(2,5). Innate immune cells and mediators have
important roles in the pathogenesis of AP (6,7), and
prognosis of the disease is directly associated with the intensity
of inflammation. Immune cell infiltration and elevated serum levels
of mediators, including tumor necrosis factor (TNF)-α and
interleukin (IL)-1β, are used as markers of inflammatory responses
(8). Recently, antimicrobial
peptides (AMPs), particularly α-defensins, have been implicated in
AP (9). AMPs are innate
immunity-derived peptides, which are primarily expressed by
epithelial cells and infiltrating immune cells in mammals under
steady state or during inflammation (10). AP is an inflammatory disorder, and
is therefore associated with altered permeability of the
AMP-producing cells, thus suggesting a potential role for AMPs in
this condition.
Among AMPs, cathelicidins are pleiotropic AMPs that
possess broad-spectrum antimicrobial activities and have a major
role in regulating local inflammation and immunity (11,12).
Cathelicidins are characteristically cationic and share a conserved
N-terminal pro-region, which is termed the cathelin domain, and a
variable C-terminal antimicrobial domain. A single cathelicidin is
found in humans (hCAP18/LL-37) and its orthologs in the rat and
mouse are rat cathelicidin-related antimicrobial peptide (CRAMP)
and mouse CRAMP, respectively (13,14).
In addition to their antimicrobial activities, cathelicidins have
been reported to exert modulatory effects on various host cells,
notably epithelial and immune cells (15–18).
Cathelicidins contribute to immune cell recruitment and activation,
cytokine production, modulation of inflammatory responses during
inflammatory bowel diseases and gastrointestinal inflammation
(12,18). However, the role of CRAMP in AP
remains clear.
AP is associated with complex episodes of
inflammation of the pancreatic acinar cells and distant organs.
While the cellular and molecular regulatory mechanisms underlying
AP pathogenesis remain to be fully elucidated for the
identification of a curative treatment, exploration of novel innate
immunomodulatory mediators may yield a promising outcome (19). Therefore, the present study
investigated the potential effects of CRAMP on caerulein-induced
experimental AP in mice. The results support a modulatory role of
CRAMP in AP, and suggest CRAMP may be a potential therapeutic
target for future investigation.
Materials and methods
Animals
Male C57BL/6J (Su Pu Si Biotechnology Co., Ltd.,
Suzhou, China) and CRAMP-deficient cnlp−/− mice
(C57BL/6J background; age, 8 weeks; The Jackson Laboratory,
Sacramento, CA, USA) were maintained at the Animal Housing Unit of
Jiangnan University (Wuxi, China) under a controlled temperature
(23–25°C) and a 12 h light/12 h dark cycle. All of the mice were
provided with standard laboratory chow and water ad libitum.
All experimental protocols were approved by the Animal Ethics
Committee of Jiangnan University, and were performed in accordance
with the guidelines therein.
Reagents
Caerulein and tetramethylbenzidine substrate were
used for enzyme-linked immunosorbent assay (ELISA) assays and were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Amylase and
myeloperoxidase (MPO) activity measurement kits were purchased from
the Jiancheng Bioengineering Institute (Nanjing, China). Mice TNF-α
and mice monocyte chemotactic protein (MCP)-1 ELISA kits were
obtained from Biolegend, Inc. (San Diego, CA, USA). All other
reagents were supplied locally by the material library of Jiangnan
University and were purchased from National Medicine Group Chemical
Reagent Co., Ltd. (Shanghai, China).
Induction of AP
Mice were randomly assigned into the control and
experimental groups (n=8). The groups were as follows: CRAMP gene
knockout (cnlp−/−) mice and wild-type C57BL/6J mice with
the same genetic background were randomly assigned into the
cnlp−/− control, cnlp−/− mice with AP group
[AP (cnlp−/−)] and C57BL/6J mice with AP group [AP
(C57)]. The mice received hourly intraperitoneal injections with
normal saline or saline containing caerulein (50 µg/kg) for
10 h to induce AP. A total of 1 h after the final injection, the
mice were sacrificed with a lethal dose of pentobarbitone sodium
(100 mg/kg). The experiments were repeated three times. Blood
samples were collected in sterilized centrifuge tubes, were
centrifuged to separate the serum, and were stored at −80°C. The
harvested pancreatic tissue samples were stored at −80°C for
subsequent measurements of MPO activity, and cytokine and chemokine
levels.
Serum amylase measurements
Serum was collected by allowing the blood to
coagulate at ambient temperate for 25 min, and subsequently
centrifuging the samples at 3,000 × g for 10 min at 4°C. The
supernatant was then collected for analysis. An iodine-starch
colorimetric method was used to measure serum amylase levels.
Briefly, serum samples were incubated with 0.5 ml pre-warmed
substrate buffer for 7.5 min at 37°C. Absorbance was measured,
following the addition of 0.5 ml iodine and 3 ml ddH2O
to the mixture, at 660 nm using a UV-2450 UV-VIS spectrophotometer
(Shimadzu Corporation, Kyoto, Japan).
MPO activity
MPO activity was evaluated using an MPO assay kit
according to the manufacturer's protocol. Pancreatic tissues (5%)
were homogenized in 0.9% saline using an IKA homogenizer (Straufen,
Germany). Absorbance was analyzed using a UV-2450 UV-VIS
spectrophotometer (Shimadzu Corporation) at 460 nm within 10 min
and MPO activities are expressed as units/g tissue.
ELISA assays of inflammatory
mediators
Pancreatic homogenates were assayed for MCP-1 and
TNF-α levels using sandwich ELISA kits. Previous procedures
validated by our group were adopted, according to the
manufacturer's protocols. Absorbance was measured at 450 nm within
30 min, using an automated microplate reader (Multiskan™ GO; Thermo
Fisher Scientific Oy, Vantaa, Finland). TNF-α and MCP-1
concentrations were calculated based on the absorbance
measurements, and are expressed as pg/ml.
Histological examination
Freshly harvested pancreatic samples were fixed with
4% paraformaldehyde overnight. The tissues were then washed with
ddH2O, dehydrated with gradient ethanol solutions and
embedded in paraffin and cut into 5 µm sections. The
sections were subsequently stained with hematoxylin/eosin
(H&E). Pancreatic injury was examined under a DM2000 light
microscope (Leica Microsystems GmbH, Wetzlar, Germany) at ×200
magnification, and was evaluated based on acinar cell injury,
inflammatory cell infiltration and structural changes, which are
markers of tissue damage and inflammation.
Statistical analysis
Statistical analysis was performed by independent
t-test to determine if there was a difference between MPO activity
levels in the control and AP (C57) group, AMY levels between AP
(cnlp−/−) and AP (C57) groups. When multiple comparisons
were made, by one-way analysis of variance using GraphPad Prism
(version 5; GraphPad Software Inc., San Diego, CA, USA). Tukey's
honest significant difference test was performed as a post-hoc
test. P<0.05 were considered to indicate a statistically
significant difference.
Results
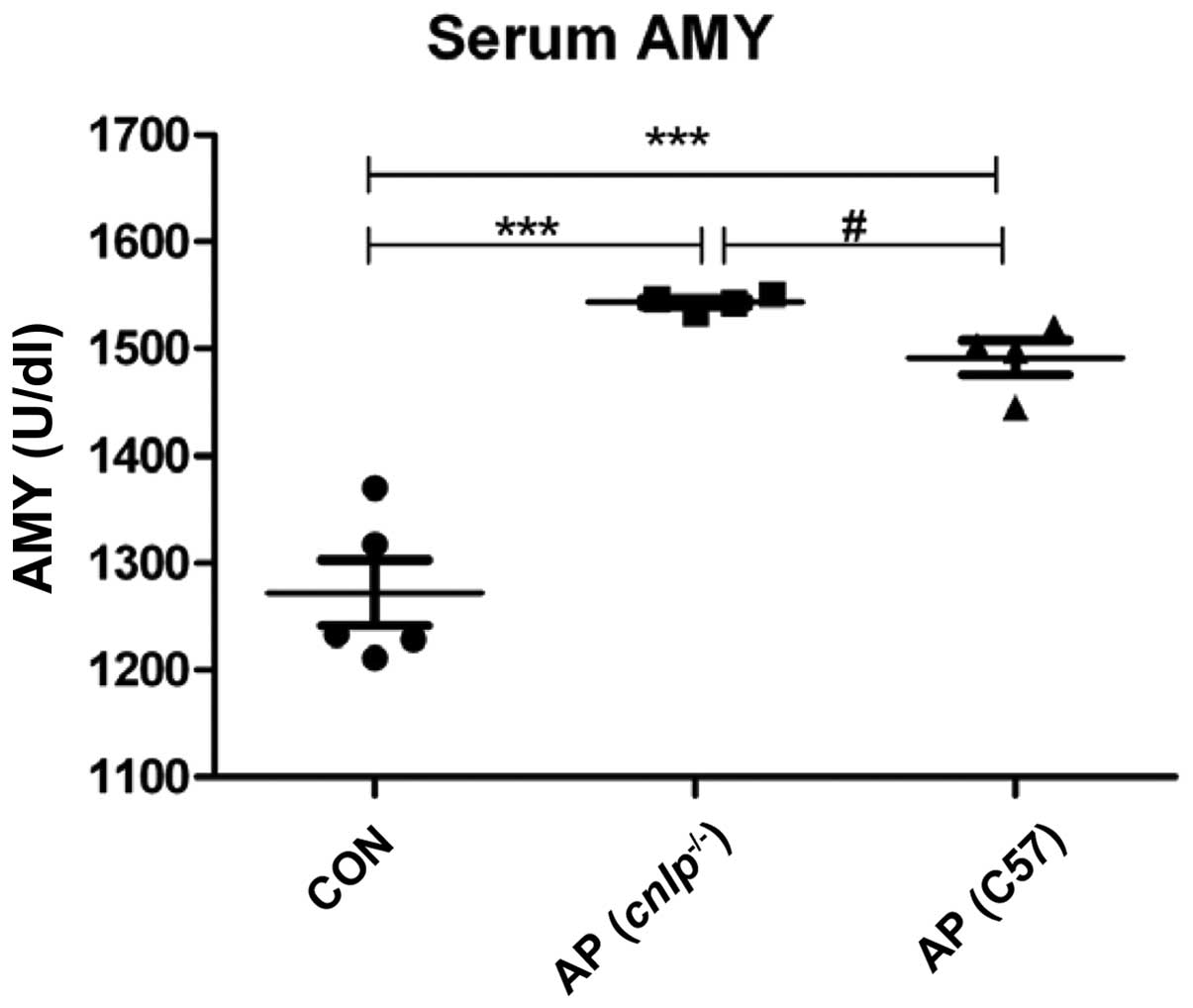
Effects of CRAMP deficiency on serum
amylase levels during AP
Serum amylase is measured as a sensitive biochemical
marker of AP, which is released due to pancreatic acinar cell
damage. Serum amylase levels were elevated in the wild-type
(P<0.001) and cnlp−/− AP (P<0.001) mice
compared with in the control mice. In addition, CRAMP-deficient
mice exhibited more pronounced serum amylase levels compared with
the wild-type mice (P<0.05; Fig.
1). These findings suggest that CRAMP may have a beneficial
role in protecting mice against AP.
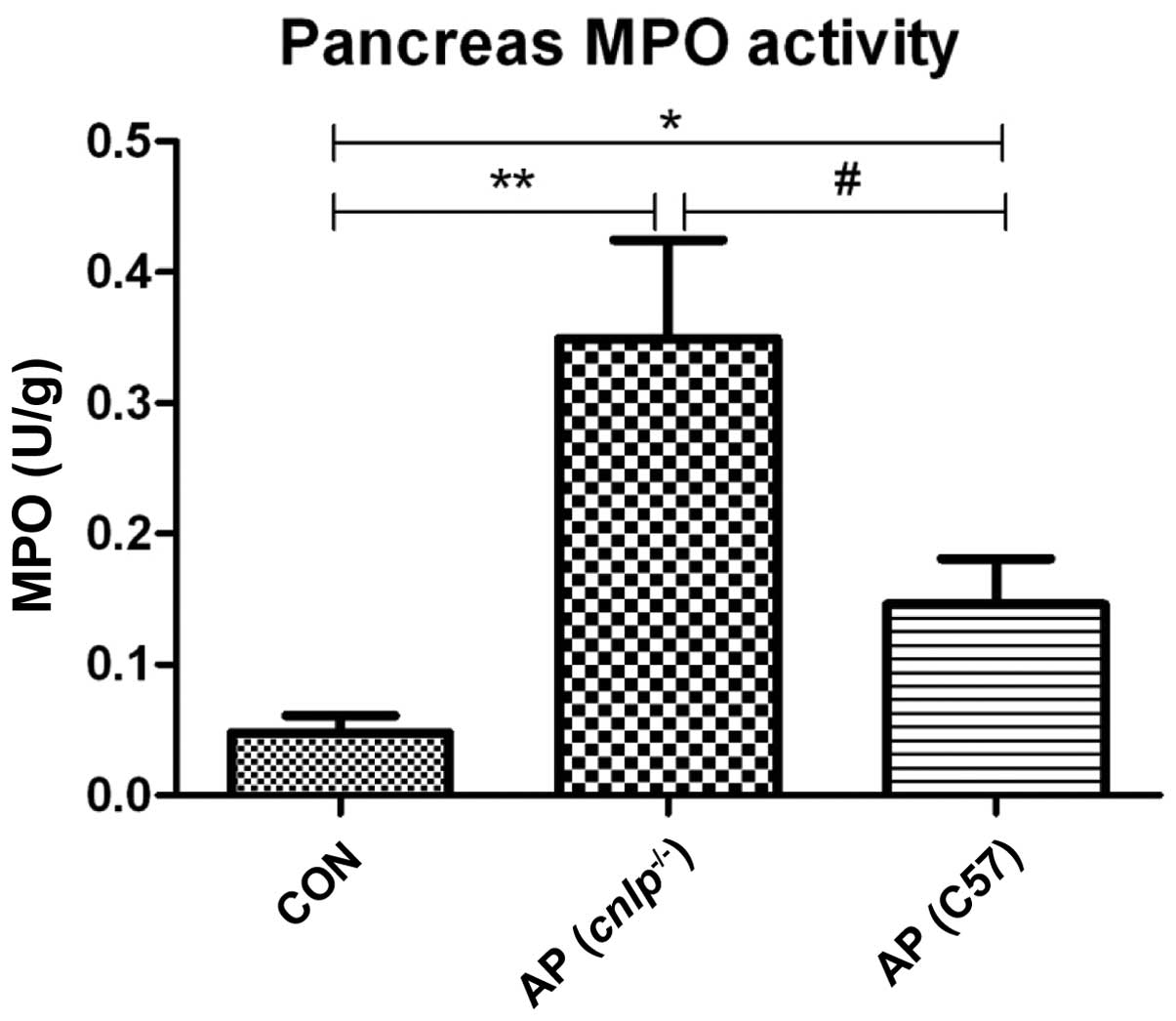
Effects of CRAMP deficiency on pancreatic
MPO release
Pancreatic MPO levels were evaluated as an indicator
of neutrophil infiltration into tissues during AP. Caerulein
hyperstimulation resulted in increased pancreatic MPO levels in the
wild-type (P<0.05) and cnlp−/− mice
(P<0.01; Fig. 2). This increase
in MPO was more significant in the pancreatic tissues of the
cnlp−/− mice compared with the wild-type mice
(P<0.05; Fig. 2). These results
suggest that CRAMP may be involved in early neutrophil recruitment
to the pancreas and regulating MPO activities during AP.
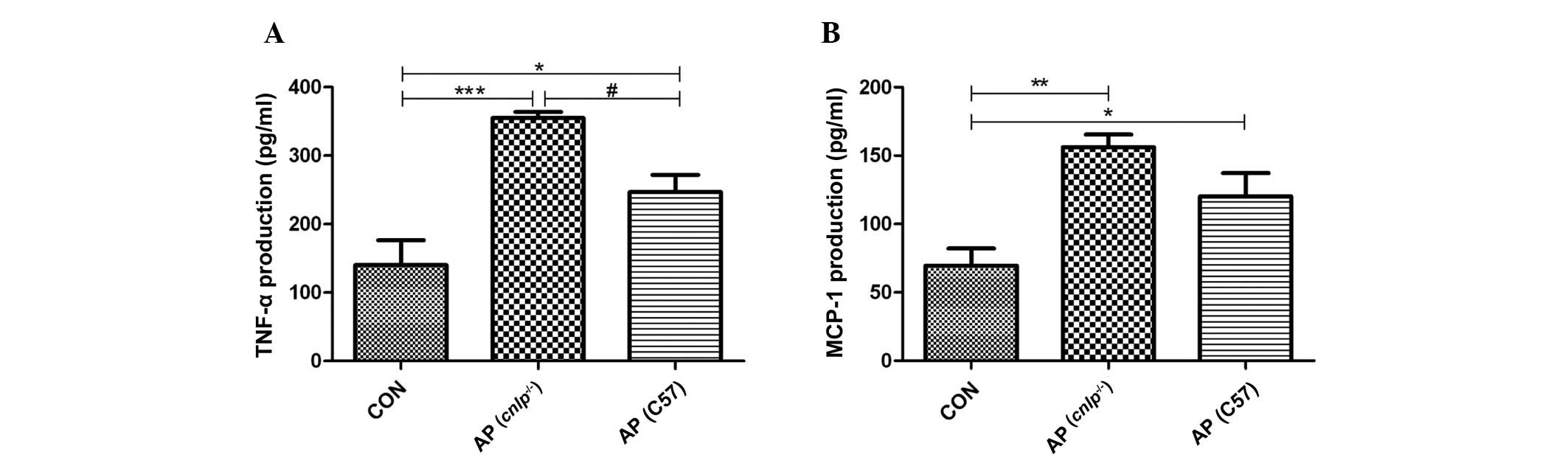
Effects of CRAMP deficiency on pancreatic
production of inflammatory mediators
As AP propagates it is associated with enhanced
production of inflammatory cytokines and chemokines at the tissue
level. Therefore, the present study examined the levels of known
early mediators of AP, including TNF-α and MCP-1. As shown in
Fig. 3A and B, experimental AP is
associated with increased levels of pancreatic TNF-α (P<0.001)
and MCP-1 (P<0.01). A further increase in TNF-α levels (all
P<0.05), but not MCP-1 levels, was observed in the pancreas of
cnlp−/− mice compared with in the C57BL/6J
wild-type AP mice (Fig. 3A and B).
These results indicate that CRAMP selectively modulates pancreatic
cytokine production during AP.
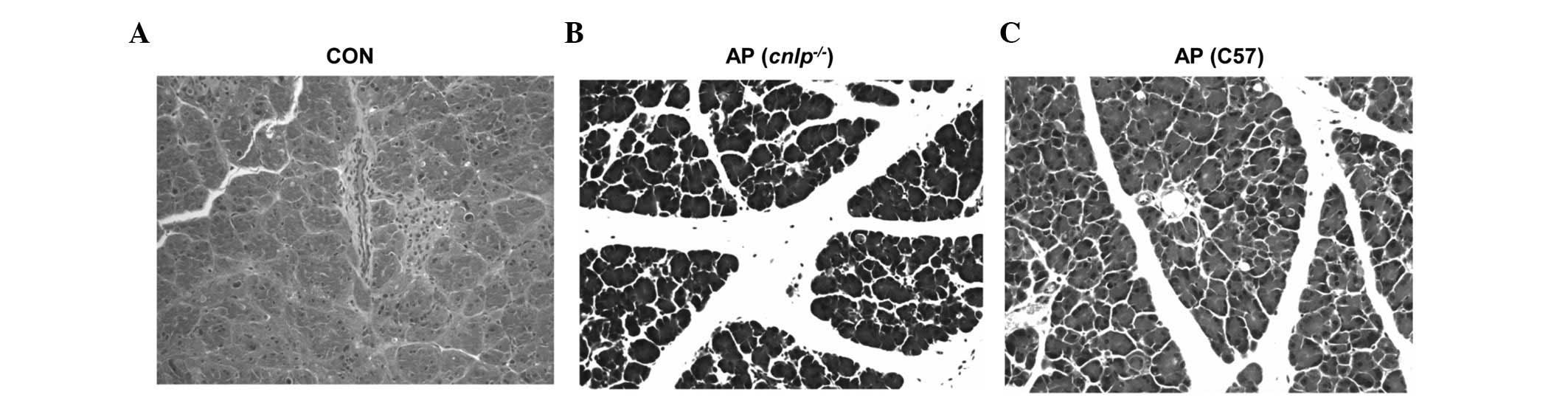
Effects of CRAMP deficiency on tissue
damage during AP
Pancreatic injury in mice with AP was evaluated by
H&E histological examination. The wild-type and CRAMP-deficient
cnlp−/− mice induced with caerulein
hyperstimulation exhibited pronounced pancreatic edema, enhanced
neutrophil infiltration into the pancreas, and pancreatic
morphological injuries, as compared with the control mice (Fig. 4A–C). Furthermore, pancreatic injury
in the cnlp−/− mice (Fig. 4B) was more severe than in the
wild-type mice (Fig. 4C). This
histological analysis confirmed that CRAMP alleviates pancreatic
injury and inflammation.
Discussion
The present study demonstrated that mouse CRAMP is a
novel modulatory mediator during AP, which is particularly
associated with local pancreatic inflammation. The
cnlp−/− C57BL/6J mice developed a more severe
phenotype and inflammatory responses, as compared with their
wild-type littermates induced with AP. In addition, the
cnlp−/− C57BL/6J mice exhibited more pronounced
serum amylase levels, pancreatic MPO release and TNF-α production
than the wild-type mice. These data indicated that CRAMP may have a
modulatory role in experimental AP.
A cascade of cellular events, including activation
of intra-acinar enzymes, release of inflammatory mediators and
cytokines, acinar cell apoptosis, and pancreatic microcirculation
disorder have been reported to underlie the pathogenesis of AP
(20–23). However, AP pathogenesis remains to
fully elucidated. AMPs represent novel mediators of the condition.
Intestinal α-defensin (α-defensin-5 and α-defensin-7) levels, but
not CRAMP, were shown to be elevated in aged rats with AP, and were
associated with more severe local inflammation (9). The role of cathelicidins (human LL-37
and mouse CRAMP) has been particularly documented in other
inflammatory processes (24);
however, its role is unknown in AP. Therefore the present study
investigated cathelicidins as a potential immunomodulatory mediator
in a mouse model of experimental AP, using CRAMP-deficient
cnlp−/− mice.
The results of the present study demonstrated that
cnlp−/− mice with AP exhibited more pronounced
acinar cell injury, as measured by serum amylase levels, MPO
activity and TNF-α production, as compared with the wild-type AP
mice. Immune cell infiltration into the tissues and production of
inflammatory cytokines (chemokines) are key pathological events
that determine, to a large extent, disease severity (25,26).
During AP, neutrophils are the first-line innate immune cells
recruited to the pancreas. TNF-α, which is derived predominantly
from activated macrophages, acts via cell membrane-bound receptors
(27) to induce proinflammatory
gene expression of IL-1, IL-6, IL-8 and itself, thereby causing
pancreatic tissue necrosis and further migration of leukocytes
(28). The prototypic CC chemokine
MCP-1 is an early mediator associated with chemo-attraction of
macrophages and further propagation of local to systemic
inflammation (29). Although both
TNF-α and MCP-1 are significantly elevated in the pancreas of
cnlp−/− and wild-type AP mice (Fig. 3), only TNF-α levels were
significantly higher in the cnlp−/− mice than the
wild-type mice, thus suggesting that the regulatory effects of
CRAMP may be selective towards TNF-α-producing cells in the
pancreas.
The present study detected a more resistant
phenotype during AP due to the C57BL/6J genetic background of
cnlp−/− and wild-type mice. Different mouse
strains have exhibited varied susceptibility to caerulein-induced
AP. Serum amylase and TNF-α production in BALB/c mice are
significantly higher than in C57BL/6J mice upon AP induction
(unpublished data). Despite this intrinsic variation of responses,
CRAMP deficiency still worsens pancreatic inflammatory conditions,
further suggesting its modulatory role in AP.
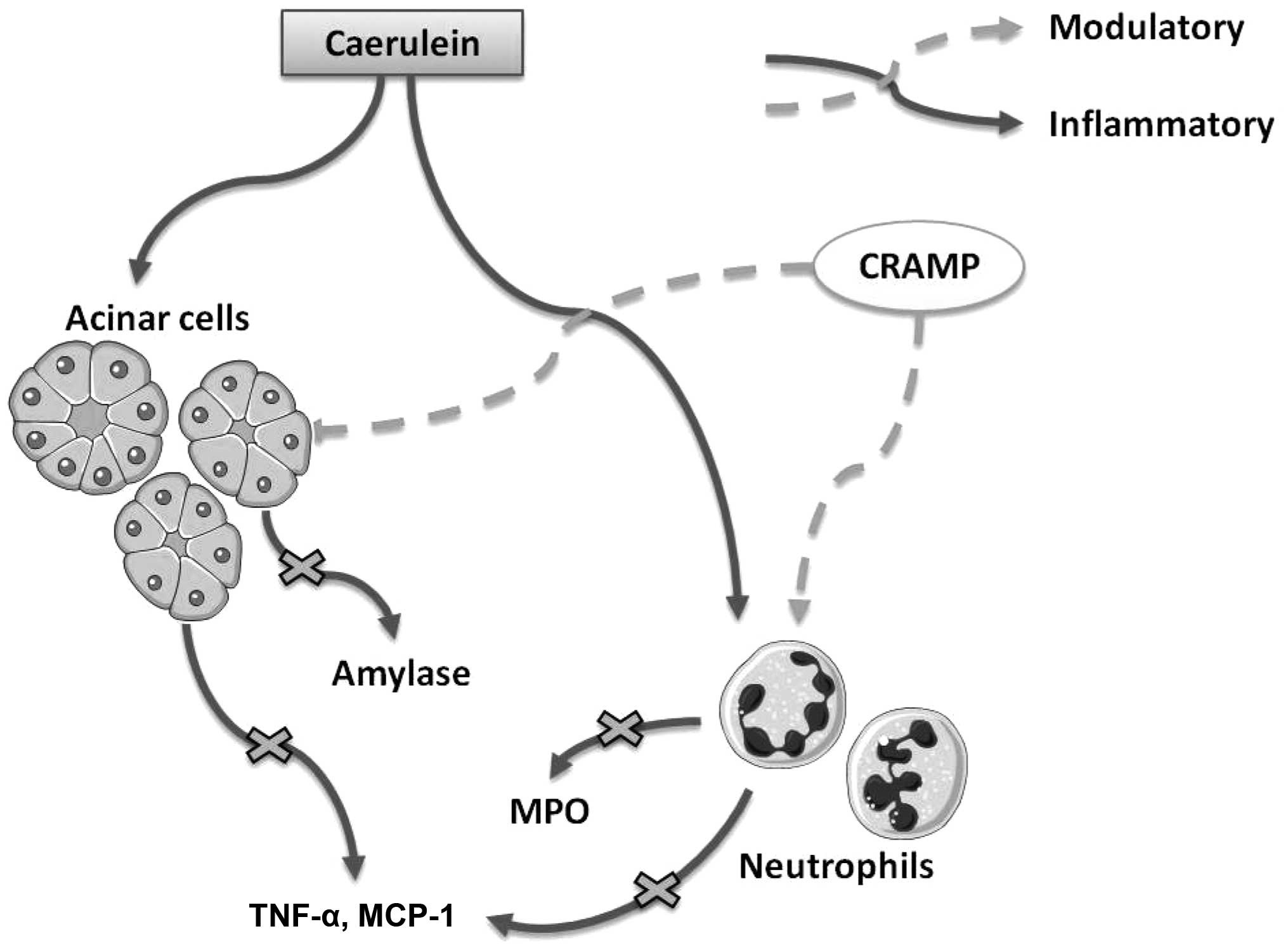
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that cathelicidins exert
immune regulatory effects on AP in mice (Fig. 5). In addition, the present study
provides novel evidence for the effective prevention and treatment
of AP in clinical practice.
Acknowledgments
The present study was supported by funds from the
National Natural Science Foundation of China (grant nos. 31400779
and 31570915; National Youth 1000 Talents Plan), the Provincial
Natural Science Foundation of Jiangsu (grant no. BK20130133), and
the Jiangsu Provincial Shuang Chuang Innovator Plan, Jiangsu
Province Recruitment Plan for High-level, Innovative and
Entrepreneurial Talents and Jiangsu Province 'Six Summit Talents'
Program (grant no. 2014-SWYY-035) to J.S.
Abbreviations:
|
AP
|
acute pancreatitis
|
|
AMP
|
antimicrobial peptide
|
|
CRAMP
|
cathelicidin-related antimicrobial
peptide
|
|
MCP
|
monocyte chemotactic protein
|
|
MPO
|
myeloperoxidase
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
References
|
1
|
Bell D, Keane MG and Pereira SP: Acute
pancreatitis. Medicine. 43:174–181. 2015. View Article : Google Scholar
|
|
2
|
Petrov M: Nutrition, inflammation, and
acute pancreatitis. ISRN Inflamm. 2013:3414102013. View Article : Google Scholar
|
|
3
|
Kylänpää L, Rakonczay Z Jr and O'Reilly
DA: The clinical course of acute pancreatitis and the inflammatory
mediators that drive it. Int J Inflam. 2012:3606852012. View Article : Google Scholar
|
|
4
|
Johnson CD and Abu-Hilal M: Persistent
organ failure during the first week as a marker of fatal outcome in
acute pancreatitis. Gut. 53:1340–1344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DiMagno MJ and DiMagno EP: New advances in
acute pancreatitis. Curr Opin Gastroenterol. 23:494–501.
2007.PubMed/NCBI
|
|
6
|
Abdulla A, Awla D, Thorlacius H and Regnér
S: Role of neutrophils in the activation of trypsinogen in severe
acute pancreatitis. J Leukoc Biol. 90:975–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akinosoglou K and Gogos C:
Immune-modulating therapy in acute pancreatitis: Fact or fiction.
World J Gastroenterol. 20:15200–15215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhatia M, Neoptolemos JP and Slavin J:
Inflammatory mediators as therapeutic targets in acute
pancreatitis. Curr Opin Investig Drugs. 2:496–501. 2001.PubMed/NCBI
|
|
9
|
Cunha DM, Koike MK, Barbeiro DF, Barbeiro
HV, Hamasaki MY, Coelho Neto GT, Machado MC and da Silva FP:
Increased intestinal production of α-defensins in aged rats with
acute pancreatic injury. Exp Gerontol. 60:215–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallo RL and Hooper LV: Epithelial
antimicrobial defence of the skin and intestine. Nat Rev Immunol.
12:503–516. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiemstra PS: Defensins and cathelicidins
in inflammatory lung disease: Beyond antimicrobial activity.
Biochem Soc Trans. 34:276–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hilchie AL, Wuerth K and Hancock RE:
Immune modulation by multifaceted cationic host defense
(antimicrobial) peptides. Nat Chem Biol. 9:761–768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sørensen OE, Follin P, Johnsen AH, Calafat
J, Tjabringa GS, Hiemstra PS and Borregaard N: Human cathelicidin,
hCAP-18, is processed to the antimicrobial peptide LL-37 by
extracellular cleavage with proteinase 3. Blood. 97:3951–3959.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zanetti M, Gennaro R and Romeo D:
Cathelicidins: A novel protein family with a common proregion and a
variable C-terminal antimicrobial domain. FEBS Lett. 374:1–5. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zanetti M: The role of cathelicidins in
the innate host defenses of mammals. Curr Issues Mol Biol.
7:179–196. 2005.PubMed/NCBI
|
|
16
|
Shaykhiev R, Beisswenger C, Kändler K,
Senske J, Püchner A, Damm T, Behr J and Bals R: Human endogenous
antibiotic LL-37 stimulates airway epithelial cell proliferation
and wound closure. Am J Physiol Lung Cell Mol Physiol.
289:L842–L848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagaoka I, Tamura H and Hirata M: An
antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses
neutrophil apoptosis via the activation of formyl-peptide
receptor-like 1 and P2X7. J Immunol. 176:3044–3052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wehkamp J, Harder J, Weichenthal M,
Mueller O, Herrlinger KR, Fellermann K, Schroeder JM and Stange EF:
Inducible and constitutive beta-defensins are differentially
expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel
Dis. 9:215–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dawra R, Sah RP, Dudeja V, Rishi L,
Talukdar R, Garg P and Saluja AK: Intra-acinar trypsinogen
activation mediates early stages of pancreatic injury but not
inflammation in mice with acute pancreatitis. Gastroenterology.
141:2210–2217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhatia M: Apoptosis versus necrosis in
acute pancreatitis. Am J Physiol Gastrointest Liver Physiol.
286:G189–G196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sah RP and Saluja A: Molecular mechanisms
of pancreatic injury. Curr Opin Gastroenterol. 27:444–451. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sah RP, Dawra RK and Saluja AK: New
insights into the pathogenesis of pancreatitis. Curr Opin
Gastroenterol. 29:523–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Criddle DN, McLaughlin E, Murphy JA,
Petersen OH and Sutton R: The pancreas misled: Signals to
pancreatitis. Pancreatology. 7:436–446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meguro S, Tomita M, Katsuki T, Kato K, Oh
H, Ainai A, Ito R, Kawai T, Itoh H and Hasegawa H: Plasma
antimicrobial peptide LL-37 level is inversely associated with HDL
cholesterol level in patients with type 2 diabetes mellitus. Int J
Endocrinol. 2014:7036962014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xue J, Sharma V and Habtezion A: Immune
cells and immune-based therapy in pancreatitis. Immunol Res.
58:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayerle J, Dummer A, Sendler M, Malla SR,
van den Brandt C, Teller S, Aghdassi A, Nitsche C and Lerch MM:
Differential roles of inflammatory cells in pancreatitis. J
Gastroenterol Hepatol. 27(Suppl 2): 47–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhatia M, Brady M, Shokuhi S, Christmas S,
Neoptolemos JP and Slavin J: Inflammatory mediators in acute
pancreatitis. JPathol. 190:117–125. 2000. View Article : Google Scholar
|
|
28
|
Norman JG, Fink GW and Franz MG: Acute
pancreatitis induces intrapancreatic tumor necrosis factor gene
expression. Arch Surg. 130:966–970. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun J and Bhatia M: Blockade of
neurokinin-1 receptor attenuates CC and CXC chemokine production in
experimental acute pancreatitis and associated lung injury. Am J
Physiol Gastrointest Liver Physiol. 292:G143–G153. 2007. View Article : Google Scholar
|