Introduction
Glycosylation is the most frequent
post-translational modification of proteins and lipids, which adds
to the large structural diversity of cellular molecules (1). Functionally, this process contributes
to altering cell adhesion properties (2,3) and
influencing intra- and intercellular communication (4). Furthermore, protein glycosylation can
be essential in antigen recognition by the immune system (5).
Malignant transformation of cells is frequently
accompanied by alterations in the post-translational modification
of proteins (6–8). This was substantiated first in 1985
by the use of antibodies against altered carbohydrate chains
(9) and was more recently
confirmed in cell culture models (10). The most frequent alterations,
particularly in adenocarcinomas (11), are mucin-type-O-glycosylations,
giving rise to glycosylation patterns that can only be found in
cancer, not in normal cells (8,12).
Cancer-associated glycans form novel glycopeptide epitopes that can
be targeted by the immune system (13,14).
Hence, these altered glycopeptides can exhibit diverse and even
opposing roles in immunogenicity (15). Conversely, they can either have
immunogenic character themselves or new conformational changes
(16,17), or can provoke anti-tumour responses
(18). These activities can then
lead to elevated antibody levels against the pancarcinoma antigen,
which is regarded as a marker for increased survival (19). As a consequence, altered
glycosylation is critical in early phases of tumourigenesis as well
as in tumour progression. However, it also offers novel angles for
diagnostic, prognostic and therapeutic strategies in cancer
(20–22).
In breast cancer, altered glycosylation is
frequently associated with a worse prognosis and shorter overall
survival (23). Shortened
O-glycans and increased sialylation are prevalently found in this
tumour type. Furthermore, morphological and phenotypic
transformation of epithelial cells caused by alterations in
glycosylation patterns was shown, which ultimately leads to a
higher incidence of remote metastasis (4).
Changes in glycosylation are predominantly caused by
changes in the expression of glycosyltransferases, a class of
enzymes that are responsible for the transfer of carbohydrate
chains to acceptor sites on proteins and lipids (24,25).
Expression profiles of glycosyltransferases have been extensively
investigated (17,26–29)
and it was reported that they may serve as an important marker for
tumour characterization (30–32).
The present study aimed to determine the specific
role of glycosyltransferase N-acetylgalactosaminyltransferase 6
(GALNT6) in breast tumourigenesis. Accordingly, paraffin-embedded
tissue samples of primary tumours including adjacent healthy tissue
as well as paired metastatic sites were immunohistochemically
stained. It was demonstrated that GALNT6 is correlated with the
occurrence of lymph node metastasis, local recurrence and remote
metastasis (33). Moreover, GALNT6
is known to be involved in the first steps of O-glycosylation
(33). The mRNA for this enzyme
was shown to be expressed in breast cancer and associated with
smaller tumours (T1) (34). This
association was confirmed in the present study. It was hypothesised
that certain glycosylation motifs may enable immune escape
mechanisms of small tumours, as well as remote metastasis formation
to different sites since the latter requires alterations in cell
adhesion and detachment that partially depend on glycosylation
changes (35,36).
Materials and methods
Tissue samples
Tissue samples for the study were obtained from the
pathology archive of the Department of Gynaecology and Obstetrics,
Ludwig Maximilians University of Munich, (Munich, Germany).
In total, 44 patients diagnosed and treated with
surgery for primary breast cancer between 1998 and 2002 were
followed up for 8 years and included in the study. Research
conducted on patients was in compliance with the Helsinki
declaration. The present study was approved by the ethical
committee of Ludwig-Maximilians University of Munich (approval nos.
148–12 and 048–08). Patients were asked to sign written informed
consent prior to the use of tissue samples for investigation by the
current study.
For the current study, 10 breast cancer samples from
patients with lymph node metastasis, 10 samples from patients with
local recurrence and 8 samples from patients with metastasized
breast cancer were included. For all these samples, tissue sections
of the primary tumour as well as diseased lymph node, local
recurrent tumour or remote metastases were available. The
histological classification, and analyses of grading and hormone-
and Her2-receptor-status were performed pathologically, subsequent
to surgery.
As controls, 8 samples of breast cancer diagnosed as
ductal carcinoma in situ and 8 samples of primary tumours
without local recurrence or remote metastasis were used.
Patients had an average age of 57 years (range from
33–87 years) at time of surgery. TNM-staging of the tumours and
their histology were determined postoperatively by a pathologist
and location of recurrence or metastases were also noted. The
intensity and amount of staining of the tumour tissues for ER, PR
and Her2 were asserted and categorized (− no staining, + slight
staining, ++ moderate staining, +++ strong staining). The
determination and classification of staining intensity for GALNT6
for the primary tumour (PT) and tissue of affected lymph nodes
(LN), recurrence (Rec.) or metastasis (Met.) were determined as
described in Table I.
 | Table IPatient/tumour data in correlation to
IRS for GALNT6. |
Table I
Patient/tumour data in correlation to
IRS for GALNT6.
| Patient no. | Age (years) | Histology | TNM | Size (cm) | Loc. Met/Rec. | ER | PR | Her2 | IRS PT | IRS LK./Rec/Met. |
|---|
| 1 | 56 | Duct. Lob. | pT1a, pN0, MX | 1,2 mult. | None | − | + | ND | 8 | None |
| 2 | 67 | Duct. | pTis, pNX, MX | 0,8 | None | ND | ND | ND | ND | None |
| 3 | 49 | Duct. | pTis, NX, MX | 0,9 | None | ND | ND | ND | 8 | None |
| 4 | 47 | Duct. | pTis, NX, MX | 1,1 | None | + | + | ND | 8 | None |
| 5 | 55 | Duct. | pTis, NX, MX | 0,3 | None | ND | ND | ND | 4 | None |
| 6 | 45 | Duct. | pTis, NX, MX | 0,7 | None | ND | ND | ND | 12 | None |
| 7 | 57 | Duct. | pTis, NX, MX | 4,0 mult. | None | ND | ND | ND | 8 | None |
| 8 | 72 | Duct. | pTis, NX, MX | 0,9 | None | ND | ND | ND | 8 | None |
| 9 | 51 | Duct. + Cis | pT1c, pN0, MX,
G3 | 1,5 mult. | None | + | + | ND | 4 | None |
| 10 | 55 | Duct. | pT1c, pN0, MX,
G3 | 1,2 | None | + | + | ND | ND | None |
| 11 | 57 | Duct. | pT1c, pN0, MX,
G2 | 1,4 mult. | None | + | + | ND | 4 | None |
| 12 | 59 | Lob. | pT2, pN0, MX | 2,8 mult. | None | − | + | ND | 4 | None |
| 13 | 75 | Duct. | pT1c, pN0, MX,
G3 | 1,3 | None | + | + | ND | 4 | None |
| 14 | 49 | Duct. | pT2, pN0, MX,
G3 | 2,7 | None | − | − | ND | 4 | None |
| 15 | 77 | Duct. + Cis | pT2, pN0, MX,
G2 | 2 | None | + | + | ND | 4 | None |
| 16 | 55 | Duct. | pT2, pN0, MX,
G2 | 3,2 | None | + | + | ND | 4 | None |
| 17 | 58 | Lob. | pT1c, pN2, MX | 1,4 mult. | LN | + | + | ND | 4 | ND |
| 18 | 33 | Duct. | pT1c, pN1bi, MX,
G2 | 1,5 | LN | + | + | ND | 4 | 4 |
| 19 | 63 | Duct. | pT2, pN1biii, MX,
G3 | 2,5 | LN | + | + | ND | 12 | 8 |
| 20 | 60 | Lob. | pT1c, pN2, MX,
G3 | 3,5 | LN | + | − | ND | 8 | 8 |
| 21 | 45 | Duct. | pT1c, pN2, MX,
G3 | 1,2 | LN | − | + | ND | 4 | 4 |
| 22 | 54 | Duct. | pT2, pNbiii, MX,
G2 | 3,3 | LN | + | + | ND | 12 | 8 |
| 23 | 77 | Duct. | pT1c, pN1biii, MX,
G2 | 1,2 | LN | + | + | ND | 8 | 8 |
| 24 | 48 | Duct. + Cis | pT1b, pN1bi, MX,
G3 | 1 | LN | − | − | ND | 0 | 4 |
| 25 | 72 | Duct. | pT2, pN1biv, MX,
G3 | 2,1 | LN | − | − | ND | 8 | 8 |
| 26 | 65 | Duct. | pT2, pN1biii, MX,
G3 | 2 | LN | + | + | ND | 8 | 8 |
| 27 | 45 | Duct. + Cis | pT1c, pN1bi, MX,
G2 | 1,5 mult. | Thor. Wall | − | − | +++ | 2 | 4 |
| 28 | 64 | Duct. | pT1c, pN0, MX,
G2 | 1,9 mult. | Intra-mam. | ++ | − | + | 12 | 12 |
| 29 | 57 | Duct. + Cis | Post CTx | 1 | Intra-mam. | − | + | + | 3 | n.d. |
| 30 | 87 | Lob. | pT4, pNX, MX | 1,5 | Thor. Wall | +++ | − | ++ | 2 | 1 |
| 31 | 74 | Duct. | pT1c, pN0, MX,
G2 | 1,8 | Thor. Wall | + | + | − | 8 | 3 |
| 32 | 53 | Duct. + Cis | pT1a, pNX, MX | 0,1 mult. | Ulc. | − | − | +++ | 8 | 4 |
| 33 | 39 | Duct. + Cis | pT2, pNbiii, MX,
G2 | 3 | Intra-mam. | +++ | ++ | +++ | 4 | 4 |
| 34 | 38 | Duct. | pT1c, pNX, MX,
G2 | 1,5 mult. | Thor. Wall | +++ | + | +++ | 4 | 8 |
| 35 | 58 | Med. | pT1c, pN0, MX | 1,8 | Intra-mam. | − | − | +++ | 1 | 4 |
| 36 | 52 | Duct. + Cis | pT1, pN1bi, MX | 1 | Intra-mam. | − | − | +++ | 4 | 4 |
| 37 | 51 | Lob. + LCIS | pT1c, pN1a, M1 | 1,8 | Skin | − | + | ++/+++ | 3 | 4 |
| 38 | 78 | Lob. | pT1c, pN1biii, M1,
GND | 1,5 mult. | Contr. Ax. LN | + | − | +++ | 4 | 12 |
| 39 | 45 | Duct. + Cis | pT1c, pN1bi, M1,
G2 | 1,5 mult. | Contr. Ax. LN | − | − | +++ | 2 | 4 |
| 40 | 71 | Duct. + Cis | pT2, pN1biv, M1,
G2 | 2 | Contr. Ax. LN | − | − | +++ | 8 | 8 |
| 41 | 33 | Small cell Ca. | pT1c, pN1bii,
M1 | 10 | Ovary | − | + | + | 12 | 12 |
| 42 | 38 | Duct. | pT2, nP1a, M1,
G2 | 2,5 | Contr. Ax. LN | − | − | ++ | ND | 12 |
| 43 | 58 | Med. | pT1c, pN0, M1 | 1,8 | Contr. Ax. LN | − | − | +++ | 3 | 1 |
| 44 | 41 | Duct. | pT4d, pN1bii,
M1 | 1,3 mult. | Contr. Ax. LN | + | + | +++ | 8 | 8 |
Immunohistochemistry
Paraffin-embedded (Merck Milipore, Darmstadt,
Germany) tumour blocks were cut into 2–3 µm sections with a
sliding microtome (Leica Microsystems GmbH, Wetzlar, Germany),
placed on covered microscope slides (Menzel GmbH, Braunschweig,
Germany) and air-dried overnight. Samples were then incubated in
Xylol (Merck Milipore). After Xylol removal, endogenous tissue
peroxidase activity was inhibited by incubation of the samples in
3% H2O2 (VWR International, Wayne, PA, USA).
Then, samples were heated for 5 min in a pressure cooker in a
Na-citrate buffer (Merck Milipore) at pH 6 in order to dissolve
protein crosslinks that arise from the fixation procedure. The
tissue samples were washed in water and phosphate-buffered saline
(Biochrom GmbH, Berlin, Germany).
The prepared slides were first incubated in normal
goat serum (Vector Laboratories, Inc., Burlingame, CA, USA) to
prevent unspecific binding of the primary antibody. After removal
of the blocking solution (Vector Laboratories, Inc.), primary
antibodies were added at concentrations determined in positive
control sample staining.
Incubation with the polyclonal IgG rabbit
anti-GALNT6 (1:1,000; cat. no. GTX104602; GeneTex, Inc., Irvine,
CA, USA) primary antibody was conducted for 18 h at 4°C. Slides
were then washed three times with phosphate-buffered saline (PBS)
for 5 min and subsequently incubated with biotinylated secondary
antibody solution (from the HRP100 staining kit; Zytomed Systems
GmbH, Berlin, Germany) for 30 min at room temperature. When the
secondary antibody (Vector Laboratories, Inc.) was removed by a
subsequent wash with PBS, ABC-reagent (Vector Laboratories, Inc.)
was applied to the slides for 30 min. Next, a solution of
DAB-reagent (Dako; Agilent Technologies, Santa Clara, CA, USA) was
added to the slides for 1 min. DAB is the substrate for
biotin-coupled peroxidase, the reaction results in a brown
precipitate, which can then be evaluated by Leitz Diaplan light
microscope (Ernst Leitz GmbH, Wetzlar, Germany). The slides were
washed under running tap water to arrest the enzyme reaction.
Nuclei were counterstained with Mayer's hemalum (AppliChem,
Darmstadt, Germany) for 5 min and samples were then dehydrated and
embedded in Eukitt (Medite, Burgdorf, Germany). The stained samples
were then evaluated or stored at room temperature.
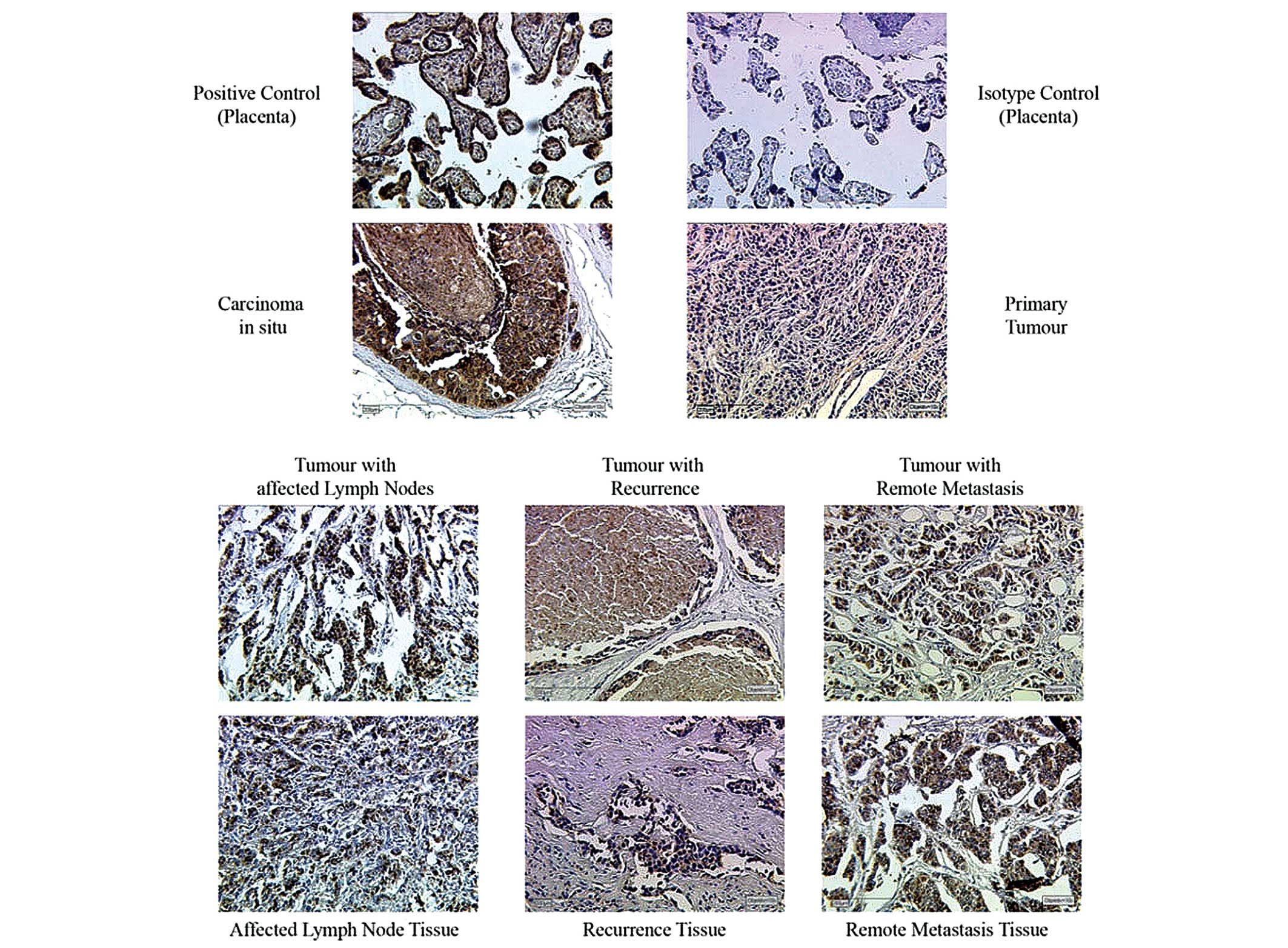
Prior to the staining procedure on tumour tissue
samples, positive and isotype control samples were stained
(Fig. 1). For the positive
control, a sample from a tissue (placenta, collected after birth)
known to overexpress the antigen of interest was stained to test
antibody function and to determine an ideal dilution of the
antibody. The isotype control should reveal background staining due
to primary antibody. Therefore the tissue was stained, which was
also used for positive control, with an isotype control serum
instead of a primary antibody solution.
Microscopy and evaluation of
staining
Samples were visualized with a Leitz Diaplan light
microscope. Four objectives with different magnifications (6,3X,
10X, 25X and 40X) were used.
Staining was evaluated according to the
immune-reactive-score (IRS) described by Remmele and Stegner
(37). The IRS is obtained by
multiplication of the staining intensity with the number of stained
cells. Briefly, staining intensity is classified into groups from 0
to 3 (0 means 'no staining reaction' and 3 means 'strong color
reaction') and the number of stained cells is similarly classified
(0 means '0% stained cells' to 4 '81–100% stained cells'). Thus,
the IRS is in a range of 0 to 12.
Statistical analysis
Statistical analysis was conducted using SPSS
version 20.0 (IBM, Armonk, NY, USA). Since patient samples are not
normally distributed, non-parametric Mann-Whitney-U-Test comparing
two variables was applied; in cases of more variables,
Kruskal-Wallis Test was used. A P≤0.05 was considered to indicate a
statistically significant difference.
Results
Staining intensity correlation with
patient data
Breast cancer tissues at different phases of disease
progression (Cis, primary tumour tissue, tumour with lymph node
infiltration, recurrent tumours and metastasized tumours) were
immunhistochemically stained for GALNT6 (Fig. 1). IRS was determined by light
microscopy by two independent investigators. Subsequently, IRS was
correlated with patient and tumour characteristics in order to
detect associations between tumour features and the GALNT6 staining
intensity (Table I). The following
parameters were included in the study: Age of the patient at
primary diagnosis, tumour histology, TNM-staging of the tumour,
tumour size and hormone (estrogen and progesterone)- and
Her2-receptor status (if available). Extensive statistical analysis
revealed no significant correlation between these investigated
patient-related factors and the staining intensity of GALNT6.
Staining intensity of different tumour
stages
It was then evaluated whether mean IRS-values for
GALNT6 of the samples was correlated with different developmental
stages of the tumour samples (Table
II). Notably, the highest staining intensity was demonstrated
in precursor lesions (Cis-tumours) with an average IRS of 8.00 and
in metastases tissue samples with an average IRS of 7.63. Thus, the
pre-invasive form of breast cancer intraductal carcinoma (also
Cis), newly arising small tumours and metastatic lesions display
the highest incidence of GALNT6.
 | Table IIGALNT6 staining intensity of tissue
samples. |
Table II
GALNT6 staining intensity of tissue
samples.
| Type of sample | Number of
samples | Average IRS | Minimum IRS | Maximum IRS | Standard
deviation |
|---|
| DCIS | 7 | 8.00 | 4.00 | 12.00 | 2.309 |
| Primary tumour | 7 | 4.00 | 4.00 | 4.00 | 0.000 |
| Tumour with lymph
node affection | 10 | 6.80 | 0.00 | 12.00 | 3.795 |
| Affected lymph
nodes | 10 | 6.00 | 0.00 | 8.00 | 2.828 |
| Tumour with
recurrence | 10 | 4.80 | 1.00 | 12.00 | 3.458 |
| Recurrence
tissue | 9 | 4.89 | 1.00 | 12.00 | 3.219 |
| Tumour with remote
metastasis | 7 | 5.71 | 2.00 | 12.00 | 3.684 |
| Metastasis
tissue | 8 | 7.63 | 1.00 | 12.00 | 4.274 |
Statistical analysis of
GALNT6-staining
Lastly, the IRS values of the control group (DCIS-
and primary tumours) were statistically compared with the more
advanced tumour stages (Table
III). No significant differences were identified between the
control group and tumours with infiltrated lymph nodes (P=0.546).
In addition, no statistical significance was identified when
comparing the control group with the recurrence-group (P=0.172) or
the metastasis group (P=0.443). Finally, comparisons of the control
group with all other tumour stages together did not reveal any
statistically significant results (P=0.541).
 | Table IIIStatistical comparison of
GALNT6-IRS. |
Table III
Statistical comparison of
GALNT6-IRS.
| Group | P-valuea |
|---|
| Tumours with
affected lymph nodes | 0.546 |
| Tumours with
recurrence | 0.172 |
| Tumours with remote
metastasis | 0.443 |
| Tumours with
affected lymph nodes/recurrence/metastasis | 0.541 |
Discussion
The present data did not identify a direct
correlation between GALNT6-staining and the age of primary
diagnosis, TNM-staging, tumour size and histology, and hormone
(estrogen and progesterone)-receptor and Her2-status. However, the
results presented here may be regarded as preliminary, as they were
collected from a small patient collective.
Notably a coherence between GALNT6-staining and
different developmental stages of the tumours was identified, as
Cis-tumours and metastatic tumour mass are highly stained. This is
consistent with a previous study (38) that demonstrated a correlation
between the occurrence of glycosyltransferases and tumour size and
grade. It could be shown, that glycosyltransferases, particularly
GALNT6, are identified in recently formed tumours. Knockdown of
GALNT6 by small interfering RNA slowed breast cancer cell growth by
increasing cell adhesion. Furthermore GALNT6 was shown to be
important for the stabilization of the MUC1-oncoprotein (33). GALNT6 is critical in the
fibronectin pathway, regulating cellular morphology and
morphological changes, which are required for the transformation of
a normal epithelial cell into a tumour cell, and is thereby an
important component for breast cancer development and progression
(39). Consistently, a recent
study in lung cancer research demonstrated that cigarette smoke
induces the glycosylation of MUC1 via GALNT6, and that inhibition
of GLANT6 leads to the maintenance of cellular polarity and cell
adhesion (33), which are critical
steps in the early stages of tumourigenesis (40).
A possible reason for the correlation identified may
be that at early stages of development, tumour cells activate an
immune response. In order to spread and grow, the cells use
glycosylation alterations to escape these mechanisms. By contrast,
glycosylation may also be key in ensuring that an established
tumour is no longer attacked by the immune system. Therefore, the
presence of GALNT6 may also be an important marker in tumour
diagnosis and tumour staging. To further address this hypothesis,
our future studies will investigate a larger panel of tumour
samples of different stages with matched metastasis in order to
have a sample that is large enough for meaningful statistical
analysis which in turn would consolidate these working hypotheses.
Furthermore, we plan on staining a different and broader panel of
glycosylation enzymes in the tumour samples. With the aim of
identifying different steps in glycosylation, which are important
for tumourigenesis and immune escape of the tumour. Thereby,
mechanisms of tumourigenesis may be further clarified and could
lead to the development of novel treatment strategies for patients
with breast cancer. Furthermore, it is desirable to identify the
precise chronology of glycosylation enzyme activation and
expression during the course of breast cancer tumourigenesis, as
this knowledge may aid in staging tumours.
Acknowledgments
The authors would like to thank Dr Sabine Heublein
for help with the statistical analysis.
References
|
1
|
Skelton TP, Zeng C, Nocks A and
Stamenkovic I: Glycosylation provides both stimulatory and
inhibitory effects on cell surface and soluble CD44 binding to
hyaluronan. J Cell Biol. 140:431–446. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diamond MS, Staunton DE, Marlin SD and
Springer TA: Binding of the integrin Mac-1 (CD11b/CD18) to the
third immunoglobulin-like domain of ICAM-1 (CD54) and its
regulation by glycosylation. Cell. 65:961–971. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McEvoy LM, Sun H, Frelinger JG and Butcher
EC: Anti-CD43 inhibition of T cell homing. J Exp Med.
185:1493–1498. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dennis JW, Granovsky M and Warren CE:
Glycoprotein glycosylation and cancer progression. Biochim Biophys
Acta. 1473:21–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rudd PM, Elliott T, Cresswell P, Wilson IA
and Dwek RA: Glycosylation and the immune system. Science.
291:2370–2376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hakomori S: Aberrant glycosylation in
cancer cell membranes as focused on glycolipids: Overview and
perspectives. Cancer Res. 45:2405–2414. 1985.PubMed/NCBI
|
|
7
|
Hakomori S: Tumor malignancy defined by
aberrant glycosylation and sphingo (glyco)lipid metabolism. Cancer
Res. 56:5309–5318. 1996.PubMed/NCBI
|
|
8
|
Springer GF: T and Tn, general carcinoma
autoantigens. Science. 224:1198–1206. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feizi T: Demonstration by monoclonal
antibodies that carbohydrate structures of glycoproteins and
glycolipids are onco-developmental antigens. Nature. 314:53–57.
1985. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Julien S, Adriaenssens E, Ottenberg K,
Furlan A, Courtand G, Vercoutter-Edouart AS, Hanisch FG, Delannoy P
and Le Bourhis X: ST6GalNAc I expression in MDA-MB-231 breast
cancer cells greatly modifies their O-glycosylation pattern and
enhances their tumourigenicity. Glycobiology. 16:54–64. 2006.
View Article : Google Scholar
|
|
11
|
Taylor-Papadimitriou J, Burchell J, Miles
DW and Dalziel M: MUC1 and cancer. Biochim Biophys Acta.
1455:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hakomori S: Carbohydrate-to-carbohydrate
interaction in basic cell biology: A brief overview. Arch Biochem
Biophys. 426:173–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sørensen AL, Reis CA, Tarp MA, Mandel U,
Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R,
Taylor-Papadimitriou J, Hollingsworth MA, et al: Chemoenzymatically
synthesized multimeric Tn/STn MUC1 glycopeptides elicit
cancer-specific anti-MUC1 antibody responses and override
tolerance. Glycobiology. 16:96–107. 2006. View Article : Google Scholar
|
|
14
|
Tarp MA, Sørensen AL, Mandel U, Paulsen H,
Burchell J, Taylor-Papadimitriou J and Clausen H: Identification of
a novel cancer-specific immunodominant glycopeptide epitope in the
MUC1 tandem repeat. Glycobiology. 17:197–209. 2007. View Article : Google Scholar
|
|
15
|
Madsen CB, Petersen C, Lavrsen K, Harndahl
M, Buus S, Clausen H, Pedersen AE and Wandall HH: Cancer associated
aberrant protein O-glycosylation can modify antigen processing and
immune response. PLoS One. 7:e501392012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Apostolopoulos V, Pietersz GA, Xing PX,
Lees CJ, Michael M, Bishop J and McKenzie IF: The immunogenicity of
MUC1 peptides and fusion protein. Cancer Lett. 90:21–26. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wandall HH, Blixt O, Tarp MA, Pedersen JW,
Bennett EP, Mandel U, Ragupathi G, Livingston PO, Hollingsworth MA,
Taylor-Papadimitriou J, et al: Cancer biomarkers defined by
autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer
Res. 70:1306–1313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Gendler SJ and Franco A: Designer
glycopeptides for cytotoxic T cell-based elimination of carcinomas.
J Exp Med. 199:707–716. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blixt O, Bueti D, Burford B, Allen D,
Julien S, Hollingsworth M, Gammerman A, Fentiman I,
Taylor-Papadimitriou J and Burchell JM: Autoantibodies to
aberrantly glycosylated MUC1 in early stage breast cancer are
associated with a better prognosis. Breast Cancer Res. 13:R252011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dabelsteen E: Cell surface carbohydrates
as prognostic markers in human carcinomas. J Pathol. 179:358–369.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: Protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View
Article : Google Scholar
|
|
22
|
Kim YJ and Varki A: Perspectives on the
significance of altered glycosylation of glycoproteins in cancer.
Glycoconj J. 14:569–576. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miles DW, Happerfield LC, Smith P,
Gillibrand R, Bobrow LG, Gregory WM and Rubens RD: Expression of
sialyl-Tn predicts the effect of adjuvant chemotherapy in
node-positive breast cancer. Br J Cancer. 70:1272–1275. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joziasse DH: Mammalian
glycosyltransferases: Genomic organization and protein structure.
Glycobiology. 2:271–277. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le Marer N, Laudet V, Svensson EC,
Cazlaris H, Van Hille B, Lagrou C, Stehelin D, Montreuil J, Verbert
A and Delannoy P: The c-Ha-ras oncogene induces increased
expression of beta-galactoside alpha-2, 6-sialyltransferase in rat
fibroblast (FR3T3) cells. Glycobiology. 2:49–56. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennett EP, Hassan H, Mandel U,
Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, van Kessel AG,
Olofsson S and Clausen H: Cloning and characterization of a close
homologue of human UDP-N-acetyl-alpha-D-galactosamine: Polypeptide
N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6.
Evidence for genetic but not functional redundancy. J Biol Chem.
274:25362–25370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brooks SA, Carter TM, Bennett EP, Clausen
H and Mandel U: Immunolocalisation of members of the polypeptide
N-acetylgalactosaminyl transferase (ppGalNAc-T) family is
consistent with biologically relevant altered cell surface
glycosylation in breast cancer. Acta Histochem. 109:273–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freire T, Berois N, Sóñora C, Varangot M,
Barrios E and Osinaga E: UDP-N-acetyl-D-galactosamine: Polypeptide
N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a
potential new marker for detection of bone marrow-disseminated
breast cancer cells. Int J Cancer. 119:1383–1388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nomoto M, Izumi H, Ise T, Kato K, Takano
H, Nagatani G, Shibao K, Ohta R, Imamura T, Kuwano M, et al:
Structural basis for the regulation of
UDP-N-acetyl-alpha-D-galactosamine: Polypeptide
N-acetylgalactosaminyl transferase-3 gene expression in
adenocarcinoma cells. Cancer Res. 59:6214–6222. 1999.
|
|
30
|
Paulson JC and Colley KJ:
Glycosyltransferases. Structure, localization, and control of cell
type-specific glycosylation. J Biol Chem. 264:17615–17618.
1989.PubMed/NCBI
|
|
31
|
Hebbar M, Krzewinski-Recchi MA, Hornez L,
Verdière A, Harduin-Lepers A, Bonneterre J, Delannoy P and Peyrat
JP: Prognostic value of tumoral sialyltransferase expression and
circulating E-selectin concentrations in node-negative breast
cancer patients. Int J Biol Markers. 18:116–122. 2003.PubMed/NCBI
|
|
32
|
Recchi MA, Hebbar M, Hornez L,
Harduin-Lepers A, Peyrat JP and Delannoy P: Multiplex reverse
transcription polymerase chain reaction assessment of
sialyltransferase expression in human breast cancer. Cancer Res.
58:4066–4070. 1998.PubMed/NCBI
|
|
33
|
Park JH, Nishidate T, Kijima K, Ohashi T,
Takegawa K, Fujikane T, Hirata K, Nakamura Y and Katagiri T:
Critical roles of mucin 1 glycosylation by transactivated
polypeptide N-acetylgalactosaminyltransferase 6 in mammary
carcinogenesis. Cancer Res. 70:2759–2769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berois N, Mazal D, Ubillos L, Trajtenberg
F, Nicolas A, Sastre-Garau X, Magdelenat H and Osinaga E:
UDP-N-acetyl-D-galactosamine: Polypeptide
N-acetylgalactosaminyltransferase-6 as a new immunohistochemical
breast cancer marker. J Histochem Cytochem. 54:317–328. 2006.
View Article : Google Scholar
|
|
35
|
Casey RC, Oegema TR Jr, Skubitz KM,
Pambuccian SE, Grindle SM and Skubitz AP: Cell membrane
glycosylation mediates the adhesion, migration, and invasion of
ovarian carcinoma cells. Clin Exp Metastasis. 20:143–152. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L and Ten Hagen KG: The cellular
microenvironment and cell adhesion: A role for O-glycosylation.
Biochem Soc Trans. 39:378–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.In German. PubMed/NCBI
|
|
38
|
Andergassen U, Liesche F, Kölbl AC, Ilmer
M, Hutter S, Friese K and Jeschke U: Glycosyltransferases as
markers for early tumourigenesis. Biomed Res Int.
2015L:7926722015.
|
|
39
|
Park JH, Katagiri T, Chung S, Kijima K and
Nakamura Y: Polypeptide N-acetylgalactosaminyltransferase 6
disrupts mammary acinar morphogenesis through O-glycosylation of
fibronectin. Neoplasia. 13:320–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang L, Gallup M, Zlock L, Chen YT,
Finkbeiner WE and McNamara NA: Pivotal role of MUC1 glycosylation
by cigarette smoke in modulating disruption of airway adherens
junctions in vitro. J Pathol. 234:60–73. 2014. View Article : Google Scholar : PubMed/NCBI
|