Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the ten most common types of malignancy worldwide, and is
associated with a poor prognosis (1). In China, ESCC has fourth highest rate
of cancer-related mortality (2).
The overall 5-year survival rate is <30%, and the high
recurrence rate is the predominant reason for poor quality of life
and mortality in patients with ESCC (3,4).
Therefore, investigation into the mechanism underlying the
recurrence and metastasis of ESCC is of clinical significance for
improving the prognosis of these patients. Increased expression
levels of metastasis-associated 1 gene (MTA1) are positively
correlated with the invasion and metastasis of a variety of types
of malignant tumor (5). Toh et
al (6) observed that the
expression level of MTA1 in ESCC is associated with deacetylase
activity of the H4 histone, and that the invasion and lymph node
metastasis of tumor cells with high expression levels of MTA1 mRNA
are significantly increased.
The insulin-like growth factor (IGF) signaling
pathway is important for the proliferation, differentiation and
apoptosis of cells, among which IGF-1 and IGF binding protein 3
(IGFBP-3) are key in cell growth and tumor formation (7). Rajah et al (8) demonstrated that by blocking the
binding of IGFs to their receptors, IGFBP-3 inhibits the activity
of IGFs and induces apoptosis, indicating a protective effect. A
number of epidemiological studies have demonstrated that high
levels of circulating IGF-1 and low levels of IGFBP-1 are
associated with increased risk of several common cancers, including
breast (9), prostate (10), lung (11) and colorectal (12).
The association of MTAl and IGFBP-3 expression
levels with the clinical pathology and prognosis of ESCC is rarely
evaluated, and whether the expression levels of these two factors
are associated with ESCC remains to be elucidated. The present
study investigated the correlation of IGFBP-3 and MTA1 protein
expression and the clinicopathological features and prognosis of
197 ESCC patients, with the aim of providing an objective basis for
the diagnosis and treatment of ESCC.
Subjects and methods
Subjects
ESCC patients (148 males and 49 females; age, 41–77
years; mean age, 59.8 years) who underwent ESCC resection in the
Department of Thoracic and Cardiovascular Surgery, Beijing Luhe
Hospital Affiliated to Capital Medical University (Beijing, China)
or Department of Thoracic Surgery, Cixian People's Hospital
(Handan, China) between October 2008 and June 2010 were enrolled in
the present study. All patients were diagnosed with ESCC by
preoperative biopsy, had surgical indications and no surgical
contraindications. They did not receive preoperative adjuvant
therapies, such as radiotherapy or chemotherapy, and had no serious
perioperative complications. The pathological specimens embedded in
paraffin were preserved well and the medical records were complete.
The present study was approved by the Ethics Committee of Beijing
Luhe Hospital Affiliated to Capital Medical University (Beijing,
China) and informed consent was obtained from all patients.
Grouping of paraffin specimens and
detection of IGFBP-3 and MTA1 expression
The paraffin specimens were divided into an ESCC
group and control group, which included ESCC tissues (197 samples)
and adjacent normal tissues (>5 cm away from the tumor margin;
197 samples), respectively. The expression levels of IGFBP-3 and
MTA1 protein were detected by immunohistochemistry according to
previously described methods (13,14).
Primary antibodies used included rabbit anti-human polyclonal
antibody against IGFBP-3 (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China; cat. no. BA2162; dilution, 1:100) and goat anti-human
polyclonal antibody against MTA1 (Santa Cruz Biotechnology, Inc.,
TX, USA; cat. no. sc-9446; dilution, 1:100). Secondary antibodies
including goat anti-rabbit immunoglobulin G (IgG) conjugated to
horseradish peroxidase (HRP; cat. no. ZB-2301; dilution, 1:2,000)
and rabbit anti-goat IgG-HRP (cat. no. ZB-2306; dilution, 1:2,000)
were purchased from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China).
Pathological grading
According to the 7th edition of the ESCC staging
system (15), there were 35, 51
and 111 cases with phase I, II and III ESCC, respectively. In
total, 35 cases were well-differentiated, 123 cases were
moderately-differentiated, and 39 cases were poorly-differentiated.
Tumor size ≤3 cm was observed in 43 cases, while tumor size >3
cm was observed in 154 cases.
Criteria to judge results
The stained slides were evaluated by two independent
pathologists. The proportion of cells with positive brown staining
for MTA1 and IGFBP-3 was observed. The positive-cell scoring was as
follows: <5%, 0 points; 5–25%, 1 point; 26–50%, 2 points;
51–75%, 3 points; and >75%, 4 points. The staining intensity
with MTA1 and IGFBP-3 antibodies was scored was as follows: Minimal
staining similar to the background, 0 points; lightly stained, more
than the background and pale yellow, 1 point; moderately stained,
markedly more than the background and a brown-yellow, 2 points; and
clearly stained a dark brown-yellow or tan, 3 points. The total
scoring was as follows: Total score = number of positive cells x
staining intensity. Total score ≥5 indicated a positive result, and
<5 indicated a negative result. The nucleus and cytoplasm were
observed to perform the scoring and statistical analysis. All the
sections were judged by two pathologists blinded to the groupings
and the inconsistencies were negotiated to reach a consensus.
Follow up
All the patients were successfully discharged, and
follow up was performed once every three months for the first 2
years and subsequently once every 6 months. Follow-up included
physical examination, chest X-ray, biochemical analysis (squamous
cell carcinoma antigen, carbohydrate antigen (CA)-125, α-fetal
protein, cancer embryo antigen, CA-199, CA-153, ferritin), computed
tomography, ultrasound and gastroscopy. The postoperative tumor
recurrence and metastasis were diagnosed according to the patients'
imaging and histological findings, and the locations and times of
recurrence and metastasis were recorded. The disease-free survival
period referred to the period starting from the date of surgery to
that of tumor recurrence or mortality as a result of
non-cancer-associated disease. The overall survival period refers
to the period starting from the date of surgery to mortality or to
the follow-up deadline. The follow-up deadline of the present study
was June 30, 2013, with a median follow-up time of 12 months (2–56
months). The follow-up data was obtained from outpatient and
telephone reviewing.
Statistical analysis
SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for data analysis. The association between
positive staining for IGFBP-3 and MTA1, and the clinical
pathological characteristics were analyzed with a χ2
test. The Kaplan-Meier life-table method was performed for the
survival analysis and log-rank test was used to determine the
survival difference. The multivariate analysis used the COX
regression analysis to determine the independent risk factors of
prognosis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of IGFBP-3 and MTA1
protein in ESCC and adjacent tissues
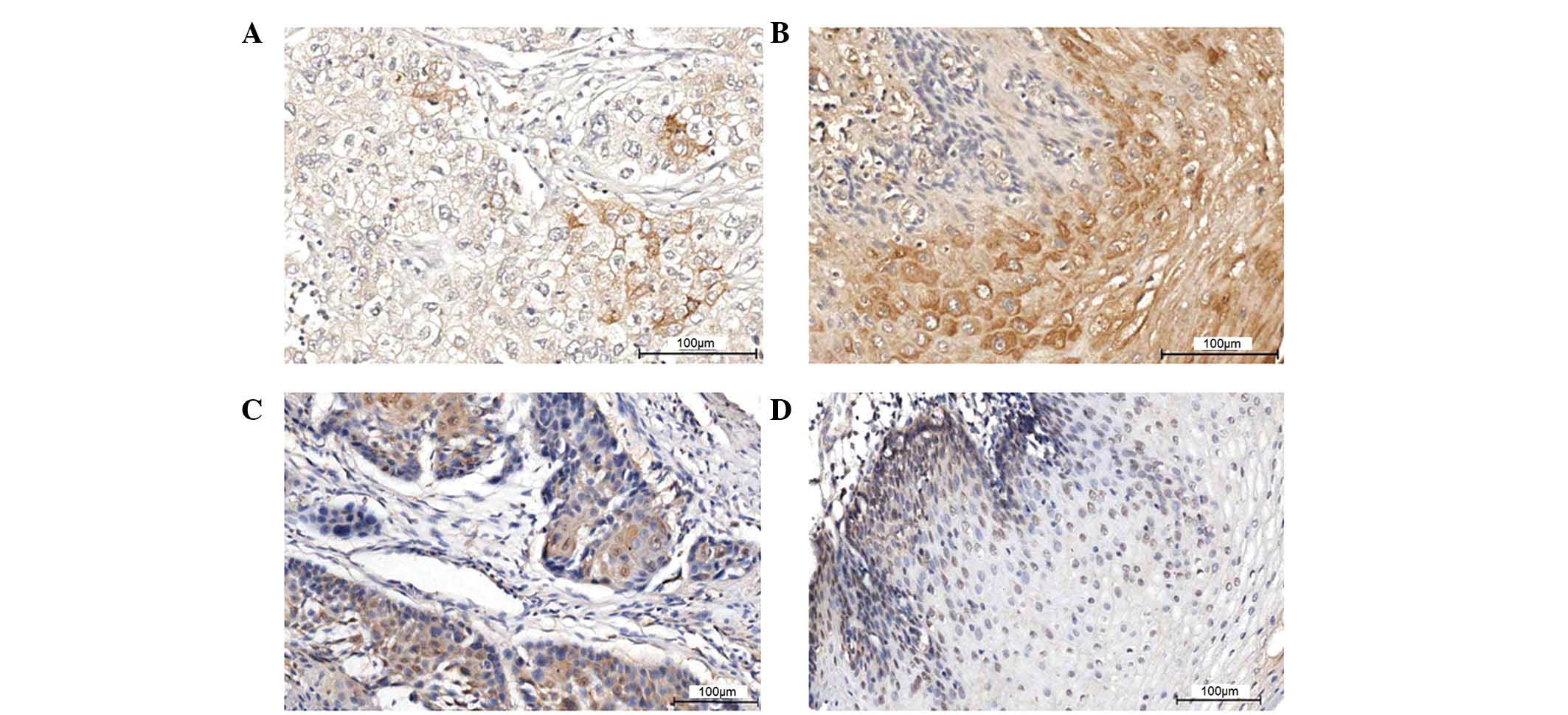
Positive staining for IGFBP-3 was predominantly
localized to the cytoplasm. Among the 197 ESCC cases, 54 cancer
tissue samples (27.4%) exhibited positive expression of IGFBP-3,
while 80 adjacent tissue samples (40.6%) exhibited expression of
IGFBP-3. The intergroup comparison indicated a statistically
significant difference (P<0.05; Table I; Fig.
1A and B).
 | Table IExpression of IGFBP-3 and MTA1
protein in cancer tissues and adjacent normal tissues. |
Table I
Expression of IGFBP-3 and MTA1
protein in cancer tissues and adjacent normal tissues.
| Tissue | IGFBP-3 (no. of
cases)
| P-value | MTA1 (no. of cases)
| P-value |
|---|
| Negative | Positive | Negative | Positive |
|---|
| Cancer | 143 | 54 | 0.008 | 114 | 83 | 0.001 |
| Adjacent
normal | 117 | 80 | | 175 | 22 | |
The staining of MTA1 protein was predominantly
localized in the nucleus. Among the 197 ESCC cases, 83 cancer
tissue samples (42.1%) exhibited positive expression of MTA1, while
22 adjacent tissue samples (11.2%) exhibited positive expression of
MTA1 protein. The intergroup comparison indicated a statistically
significant difference (P<0.05; Table I; Fig.
1C and D).
Correlation between IGFBP-3 and MTA1
protein expression levels and clinicopathological
characteristics
The expression of IGFBP-3 differed significantly
difference between male and female patients and between patients
with and without tumor family history (P<0.05), and was
negatively correlated with the smoking status, degree of tumor
differentiation and lymph node metastasis (P<0.05), but was not
correlated with age, tumor size, extent of tumor invasion or
survival status (P>0.05, χ2 test; Table II).
 | Table IICorrelation between IGFBP-3
expression and clinicopathological characteristics in 197 patients
with esophageal squa-mous cell carcinoma. |
Table II
Correlation between IGFBP-3
expression and clinicopathological characteristics in 197 patients
with esophageal squa-mous cell carcinoma.
| Parameter | No. of cases | IGFBP-3, no. of
cases (%)
| P-value |
|---|
| Negative | Positive |
|---|
| Gender | | | | <0.05 |
| Male | 148 | 101 (68.2) | 47 (31.8) | |
| Female | 49 | 42 (85.7) | 7 (14.3) | |
| Age (years) | | | | >0.05 |
| <59 | 93 | 67 (72.0) | 26 (28.0) | |
| ≥59 | 104 | 76 (73.1) | 28 (26.9) | |
| Smoking status | | | | <0.05 |
| <30
pack-years | 103 | 67 (65.0) | 36 (35.0) | |
| ≥30
pack-years | 94 | 76 (80.9) | 18 (19.1) | |
| Family history | | | | <0.05 |
| No cancer | 153 | 105 (68.6) | 48 (31.4) | |
| With cancer | 44 | 38 (86.4) | 6 (13.6) | |
| Tumor size
(cm) | | | | >0.05 |
| ≤3 | 43 | 33 (76.7) | 10 (23.3) | |
| >3 | 154 | 110 (71.4) | 44 (28.6) | |
| WHO grade | | | | <0.05 |
| G1 | 35 | 17 (48.6) | 18 (51.4) | |
| G2 | 123 | 93 (75.6) | 30 (24.4) | |
| G3 | 39 | 33 (84.6) | 6 (15.4) | |
| T status | | | | >0.05 |
| T1 | 29 | 23 (79.3) | 6 (20.7) | |
| T2 | 57 | 37 (64.9) | 20 (35.1) | |
| T3 | 107 | 80 (74.7) | 27 (25.2) | |
| T4 | 4 | 3 (75.0) | 1 (25.0) | |
| N status | | | | <0.05 |
| N0 | 109 | 69 (63.3) | 40 (36.7) | |
| N1 | 88 | 74 (84.1) | 14 (15.9 | |
| Survival
status | | | | >0.05 |
| Alive | 71 | 53 (74.6) | 18 (25.4) | |
| Deceased | 126 | 90 (71.4) | 36 (28.6) | |
The expression of MTA1 protein was positively
correlated with the tumor size, degree of tumor invasion and lymph
node metastasis (P<0.05), and negatively correlated with a
family history of cancer (P<0.05). It was not correlated with
gender, age, the smoking status, degree of tumor differentiation or
survival status (P>0.05, χ2 test; Table III).
 | Table IIICorrelation between MTA1 protein
expression and clinicopathological characteristics in 197 patients
with esophageal squamous cell carcinoma. |
Table III
Correlation between MTA1 protein
expression and clinicopathological characteristics in 197 patients
with esophageal squamous cell carcinoma.
| Parameter | Case | MTA1 protein, no.
of cases (%)
| P-value |
|---|
| Negative | Positive |
|---|
| Gender | | | | >0.05 |
| Male | 148 | 85 (57.4) | 63 (42.6) | |
| Female | 49 | 29 (59.2) | 20 (40.8) | |
| Age (years) | | | | >0.05 |
| <59 | 93 | 55 (59.1) | 38 (40.9) | |
| ≥59 | 104 | 59 (56.7) | 45 (43.3) | |
| Smoking status | | | | >0.05 |
| <30
pack-year | 103 | 61 (59.2) | 42 (40.8) | |
| ≥30 pack-year | 94 | 53 (56.4) | 41 (43.6) | |
| Family history | | | | <0.05 |
| No cancer | 153 | 81 (52.9) | 72 (47.1) | |
| With cancer | 44 | 33 (75.0) | 11 (25.0) | |
| Tumor size
(cm) | | | | <0.05 |
| ≤3 | 43 | 32 (74.4) | 11 (25.6) | |
| >3 | 154 | 82 (53.2) | 72 (46.8) | |
| WHO grade | | | | >0.05 |
| G1 | 35 | 18 (51.4) | 17 (48.6) | |
| G2 | 123 | 75 (61.0) | 48 (39.0) | |
| G3 | 39 | 21 (53.8) | 18 (46.2) | |
| T status | | | | <0.05 |
| T1 | 29 | 21 (72.4) | 8 (27.6) | |
| T2 | 57 | 37 (64.9) | 20 (35.1) | |
| T3 | 107 | 54 (50.5) | 53 (49.5) | |
| T4 | 4 | 2 (50.0) | 2 (50.0) | |
| N status | | | | <0.05 |
| N0 | 109 | 71 (65.1) | 38 (34.9) | |
| N1 | 88 | 43 (48.9) | 45 (51.1) | |
| Survival
status | | | | >0.05 |
| Alive | 71 | 41 (57.7) | 30 (42.3) | |
| Deceased | 126 | 73 (57.9) | 53 (42.1) | |
Correlation between IGFBP-3 and MTA1
protein expression
Positive expression of MTA1 and IGFBP-3 was observed
in 23 cases, while 83 cases were negative for both MTA1 and IGFBP-3
protein expression. The χ2 test indicated that there was
no association between IGFBP-3 and MTA1 (P>0.05; Table IV).
 | Table IVCorrelation between IGFBP-3 and MTA1
protein expression. |
Table IV
Correlation between IGFBP-3 and MTA1
protein expression.
| MTA1 | IGFBP-3
| Total | χ2 | P-value |
|---|
| Negative | Positive |
|---|
| Negative | 83 | 31 | 114 | | |
| Positive | 60 | 23 | 83 | | |
| Total | 143 | 54 | 197 | 0.936 | >0.05 |
Correlation of IGFBP-3 and MTA1 protein
expression with prognosis of patients with ESCC
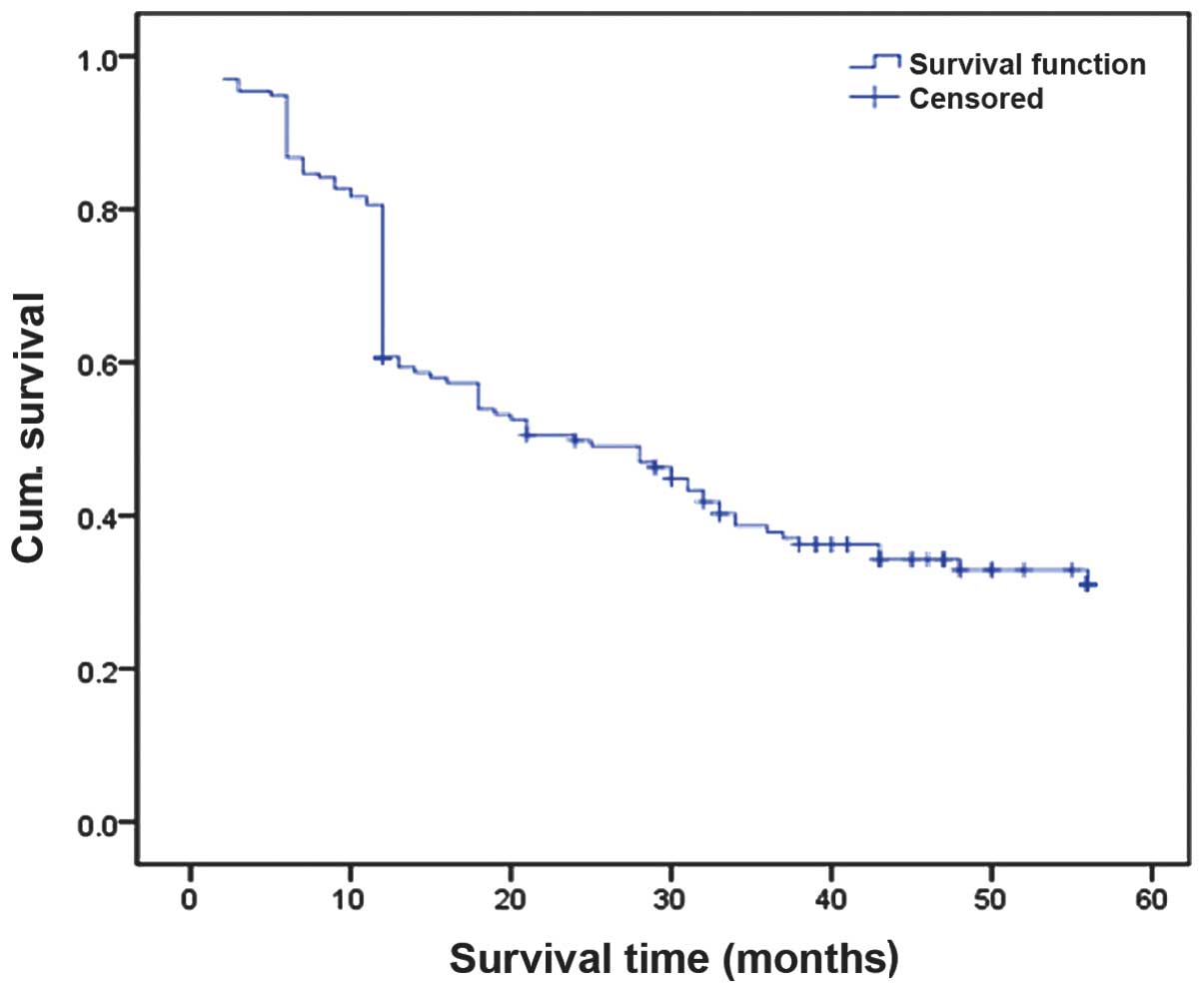
Based on the follow-up data of 197 ESCC cases, the
Kaplan-Meier survival curve analysis indicated that the 3-year
survival rate of all patients was 36.04% (Fig. 2). The 3-year survival rates of the
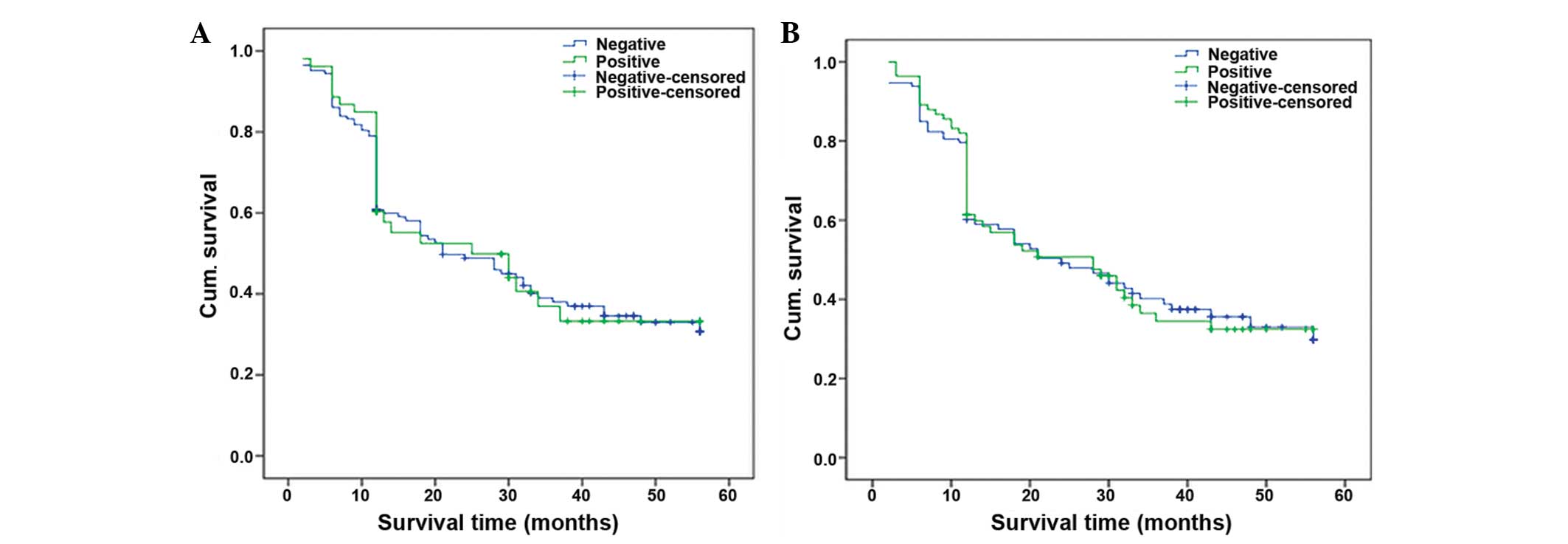
patients with positive and negative expression of IGFBP-3 and MTA1
protein indicated no significant difference by the Log-rank test
(P=0.874 and P=0.942, respectively; Fig. 3). The multivariate analysis of COX
regression demonstrated that the expression of IGFBP-3 and MTA1
were not independent risk factors of ESCC, while the tumor invasion
degree (P=0.020) and lymph node metastasis rate (P=0.027) were
(Table V).
 | Table VResults of COX multivariate
regression analysis. |
Table V
Results of COX multivariate
regression analysis.
| Parameter | B | SE | Wald | P-value | 95% CI for HR |
|---|
| Gender | 0.151 | 0.314 | 0.232 | 0.630 | 1.163
(0.628–2.154) |
| Age | 0.006 | 0.013 | 0.196 | 0.658 | 1.006
(0.980–1.033) |
| Smoking status | 0.237 | 0.262 | 0.820 | 0.365 | 1.267
(0.759–2.117) |
| Grade | −0.650 | 0.165 | 0.154 | 0.695 | 0.938
(0.679–1.294) |
| T | 0.349 | 0.150 | 5.413 | 0.020 | 1.418
(1.057–1.902) |
| N | 0.461 | 0.208 | 4.919 | 0.027 | 1.586
(1.055–2.383) |
| Tumor size | 0.306 | 0.290 | 1.109 | 0.292 | 1.358
(0.768–2.399) |
| IGFBP-3 | −0.128 | 0.221 | 0.335 | 0.563 | 0.88
(0.570–1.358) |
| MTA1 | −0.166 | 0.195 | 0.728 | 0.394 | 0.847
(0.578–1.241) |
Discussion
The occurrence, development and prognosis of ESCC
are the result of multiple factors, including genetics and
environment. Various genes that are associated with tumorigenesis,
invasion and metastasis have been identified and cloned. The
ESCC-associated genes include alcohol dehydrogenase, cytochrome
P450, family 1, member A1, IGF-1 and MTA1, which provide a
theoretical basis for improvements in the diagnosis, treatment and
prognosis of ESCC (16). The IGF
system includes IGF-1 and IGF-2, and their receptors IGF-1R and
IGF-2R. There are at least seven types of IGFBP (IGFBP1-7). IGF-1R
is the most active, and the combination of IGF-1R and IGF-1
promotes mitosis, cell transformation, anti-apoptosis, which are
insulin-like biological functions. The role of IGFBPs is to act as
the IGF carrier in the blood circulation, and IGFBP-3 predominates.
IGFBP-3 binds with >80% of the IGF-1 in the circulation, which
transports IGF-1 to the reaction site, and protects IGF-1 from
degradation by proteases. Thus, IGFBP-3 is important in the
regulation of the concentration of IGF-1 and inhibits or enhances
IGF-1 function. Therefore, IGFBP-3 and IGF-1R are key regulatory
aspects in the IGF signaling pathway (17).
IGFBP-3 may also interact with other proteins and,
thus, be important in the inhibition of proliferation and promotion
of apoptosis in various cells in a non-IGF-1-dependent manner,
therefore, IGFBP-3 exhibits a dual regulatory role in the IGF
family (18). IGFBP-3 translocates
into the nucleus and directly or indirectly interacts with the
intranuclear growth inhibition and apoptosis genes, affecting
cellular gene expression and inducing apoptosis (19). Overexpression of IGFBP-3 may
increase the cellular apoptosis (20,21),
suggesting that it may act as a tumor suppressor. Abnormal
methylation and gene silencing of the IGFBP-3 promoter has been
observed in different types of cancer, and its abnormal expression
or dysfunction has been associated with cancer development
(22–24).
It has been reported that the expression levels of
IGFBP-3 in lung cancer (25),
hepatocellular carcinoma (26),
ovarian cancer (27) and prostate
cancer (28) are reduced. Tas
et al (29,30) observed that the serum IGFBP-3
concentration did not predict the prognosis of breast and ovarian
cancer. Another previous study indicated that high expression of
IGFBP-3 is significantly associated with the recurrence of prostate
cancer (31). In addition, Kim
et al (32) investigated
191 cases of lung cancer and observed that IGFBP-3 was not
significantly correlated with the patients' clinicopathological
changes. Rohrmann et al (33) observed that low concentrations of
serum IGFBP-3 did not increase the risk of pancreatic cancer. These
contradictory studies may be due to the protective effect of
increased expression of IGFBP-3, which inhibits cell proliferation
and induces apoptosis. However, under different experimental
conditions, IGFBP-3 may stimulate cell proliferation in an IGF
signaling pathway-dependent or independent manner (34). In certain cases, IGFBP-3 exerts
positive effects toward cell growth (35). The expression levels of IGFBP-3 in
ESCC are uncertain. The present study uses the immunohistochemical
method to evaluate the IGFBP-3 expression in ESCC and its impact on
the prognosis of ESCC patients. The results demonstrate that
IGFBP-3 is expressed predominantly in the cytoplasm, and the
positive expression of IGFBP-3 in the ESCC tissue samples is
significantly lower than that in the adjacent tissue samples (27.4
vs. 40.6%; P<0.05). The low expression levels of IGFBP-3 in the
ESCC tissues may be due to IGFBP-3 as a downstream gene of p53, and
mutated p53 loses the ability to activate IGFBP-3 via its
transcriptional signaling pathway (36), thus the functions of IGFBP-3 that
inhibit tumor cell proliferation and apoptosis via the
IGF-1-dependent signaling pathway are blocked (37). This may also be associated with the
reduction of apoptosis induced by IGFBP-3 via the p53 signaling
pathway (8).
The present study also demonstrates that the
positive expression of IGFBP-3 in ESCC is associated with gender,
smoking status and family history of cancer, which is consistent
with esophageal cancer epidemiology. The expression of IGFBP-3 is
negatively correlated with the degree of ESCC differentiation and
lymph node metastasis, tumors of different grades (G1, G2 and G3).
The positive expression rates of IGFBP-3 were 51.4, 24.4 and 15.4%,
respectively, and the IGFBP-3 expression in patients with non-lymph
node metastasis was significantly higher than those with lymph node
metastasis (36.7 vs. 15.9%). This may be associated with the
anti-angiogenic and anti-metastatic roles of IGFBP-3 (38,39).
In the present study, IGFBP-3 staining was observed inside the
nuclei of ESCC cells, although it is markedly weaker compared with
in the cytoplasm. This may be due to IGFBP-3 expression inside the
nuclei directly or indirectly inducing the apoptosis of cancer
cells, thus inhibiting the tumor cell growth (40), however, this requires further
elucidation.
MTA1 is upregulated during tumor metastasis, as
observed by Toh et al in 1994 (41). The human MTA1 gene is located on
14q32.3 (42), with full-length
cDNA of 2,756 bp. The encoded protein has 703 amino acid residues,
and the product of this gene is a component of the nuclear
remodeling and deacetylation complex, which regulates gene
transcription by affecting the chromatin state (5). There is a low level of MTA1 in normal
body tissues, including the heart, kidney, lung, liver, while in a
variety of tumor tissues, such as from liver, lung and ovarian
cancer, it is highly expressed. Toh et al (6) observed that MTA1 expression in ESCC
is associated with the activity of H4 histone deacetylase. As tumor
suppressor genes, including p53, p21 and retinoblastoma, are
regulated by histone acetylation (43), the invasion and lymph node
metastasis of tumor cells that have high MTA1 mRNA expression
levels are significantly increased.
Results of the present study demonstrate that the
MTA1 protein is predominantly expressed in the nucleus. In ESCC
tissue samples, the MTA1 protein expression level was
signifi-cantly higher than in the control samples (42.1 vs. 11.2%;
P<0.05), while its expression was not associated with the
gender, age, smoking status or degree of tumor tissue
differentiation, while in the patients with no family history of
cancer the MTA1 expression is as high as 47.1%. Furthermore, MTAl
expression was positively correlated with tumor size, extent of
cancer tissue invasion and lymph node metastasis. In the patients
with tumors >3 cm, the percentage of patients that expressed
MTA1 was 46.8%, while in the patients with tumors ≤3 cm, the
percentage of patients expressing MTA1 was 25.6%. MTA1 expression
rates in different stages of invasive cancer (T1, T2 and T3) were
27.6, 35.1 and 49.5%, respectively. The expression of MTA1 in
patients with lymph node metastasis was significantly higher than
those without lymph node metastasis (51.1 vs. 34.9%; P<0.05).
This may be due to the involvement of MTA1 in changing the assembly
of the cyto-keratin filament system and location of cytoskeletal
proteins, thus increasing the cellular invasion and metastasis
(44). In the present study,
IGFBP-3 and MTA1 exhibited no interaction in the
clinicopathological features of ESCC. They were not identified to
be correlated with the prognosis of ESCC, and they were not
independent risk factors in the prognosis of ESCC; however, the
extent of tumor invasion and the rate of lymph node metastasis are
the independent risk factors in ESCC prognosis. No significant
correlation was identified between the protein expression of
IGFBP-3 and tumor size, positive expression of MTA1 and the degree
of tumor tissue differentiation, or expression of the two proteins
and prognosis. This may be affected by certain cases in the present
study coming from the ESCC-high-incidence region (Cixian, China).
The patients in this geographical area undergo regular screening
and earlier treatment due to the high prevalence of ESCC. Certain
cases were also from Beijing, China, which has a low incidence of
ESCC and a poorer screening program, thus, these patients usually
only seek medical help when clinical symptoms appear and their
staging tends to be higher.
The present study has certain limitations. First,
the samples are from different regions. A number of the cases were
obtained from a region with high-incidence of ESCC, which may have
affected the current study. Second, reverse
transcription-polymerase chain reaction or western blot analysis
were not performed to validate the immunohistochemical results,
these should be conducted in the future. Third, the follow-up
period was short and should be increased in future research.
In conclusion, low expression levels of IGFBP-3 may
be a risk factor of ESCC, and high expression levels of MTAl are
closely associated with the invasion and metastasis of ESCC. The
detection of IGFBP-3 and MTAl may have important clinical
implications for the diagnosis, treatment and prognosis of
ESCC.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suntharalingam M: Definitive
chemoradiation in the management of locally advanced esophageal
cancer. Semin Radiat Oncol. 17:22–28. 2007. View Article : Google Scholar
|
|
3
|
Rohatgi PR, Swisher SG, Correa AM, Wu TT,
Liao Z, Komaki R, Walsh G, Vaporciyan A, Lynch PM, Rice DC, et al:
Failure patterns correlate with the proportion of residual
carcinoma after preoperative chemoradiotherapy for carcinoma of the
esophagus. Cancer. 104:1349–1355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Fiore F, Lecleire S, Rigal O, Galais
MP, Ben Soussan E, David I, Paillot B, Jacob JH and Michel P:
Predictive factors of survival in patients treated with definitive
chemoradiotherapy for squamous cell esophageal carcinoma. World J
Gastroenterol. 12:4185–4190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicolson GL, Nawa A, Toh Y, Taniguchi S,
Nishimori K and Moustafa A: Tumor metastasis-associated human MTA1
gene and its MTA1 protein product: Role in epithelial cancer cell
invasion, proliferation and nuclear regulation. Clin Exp
Metastasis. 20:19–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toh Y, Ohga T, Endo K, Adachi E, Kusumoto
H, Haraguchi M, Okamura T and Nicolson GL: Expression of the
metastasis-associated MTA1 protein and its relationship to
deacetylation of the histone H4 in esophageal squamous cell
carcinomas. Int J Cancer. 110:362–367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holdaway IM, Mason BH, Lethaby AE, Singh
V, Harvey VJ, Thompson PI and Evans BD: Serum insulin-like growth
factor-I and insulin-like growth factor binding protein-3 following
chemotherapy for advanced breast cancer. ANZ J Surg. 73:905–908.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajah R, Valentinis B and Cohen P:
Insulin-like growth factor (IGF)-binding protein-3 induces
apoptosis and mediates the effects of transforming growth
factor-beta1 on programmed cell death through a p53- and
IGF-independent mechanism. J Biol Chem. 272:12181–12188. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hankinson SE, Willett WC, Colditz GA,
Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE and Pollak M:
Circulating concentrations of insulin-like growth factor I and risk
of breast cancer. Lancet. 351:1393–1396. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan JM, Stampfer MJ, Giovannucci E, Gann
PH, Ma J, Wilkinson P, Hennekens CH and Pollak M: Plasma
insulin-like growth factor-I and prostate cancer risk: A
prospective study. Science. 279:563–566. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Spitz MR, Mistry J, Gu J, Hong WK
and Wu X: Plasma levels of insulin-like growth factor-I and lung
cancer risk: A case-control study. J Natl Cancer Inst. 91:151–156.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma J, Pollak MN, Giovannucci E, Chan JM,
Tao Y, Hennekens CH and Stampfer MJ: Prospective study of
colorectal cancer risk in men and plasma levels of insulin-like
growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer
Inst. 91:620–625. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuang ST, Patton KT, Schafernak KT,
Papavero V, Lin F, Baxter RC, Teh BT and Yang XJ: Over expression
of insulin-like growth factor binding protein 3 in clear cell renal
cell carcinoma. J Urol. 179:445–449. 2008. View Article : Google Scholar
|
|
14
|
Balasenthil S, Broaddus RR and Kumar R:
Expression of metastasis-associated protein 1 (MTA1) in benign
endometrium and endometrial adenocarcinomas. Hum Pathol.
37:656–661. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rice TW, Blackstone EH and Rusch VW: 7th
edition of the AJCC cancer staging manual: Esophagus and
esophagogastric junction. Ann Surg Oncol. 17:1721–1724. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang HP, Liu JF, Rao J, Zhang XM, Qian HL,
Niu XQ and Zhao ZL: Insulin-like growth factor binding protein-3
(IGFBP-3) genetic variant and the risk of esophageal squamous cell
carcinoma in a Chinese population. Genet Mol Res. 13:4146–4153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwa V, Oh Y and Rosenfeld RG: The
insulin-like growth factor-binding protein (IGFBP) superfamily.
Endocr Rev. 20:761–787. 1999.PubMed/NCBI
|
|
18
|
Gui Y and Murphy LJ: Insulin-like growth
factor (IGF)-binding protein-3 (IGFBP-3) binds to fibronectin (FN):
Demonstration of IGF-I/IGFBP-3/fn ternary complexes in human
plasma. J Clin Endocrinol Metab. 86:2104–2110. 2001.PubMed/NCBI
|
|
19
|
Yamada PM and Lee KW: Perspectives in
mammalian IGFBP-3 biology: Local vs. systemic action. Am J Physiol
Cell Physiol. 296:C954–C976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gill ZP, Perks CM, Newcomb PV and Holly
JM: Insulin-like growth factor-binding protein (IGFBP-3)
predisposes breast cancer cells to programmed cell death in a
non-IGF-dependent manner. J Biol Chem. 272:25602–25607. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collard TJ, Guy M, Butt AJ, Perks CM,
Holly JM, Paraskeva C and Williams AC: Transcriptional upregulation
of the insulin-like growth factor binding protein IGFBP-3 by sodium
butyrate increases IGF-independent apoptosis in human colonic
adenoma-derived epithelial cells. Carcinogenesis. 24:393–401. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torng PL, Lee YC, Huang CY, Ye JH, Lin YS,
Chu YW, Huang SC, Cohen P, Wu CW and Lin CT: Insulin-like growth
factor binding protein-3 (IGFBP-3) acts as an invasion-metastasis
suppressor in ovarian endometrioid carcinoma. Oncogene.
27:2137–2147. 2008. View Article : Google Scholar
|
|
23
|
Torng PL, Lin CW, Chan MW, Yang HW, Huang
SC and Lin CT: Promoter methylation of IGFBP-3 and p53 expression
in ovarian endometrioid carcinoma. Mol Cancer. 8:1202009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang YS, Wang L, Liu D, Mao L, Hong WK,
Khuri FR and Lee HY: Correlation between insulin-like growth
factor-binding protein-3 promoter methylation and prognosis of
patients with stage I non-small cell lung cancer. Clin Cancer Res.
8:3669–3675. 2002.PubMed/NCBI
|
|
25
|
Wang Z, Wang Z, Liang Z, Liu J, Shi W, Bai
P, Lin X, Magaye R and Zhao J: Expression and clinical significance
of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues
from patients with non-small cell lung cancer. Onco Targets Ther.
6:1437–1444. 2013.PubMed/NCBI
|
|
26
|
Aishima S, Basaki Y, Oda Y, Kuroda Y,
Nishihara Y, Taguchi K, Taketomi A, Maehara Y, Hosoi F, Maruyama Y,
et al: High expression of insulin-like growth factor binding
protein-3 is correlated with lower portal invasion and better
prognosis in human hepatocellular carcinoma. Cancer Sci.
97:1182–1190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walker G, MacLeod K, Williams AR, Cameron
DA, Smyth JF and Langdon SP: Insulin-like growth factor binding
proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine
responsiveness in patients with ovarian cancer. Clin Cancer Res.
13:1438–1444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shariat SF, Lamb DJ, Kattan MW, Nguyen C,
Kim J, Beck J, Wheeler TM and Slawin KM: Association of
preoperative plasma levels of insulin-like growth factor I and
insulin-like growth factor binding proteins-2 and -3 with prostate
cancer invasion, progression, and metastasis. J Clin Oncol.
20:833–841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tas F, Karabulut S, Bilgin E, Tastekin D
and Duranyildiz D: Clinical significance of serum insulin-like
growth factor-1 (IGF-1) and insulin-like growth factor binding
protein-3 (IGFBP-3) in patients with breast cancer. Tumour Biol.
35:9303–9309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tas F, Karabulut S, Serilmez M, Ciftci R
and Duranyildiz D: Clinical significance of serum insulin-like
growth factor-1 (IGF-1) and insulin like growth factor binding
protein-3 (IGFBP-3) in patients with epithelial ovarian cancer.
Tumour Biol. 35:3125–3132. 2014. View Article : Google Scholar
|
|
31
|
Seligson DB, Yu H, Tze S, Said J, Pantuck
AJ, Cohen P and Lee KW: IGFBP-3 nuclear localization predicts human
prostate cancer recurrence. Horm Cancer. 4:12–23. 2013. View Article : Google Scholar :
|
|
32
|
Kim YH, Sumiyoshi S, Hashimoto S, Masago
K, Togashi Y, Sakamori Y, Okuda C, Mio T and Mishima M: Expressions
of insulin-like growth factor receptor-1 and insulin-like growth
factor binding protein 3 in advanced non-small-cell lung cancer.
Clin Lung Cancer. 13:385–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rohrmann S, Grote VA, Becker S, Rinaldi S,
Tjønneland A, Roswall N, Grønbæk H, Overvad K, Boutron-Ruault MC,
Clavel-Chapelon F, et al: Concentrations of IGF-I and IGFBP-3 and
pancreatic cancer risk in the European prospective investigation
into cancer and nutrition. Br J Cancer. 106:1004–1010. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Firth SM and Baxter RC: Cellular actions
of the insulin-like growth factor binding proteins. Endocr Rev.
23:824–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grimberg A: Mechanisms by which IGF-I may
promote cancer. Cancer Biol Ther. 2:630–635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buckbinder L, Talbott R, Velasco-Miguel S,
Takenaka I, Faha B, Seizinger BR and Kley N: Induction of the
growth inhibitor IGF-binding protein 3 by p53. Nature. 377:646–649.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Binoux M: Insulin-like growth factor
binding proteins (IGFBPs): Physiological and clinical implications.
J Pediatr Endocrinol Metab. 9(Suppl 3): S285–S288. 1996.
|
|
38
|
Mehta HH, Gao Q, Galet C, Paharkova V, Wan
J, Said J, Sohn JJ, Lawson G, Cohen P, Cobb LJ and Lee KW: IGFBP-3
is a metastasis suppression gene in prostate cancer. Cancer Res.
71:5154–5163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao L, He L, Zhang R, Cai MY, Liao YJ,
Qian D, Xi M, Zeng YX, Xie D and Liu MZ: Low expression of IGFBP-3
predicts poor prognosis in patients with esophageal squamous cell
carcinoma. Med Oncol. 29:2669–2676. 2012. View Article : Google Scholar
|
|
40
|
Jenkins PJ, Khalaf S, Ogunkolade W,
McCarthy K, David T, Hands RE, Davies D and Bustin SA: Differential
expression of IGF-binding protein-3 in normal and malignant colon
and its influence on apoptosis. Endocr Relat Cancer. 12:891–901.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toh Y, Pencil SD and Nicolson GL: A novel
candidate metastasis-associated gene, mta1, differentially
expressed in highly metastatic mammary adenocarcinoma cell lines.
cDNA cloning, expression, and protein analyses. J Biol Chem.
269:22958–22963. 1994.PubMed/NCBI
|
|
42
|
Cui Q, Takiguchi S, Matsusue K, Toh Y and
Yoshida MA: Assignment of the human metastasis-associated gene 1
(MTA1) to human chromosome band 14q32.3 by fluorescence in situ
hybridization. Cytogenet Cell Genet. 93:139–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roy S, Packman K, Jeffrey R and Tenniswood
M: Histone deacetylase inhibitors differentially stabilize
acetylated p53 and induce cell cycle arrest or apoptosis in
prostate cancer cells. Cell Death Differ. 12:482–491. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hofer MD, Menke A, Genze F, Gierschik P
and Giehl K: Expression of MTA1 promotes motility and invasiveness
of PANC-1 pancreatic carcinoma cells. Br J Cancer. 90:455–462.
2004. View Article : Google Scholar : PubMed/NCBI
|